Abstract
Microfluidics can be considered both a science and a technology. It is defined as the study of fluid behavior at a sub-microliter level and the investigation into its application to cell biology, chemistry, genetics, molecular biology and medicine. There are at least two characteristics of microfluidics, mechanical and biochemical, which can be influential in the field of mammalian gamete and preimplantation embryo biology. These microfluidic characteristics can assist in basic biological studies on sperm, oocyte and preimplantation embryo structure, function and environment. The mechanical and biochemical characteristics of microfluidics may also have practical and/or technical application(s) to assisted reproductive technologies (ART) in rodents, domestic species, endangered species and humans. This review will consider data in mammals, and when available humans, addressing the potential application(s) of microfluidics to assisted reproduction. There are numerous sequential steps in the clinical assisted reproductive laboratory process that work, yet could be improved. Cause and effect relations of procedural inefficiencies can be difficult to identify and/or remedy. Data will be presented that consider microfluidic applications to sperm isolation, oocyte cumulus complex isolation, oocyte denuding, oocyte mechanical manipulation, conventional insemination, intracytoplasmic sperm injection, embryo culture, embryo analysis and oocyte and embryo cryopreservation. While these studies have progressed in animal models, data with human gametes and embryos are significantly lacking. These data from clinical trials are requisite for making future evidence-based decisions regarding the application of microfluidics in human ART.
Keywords: fertilization, oocyte, embryo development, spermatozoa, microfluidics
Microfluidics: definitions, history and benefits
Microfluidics can be considered both a science and a technology. Scientifically, it is the study of fluid behavior at a sub-microliter level. Technologically, it relates to applications within analytics and diagnostics, cell biology, to single cell genomics (Whitesides, 2006). Microfluidics broadly represents a multi- and trans-disciplinary field of study incorporating engineering, physics, chemistry, biology and biotechnology whereby systems can be designed for practical applications with low fluid volumes used to multiplex, automate, integrate and facilitate cell manipulation and/or high-throughput analysis/screening (Volpatti and Yetisen, 2014). Historically, the field of microfluidics is relatively young. In the 1950s and 1960s, significant efforts focused on design and use of fluidic circuits for defense applications with the premise that such circuits would outlast destructive influences of the electromagnetic pulse of a nuclear explosion. However, miniaturization of fluidic devices proved problematic in the face of mounting success in electronic circuit miniaturization. An early insightful opinion on miniaturization, including suggestions of mini-machines for biological applications, was presented by Professor Richard P. Feynman (Nobel Laureate in Physics, 1965) at the American Physical Society meeting at Caltech (Feynman, 1960), entitled ‘There's Plenty of Room at the Bottom’. In the 1970s through 1990s pioneering work was performed and reported in micro-total analysis systems (μ-TAS) for chemistry, micro-electro-mechanical-systems for electronics and lab-on-a-chip (LOC) designs for chemical, molecular and cellular manipulations and formed the foundation for Bio-Microfluidics (Brody et al., 1996). In the late 1990s to early 2000s two pioneers in Microfluidics, Drs George Whitesides and Steven Quake, made bedrock and long-lasting contributions in use of soft lithography with the transparent elastomer poly-dimethyl siloxane (PDMS; Duffy et al., 1998) and the use of soft elastomeric materials to produce multi-layered devices with miniaturized values, channels and pumps (Unger et al., 2000).
Following completion of the Human Genome Project, a focus of using microfluidics for faster sequencing began. Hundreds of publications have dissected and investigated the use of microfluidics for components of single cell isolation and sample preparation, electrophoresis, DNA analysis, RNA analysis and epigenome analysis (Paegel et al., 2003; Matsuoka et al., 2013; Bose et al., 2015). The application of microfluidics for genomic, transcriptomic and epigenomic analysis appears well suited for this technology and has supported numerous start-up companies over the last decade. Considering the past, present and future application of microfluidics for gamete and embryo isolation, manipulation, culture and analysis within human assisted reproduction, there are at least two theoretical advantages broadly categorized as (i) mechanical and (ii) biochemical. From an impartial, experimental point-of-view these can actually be advantageous, equivalent or disadvantageous. From a practical perspective, our goals are to identify advantageous, enhanced and/or improved methods, technologies and outcomes in laboratory clinical assisted reproduction. For this reason, we will maintain the ‘advantageous’ perspective. Mechanical advantages can be defined as using characteristics of fluid dynamics at a microscale to enhance cell isolation, manipulation and analysis. Biochemical advantages can be defined as using microscale fluid control to physically regulate the in vitro biochemical environment for cell manipulation, expansion, growth and analysis. Many permutations of mechanical and/or biochemical influences, and advantageous/equivalent/disadvantageous results may exist within a single application and deciphering beneficial or detrimental outcomes and regulatory mechanism can be difficult to elucidate. However, as is the case in all areas of science, solid repeatable data will at the end of the day dictate the clinical applications of microfluidics in human assisted reproductive technologies (ART).
Current needs assessment of human clinical ART
Over the last 3–4 decades, significant improvements in human ART outcomes have been realized. Laboratory technologies, equipment, medium and the collective environments have changed over time and have positively influenced each step in human IVF, embryo culture and analysis, and embryo cryopreservation processes. As procedures move toward elective single embryo transfer (eSET), with a goal of high pregnancy rate with minimal detrimental impact of multiple implantations within a single gestation on offspring health, the efficiency of each individual laboratory step in an IVF cycle becomes increasingly important. This movement toward eSET is gaining acceptance worldwide, and while currently not universal, it will ultimately be beneficial for patients, offspring and the medical field (Dyer et al., 2016). As in the past, the collective success of a single healthy offspring from a single IVF cycle is influenced by the efficiency of each process step. Currently, none of the steps are 100% efficient, and the collective attrition reduces the overall success. Therefore, each step of the laboratory IVF and cryopreservation cycle should be evaluated for ways of improving efficiency. In addition, the potential to integrate numerous laboratory steps of an IVF cycle into a single automated procedure may be advantageous in reducing gamete/embryo handling, decreasing stresses induced through gamete/embryo manipulation and removing human subjectivity and variability in assisted reproduction. Finally, integration of an automated IVF-LOC with real-time non-invasive measures of embryo competency (morphometrics, metabolomics, secretomics, etc.) may ultimately improve the entire process and support the most efficient step forward in eSET and pregnancy establishment. This goal is not minor, nor is the pathway to accomplish this goal simple. Experiments take time, and should be designed and applied to yield interpretable data, preferably in animal models prior to testing with human gametes and embryos. As scientists, laboratorians and clinicians move toward this goal, and investigate the potential of microfluidics as an enabling technology, we must balance enthusiasm with data and practice evidence-based decision making.
Current state-of-the-art of microfluidics for non-human mammalian and human gametes and embryos
Gamete isolation and manipulation with microfluidics
Sperm
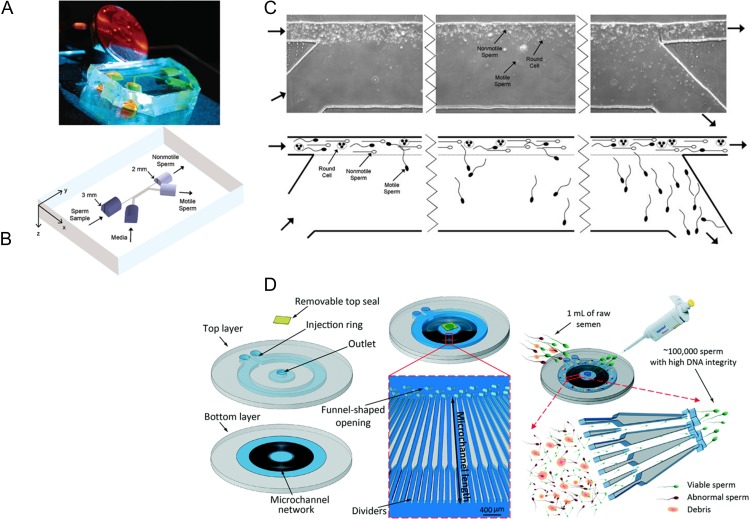
In human IVF, one goal is the isolation of sperm of sufficient quality and quantity to facilitate fertilization by either conventional insemination (IVF/CI) or ICSI (IVF/ICSI). Depending on semen characteristics (semen volume, sperm concentration, sperm motility, sperm viability, sperm morphology and presence/concentration of non-gamete cells), a laboratorian will need to make a decision relating to method of sperm processing or isolation. The objective is to remove seminal plasma from sperm, maintain and/or enrich the sperm population for motility and inseminate with numerous (IVF/CI) or one (IVF/ICSI) motile sperm with normal morphology. Classically, sperm processing for IVF has been performed with simple media washing, semen overlay with medium and swim-up of sperm out of the seminal plasma, density gradient centrifugation or a combination of above methods (WHO, 2010). In the early- to mid-1990s, Kricka et al. (1993, 1997) made and tested silicon/glass microfluidic devices as diagnostic tools for evaluating sperm motility. These investigators assessed sperm progression through branching microchannels with a suggestion that such a microfluidic device could displace conventional methods of motility testing in clinical semen analyses. The first reports of using microfluidics for human semen processing and motile sperm isolation with potential therapeutic utility occurred in 2003 (Cho et al., 2003; Schuster et al., 2003). In these initial reports, it was theorized that one could use the fluid mechanical characteristics of microfluidics for sorting motile sperm from seminal plasma, non-motile sperm and non-motile non-gamete cellular debris. The microfluidic device designed and tested relied on parallel laminar flow streams (one being semen and the other being media) present at the microscale. Flow within microchannels was generated and maintained by a gravity driven, passive, horizontally oriented pumping mechanism developed specifically for the device (Cho et al., 2003). The two parallel laminar flow streams had minimal mixing by diffusion and provided an environment where motile sperm could actively swim across the slight meniscus generated by laminar flow of the two liquid streams for collection downstream. On the other hand, seminal plasma, dead sperm and debris remained in the initial stream of flow and exited the device as waste. Microfluidic processing of human semen significantly enriched for motility (44% motility of unprocessed sperm versus 98% motility after microfluidic processing) and normal morphology. In addition, experiments were performed in which human semen was spiked with large concentrations of round, non-motile, non-gamete human cells to demonstrate the microfluidic device could isolate motile sperm from very poor quality semen samples. These data represented proof-of-principle, with human samples, that microfluidics could be used to isolate motile sperm for therapeutic application (Fig. 1). As one can imagine, the width of each channel, and the length of the parallel channels, can be changed and resulting motile sperm isolation will also change. These types of alterations, in addition to others such as distally sequential isolation channels, can provide means of scientifically evaluating sub-populations of motility within a single sample, influence of semen/sperm treatments and modified motility, and potential chemotaxis molecules and sperm motility (Kaupp et al., 2006; Strunker et al., 2011).
Figure 1.
(A) A photograph of a microfluidic sperm sorter. (B) A three-dimensional schematic of a 2-inset microchannel, 2-outlet microfluidic microchannel device with horizontally oriented fluid reservoirs that result in passively driven flow. Semen is placed in one inlet (arrow pointing into microchannel) and media placed into the other inlet. After sorting, seminal plasma, debris and non-motile sperm exit in their initial stream of flow, while motile sperm in media is collected in an outlet reservoir. (C) Micrographs and schematics illustrating the movement of semen, sperm, debris and media during sorting. Adapted from Cho et al. (2003) and Schuster et al. (2003). (D) Isolation of motile sperm with reduced DNA damage based on microfluidics and microchannels containing viscoelastic media in an attempt to recapitulate in vivo condition of the female reproductive tract. Adapted from Nosrati et al. (2014).
However, from a practical perspective, one has to ask why use microfluidics to isolate human sperm for therapeutic interventions? Currently, semen processing, such as swim-up, simple washing with centrifugation, and density gradient with centrifugation, all provide efficient means of sperm isolation for IVF/CI and/or IVF/ICSI. However, centrifugation has been reported to cause sub-lethal damage to sperm (Alvarez et al., 1993). Experimental evidence suggests that sperm DNA is exposed to high levels of reactive oxygen species during centrifugation processing (Aitken and Clarkson, 1988; Hughes et al., 1998), and this oxidative stress was positively correlated with sperm DNA damage (Barroso et al., 2000). Sperm DNA damage has been negatively correlated with sperm motility (Irvine et al., 2000). Yet, sperm motility is not always a reliable index of sperm DNA integrity as mild oxidative conditions can cause significant effect on sperm DNA fragmentation with little effect on the percentage of motile sperm (Zini et al., 2000). This results in isolation of motile sperm for IVF/CI or IVF/ICSI that can be used for insemination and fertilization that have DNA damage and will not result in embryos with developmental potential/implantation/viable offspring development. In current clinical ART laboratory work, this DNA damage is not recognizable in living sperm prior to insemination, and may contribute to developmental inefficiencies of embryos, implantation and viable offspring. However, proof-of-concept reports exist showing potential use of Raman microspectroscopy to visualize damaged sperm DNA (Mallidis et al., 2011; Sanchez et al., 2012). Although the consequences of using sperm with fragmented DNA in human ART are not completely understood, sperm DNA damage has been reported to have a negative influence on fertilization rates (Benchaib et al., 2003; Payne et al., 2005), embryo quality (Seli et al., 2004) and pregnancy rates (Virroet et al., 2004; Zini et al., 2005) during IVF. For these reasons, we postulated that passive microfluidic sperm sorting without centrifugation would yield sperm with less DNA damage and may have clinical utility in the future. Using human semen samples, it was demonstrated that microfluidic semen processing could provide sperm with high motility, enhanced percent normal morphology and significantly reduced percentage of sperm with DNA damage in comparison with simple wash and density gradient centrifugation (Schulte et al., 2007). These findings recently have been confirmed and expanded in elegant studies by Shirota et al. (2016). One of the major limitations to the early work and potential clinical application of the microfluidics sperm sorter was the sub-milliliter volumes that were processed. Normal human semen volumes are typically greater than 1.5 ml, with 50% of samples greater than 3.7 ml (WHO, 2010). Recently, a clinically applicable microfluidic device was described that isolates sperm based on the progressive motility in 500 parallel microchannels and represents a one-step procedure for sperm selection with high motility and high DNA integrity sperm (Nosrati et al., 2014) (Fig. 1). This device could process up to 1 ml of semen. These types of device improvements and multi-scaling are positive steps forward and will yield microfluidic devices that are more likely to find clinical application in the future. In the last decade, other microfluidic applications for important studies on sperm biology, function and genetics at a fundamental science level have been reported and reviewed (Knowlton et al., 2015).
Oocytes
The clinical ART laboratory task of oocyte collection is inherently a combination of macro- and micro-processing using needles, tubing, vacuum, test tubes, petri-dishes and microscopic observations to search follicular aspirates and isolate individual oocyte cumulus masses. Once oocyte cumulus complexes are isolated, the amount of cumulus cells can be left as is for IVF/CI, or a portion of the cumulus can be mechanically removed. This partial removal of cumulus prior to IVF/CI has questionable benefit, yet is likely not detrimental. The maintenance of some cumulus surrounding the oocyte is beneficial for optimal fertilization in IVF/CI. Thus, oocyte isolation and manipulation in IVF/CI works well and would not likely benefit from microfluidic applications. In IVF/ICSI, there is a requirement of removing the majority of cumulus cells to allow determination of oocyte maturity, positioning of the polar body in relation to sperm injection, and the process of injecting sperm in IVF/ICSI. Classically, these cumulus cells are removed with a combination of enzymatic treatment and mechanical pipetting (Van de Velde et al., 1997). For the last two decades, this method of cumulus cell removal was been used successfully in human IVF/ICSI and is not considered a significant problem in fertilization or subsequent embryo development success. However, it was demonstrated that microfluidics could be used for mechanical cumulus cell removal of bovine oocyte cumulus complexes without enzyme exposure (Zeringue and Beebe, 2004). Microfluidic human oocyte cumulus cell removal, or its benefit, has not been reported.
It has been demonstrated that microfluidics can be used to trap and squeeze murine oocytes (Luo et al., 2015). While this provides a technical ability for study of oocyte structure and function, its application to clinical human ART is of question. One must also consider the potential detriment of oocyte manipulation. However, recently it was reported that non-microfluidic mechanical assessment of murine oocytes and zygotes, as well as cryopreserved human zygotes, viscoelasticity properties could nondestructively provide information about embryo development (Yanez et al., 2016). These two technologies might merge in the future and could provide insightful non-invasive information on oocyte/zygote/embryo developmental potential in the human ART laboratory.
IVF/CI with microfluidics
Conventional insemination
Macroscopic placement of sperm in a constricted volume (10 μl–1 ml) of media with single or numerous oocyte cumulus masses is a long-standing method of human IVF/CI. While this approach has been tweaked and refined over the last 3–4 decades, the principles have remained. Contemporary human IVF/CI fertilization rates vary greatly, from 50 to 70% (Bhattacharya et al., 2001; Foong et al., 2006) and a ‘true fertilization rate’ following IVF/CI is unknown due to the presence of cumulus cells and inability to fully determine maturity of the oocyte within the cumulus mass. Since immature oocytes can be penetrated by sperm at the time of insemination, lack ability to form pronuclei or developmentally competent embryos, and yet still can progress to metaphase II (Van Blerkom et al., 1994), this interferes with a calculation of ‘true fertilization’. Thus, the inefficiencies of human oocyte IVF/CI and fertilization can be difficult to quantify. Microfluidics for IVF/CI in the porcine model (Clark et al., 2005) was proposed to limit of incidence of polyspermic fertilization, a significant limitation in porcine in vitro production of embryos. It was demonstrated that this microfluidic device resulted in significantly more monospermic fertilization compared with traditional microdrop insemination. However, in contemporary human IVF/CI the incidence of polyspermic fertilization is very low (typically 2–7%; Aoki et al., 2005; Xia, 2013); therefore, use of microfluidic IVF/CI to circumvent polyspermic fertilization in human IVF may not be justified. It was also demonstrated in the murine model that microfluidic insemination could significantly enhance fertilization rates compared with traditional insemination at low sperm concentrations (0.01–0.08 × 106 sperm/ml) (Suh et al., 2006). In human IVF, if sperm concentrations are low a decision will be made to inseminate by ICSI, thus overcoming this issue of low sperm concentration and compromised fertilization. Yet, with ICSI there is a risk of oocyte lysis, or degeneration, due to the mechanical invasiveness of inserting a glass needle into the oocyte. Typically, an experienced ICSI technician can expect an average of 7% of injected oocytes to lyse or degenerate (Rosen et al., 2006). If microfluidic human IVF/CI could be refined to provide high fertilization rates at low sperm concentrations, this could reduce the need for ICSI in many human IVF cases, and consequently, reduce the incidence of ICSI-associated oocyte degeneration in these cases. Whether such studies will be performed, or will lead to a conversion of ICSI to microfluidic IVF/CI cycles, might be highly questionable because current use of ICSI in non-male factor cases is on the rise (Dyer et al., 2016), and many programs use ICSI quite liberally, even with the 2012 American Society of Reproductive Medicine Committee Opinion article that concluded there were no data to support the routine used of ICSI for non-male factor infertility (Practice Committees of the American Society for Reproductive and Society for Assisted Reproductive, 2012).
Microfluidic IVF/CI in animal model studies has also emerged as a step in the process of ‘LOC’ IVF. A sophisticated microfluidic device was used for trapping (spatially maintaining) mouse oocytes, motile sperm isolation, microinsemination and embryo development for 96 h (Han et al., 2010). This report demonstrated that all these steps in the mouse IVF process could be performed on a single device. However, no significant improvements were reported compared with the non-microfluidic steps. These types of studies set the stage for future use in human ART where integration and automation systems of IVF will need to be evaluated. Recently work toward evaluating the use of microfluidics for ICSI (Matsuura et al., 2013) have been reported. Working with porcine ICSI, Matsuura and colleagues demonstrated that microfluidics could be used for the ICSI process and this reduced time for procedure completion. These proof-of-concept studies in animal models are the beginning. While human ICSI is widely used, and highly successful, it does entail multiple highly technical steps that are subject to human error or individual technician variability. In addition, contemporary human ICSI can be expensive and a barrier to wide-spread use in infertility treatment. If integration of microfluidic IVF/CI or ICSI can reduce laboratory human error or variability, and/or reduce the cost, while maintaining similar or improved results, then these microfluidic applications may find use in the future. With that said, these microfluidic IVF/CI or ICSI methods and devices themselves will need to show more consistency, great or equivalent outcome success, must not be cost prohibitive, and technical staff will need to embrace their use. All of the above will take time, rigorous studies, and personal flexibility to achieve.
Embryo culture with microfluidics
The preimplantation embryo, whether it is rodent, domestic species, non-human primate or human, develops in a moving environment within the oviduct (fallopian tube) and uterus. This has been termed a dynamic environment, with dynamic used as an adjective meaning ‘characterized by constant change’. This is in contrast to how preimplantation embryos are grown in the laboratory. In relation of embryo culture, microfluidics can provide at least three unique characteristics that can be tested for equivalence, benefit or detriment: (i) microenvironment, (ii) dynamic fluid environment and/or (iii) dynamic chemical environment.
The cell microenvironment is composed of individual and interactive factors that consequently influence the conditions surrounding the preimplantation embryo and directly or indirectly impact embryo growth, structure or function through biophysical or biochemical means. One of the first reports on regulation of embryo microenvironment was the work using agar encapsulation of ovine embryos (Willadsen, 1979). Microfluidics was first applied to regulation and testing of microenvironment on mouse embryos within no or minimal fluid flow in microchannels (Raty et al., 2004). It was demonstrated that mouse embryos culture in microchannels displayed faster cleavage rates, yielded more blastocysts, and had reduced embryo degeneration compared with control microdrop culture. However, when media flow was applied to this microchannel embryo culture platform (flow rates of 0.1 and 0.5 ml/h) this did not improve embryo development and was reported to be detrimental to mouse embryo growth and resulted in a high incidence of abnormal embryos (Hickman et al., 2002). From a practical perspective of future culture of human embryos in microchannels, there is/was a concern of retrieval of cultured embryos from microchannels within a microfluidic device. In addition, this detrimental influence of fluid flow on embryo development was interesting, seems counterintuitive to the in vivo situation and leads to queries of ways to precisely control fluid movement in a microfluidic device.
In 2004, Dr Takayama and colleagues reported on a computer-controlled refreshable Braille display platform using vertically moving pins to facilitate integrated pumping of channel-contained fluids through localized deformation of channels made of elastic compounds (Gu et al., 2004). This provided a means to precisely regulate fluid flow in microchannels, with computer programming, that could be applied to embryo culture platforms. Inherent in this platform was the need for thin elastomeric compounds that could be deformed by Braille pins. This lead to studies of culturing mouse embryos in microchannels, with thin elastomeric bottoms, that would be amendable the Braille pin actuator and controlled fluid flow. In these studies, it became apparent that evaporation was problematic when handling sub-microliter volumes of media in thin flexible elastomer, PDMS, even in a humidified environment (Heo et al., 2007). This media evaporation resulted in significant osmolality shifts that were detrimental to mouse embryo development. In essence, we had discovered a new way to cause embryo demise, a result we neither desired nor embraced. Experimental and mathematical studies were then performed to measure and predict evaporation and osmolality shifts. Interestingly, we made an observation of an osmolality change (~15 mmol/kg) could not be explained by evaporation of water through PDMS, and that this evaporation appeared to be happening during initial handling of media from its macroscopic media vessel to a microdrop or well prior to covering with oil. As a side note, this observation led to non-microfluidic experiments that assessed ways in which research and clinical embryologists prepare oocyte and embryo culture media wells and microdrops, and how different environmental and technical activities can influence media osmolality and embryo development (Swain et al., 2012). This report provides some very practical consideration in media preparations for human clinical ART, independent of microfluidics.
To maintain a thin/flexible membrane to enable Braille pin actuation, yet circumvent evaporation and osmolality shifts that were not compatible with embryo viability, a potential solution with tested whereby a PDMS-parylene-PDMS hybrid membrane was developed (Heo et al., 2007). This hybrid membrane greatly reduced evaporation and media osmolality changes, maintained flexibility needed to interface with the Braille deformation-based microfluidic actuation system, with necessary clarity for microscopic optical clarity, and enabled successful development of mouse embryos. This is one of many examples of the multiple levels of hurdles encountered in investigating novel systems for embryo culture.
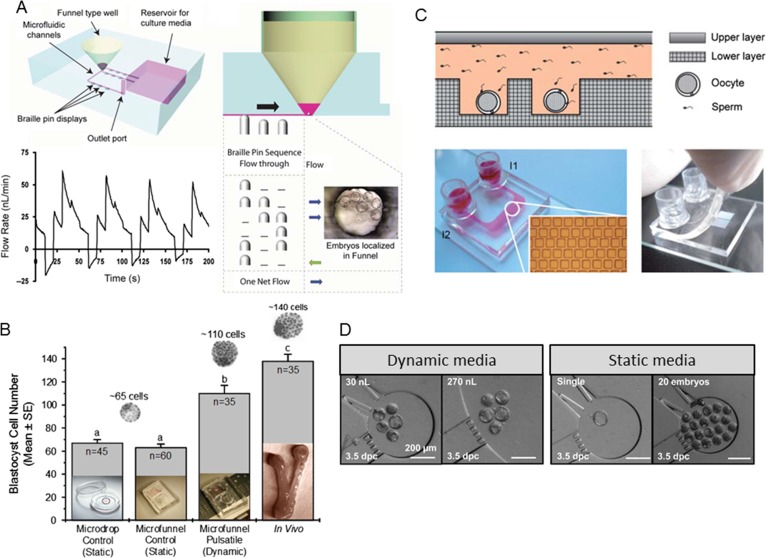
With a means of precisely regulating fluid flow that was programmable and compatible with culturing embryos in an incubator, and a partial chip design that did not result in embryo demise, we then focused on microfluidic chip architecture that would allow dynamic fluid culture of embryos without placement of embryos in microchannels. From practical experience, it was found that when oocytes/zygotes/embryos were placed into microchannels and allowed to travel by passive capillary flow to a point of movement impedance, that the retrieval of said cells was not 100%. Recognizing that this would not be acceptable in clinical assisted reproduction, efforts were focused on integration of a microfunnel for media and embryo placement into a PDMS cartridge with microchannel for media movement into and out of the bottom of the microfunnel. When placed on the Braille pin actuator, the sequential computer-programed movement of pins could continually and precisely provided periodic pulses of media at physiological frequencies of ~0.1 Hz, and an average flow rate of 17.9 nl/min (Heo et al., 2010; Fig. 2). Culture of mouse embryos in this microfluidic dynamic fluid system, and comparison with static microdrop and microfunnel culture controls, resulted in faster rates of preimplantation embryo development, development of blastocysts with more cells, and significantly improved implantation. In addition, an in silico modelling experiment was performed to begin explaining potential mechanisms of benefit of culturing in a microfunnel with dynamic media flow compared with microchannels with gravity flow (detrimental to embryo development; Hickman et al., 2002).
Figure 2.
(A) Schematic drawing of a microfunnel cartridge used for culturing mouse embryos. The cartridge is placed on an array of piezoelectric pins with actuation provided by Braille pins. Embryos are placed into media, overlaid with oil, within microfunnels under the flow-through condition created by the pin actuation sequence. The flow pattern generated over four cycles of the five-step Braille pin actuation sequence at 0.1 Hz. (B) Dynamic fluid condition (microfunnel-pulsatile) shows a greater number of cells per blastocyst compared with static culture (in either media microdrops under oil or media in microfunnels under oil with no flow) within the same amount of culture time, with results closer to in vivo conditions (when flushed out of the uterus at a time corresponding to culture length; a,b,c = P < 0.01). Adapted from Heo et al. (2010). (C) Illustration of the cross-section of the double-layer microfluidic device used for culturing mouse embryos. A view of the microfluidic device fixed on a 76.2 mm × 25.4 mm microscopic slide, as well as the microwell array micrograph. The microchannel and the two reservoirs were filled with red dye. Scale bar is 500 mm. Finally, a photograph of the microfluidic device with the upper layer lifted. Adapted from Han et al. (2010). (D) Micrographs of mouse embryos cultured in nL chambers under various conditions. Adapted from Esteves et al. (2013).
Han et al. (2010) also reported a microwell structure microfluidic device that performed multiple steps of the IVF process. Using mouse embryos they demonstrated that this microfluidic cartridge could trap oocytes in microwells, integrate insemination and fertilization and support embryo development to the blastocyst stage. However, in assessing preimplantation embryo development this microfluidic system did not improve the rate of embryo development, stage of embryo development over set times, or number of cells per blastocyst, compared with controls. Similarly, a microfluidic platform allowing precise nanoliter culture of mouse embryos supported embryo development to the blastocyst stage, without significant improvement, yet importantly provided ability to dissect physical requirement of single and group culture (Melin et al., 2009). Esteves et al. (2013) also reported that mouse preimplantation embryos could be cultured in microfluidics and studied the impact of fluid flow, embryo density and media volume. Collectively, these experiments using mouse embryos document that microfluidics can be used for preimplantation embryo culture, they provide experimental power in precise regulation of culture environment and informative data on embryo development, and provided benefits for embryo development. There have been numerous reviews discussing the future of microfluidics for domestic animal and human assisted reproduction (Krisher and Wheeler, 2010; Meseguer et al., 2012; Swain et al., 2013). Kieslinger et al. (2015) have demonstrated that human embryos can be grown in microfluidic devices, yet to our knowledge, no peer-reviewed published manuscript currently exist demonstrating a benefit of microfluidic embryo culture with human embryos. These studies are wanting and will be necessary to support the use of microfluidics in the future for human assisted reproduction.
Embryo analysis and selection with microfluidics
Over the last 3–4 decades of human laboratory assisted reproduction, little has changed in embryo evaluation and selection for transfer, with the exceptions of recent time-lapse micro-videography of morphometrics and morphokinetics (Wong et al., 2010; Kirkegaard et al., 2012, 2013; Paternot et al., 2013; Ziebe, 2013; Molina et al., 2014; Basile et al., 2015) and preimplantation genetic screening for aneuploidy (Lyet al., 2011; Franasiak et al., 2014). Classically, embryo analysis and selection has been performed manually with microscopic observations of cleavage rates, embryo cell number (in relation to time of development), degree of cellular fragmentation, blastocyst formation, stage of blastocyst development (early, full, expanding, expanded or hatching) and/or cellular contributions of the blastocyst inner cell mass and trophectoderm. These manual microscopic observations can be subjective, lack predictive value and represent within and between laboratory inconsistencies (Alpha Scientists in Reproductive and Embryology, 2011). Without suggesting that microscopic observations of embryo development lack informative power, or that we should halt these observations, a long-standing goal has been to identify biochemical indicators of embryo developmental competence and implantation potential. The ability to gain informative data on embryo morphology, morphokinetics, genetic normalcy and biochemical indicators of embryo health would likely lead to selection of embryos with the greatest developmental competence, implantation potential, and facilitate the most efficient means of obtaining a single healthy offspring from an eSET.
To enable biochemical non-invasive analysis and selection of embryos, one needs both biomarkers of embryo health and a means to measure such bio-molecules. Over time significant data have accumulated for embryo biomarker measurements; a non-exhaustive list includes: (i) oxygen update (O'Donovan et al., 2006), (ii) amino acid turnover (Houghton et al., 2002; Brison et al., 2004) and (iii) energy metabolism (Gardner et al., 2001). In relation to energy metabolism, it has been demonstrated that non-invasive means of measuring glucose consumption from media and lactate production and release into media, as an estimate of embryo glycolytic activity, can be useful in selection of human embryos. To date, there have been at least three reports using microfluidics as an enabling platform to perform non-invasive measures of embryo biomarkers. O'Donovan et al. (2006) developed and tested a respirometric microfluidic cartridge to monitor oxygen consumption of 2-cell and blastocyst stage mouse embryos. This microfluidic chip incorporated embryo loading with capillary passive fluid flow, embryo trapping, non-invasive oxygen sensing and could reproducibly measure oxygen consumption by monitoring 10 preimplantation embryos over a 1-h period. While these data represent a significant step forward in on-chip assessment of embryo metabolism, the use of passive capillary loading of embryos, and group assessment, needs to be considered as barriers to practical implementation to clinical human embryo analysis and selection.
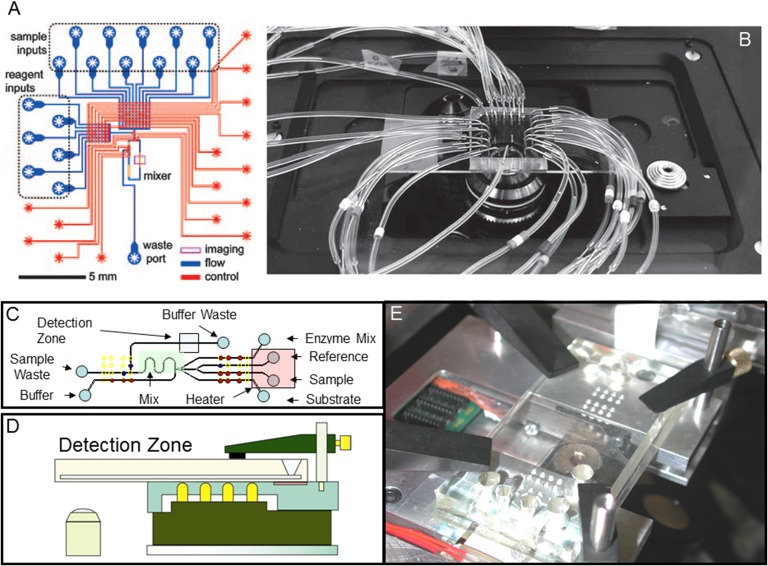
Urbanski et al. (2008) performed proof-of-concept experiments to demonstrate the use of microfluidics to measure embryo metabolism. They designed and tested a microfluidic chip that performed automated metabolic assays to measure glucose, pyruvate and lactate from sub-microliter volumes. This microfluidic assay system allowed on-chip sample and enzyme aliquotting, reagent mixing, data collection and analysis in an automated fashion without operator involvement (Fig. 3). The microfluidic device performed preimplantation embryo metabolism measures by injecting embryo culture media supernatant into the system. Thus, this microfluidic device functioned as a stand-alone device, without embryo culture on-chip and required periodic manual placement of spent embryo culture media from a separated embryo culture well. This statement is not intended to be critical, yet emphasizes the step-wise way in which new microfluidic platforms and assays are developed, tested, and the future requirements for non-simplistic integration which is needed for practical application.
Figure 3.
(A) A schematic representation of a prototype and tested microfluidic device for automated metabolic analysis that was fabricated with soft lithography. Red and blue features represent control and flow layers of PDMS, respectively. Adapted from Urbanski et al. (2008). (B) A microfluidic cartridge used to analyze spent mouse embryo culture media for metabolic substrates and metabolites. External tubing provides necessary pneumatics for fluid flow. Adapted from Urbanski et al. (2008). (C) A schematic representation of the metabolic assay integrated with embryo culture on a cartridge. (D) An illustration and schematic cross-section demonstrating an integrated Braille display-based microfluidic cell culture and metabolic analysis system. The microfluidic cartridge is held on a flat surface consisting of the fingerplate and the heater unit. The Braille pin arrays (pins—yellow) are fixed on the bottom plate so that the pins are aligned with the holes of the fingerplate. (E) Photograph of the entire system placed. Adapted from Heo et al. (2012).
In an attempt to move these microfluidic embryo metabolic assays toward clinical application, Heo et al. (2012) reported on the design and testing of an automated computer-controlled microfluidic platform for both embryo culture and metabolic analysis on a single integrated device. Past methods of measuring embryo glucose consumption and lactate or pyruvate secretion utilized constriction pipets and UV light-mediated detection (Gardner et al., 2001). Considering that UV light exposure on-cartridge would be detrimental to embryo DNA integrity, an on-cartridge assay system was developed allowing real-time glucose measurements using fluorescent wavelengths that would not cause DNA damage to embryos. This assay already existed as an off-cartridge sensitive method of quantifying glucose through a glucose oxidase-peroxidase mediated reaction that produces red-fluorescent resorufin from colorless Amplex Red (10-acetyl-3,7-dihydroxyphenoxazine; Molecular Probes Eugene, OR, USA). This assay allowed fluorescent quantification of glucose in media automatically sampled from embryos culture on the microfluidic cartridge with little interference from autofluorescence in culture media. By utilizing the Braille pin actuation discussed earlier, with this embryo culture and metabolism assay cartridge, it enabled on-cartridge real-time automated embryo culture, spent media sampling, pumping, mixing, washing and detection of glucose from individual preimplantation embryos every hour, over a 6.3-h interval (Fig. 3). While these microfluidic embryo assays have been used with mouse embryos, they have not been applied to human embryos. Further refinement of assays, integration with embryo culture and studies on biomarkers to indicated utility for human embryo analysis and selection continue to be needed to support and facilitate the potential future use of microfluidics for preimplantation embryos selection in the human ART laboratory.
Cryopreservation with microfluidics
Cryopreservation of oocytes, zygotes and embryos has significantly expanded the scope of infertility treatment and recently oocyte cryopreservation has enabled fertility preservation for women at risk of losing fertility due to chronic disease, cancer treatment or genetic predisposition to infertility. Two cryopreservation methods exist; slow-rate freezing and vitrification. Vitrification is surpassing slow-rate freezing as the dominant means of oocyte, zygote and embryo cryopreservation because of its high (>90%) cryosurvival rates. However, a major concern in cryobiology is the damage that cells receive as their water content is exchanged with cryoprotectant agents (CPAs) before cooling and back to water during warming. CPAs are necessary to eliminate formation of damaging intracellular ice crystals, yet CPA exchange procedures exert osmotic stress. Osmotic stress refers to the stress experienced by cells due to changes in osmotic pressure within and without the cell. While lethal osmotic stress during vitrification and warming has largely been overcome (high cryosurvival rates), sub-lethal effects remain and affect oocyte function, embryo development and treatment outcomes. Osmotic stress is especially a challenge for oocyte and zygote cryopreservation because their fluid volumes, that need to be exchanged with CPAs, are several orders of magnitude larger than other mammalian cells. In addition, the high concentration of CPAs used in vitrification can also be problematic in relation to sub-lethal osmotic stress. Initial work using microfluidics and cryopreservation was focused on freezing of HepG2 (hepatocellular carcinoma human liver) cells and demonstrated benefit in cell survival (Song et al., 2009).
Many advancements in gamete/embryo cryopreservation of the past have been based on theoretical or empirical analyses (Leibo, 2008). In cryopreservation, independent of it being slow-rate freezing/thawing or vitrification/warming, there are more CPA exchange protocol possibilities (concentrations of CPA, timing of exchange, etc.) than can be efficiently and reliably tested by trial and error experiments alone. Therefore, utilizing mathematically/computer modelling and theoretical principles to derive potentially optimal CPA exchange protocols have found favor. Early investigations of cryopreservation-related osmotic stress indicated the presence of a threshold minimum cell volume, with shrinkage beyond that volume resulting in cell death (‘Meryman's Minimum Cell Volume Theory’, Meryman, 1971; Agca et al., 2000). In attempts to avoid the critical minimum cell volume, common methods for oocyte and zygote vitrification have incorporated at least three-step equilibration processes whereby cells are manually pipetted into subsequently higher levels of permeating CPA concentrations (Kuwayama et al., 2005a, 2005b; Pegg, 2010; Smith et al., 2010) allowing the cell to shrink in a specific interval. It has been experimentally demonstrated that the use of a higher number of ‘steps’ leads to enhanced cryosurvival and developmental outcomes (Kuwayama et al., 1992; Otoi et al., 1998); however, such protocols have not been adopted due to impracticality. The use of an automated, continuously and gradually changing CPA concentration exposure is a logical extension of this previous work, as a continuously gradual change in CPA concentration is fundamentally an ‘unlimited amount of steps’. Such a continuous protocol requires bioengineering concepts to design and execute which could not be practically performed by conventional manual pipetting procedures.
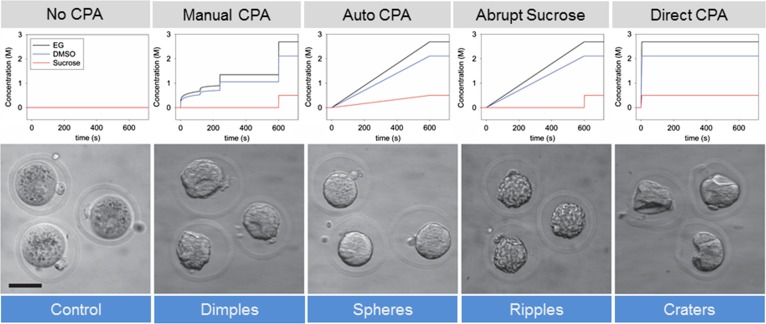
Heo et al. (2011) recognized this potential application of microfluidics for cryopreservation CPA exchange and reported precise fluid control, its relation to cell volume and utility of microfluidics for exchange of permeating CPAs. However, these investigators did not include non-permeating CPAs in mathematical modelling of fluid exchange studies, nor did they cool or cryopreserve gametes, zygotes or embryos. Furthering these studies, Lai et al. (2015) applied the Kedem-Katchalsky equations to mathematically model, and experimentally measure oocyte/zygote cell shrinkage when exposed to vitrification solutions with both permeating and non-permeating CPAs with manual pipetting/no equilibration (direct plunge in vitrification solution; knows to be detrimental; negative control); manual pipetting using equilibration steps (standard manual contemporary control), and automated continual/gradual microfluidic equilibration (Fig. 4). It was demonstrated that strain and strain rate of the cell during CPA exposure can be independently regulated, in a fixed sum of time, in relation to the way cells are exposed to CPAs. These investigators then applied mathematically derived CPA exposure profiles, measured stain and reported that for a given strain, a decrease in strain rate yields enhanced outcomes in oocyte and/or zygote cryopreservation. Measures of improved outcomes included qualitative and quantitative measures of oocyte/zygote morphometrics, including cell surface smoothness, overall sphericity and viscoelastic buckling of membranes. In addition, mouse zygotes vitrified using the microfluidic automated gradual/continuously changing CPA exposure not only have lower strain rates compared with control groups, but had quantifiably less sub-lethal membrane damage, higher retention of cytoplasmic lipids (likely as a consequence of reduced need for membrane repair) and significantly better embryonic developmental potential (Lai et al., 2015, Fig. 4). These experiments emphasize the need to dissect out individual steps in cryopreservation, a way of designing experiments to address new concepts, and the need record logical, classical and non-classical outcome measures and to test the equivalence, superiority or inferiority of a new technology. Other investigators have also recently made advances in non-microfluidic semi-automated vitrification (Roy et al., 2014) and microfluidic processing of embryos for vitrification (Pyne et al., 2014). Currently, we are addressing similar questions on the warming side of the cryopreservation procedure, and finally we will need to experimentally test the combinations. In this manuscript, the development of a precise microfluidic CPA exchange system was described that could eliminate user-to-user and program-to-program variability in cryopreservation solution exposure.
Figure 4.
Use of a microfluidic cryoprotectant exchange system to generate automated, continuous and gradual permeable and impermeable (sucrose) cryoprotectant addition and test the influence on mouse oocyte and zygote osmotic stress, morphology, sub-lethal cell damage and development. CPA exchange profiles and representative morphology for: No CPA exposure (control), commonly used manual pipetting protocol (Manual CPA), automated microfluidic protocol with gradual addition of all CPA components (Auto), and alternative CPA exposure protocols, gradual exposure of permeable CPA components but abrupt exposure to the impermeable component sucrose (Abrupt Sucrose) and direct and sudden exposure from culture media to VS (known to be detrimental to oocyte viability). Scale bar: 50 mm. Adapted from Lai et al. (2015).
Removing this variability will facilitate future discovery and validation of new and improved cryopreservation solution formulations and procedures and may find usefulness and favor in clinical human assisted reproduction. This may be of practical utility, especially in warming of cryopreserved donor oocytes. Currently, there are a few donor cryopreserved oocyte banks that vitrify oocytes, train end-user laboratories to warm using their ‘egg bank protocols’, sale and distribute vitrified eggs to end-user laboratories, and then warming and donor egg insemination, embryo development and transfers are performed at the end-user laboratory. If oocytes do not survive or embryo development is sub-optimal or no pregnancy is achieved; it might be queried as to whether ‘technical drift’ in the warming procedure is involved? One could suggest that automating and removing ‘technical signatures’ would be advantageous for outcomes, and at least help in identification of causative factors in sub-par results.
Conservative future projection of microfluidics and human assisted reproduction
In the last decade, significant advances have been made in evaluating the potential utility of microfluidics in isolation, manipulation, analysis and cryopreservation of mammalian gametes and embryos. There is still a lot of work to do. As evidenced by the authorship of these reports, the experimental advancements have been facilitated by true interdisciplinary work, usually involving a biologist and a bioengineer, and their respective groups. These interactions can be quite intellectually rewarding, yet take time, patience, understanding and good communications are required. In a broad scientific sense, microfluidic can be useful in gamete/embryo biology from an experimental/investigative perspective and/or a practical perspective. One advantage is not more important than the other. There is always the question of when will microfluidics be applied to human ART? From a practical perspective, the future utility of microfluidics in human ART will depend on data. It will take time to design new systems, test them for safety, test them for efficacy in animal model systems, have it repeated by others, and finally to use them in randomized prospective controlled clinical trials. This is the proper way of generating evidence and applying evidence-based decision making in clinical laboratory work. At the end of the day, experimental data from numerous levels of testing, and repeated by numerous laboratories, will dictate the use, or lack-of use, of microfluidics in human assisted reproduction. It is exciting and encouraging to see numerous interdisciplinary groups worldwide working in this area.
Acknowledgements
We appreciate the collaborations, dedication, hardwork and imagination within past and present members of the Smith and Takayama laboratories. The authors would also like to thank Dr Shawn Chavez for the invitation to participate in this collection of articles. We apologize to those whose work we have not cited because of space limitation.
Authors’ roles
Gary D. Smith and Shuichi Takayama co-wrote this review.
Funding
We thank the National Institutes of Health, United States (HD049607), National Research Initiative Competitive Grant from the USDA National Institute of Food and Agriculture, United States (2005-35203-16148), Michigan Economic Development Corporation (MEDC; GR696), and the Coulter Foundation for financial support.
Conflict of interest
None declared.
References
- Agca Y, Liu J, Rutledge JJ, Critser ES, Critser JK. Effect of osmotic stress on the developmental competence of germinal vesicle and metaphase II stage bovine cumulus oocyte complexes and its relevance to cryopreservation. Mol Reprod Dev 2000;55:212–219. [DOI] [PubMed] [Google Scholar]
- Aitken RJ, Clarkson JS. Significance of reactive oxygen species and antioxidants in defining the efficacy of sperm preparation techniques. J Androl 1988;9:367–376. [DOI] [PubMed] [Google Scholar]
- Alpha Scientists in Reproductive Medicine, ESHRE Special Interest Group of Embryology The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod 2011; 26:1270–1283. [DOI] [PubMed] [Google Scholar]
- Alvarez JG, Lasso JL, Blasco L, Nunez RC, Heyner S, Caballero PP, Storey BT. Centrifugation of human spermatozoa induces sublethal damage; separation of human spermatozoa from seminal plasma by a dextran swim-up procedure without centrifugation extends their motile lifetime. Hum Reprod 1993;8:1087–1092. [DOI] [PubMed] [Google Scholar]
- Aoki VW, Peterson CM, Parker-Jones K, Hatasaka HH, Gibson M, Huang I, Carrell DT. Correlation of sperm penetration assay score with polyspermy rate in in-vitro fertilization. J Exp Clin Assist Reprod 2005;2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroso G, Morshedi M, Oehninger S. Analysis of DNA fragmentation, plasma membrane translocation of phosphatidylserine and oxidative stress in human spermatozoa. Hum Reprod 2000;15:1338–1344. [DOI] [PubMed] [Google Scholar]
- Basile N, Vime P, Florensa M, Aparicio Ruiz B, Garcia Velasco JA, Remohi J, Meseguer M. The use of morphokinetics as a predictor of implantation: a multicentric study to define and validate an algorithm for embryo selection. Hum Reprod 2015;30:276–283. [DOI] [PubMed] [Google Scholar]
- Benchaib M, Braun V, Lornage J, Hadj S, Salle B, Lejeune H, Guerin JF. Sperm DNA fragmentation decreases the pregnancy rate in an assisted reproductive technique. Hum Reprod 2003;18:1023–1028. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S, Hamilton MP, Shaaban M, Khalaf Y, Seddler M, Ghobara T, Braude P, Kennedy R, Rutherford A, Hartshorne G et al. Conventional in-vitro fertilisation versus intracytoplasmic sperm injection for the treatment of non-male-factor infertility: a randomised controlled trial. Lancet 2001;357:2075–2079. [DOI] [PubMed] [Google Scholar]
- Bose S, Wan Z, Carr A, Rizvi AH, Vieira G, Pe'er D, Sims PA. Scalable microfluidics for single-cell RNA printing and sequencing. Genome Biol 2015;16:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brison D, Houghton F, Falconer D, Roberts S, Hawkhead J, Humpherson P, Lieberman B, Leese H. Identification of viable embryos in IVF by non-invasive measurement of amino acid turnover. Hum Reprod 2004;19:2319–2324. [DOI] [PubMed] [Google Scholar]
- Brody JP, Yager P, Goldstein RE, Austin RH. Biotechnology at low Reynolds numbers. Biophys J 1996;71:3430–3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho BS, Schuster TG, Zhu X, Chang D, Smith GD, Takayama S. Passively driven integrated microfluidic system for separation of motile sperm. Anal Chem 2003;75:1671–1675. [DOI] [PubMed] [Google Scholar]
- Clark SG, Haubert K, Beebe DJ, Ferguson CE, Wheeler MB. Reduction of polyspermic penetration using biomimetic microfluidic technology during in vitro fertilization. Lab Chip 2005;5:1229–1232. [DOI] [PubMed] [Google Scholar]
- Duffy DC, McDonald JC, Schueller OJ, Whitesides GM. Rapid prototyping of microfluidic systems in poly(dimethylsiloxane). Anal Chem 1998;70:4974–4984. [DOI] [PubMed] [Google Scholar]
- Dyer S, Chambers GM, de Mouzon J, Nygren KG, Zegers-Hochschild F, Mansour R, Ishihara O, Banker M, Adamson GD. International Committee for Monitoring Assisted Reproductive Technologies world report: Assisted Reproductive Technology 2008, 2009 and 2010. Hum Reprod 2016;31:1588–1609. [DOI] [PubMed] [Google Scholar]
- Esteves T, van Rossem F, Nordhoff V, Schlatt S, Boiani M, Le Gac S. A microfluidic system supports single mouse embryo culture leading to full-term development. Royal Soc Chem Adv 2013;3:26451–26458. [Google Scholar]
- Feynman R. There's plenty of room at the bottom: an invitation to enter a new field of physics. Eng Sci 1960;23:22–36. [Google Scholar]
- Foong SC, Fleetham JA, O'Keane JA, Scott SG, Tough SC, Greene CA. A prospective randomized trial of conventional in vitro fertilization versus intracytoplasmic sperm injection in unexplained infertility. J Assist Reprod Genet 2006;23:137–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franasiak JM, Forman EJ, Hong KH, Werner MD, Upham KM, Treff NR, Scott RT Jr. The nature of aneuploidy with increasing age of the female partner: a review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril 2014;101:656–663. e1. [DOI] [PubMed] [Google Scholar]
- Gardner DK, Lane M, Stevens J, Schoolcraft WB. Noninvasive assessment of human embryo nutrient consumption as a measure of developmental potential. Fertil Steril 2001;76:1175–1180. [DOI] [PubMed] [Google Scholar]
- Gu W, Zhu X, Futai N, Cho B, Takayama S. Computerized microfluidic cell culture using elastomeric channels and Braille displays. PNAS 2004;101:15861–15866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C, Zhang Q, Ma R, Xie L, Qiu T, Wang L, Mitchelson K, Wang J, Huang G, Qiao J et al. Integration of single oocyte trapping, in vitro fertilization and embryo culture in a microwell-structured microfluidic device. Lab Chip 2010;10:2848–2854. [DOI] [PubMed] [Google Scholar]
- Heo YS, Cabrera LM, Bormann CL, Shah CT, Takayama S, Smith GD. Dynamic microfunnel culture enhances mouse embryo development and pregnancy rates. Hum Reprod 2010;25:613–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo YS, Cabrera LM, Bormann CL, Smith GD, Takayama S. Real time culture and analysis of embryo metabolism using a microfluidic device with deformation based actuation. Lab Chip 2012;12:2240–2246. [DOI] [PubMed] [Google Scholar]
- Heo YS, Cabrera LM, Song JW, Futai N, Tung YC, Smith GD, Takayama S. Characterization and resolution of evaporation-mediated osmolality shifts that constrain microfluidic cell culture in poly(dimethylsiloxane) devices. Anal Chem 2007;79:1126–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo YS, Lee HJ, Hassell BA, Irimia D, Toth TL, Elmoazzen H, Toner M. Controlled loading of cryoprotectants (CPAs) to oocyte with linear and complex CPA profiles on a microfluidic platform. Lab Chip 2011;11:3530–3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman D, Beebe D, Rodriguez-Zas S, Wheeler M. Comparison of static and dynamic medium enviornments for culturing of pre-implantation mouse embryos. Comp Med 2002;52:122–126. [PubMed] [Google Scholar]
- Houghton FD, Hawkhead JA, Humpherson PG, Hogg JE, Balen AH, Rutherford AJ, Leese HJ. Non-invasive amino acid turnover predicts human embryo developmental capacity. Hum Reprod 2002;17:999–1005. [DOI] [PubMed] [Google Scholar]
- Hughes CM, Lewis SE, McKelvey-Martin VJ, Thompson W. The effects of antioxidant supplementation during Percoll preparation on human sperm DNA integrity. Hum Reprod 1998;13:1240–1247. [DOI] [PubMed] [Google Scholar]
- Irvine DS, Twigg JP, Gordon EL, Fulton N, Milne PA, Aitken RJ. DNA integrity in human spermatozoa: relationships with semen quality. J Androl 2000;21:33–44. [PubMed] [Google Scholar]
- Kaupp UB, Hildebrand E, Weyand I. Sperm chemotaxis in marine invertebrates–molecules and mechanisms. J Cell Physiol 2006;208:487–494. [DOI] [PubMed] [Google Scholar]
- Kieslinger DC, Hao Z, Vergouw CG, Kostelijk EH, Lambalk CB, Le Gac S. In vitro development of donated frozen-thawed human embryos in a prototype static microfluidic device: a randomized controlled trial. Fertil Steril 2015;103:680–6 e2. [DOI] [PubMed] [Google Scholar]
- Kirkegaard K, Agerholm IE, Ingerslev HJ. Time-lapse monitoring as a tool for clinical embryo assessment. Hum Reprod 2012;27:1277–1285. [DOI] [PubMed] [Google Scholar]
- Kirkegaard K, Kesmodel US, Hindkjaer JJ, Ingerslev HJ. Time-lapse parameters as predictors of blastocyst development and pregnancy outcome in embryos from good prognosis patients: a prospective cohort study. Hum Reprod 2013;28:2643–2651. [DOI] [PubMed] [Google Scholar]
- Knowlton SM, Sadasivam M, Tasoglu S. Microfluidics for sperm research. Trends Biotechnol 2015;33:221–229. [DOI] [PubMed] [Google Scholar]
- Kricka LJ, Faro I, Heyner S, Garside WT, Fitzpatrick G, McKinnon G, Ho J, Wilding P. Micromachined analytical devices: microchips for semen testing. J Pharm Biomed Anal 1997;15:1443–1447. [DOI] [PubMed] [Google Scholar]
- Kricka LJ, Nozaki O, Heyner S, Garside WT, Wilding P. Applications of a microfabricated device for evaluating sperm function. Clin Chem 1993;39:1944–1947. [PubMed] [Google Scholar]
- Krisher RL, Wheeler MB. Towards the use of microfluidics for individual embryo culture. Reprod Fertil Dev 2010;22:32–39. [DOI] [PubMed] [Google Scholar]
- Kuwayama M, Hamano S, Nagai T. Vitrification of bovine blastocysts obtained by in vitro culture of oocytes matured and fertilized in vitro. J Reprod Fertil 1992;96:187–193. [DOI] [PubMed] [Google Scholar]
- Kuwayama M, Vajta G, Ieda S, Kato O. Comparison of open and closed methods for vitrification of human embryos and the elimination of potential contamination. Reprod Biomed Online 2005. a;11:608–614. [DOI] [PubMed] [Google Scholar]
- Kuwayama M, Vajta G, Kato O, Leibo SP. Highly efficient vitrification method for cryopreservation of human oocytes. Reprod Biomed Online 2005. b;11:300–308. [DOI] [PubMed] [Google Scholar]
- Lai D, Ding J, Smith GW, Smith GD, Takayama S. Slow and steady cell shrinkage reduces osmotic stress in bovine and murine oocyte and zygote vitrification. Hum Reprod 2015;30:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibo SP. Cryopreservation of oocytes and embryos: optimization by theoretical versus empirical analysis. Theriogenology 2008;69:37–47. [DOI] [PubMed] [Google Scholar]
- Luo Z, Guven S, Gozen I, Chen P, Tasoglu S, Anchan RM, Bai B, Demirci U. Deformation of a single mouse oocyte in a constricted microfluidic channel. Microfluid Nanofluidics 2015;19:883–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly KD, Agarwal A, Nagy ZP. Preimplantation genetic screening: does it help or hinder IVF treatment and what is the role of the embryo. J Assist Reprod Genet 2011;28:833–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallidis C, Wistuba J, Bleisteiner B, Damm OS, Gross P, Wubbeling F, Fallnich C, Burger M, Schlatt S. In situ visualization of damaged DNA in human sperm by Raman microspectroscopy. Hum Reprod 2011;26:1641–1649. [DOI] [PubMed] [Google Scholar]
- Matsuoka T, Choul Kim B, Moraes C, Han M, Takayama S. Micro- and nanofluidic technologies for epigenetic profiling. Biomicrofluidics 2013;7:41301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura K, Uozumi T, Furuichi T, Sugimoto I, Kodama M, Funahashi H. A microfluidic device to reduce treatment time of intracytoplasmic sperm injection. Fertil Steril 2013;99:400–407. [DOI] [PubMed] [Google Scholar]
- Melin J, Lee A, Foygel K, Leong DE, Quake SR, Yao MW. In vitro embryo culture in defined, sub-microliter volumes. Dev Dynamics 2009;238:950–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meryman HT. Osmotic stress as a mechanism of freezing injury. Cryobiology 1971;8:489–500. [DOI] [PubMed] [Google Scholar]
- Meseguer M, Kruhne U, Laursen S. Full in vitro fertilization laboratory mechanization: toward robotic assisted reproduction. Fertil Steril 2012;97:1277–1286. [DOI] [PubMed] [Google Scholar]
- Molina I, Lazaro-Ibanez E, Pertusa J, Debon A, Martinez-Sanchis JV, Pellicer A. A minimally invasive methodology based on morphometric parameters for day 2 embryo quality assessment. Reprod Biomed Online 2014;29:470–480. [DOI] [PubMed] [Google Scholar]
- Nosrati R, Vollmer M, Eamer L, San Gabriel MC, Zeidan K, Zini A, Sinton D. Rapid selection of sperm with high DNA integrity. Lab Chip 2014;14:1142–1150. [DOI] [PubMed] [Google Scholar]
- O'Donovan C, Twomey E, Alderman J, Moore T, Papkovsky D. Development of a respirometric biochip for embryo assessment. Lab Chip 2006;6:1438–1444. [DOI] [PubMed] [Google Scholar]
- Otoi T, Yamamoto K, Koyama N, Tachikawa S, Suzuki T. Cryopreservation of mature bovine oocytes by vitrification in straws. Cryobiology 1998;37:77–85. [DOI] [PubMed] [Google Scholar]
- Paegel BM, Blazej RG, Mathies RA. Microfluidic devices for DNA sequencing: sample preparation and electrophoretic analysis. Curr Opin Biotechnol 2003;14:42–50. [DOI] [PubMed] [Google Scholar]
- Paternot G, Debrock S, De Neubourg D, D'Hooghe TM, Spiessens C. Semi-automated morphometric analysis of human embryos can reveal correlations between total embryo volume and clinical pregnancy. Hum Reprod 2013;28:627–633. [DOI] [PubMed] [Google Scholar]
- Payne JF, Raburn DJ, Couchman GM, Price TM, Jamison MG, Walmer DK. Redefining the relationship between sperm deoxyribonucleic acid fragmentation as measured by the sperm chromatin structure assay and outcomes of assisted reproductive techniques. Fertil Steril 2005;84:356–364. [DOI] [PubMed] [Google Scholar]
- Pegg DE. The relevance of ice crystal formation for the cryopreservation of tissues and organs. Cryobiology 2010;60:S36–S44. [DOI] [PubMed] [Google Scholar]
- Practice Committees of the American Society for Reproductive Medicine, Society for Assisted Reproductive Technology Intracytoplasmic sperm injection (ICSI) for non-male factor infertility: a committee opinion. Fertil Steril 2012;98:1395–1399. [DOI] [PubMed] [Google Scholar]
- Pyne DG, Liu J, Abdelgawad M, Sun Y. Digital microfluidic processing of mammalian embryos for vitrification. PloS One 2014;9:e108128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raty S, Walters EM, Davis J, Zeringue H, Beebe DJ, Rodriguez-Zas SL, Wheeler MB. Embryonic development in the mouse is enhanced via microchannel culture. Lab Chip 2004;4:186–190. [DOI] [PubMed] [Google Scholar]
- Rosen MP, Shen S, Dobson AT, Fujimoto VY, McCulloch CE, Cedars MI. Oocyte degeneration after intracytoplasmic sperm injection: a multivariate analysis to assess its importance as a laboratory or clinical marker. Fertil Steril 2006;85:1736–1743. [DOI] [PubMed] [Google Scholar]
- Roy TK, Brandi S, Tappe NM, Bradley CK, Vom E, Henderson C, Lewis C, Battista K, Hobbs B, Hobbs S et al. Embryo vitrification using a novel semi-automated closed system yields in vitro outcomes equivalent to the manual Cryotop method. Hum Reprod 2014;29:2431–2438. [DOI] [PubMed] [Google Scholar]
- Sanchez V, Redmann K, Wistuba J, Wubbeling F, Burger M, Oldenhof H, Wolkers WF, Kliesch S, Schlatt S, Mallidis C. Oxidative DNA damage in human sperm can be detected by Raman microspectroscopy. Fertil Steril 2012;98:1124–1129. e1-3. [DOI] [PubMed] [Google Scholar]
- Schulte RT, Chung YK, Ohl DA, Takayama S, Smith GD. Mircofluidic sperm sorting device provides a novel method for selecting motile sperm with higher DNA integrity. Fertil Steril 2007;88:S76. [Google Scholar]
- Schuster TG, Cho B, Keller LM, Takayama S, Smith GD. Isolation of motile spermatozoa from semen samples using microfluidics. Reprod Biomed Online 2003;7:75–81. [DOI] [PubMed] [Google Scholar]
- Seli E, Gardner DK, Schoolcraft WB, Moffatt O, Sakkas D. Extent of nuclear DNA damage in ejaculated spermatozoa impacts on blastocyst development after in vitro fertilization. Fertil Steril 2004;82:378–383. [DOI] [PubMed] [Google Scholar]
- Shirota K, Yotsumoto F, Itoh H, Obama H, Hidaka N, Nakajima K, Miyamoto S. Separation efficiency of a microfluidic sperm sorter to minimize sperm DNA damage. Fertil Steril 2016;105:315–21 e1. [DOI] [PubMed] [Google Scholar]
- Smith GD, Serafini PC, Fioravanti J, Yadid I, Coslovsky M, Hassun P, Alegretti JR, Motta EL. Prospective randomized comparison of human oocyte cryopreservation with slow-rate freezing or vitrification. Fertil Steril 2010;94:2088–2095. [DOI] [PubMed] [Google Scholar]
- Song YS, Moon S, Hulli L, Hasan SK, Kayaalp E, Demirci U. Microfluidics for cryopreservation. Lab Chip 2009;9:1874–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strunker T, Goodwin N, Brenker C, Kashikar ND, Weyand I, Seifert R, Kaupp UB. The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature 2011;471:382–386. [DOI] [PubMed] [Google Scholar]
- Suh RS, Zhu X, Phadke N, Ohl DA, Takayama S, Smith GD. IVF within microfluidic channels requires lower total numbers and lower concentrations of sperm. Hum Reprod 2006;21:477–483. [DOI] [PubMed] [Google Scholar]
- Swain JE, Cabrera L, Xu X, Smith GD. Microdrop preparation factors influence culture-media osmolality, which can impair mouse embryo preimplantation development. Reprod Biomed Online 2012;24:142–147. [DOI] [PubMed] [Google Scholar]
- Swain JE, Lai D, Takayama S, Smith GD. Thinking big by thinking small: application of microfluidic technology to improve ART. Lab Chip 2013;13:1213–1224. [DOI] [PubMed] [Google Scholar]
- Unger MA, Chou HP, Thorsen T, Scherer A, Quake SR. Monolithic microfabricated valves and pumps by multilayer soft lithography. Science 2000;288:113–116. [DOI] [PubMed] [Google Scholar]
- Urbanski JP, Johnson MT, Craig DD, Potter DL, Gardner DK, Thorsen T. Noninvasive metabolic profiling using microfluidics for analysis of single preimplantation embryos. Analy Chem 2008;80:6500–6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Blerkom J, Davis PW, Merriam J. A retrospective analysis of unfertilized and presumed parthenogentically activated human oocytes demonstrates a high frequency of sperm penetration. Hum Reprod 1994;9:2381–2388. [DOI] [PubMed] [Google Scholar]
- Van de Velde H, Nagy ZP, Joris H, De Vos A, Van Steirteghem AC. Effects of different hyaluronidase concentrations and mechanical procedures for cumulus cell removal on the outcome of intracytoplasmic sperm injection. Hum Reprod 1997;12:2246–2250. [DOI] [PubMed] [Google Scholar]
- Virro MR, Larson-Cook KL, Evenson DP. Sperm chromatin structure assay (SCSA) parameters are related to fertilization, blastocyst development, and ongoing pregnancy in in vitro fertilization and intracytoplasmic sperm injection cycles. Fertil Steril 2004;81:1289–1295. [DOI] [PubMed] [Google Scholar]
- Volpatti LR, Yetisen AK. Commercialization of microfluidic devices. Trends Biotechnol 2014;32:347–350. [DOI] [PubMed] [Google Scholar]
- Whitesides GM. The origins and the future of microfluidics. Nature 2006;442:368–373. [DOI] [PubMed] [Google Scholar]
- WHO World Health Organization (WHO) Laboratory Manual for the Examination and Processing of Human Semen, 5th edn WHO, 2010. [Google Scholar]
- Willadsen SM. A method for culture of micromanipulated sheep embryos and its use to produce monozygotic twins. Nature 1979;277:298–300. [DOI] [PubMed] [Google Scholar]
- Wong CC, Loewke KE, Bossert NL, Behr B, De Jonge CJ, Baer TM, Reijo Pera RA. Non-invasive imaging of human embryos before embryonic genome activation predicts development to the blastocyst stage. Nat Biotechnol 2010;28:1115–1121. [DOI] [PubMed] [Google Scholar]
- Xia P. Biology of polyspermy in IVF and its clinical indication. Curr Obstet Gynecol Rep 2013;2:226–231. [Google Scholar]
- Yanez LZ, Han J, Behr BB, Pera RA, Camarillo DB. Human oocyte developmental potential is predicted by mechanical properties within hours after fertilization. Nat Commun 2016;7:10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeringue HC, Beebe DJ. Microfluidic removal of cumulus cells from Mammalian zygotes. Methods Mol Biol 2004;254:365–374. [DOI] [PubMed] [Google Scholar]
- Ziebe S. Morphometric analysis of human embryos to predict developmental competence. Reprod Fertil Dev 2013;26:55–64. [DOI] [PubMed] [Google Scholar]
- Zini A, Finelli A, Phang D, Jarvi K. Influence of semen processing technique on human sperm DNA integrity. Urology 2000;56:1081–1084. [DOI] [PubMed] [Google Scholar]
- Zini A, Meriano J, Kader K, Jarvi K, Laskin CA, Cadesky K. Potential adverse effect of sperm DNA damage on embryo quality after ICSI. Hum Reprod 2005;20:3476–3480. [DOI] [PubMed] [Google Scholar]