Abstract
X-ray diffraction, infrared and electron microscope studies of avian and reptilian keratins, and of stretched wool and hair, have played a central role in the development of models for the β-conformation in proteins. Both α- and β-keratins contain sequences that are predicted to adopt a β-conformation and these are believed to play an important part in the assembly of the filaments and in determining their mechanical properties. Interactions between the small β-sheets in keratins provide a simple mechanism through which shape and chemical complementarity can mediate the assembly of molecules into highly specific structures. Interacting β-sheets in crystalline proteins are often related to one another by diad symmetry and the data available on feather keratin suggest that the filament is assembled from dimers in which the β-sheets are related by a perpendicular diad. The most detailed model currently available is for feather and reptilian keratin but the presence of related β-structural forms in mammalian keratins is also noted.
Electronic supplementary material
The online version of this article (doi:10.1007/s12551-008-0005-0) contains supplementary material, which is available to authorized users.
Keywords: α-keratin, β-keratin, Feather keratin, Reptilian keratin, Twisted β-sheets, Amyloid filaments
Introduction
In the early 1930s, Astbury and co-workers studied the molecular structure of keratins using high-angle X-ray diffraction methods. They observed a meridional arc at a spacing of 0.51 nm in mammalian keratins such as wool, noting that this value was inconsistent with that expected from an extended polypeptide chain. Consequently, they proposed that the chain was folded. This led to the discovery of the α-helix and the coiled-coil α-helical rope. They also found that when wool was stretched to approximately double its original length in steam the original X-ray diffraction pattern (the α-pattern) was transformed into a second pattern resembling that obtained from silk. This they designated the β-pattern. In a further study, the chains in the β-form were shown to be antiparallel and packed together in sheets with three-dimensional regularity (Astbury and Woods 1933). The axial separation between residues (0.31 nm), however, was somewhat less than that seen in silk (0.334 nm). Later, a detailed pleated sheet model containing antiparallel chains was devised (Pauling and Corey 1951) and this was shown to be consistent with the intensities observed in the X-ray diffraction pattern of β-keratin (Fraser et al. 1969).
Much has been learned about the β-conformation through the study of synthetic polypeptides (Fraser and MacRae 1973) and, in more recent times, a range of complex assemblies derived from the pleated sheet have been observed directly in both fibrous and globular proteins. These include the β-solenoid, the triple-stranded β-solenoid, the cross β-prism, the triple β-spiral and the spiral β-hairpin staircase (Kajava et al. 2006). To date, the only modification to the simple pleated sheet that has been shown to exist in keratins is the right-handed twist commonly observed in small sheets.
Avian and reptilian keratins
Although epidermal appendages such as the claws of mammals have similar functions and mechanical properties to those found in birds and reptiles their chemical compositions and molecular structures are quite different. In particular, mammalian hard keratins contain three disparate groups of proteins, each with many members (Gillespie 1990), whereas feathers contain only one type of protein with very few members (O'Donnell 1973). The first indication of differences in molecular structure was revealed in X-ray diffraction studies on feather keratin which indicated that the dominant secondary structure was based on the β-conformation (Marwick 1931; Astbury and Marwick 1932). Although the axial rise per residue (0.31 nm) was significantly lower than that observed in steam-stretched wool (0.334 nm) it was found possible to increase this to the “normal” value by stretching in steam, thereby adding credence to the designation of a β-type conformation.
Several models were suggested for feather keratin including a tubular β-helical structure (Schor and Krimm 1961). The crystallinity evident in high-resolution X-ray diffraction patterns, however, was not sufficient for classical crystallographical approaches to succeed; in addition the external interference between the filaments was too great for the pattern to be analyzed as the cylindrically averaged intensity transform of a helix. The external interference could be reduced, nonetheless, by selectively destroying the longitudinal and rotational correlation between filaments by mechanical treatments, thereby producing a ‘simplified’ X-ray diffraction pattern characteristic of a helical structure (Bear and Rugo 1951; Fraser and MacRae 1963). The helix had four units per turn and a pitch length of 9.5 nm. Using electron microscopy (Filshie and Rogers 1962) and infrared dichroism, it proved possible to develop a detailed model (Fraser et al. 1971). Individual molecules, each consisting of about 100 residues, were predicted to contain a β-favoring segment around 32 residues long that collectively formed the filaments observed in the electron micrographs. It was further proposed that each 32-residue segment folded into a four-stranded β-sheet that was twisted in a sense opposite to that of the helix and that the molecules associated in pairs to form antiparallel dimers. Axial polymerization of these dimers then formed the filaments. The concept of a twisted β-sheet in proteins, rather than a planar one, was unique at that time, but it was shown later that small twisted sheets are energetically more stable than planar ones and are now regarded as the norm.
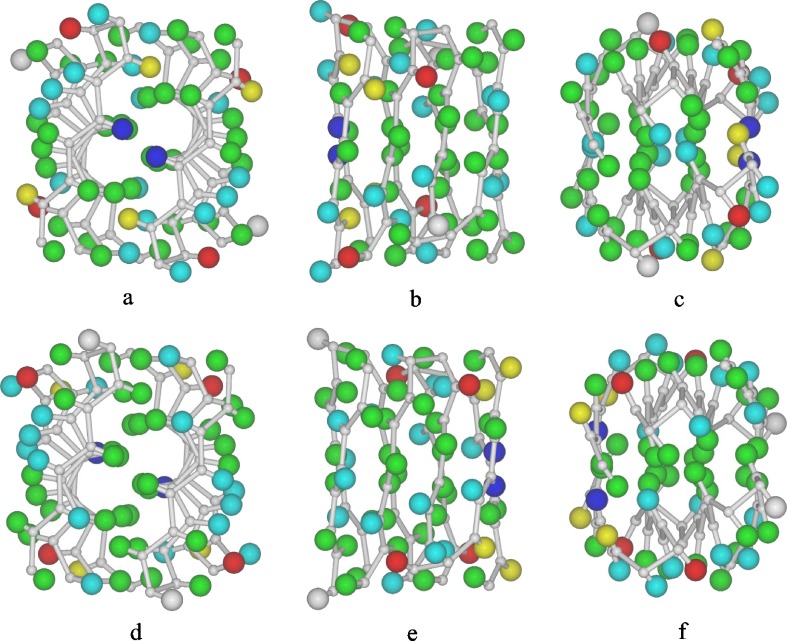
Unfortunately, the observed intensities in the X-ray diffraction pattern obtained from feather keratin cannot be phased using the methods employed with crystalline proteins. Consequently, it is not possible at present to obtain electron density maps showing the positions of the individual atoms and so the structure cannot be solved directly. However, following the determination of the amino acid sequence of emu feather (O'Donnell 1973), the original model has been progressively refined by trial-and-error methods (Fraser and MacRae 1976; Fraser and Parry 1996, 2008) and its current status is shown in Fig. 1.
Fig. 1.
Two models for the repeating unit in the feather keratin filament. The colored spheres delineate the approximate localities of the sidechains of emu feather (green, hydrophobic; pale blue, hydrogen-bonding; red, acidic; dark blue, basic; yellow, half-cystine; grey, glycine). The upper views correspond to Structure A and the lower views to a possible alternative Structure B. a and d are projections down the diad (x-axis), b and e are projections down the y-axis, and c and f are projections down the axis of the filament (z-axis)
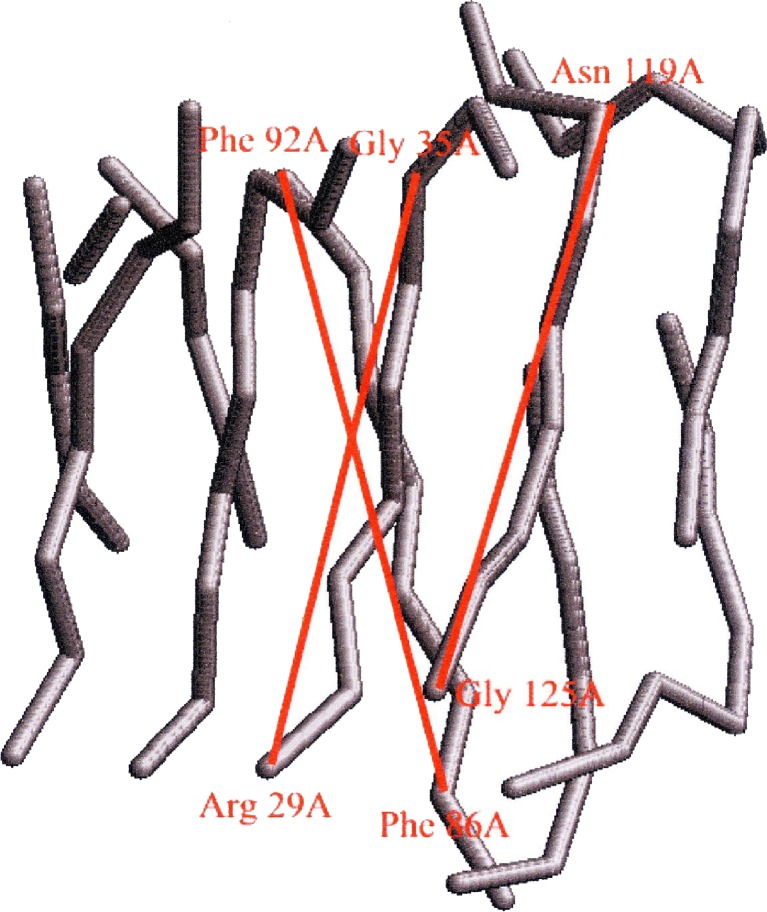
The mainchain coordinates are based on the original ‘ruled surface’ type of distortion used to introduce a twist into the β-sheet (Fraser et al. 1971). There is a current need for a more realistic set of atomic coordinates for the mainchain atoms probably based on the large amount of data on twisted β-sheets that has been collected from crystallographic studies. The reduced length of the axial repeat length observed in feather keratin provides a benchmark for selecting possible conformations of the twisted β-sheet and we have recently explored known structures which contain similar pairs of interacting twisted β-sheets. For example, Fig. 2 shows the mainchain in the β-sheet segments in the sugar-binding protein Congerin II (Kamiya et al. 2002). This contains a pair of twisted β-sheets packed together in a manner similar to that envisaged in the feather keratin dimer (Fig. 1b). The inclinations of the central chains in the front and rear sheets of Congerin II to what would be the fiber axis (z-axis) in feather can be calculated from the expressions
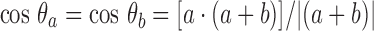 |
1 |
where a and b are unit vectors parallel to the intersecting red lines in Fig. 2, a · denotes a vector dot product, a + sign denotes a vector addition and || a magnitude. Similarly, the inclination to the fiber axis of the adjacent chain, also shown in red, is given by
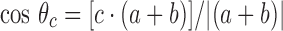 |
2 |
where c is a unit vector parallel to the direction of this chain.
Fig. 2.
The mainchain segments in the β-sheet “sandwich” in the sugar-binding protein Congerin II (Kamiya et al. 2002) provide a possible model for the geometry of the β-sheet sandwich in feather keratin
The mean separation per residue along the length of the chain in the inner chains is 0.328 nm and that in the adjacent chain is 0.333 nm. These values are close to the 0.334 nm spacing observed in the extended planar β-sheets derived from stretched α-keratin. The inner chains are inclined at 14.3° to the z-axis and the adjacent chain is inclined at 20.3°. The mean axial rise per residue parallel to the axis in a twisted sheet with two inner and two outer chains would therefore be predicted to be 0.316 nm. Thus, a conformation similar to that observed in Congerin II, if present in feather keratin, would provide a plausible explanation for the observed axial spacing of 0.31 nm (Astbury and Marwick 1932).
Detailed studies of the twisted β-sheets in crystalline proteins have revealed that the ϕ and ψ values that characterize the progression of the chain from one residue to the next oscillate between two sets of values depending on the side of the sheet from which the sidechain projects, and that the values vary according to the lateral position of the chain in the sheet. These factors need to be incorporated into any procedure for predicting the atomic coordinates in twisted β-sheets of feather keratin.
Sequence comparisons across avian and reptilian keratins
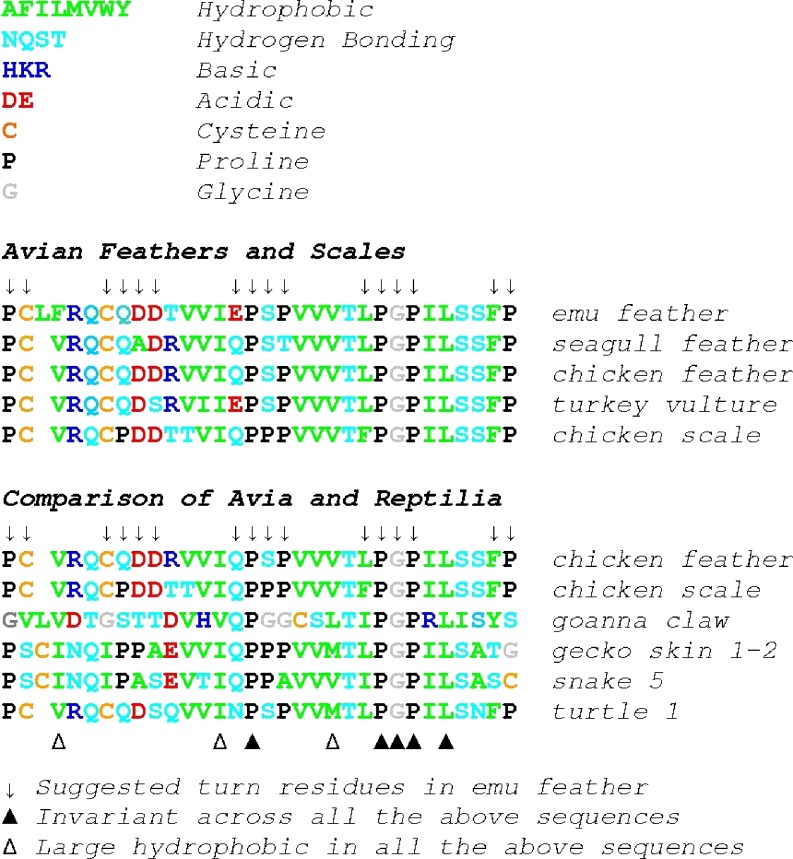
Many data are now available for the β-keratins from both birds and reptiles. The distribution and variability of residues across the 32-residue segment (including the composition of the β-bends) have been analyzed (Fraser and Parry 2008; Alibardi et al. 2008; Table 1 and Fig. 3) and notable features include:
The conservation of a P-G-P motif that is part of one of the proposed hairpin turns and, as such, may provide the nucleation site for the β-sheet,
A preponderance of apolar residues on the inner face of the β-sheet (Fig. 4) and of charged and cysteine residues on the other,
The frequent occurrence of a charged residue in the first strand of the β-sheet thereby preventing the exposed edge of one sheet inappropriately bonding to the exposed edge of another sheet (Richardson and Richardson 2002), and
The unusual proline-rich sequences that are believed to play an important role in the formation of the three proposed hairpin turns.
Table 1.
An alignment of sequences in the β-sheet segments of avian and reptilian keratins that maximizes the coincidence of proline residues
The arrows show the original assignment of turn residue locations in emu feather (Fraser and MacRae, 1976). The alignment of the other sequences is one of several alternative possibilities. That shown maximizes the alignment of proline residues and is similar to the one used in our earlier studies
aO'Donnell (1973)
bO'Donnell and Inglis (1974)
cPresland et al (1989): the same sequences is present in duck (Anas platyrhynchos) and pigeon (Columba livia)—Arai et al. (1986)
d Sawyer et al. (2003)
eWalker and Bridgen (1976)
fInglis et al. (1987)
gWhitbread et al. (1991)
hToni et al. (2007a)
iToni et al. (2007b)
jDalla Valle et al. (2008)
Fig. 3.
Comparison of representative examples from the database of sequences of avian and reptilian keratins (see Table 1 for references). The residue letters are colored to indicate their special property (see key at top of the diagram). Patterns of proline and large hydrophobic residues are evident as is the constancy of a PGP sequence. Greater variability occurs in the early part of the segment and the alignment of the sequences in this region is less certain. The alignment shown maximizes the coincidence of proline residues (Fraser and MacRae 1976)
Fig. 4.
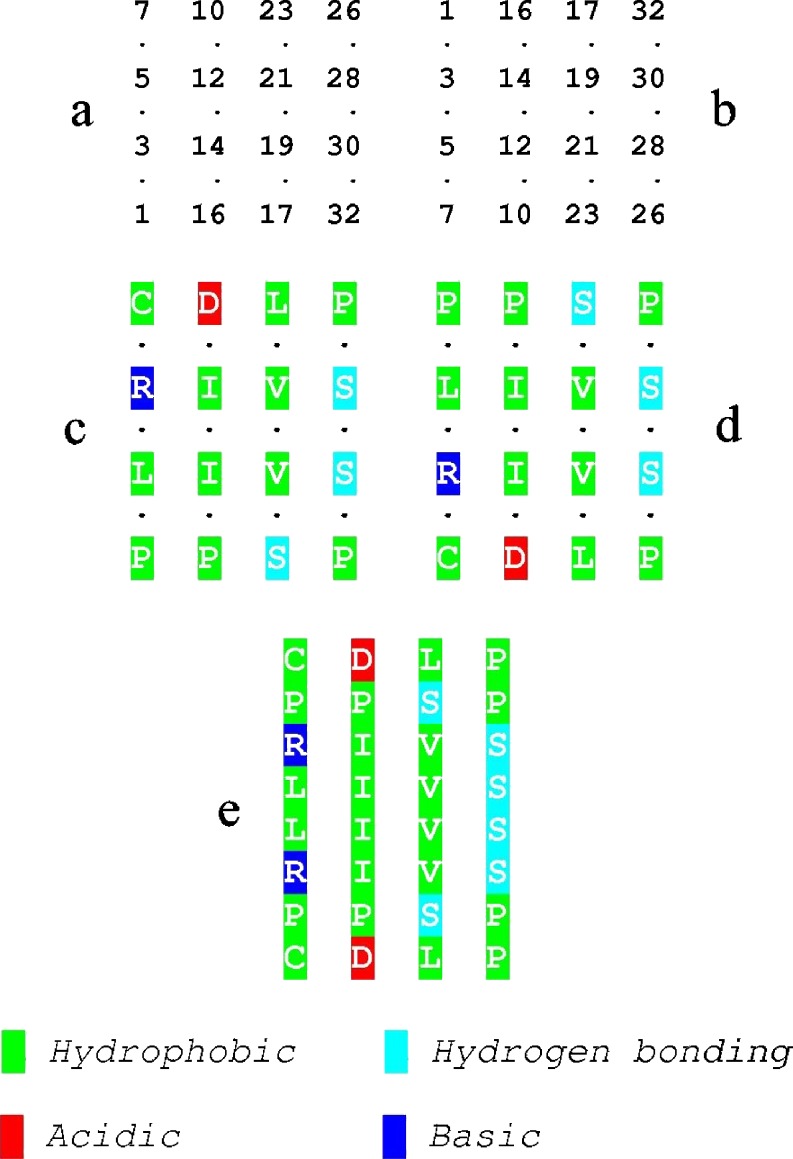
The distribution of sidechains in the interior of the model for the feather keratin dimer shows a preponderance of large hydrophobic residues but also opportunities for hydrogen bond formation towards the edges. The half-cystine residue (7) has been shown as hydrophobic since this aspect of its character is important here. Also evident are the negatively charged residues (5), which may prevent unwanted lateral aggregation (Richardson and Richardson 2002). a Residues on the inner surface of the Up component of the dimer and b the inner surface of the Down component viewed from the same direction. c and d show the nature of the residues in emu feather and e shows the two surfaces superposed
The arrows in Table 1 show the original assignment of turn residue locations in emu feather (Fraser and MacRae 1976). The alignment of the other sequences is one of several alternative possibilities, maximizing the alignment of proline residues and is similar to that used in earlier studies. An alignment based on general sequence homology is similar except for the first few residues where there is a deletion located beneath the fourth residue of the emu sequence (F) in all the other sequences. The emu sequence was determined when methods were less reliable than those today and an independent determination is advisable.
In fibrous proteins containing large β-sheets with repeating patterns of sidechains, such as Bombyx mori silk fibroin, complementarity of shape plays an important part in determining the way in which the sheets pack together. With smaller twisted sheets that lack such regularity, shape complementarity plays a less important part and the chain directions in pairs of sheets are commonly inclined to each other at an angle of around 30° (Chothia and Janin 1981). Because of the short lengths of chains involved, the maximum lateral displacement is only around ±0.2 nm and so a simple linear display of chemical properties such as that shown in Fig. 4 can be used to search for complementarity of chemical properties at the interface between the two sheets related by the diad. This reveals a concentration of hydrophobic residues in the inner region and a cluster of hydrogen-bonding residues towards the periphery.
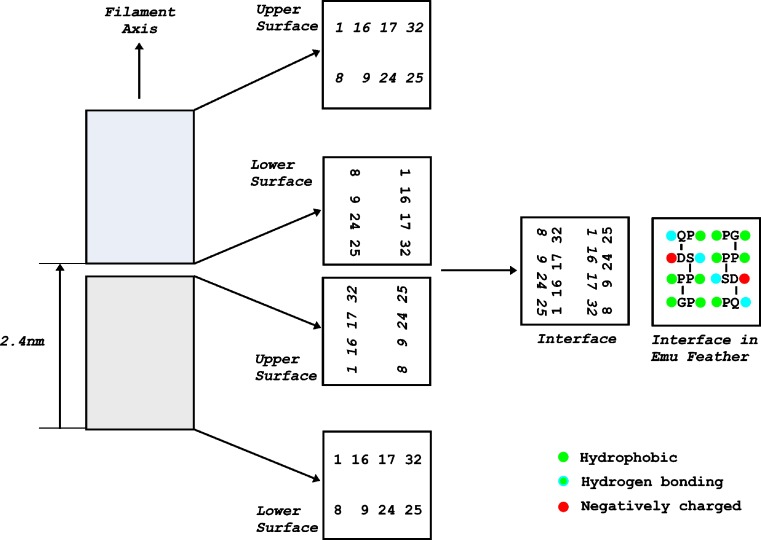
The calculated length of the β-sheet is very close to that of the unit height of the helix, indicating that the ends of dimers must be in close contact. The nature of this interface, which is important in axial polymerization, is more difficult to investigate since there is no requirement that the twist of the sheet is exactly 90° in the unit height. If it was, in fact, 90°, and allowing for the fact that the hand of the helix is opposite to that of the sheet twist the situation at the interface would be as illustrated in Fig. 5. The proximity between hydrophobic sidechains on the upper surface of a dimer and those on the lower surface of the dimer above will contribute to the stability of the polymer as will hydrogen-bonding residues. However, there will be repulsion between the negatively charged residues in position 9. Assignments of structural positions in the first and fourth chains are subject to some uncertainty (Fraser and Parry 2008). Also, the β-sheets constitute only about one third of the residues in feather keratin and it is likely that the residues adjacent to the 32-residue segment will also be involved in polymerization.
Fig. 5.
The area of contact between successive repeating units along the length of the feather keratin filament. The repeating units (shaded) are spaced at axial intervals of 2.4 nm and the left-handed helical symmetry means that when viewed down the filament axis, there is a 90° rotation about the filament axis in a clockwise direction between successive repeating units. Each repeating unit comprises a pair of twisted β-sheets related by a perpendicular diad and the distribution of residues in the upper and lower surface of each of the repeating units (viewed down the axis) is shown on the right. Allowance must be made for the fact that the β-sheets have a right-handed twist (i.e., a counterclockwise rotation when viewed down the filament axis) and the phasing in the end views has been adjusted appropriately. A right-handed twist of 90° was used although there is no requirement that that it be exactly this value and is likely to be slightly less. The upper surface of the lower repeating unit and the lower surface of the upper repeating unit are combined to show the residues that populate the interface between the two repeating units in emu feather. The majority of these residues are hydrophobic, with some having hydrogen-bonding potential. Two negatively charged sidechains occur near the periphery. Parts of the remainder may also contribute to the interactions regulating end-to-end polymerization
Amyloid-like filaments from feather keratin proteins
Another manifestation of the β-pattern reported by Astbury et al. was one in which the high-angle X-ray diffraction pattern indicated that the polypeptide chains were oriented perpendicular to the fiber axis. This was termed the cross-β pattern (Astbury et al. 1935). The original observations were made on denatured globular proteins but subsequently the cross-β pattern was observed in low-molecular-weight synthetic polypeptides (Fraser and MacRae 1973) and in the naturally occurring egg stalk of the green lacewing fly Chrysopa flava (Parker and Rudall 1957).
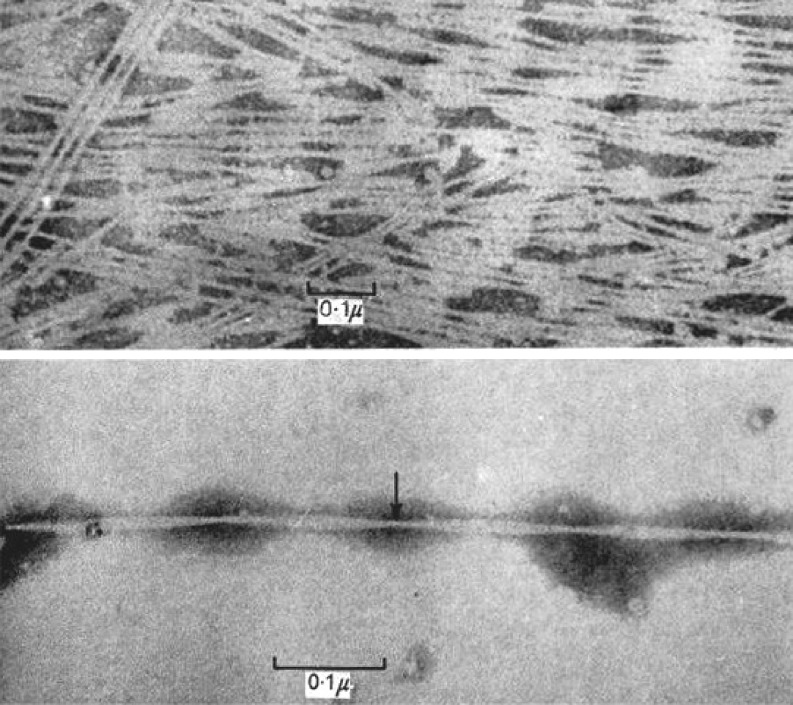
Keratins are extensively crosslinked by disulphide bonds formed between cysteine residues during the final stages of biosynthesis. Feather keratin can be solubilized by cleaving the disulphide linkages and chemically modifying the cysteine residues. X-ray diffraction patterns obtained from oriented films of these derivatives were found to be of two distinct types. In the first, the original filamentous structure appeared to have been restored (Rougvie 1954), suggesting that the disulfide linkages were not involved in the assembly process. In the second, the X-ray pattern was of the cross-β type suggesting that polymerization occurred via the hydrogen bonds of the β-sheets, so that the chains became oriented perpendicular to the fiber axis (Filshie et al. 1964). Electron microscope studies of the latter revealed the presence of filaments around 4 nm in diameter that were twisted around each other in pairs (Fig. 6, lower micrograph) and assembled into ordered sheets (Fig. 6, upper micrograph). The filaments bear a close resemblance to the cross-β structures commonly referred to as amyloid filaments.
Fig. 6.
Amyloid-like filaments regenerated from a purified fraction of solubilized fowl feather (Filshie et al, 1964). X-ray diffraction studies suggest that feather keratin dimers in this preparation are aligned with the polypeptide chains oriented perpendicular to the axis of the filament giving a cross-β structure
Other β-sheets
The β-sheets thus far described have been confirmed through high-angle X-ray studies. However, there are segments of the amino acid sequence in mammalian, avian, and reptilian keratins for which there is a high expectation that the polypeptide chain will also adopt an extended conformation. In these cases, the orientation of the sheets is probably such that their contribution to the X-ray diffraction pattern cannot easily be recognized.
An additional β-sheet in chicken leg scale
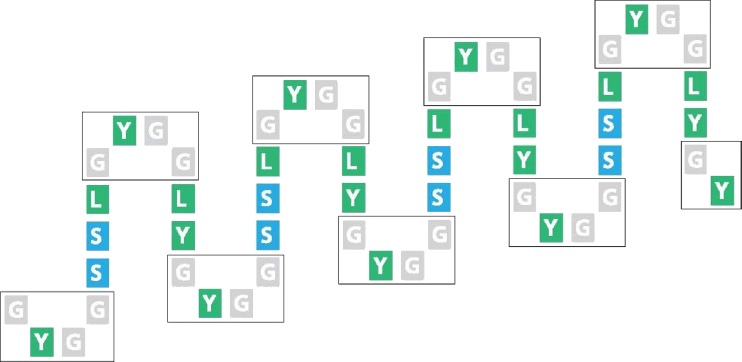
The molecular weights of chicken scale and feather keratins differ significantly from one another (about 15 and 10.5 kDa respectively) and this is almost entirely accounted for by the presence in scale, but not in feather, of a fourfold repeat (residues 77–128) of a 13-residue, glycine–tyrosine-rich motif (G-Y-G-G-S-S-L-G-Y-G-G-L-Y). X-ray diffraction studies of powdered scale keratin corresponding to this particular region (Stewart 1977) have indicated a β-structure. The 52-residue segment (Fig. 7) is located C-terminally with respect to the 32-residue β-favoring motif discussed earlier (residues 25–56). The latter lacks tyrosine residues and contains but a single glycine, thereby illustrating the very non-uniform distribution of the glycine and tyrosine residues in scale keratin. In fact, in scale, the bulk of both residue types lies in the C-terminal half of the chain (29 glycines and 20 tyrosines are found in residues 77–155 and only seven glycines and two tyrosines occur in residues 1–76).
Fig. 7.
Diagrammatic representation of the potentiality for β-sheet formation in the 52-residue segment in chick scale keratin that comprises four consecutive 13-amino acid residue repeats
Secondary structure prediction techniques suggest that the 52-residue, fourfold repeat will adopt an eight-stranded, antiparallel, twisted β-pleated sheet conformation with the β-turns each having a G-Y-G-G sequence (Gregg et al. 1984). This would give rise to rows of highly interactive tyrosine residues, especially in the turn regions. Glycine–tyrosine-rich proteins play an important part in mediating the properties of mammalian keratins but virtually nothing is known about the way in which they interact with the filaments. A similar situation exists as regards the glycine–tyrosine rich segment in avian scale keratins although some possibilities have been discussed (Fraser and Parry 1996).
An additional β-sheet in lizard claw
The first reptilian hard keratin to be analyzed in detail was from lizard claw (Varanus varius). A 32-residue segment of similar composition and sequence to that found in feather keratin was also found to be present in this reptile (Fraser and Parry 1996). In addition, a second “ghost” segment was also observed in the sequence, though its degree of similarity with the feather repeat was less well-developed. It is not yet clear whether this second segment represents a structurally significant feature of reptilian keratins.
β-sheets in mammalian keratin
In each of the examples below, there are no X-ray diffraction data that provide experimental evidence for the existence of β-sheets. In every case, the conformation is predicted on the basis of the amino acid sequences and each must, therefore, be treated with caution until supported by experimental data.
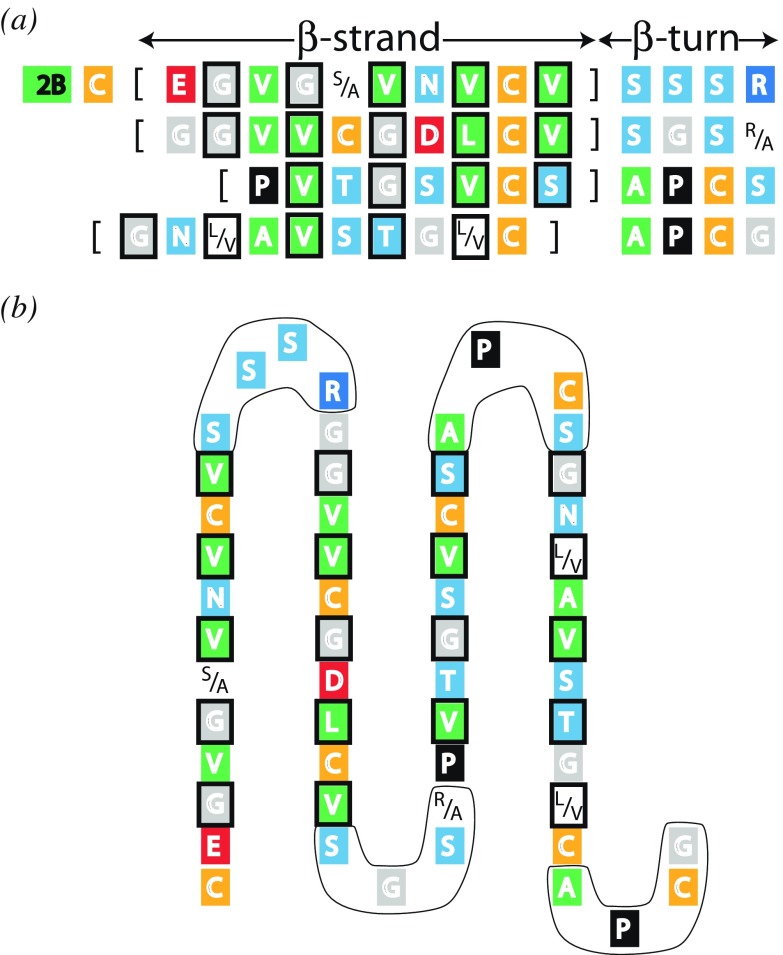
In the tail region (C-terminal domain) of the Type II trichocyte keratin chain the sequence immediately adjacent to the coiled-coil rod domain is predicted to adopt a four-stranded antiparallel β-pleated sheet (Fig. 8; Parry and North 1998). The first (S-S-S-R) and second β-bends (S-G-S-R/A) have near identical sequences as do the third (A-P-C-S) and fourth (A-P-C-G). One face of this putative structure would be composed almost entirely of apolar residues whereas the other would contain predominantly cysteine and charged residues. Since the companion (parallel, in-register) Type I chain lacks this feature, the most likely partner for the β-pleated sheet would be one from the same chain in different molecule. The apolar faces would be expected to come together to stabilize the assembly. The topology of the intermediate filament makes it most probable that the partner would arise from an antiparallel rather than a parallel molecule within the filament structure itself.
The head region (N-terminal domain) of the Type II trichocyte keratin chain contains a fourfold contiguous repeat of nine residues (G-G-F-G-Y-R-S-X-G). This too is thought to adopt a β-pleated sheet conformation but in this case, there is not a clear chemical sidedness to the two faces (Parry et al. 2002). It would still be expected, nonetheless, that this sheet would interact with the same region emanating from a second molecule. Some similarities with the 13-residue repeat in scale keratin are worth noting, most notably the high glycine and aromatic residue content in both motifs.
The two-stranded rod domain of keratin intermediate filament molecules consists of long segments of α-helical coiled-coil structure (1A, 1B, 2A, and 2B) interrupted by short, non-helical linkers known as L1, L12, and L2. Of these, a portion of L12 has a repeat of the form (apolar-hydrophilic)4 (Parry and Fraser 1985). As such, the possibility has been raised this might form a pair of parallel β-strands with an apolar aspect (Conway and Parry 1988) and that a mini β-sheet could be formed with the same region in an oppositely directed molecule in the A12 mode of aggregation. Predicting the conformation of such a short piece of sequence is extremely difficult and the model postulated must remain very speculative.
Fig. 8.
a The consensus sequence of the first 57 residues in the tail domain of Type II trichocyte keratins laid out to illustrate the potentiality for four β-strands and four connecting β-turns (Parry and North, 1998). One face is predominantly occupied by apolar residues and the other by cysteine and charged residues (amongst others). The apolar residues are boxed. b Schematic diagram of the proposed four-stranded antiparallel β-sheet. The β-turns are ringed. Figure modified from Parry and North (1998)
Summary
We have drawn attention to the diverse ways in which β-sheets contribute to the structure of keratins. The formation and subsequent interactions between small β-sheets in both fibrous and globular proteins appears to play an important role in the assembly of the molecules into well-defined aggregates. The characteristic right-handed twist, and the commonly observed partitioning of different residue types on the two faces through sequence regularities, provides a versatile mechanism for introducing a high degree of specificity into the assembly process. Assembly will be mediated by both shape and chemical complementarity, and also through the pairing of sheets, whereby sequence regularities internalize the hydrophobic residues and shield them from the aqueous environment.
Apart from the results obtained with experiments on the reassembly of solubilized feather keratin derivatives, there is little direct experimental evidence to support the involvement of interactions between β-sheets as being involved in the polymerization of fibrous proteins molecules. However, evidence from crystallographic studies of β-sheet rich proteins suggest that interactions between β-sheets will prove to play an important role in the higher levels of assembly in fibrous proteins.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Annotated references for inclusion with web version of the review (70.0 KB)
Acknowledgment
The authors wish to express their sincere appreciation to Lorenzo Alibardi for his permission to include sequence data on β-keratins that are currently in press.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s12551-008-0005-0) contains supplementary material, which is available to authorized users.
References
- Arai KM, Takahashi R, Yokote Y, Akahane K. The primary structure of feather keratin from duck (Anas platyrhynchos) and pigeon (Columba livia) Biochim Biophys Acta. 1986;873:6–12. [Google Scholar]
- Alibardi L, Dalla Valle L, Nardi A et al. (2008) Evolution of hard proteins in sauropsid integument in relation to cornification of skin derivatives in amniotes. J Anat (in press) [DOI] [PMC free article] [PubMed]
- Astbury WT, Marwick TC. X-ray interpretation of the molecular structure of feather keratin. Nature. 1932;130:309–310. doi: 10.1038/130309b0. [DOI] [Google Scholar]
- Astbury WT, Woods HJ. X-ray studies on the structure of hair, wool and related fibres II: the molecular structure and elastic properties of hair keratin. Philos Trans R Soc. 1933;A232:333–394. [Google Scholar]
- Astbury WT, Dickson S, Bailey K. The X-ray interpretation of denaturation and the structure of the seed globulins. Biochem J. 1935;29:2351–2360. doi: 10.1042/bj0292351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear RS, Rugo HJ. The results of X-ray diffraction studies on keratin fibers. Ann N Y Acad Sci. 1951;53:627–648. doi: 10.1111/j.1749-6632.1951.tb31964.x. [DOI] [PubMed] [Google Scholar]
- Chothia C, Janin J. Relative orientation of close-packed β-pleated sheets in proteins. Proc Natl Acad Sci USA. 1981;78:4146–4150. doi: 10.1073/pnas.78.7.4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway JK, Parry DAD. Intermediate filament structure 3. Analysis of sequence homologies. Int J Biol Macromol. 1988;10:79–98. doi: 10.1016/0141-8130(88)90015-3. [DOI] [Google Scholar]
- Dalla Valle L, Nardi A, Gelmi C, et al. β-keratins of the crocodilian epidermis: composition, structure, and phylogenetic relationships. J Exp Zool. 2008;310B:1–16. doi: 10.1002/jez.b.21199. [DOI] [PubMed] [Google Scholar]
- Filshie BK, Rogers GE. An electron microscope study of the fine structure of feather keratin. J Cell Biol. 1962;13:1–12. doi: 10.1083/jcb.13.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filshie BK, Fraser RDB, MacRae TP, et al. X-ray diffraction and electron-microscope observations on soluble derivatives of feather keratin. Biochem J. 1964;92:19. doi: 10.1042/bj0920018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser RDB, MacRae TP. Structural organization in feather keratin. J Mol Biol. 1963;7:272–280. doi: 10.1016/S0022-2836(63)80021-2. [DOI] [PubMed] [Google Scholar]
- Fraser RDB, MacRae TP. Conformation in Fibrous Proteins and Related Synthetic Polypeptides. New York: Academic; 1973. [Google Scholar]
- Fraser RDB, MacRae TP (1976) The molecular structure of feather keratin. Proc 16th Int Ornith Congress, Canberra, pp 443–451
- Fraser RDB, MacRae TP, Parry DAD, et al. The structure of beta keratin. Polymer (Guildf) 1969;10:810–826. doi: 10.1016/0032-3861(69)90110-4. [DOI] [Google Scholar]
- Fraser RDB, MacRae TP, Parry DAD, et al. The structure of feather keratin. Polymer (Guildf) 1971;12:35–56. doi: 10.1016/0032-3861(71)90011-5. [DOI] [Google Scholar]
- Fraser RDB, Parry DAD. The molecular structure of reptilian keratin. Int J Biol Macromol. 1996;19:207–211. doi: 10.1016/0141-8130(96)01129-4. [DOI] [PubMed] [Google Scholar]
- Fraser RDB, Parry DAD. Molecular packing in the feather keratin filament. J Struct Biol. 2008;162:1–13. doi: 10.1016/j.jsb.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Gillespie JM. The proteins of hair and other hard α-keratins. In: Goldman RD, Steinert PM, editors. Cellular and molecular biology of intermediate filaments. New York: Plenum; 1990. pp. 95–128. [Google Scholar]
- Gregg K, Wilton SD, Parry DAD, et al. A comparison of genomic sequences for feather and scale keratins: structural and evolutionary implications. EMBO J. 1984;3:175–178. doi: 10.1002/j.1460-2075.1984.tb01779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis A, Gillespie JM, Roxburgh C, Whittaker L, Casagranda F. Protein, structure and function. New York: Plenum; 1987. Sequence of a glycine-rich protein from lizard claw: unusual dilute acid and heptafluorobutyric acid cleavage; pp. 757–764. [Google Scholar]
- Kajava AV, Squire JM, Parry DAD. β-Structures in fibrous proteins. Adv Protein Chem. 2006;73:1–15. doi: 10.1016/S0065-3233(06)73001-7. [DOI] [PubMed] [Google Scholar]
- Kamiya H, Ishii C, Ogawa T, et al. Crystal structure of a conger eel galectin congerin II at 1.45 Å resolution: implication for the accelerated evolution of a new ligand-binding site following gene duplication. J Mol Biol. 2002;321:879–889. doi: 10.1016/S0022-2836(02)00700-3. [DOI] [PubMed] [Google Scholar]
- Marwick TC. The X-ray classification of epidermal proteins. J Text Sci. 1931;4:31–33. [Google Scholar]
- O'Donnell IJ. The complete amino acid sequence of a feather keratin from emu (Dromaius novae-hollandiae) Aust J Biol Sci. 1973;26:415–437. doi: 10.1071/bi9730415. [DOI] [PubMed] [Google Scholar]
- O'Donnell IJ, Inglis AS. Amino acid sequence of a feather keratin from silver gull (Larus Novae-Hollandiae) and comparison with one from emu (Dromaius nova-hollandiae) Aust J Biol Sci. 1974;27:369–382. doi: 10.1071/bi9740369. [DOI] [PubMed] [Google Scholar]
- Parker KD, Rudall KM. Structure of the silk of chrysopa egg-stalks. Nature. 1957;179:905–906. doi: 10.1038/179905a0. [DOI] [Google Scholar]
- Parry DAD, Fraser RDB. Intermediate filament structure 1. Analysis of IF protein sequence data. Int J Biol Macromol. 1985;7:203–213. doi: 10.1016/0141-8130(85)90003-0. [DOI] [Google Scholar]
- Parry DAD, North ACT. Hard α-keratin intermediate filament chains: substructure of the N- and C-terminal domains and the predicted structure and function of the C-terminal domains of type I and II chains. J Struct Biol. 1998;122:67–75. doi: 10.1006/jsbi.1998.3967. [DOI] [PubMed] [Google Scholar]
- Parry DAD, Marekov LN, Steinert PM, et al. A role for the 1A and L1 rod domain segments in head domain organization and function of intermediate filaments: structural analysis of trichocyte keratin. J Struct Biol. 2002;137:97–108. doi: 10.1006/jsbi.2002.4437. [DOI] [PubMed] [Google Scholar]
- Pauling L, Corey RB. The pleated sheet, a new layer configuration of polypeptide chains. Proc Natl Acad Sci USA. 1951;37:251–256. doi: 10.1073/pnas.37.5.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presland RB, Gregg K, Molloy PL, et al. Avian keratin genes. I. A molecular analysis of the structure and expression of a group of feather keratin genes. J Mol Biol. 1989;209:549–559. doi: 10.1016/0022-2836(89)90593-7. [DOI] [PubMed] [Google Scholar]
- Richardson JS, Richardson DC. Natural β-sheet proteins use negative design to avoid edge-to-edge aggregation. Proc Natl Acad Sci USA. 2002;99:2754–2760. doi: 10.1073/pnas.052706099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougvie MA (1954) Ph.D. Thesis, Massachusetts Institute of Technology
- Sawyer RH, Salvatore BA, Potylicki T-TF, et al. Origin of feathers: feather beta (β) keratins are expressed in discrete epidermal cell populations of embryonic scutate scales. J Exp Zool (Mol Dev Evol) 2003;295B:12–24. doi: 10.1002/jez.b.5. [DOI] [PubMed] [Google Scholar]
- Schor R, Krimm S. Studies on the structure of feather keratin II. A β-helix model for the structure of feather keratin. Biophys J. 1961;1:489–515. doi: 10.1016/S0006-3495(61)86904-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart M. The structure of chicken scale keratin. J Ultrastruct Res. 1977;60:27–33. doi: 10.1016/S0022-5320(77)80038-5. [DOI] [PubMed] [Google Scholar]
- Toni M, Dalla Valle L, Alibardi L. The epidermis of scales in gecko lizards contains multiple forms of β-keratins including basic glycine–proline–serine-rich proteins. J Proteome Res. 2007;6:1792–1805. doi: 10.1021/pr060626+. [DOI] [PubMed] [Google Scholar]
- Toni M, Dalla Valle L, Alibardi L. Hard (β−) keratins in the epidermis of reptiles: Composition, sequence, and molecular organization. J Proteome Res. 2007;6:3377–3392. doi: 10.1021/pr0702619. [DOI] [PubMed] [Google Scholar]
- Walker ID, Bridgen J. The keratin chains of avian scale tissue. J Biochem. 1976;67:283–293. doi: 10.1007/BFb0116517. [DOI] [PubMed] [Google Scholar]
- Whitbread LA, Gregg K, Rogers GE. The structure and expression of a gene encoding chick claw keratin. Gene. 1991;101:223–229. doi: 10.1016/0378-1119(91)90415-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Annotated references for inclusion with web version of the review (70.0 KB)