Abstract
Basement membranes are delicate, nanoscale and pliable sheets of extracellular matrices that often act as linings or partitions in organisms. Previously considered as passive scaffolds segregating polarized cells, such as epithelial or endothelial cells, from the underlying mesenchyme, basement membranes have now reached the center stage of biology. They play a multitude of roles from blood filtration to muscle homeostasis, from storing growth factors and cytokines to controlling angiogenesis and tumor growth, from maintaining skin integrity and neuromuscular structure to affecting adipogenesis and fibrosis. Here, we will address developmental, structural and biochemical aspects of basement membranes and discuss some of the pathogenetic mechanisms causing diseases linked to abnormal basement membranes.
Keywords: Collagen, laminin, heparan sulfate proteoglycan, discoidin domain receptor, integrin
Introduction
Basement membranes (BMs) are cell-adherent extracellular matrices widely distributed in metazoan tissues. First identified in skeletal muscle 176 years ago [1], elucidation of BM constituents, structure, functions and genetics has required advances in multiple fields stretched over many years. The beginning of a molecular understanding of BMs dates to the 1970s and 1980s when Kefalides discovered collagen IV and Kuhn, Timpl, Martin, Bruckner, Robey, Rhode and others exploited the Engelbreth-Holm-Swarm tumor as a source for obtaining soluble BM components for analysis. Since then, a combination of biochemical, biophysical, cell biological, genetic, bioengineering and other approaches led to our current understanding of BMs. We are pleased to present a special edition of Matrix Biology entitled “Basement Membranes in Health and Disease” containing a collection of twenty-six articles from specialists in the field where the physiological and pathological functions of BM components are critically assessed.
How many proteins are incorporated into a typical basement membrane?
The core structural components of BMs are laminins, collagen IV, nidogens, and the heparan sulfate proteoglycans (HSPGs) perlecan and agrin (Fig. 1). We envision core components as those macromolecules for which there is an embryonic phenotype of failed or structurally-defective BM assembly upon knockout with the provision that compensation can sometimes mask a structure-forming role. These glycoproteins and proteoglycans, initially secreted in a soluble state, become organized into insoluble cell scaffoldings and constitute a complex meshwork of proteins present in multicellular organisms [2]. In addition, BMs contain “matricellular proteins” that provide additional, often tissue-specific, functions but are not essential for BM assembly or architecture [3]. Examples are SPARC and nephronectin. Various growth factors/morphogens, many belonging to the TGFβ superfamily, are found tethered to BMs (in particular to proteoglycan HS chains) and act to provide specific signals to BM-adherent cells. Additionally, collagen XV and XVIII are found at the stromal interface. Finally, proteinases and their inhibitors, regulatory macromolecules and serum factors are found associated with BMs. Collectively, these components, organized into or associated with BMs, provide cell and tissue support and act as complex signaling platforms.
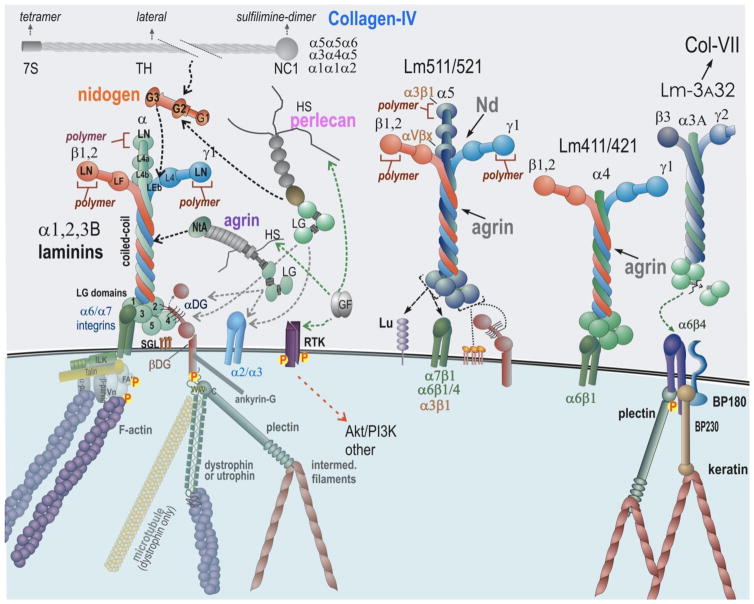
Fig. 1.
Core Basement Membrane Components and Binding Interactions. Laminins (Lms) are central organizers of BMs. Lm111, a prototypical laminin expressed in embryogenesis, binds to cell surface sulfated glycolipids (SGL), several integrins, α-dystroglycan (αDG), nidogens (Nd), agrin, and polymerizes via its LN domains. Collagen-IV (three isoforms) and perlecan bind to nidogen, completing the core basement membrane scaffold. Agrin and perlecan HS chains attach to growth factors, promoting their interactions with receptor tyrosine kinases (RTK). Integrin and αDG attach through adaptor proteins to the cytoskeleton. Lm411, an isoform that does not polymerize, exhibits weak integrin and αDG binding but strong binding to SGLs (gal sulfatide). Lm511/521, polymerizing laminins, binds to multiple integrins both in the LG domains and α5 short arm, to the Lutheran receptor (Lu), and moderately well to αDG. Lm3A32, a non-polymerizing laminin found in epithelia, binds strongly to α6β4 integrin of hemidesmosomes and links to collagen VII of the anchoring fibrils.
Evolution and embryogenesis of basement membranes
Laminin domains integrated within proteins such as cadherins exist in single-cell motile choanoflagellates representing primitive species that pre-date the evolutionary emergence of BMs [4]. These unicellular organisms can aggregate to form clusters, suggesting BM/cadherin precursors serve cell-cell adhesive functions. BMs are thought to have first emerged in metazoans as a requirement for organizing epithelial tissues. Laminin α-like, β-like and γ-like subunits, each with a laminin N-terminal (LN) and coiled-coil domains, and collagen IV-like subunits have been identified in the sponges Amphimedon and Chondrosia [5].
It is said that ontogeny recapitulates phylogeny. BMs first appear in mouse embryos at embryonic (E) day 4–4.5 as the primitive endoderm differentiates into an epithelial layer from the inner cell mass and secretes two laminins, collagen IV, nidogen-1 and perlecan [6,7] that assemble into a BM. The BM, in turn, induces conversion of the inner cell mass into the epiblast, a polarized epithelium. Inactivation of either the common LAMC1 or LAMB1 genes in mice results in a failure of all BM assembly with peri-implantation lethality by E5.5 [8]. By this stage and thereafter, laminin expression and BM assembly are critical for morphogenesis extending through gastrulation and organogenesis. Although collagen IV is present in the earliest BMs in mammals, an absolute requirement for collagen IV does not occur until later during vasculogenesis (E10.5–11.5), likely when BMs capable of resisting mechanical stresses are needed. Nidogens and perlecan are both essential components before or around birth, but not during the earliest stages of differentiation. Loss of these components affects morphogenesis at various stages [7,9].
Laminins are a fairly large family of heterotrimers each consisting of an α-, a β- and a γ-subunit joined together through a long coiled-coil domain. There are five α, four β and three γ chains that have been identified in mammals. Not all αβγ combinations are allowed and only 16 different mammalian laminin isoforms have been shown to assemble from the possible combinations. Common to most laminins is the γ1-subunit, first expressed in the peri-implantation period and essential for both early and late morphogenesis [8]. Laminins are either a cross-shaped (three short arms and one long coiled-coil arm; e.g. Lm111, Lm211, Lm511), Y-shaped (due to absence of the α-short arm; e.g., Lm411), or rod-shaped (with truncated short arms; Lm3A32). The major receptor-binding domains are the C-terminal laminin-type globular (LG) domains located at the C-terminal moiety of the α-subunit. These can interact with laminin-class integrins (α3β1, α6β1, α7β1, α6β4), α-dystroglycan, sulfated glycolipids, heparin, and, in the case of Lm511, the Lutheran receptor. The polymerization loci are located in the N-terminal LN domains. Nidogens and agrin bind to internal domains within the Lmγ subunit while perlecan and collagen IV bind to nidogen [9].
Collagen IV is composed of 6 α chains named α1-α6 that assemble in order to form heterotrimers. Three major type IV collagens, each a heterotrimer, are present in different BMs [10]. The most ubiquitous trimer consists of two α1 and one α2 chains and is present in nearly all BMs. The α3α4α5 and α5α5α6 heterotrimers, on the other hand, have restricted distributions; notably in the renal glomeruli, neuromuscular junctions, eyes, inner ears and other locations.
A given BM contains at least one laminin, one type of collagen IV heterotrimer, nidogen, and HSPGs. Proteomic analyses of the internal limiting membrane, a thick BM adjacent to the retina that could be cleanly isolated, reported considerable complexity with sixty one components identified [11]. These included different laminins, collagens, nidogens, proteoglycans, growth factors, proteases, and other secreted factors. Using a proteomic approach to compare different organs, considerable variability of tissue distribution of known BM proteins has been found [11].
Basement membrane assembly
Basement membrane formation is largely one of self-assembly involving cell surface adhesion, inter-component binding and polymerization. Laminins, by binding to cells, self, and other BM components, are essential for initiating this process. Laminin assembly is receptor-facilitated, occurs on “competent” cell surfaces that express laminin-binding molecules and is accompanied by polymerization mediated by the LN domains [12]. The BM, in good part through laminins, is anchored to integrin and dystroglycan receptors, connecting the nascent BM to the underlying cytoskeleton. This anchorage is crucial for activation of signaling cascades in the case of integrins in all tissues and likely for dystroglycan as well in select tissues. Other cell surface components (e.g. sulfatides) can promote laminin adhesion without cytoskeletal anchorage and have been found to play an important role in Schwann cell BM assembly. Specialized receptors such as the Lutheran protein uniquely interact with Lm511, while receptor tyrosine kinases bind to growth factors/morphogens tethered to the HS chains of HSPGs to affect cell functions. The laminin scaffold enables recruitment and assembly of the remaining components. Nidogen-1 and the less common nidogen-2 bind to the laminin through the γ-subunit short arm [12], acting as bridge to the collagen IV network. Of note, deletion of epidermal γ-subunit of laminin leads to defects in hair morphogenesis [13], whereas laminin α5 subunit in keratinocyte BM is required epidermal/dermal communication [14]. Perlecan binds to nidogen, αDG and collagen IV, and the last through the HS chains. Collagen IV self-assembles into a network-like polymer through N-terminal (7S), lateral, and C-terminal (NC1) domain interactions that become covalently stabilized through the 7S and NC1 domains. In epithelia, Lm3A32 plays a key role in forming strong attachments between integrin α6β4 and collagen VII of anchoring fibrils at the stromal-BM interface [12].
Mechano/chemical signaling mechanisms
Both mechanical and chemical interactions of BM and integrins and other receptors are required for cell signaling [15]. A breakthrough in our understanding of the mechanical role came from a study showing that matrix stiffness is capable of driving cell differentiation through different pathways [16]. This contribution has largely been explored with synthetic gels whose viscoelastic properties can be manipulated and that are attached to integrin ligands at known density. An emerging concept is that integrin-ligated ECMs such as BMs exert their mechanical effect by “pulling” integrins against resistance created by the viscoelastic nature of the ECM polymer and by the cell glycocalyx that is compressed between the ECM ligands and short integrin ectodomains [51]. An intracellular talin-based molecular clutch mediates mechano-transmission of the signal to the actin cytoskeleton that is modulated by the motor activity of non-muscle myosin-II.
Human genetic diseases linked to basement membrane constituents: Can we survive without basement membranes?
Defects of BM components such as the Lmγ1 chain, essential for embryogenesis, are presumed to result in embryonic death well before diseases might become manifest. Non-embryonic-lethal mutations in BM component genes are found for other laminin subunits, collagen IV, and perlecan. Several of these diseases are briefly discussed below and summarized in Table 1.
Table 1.
Human genetic diseases associated with mutations in basement membrane constituents
| Gene | Mutation type | Mutation effects | Disease | References |
|---|---|---|---|---|
|
| ||||
| LAMA2 | Substitutions, deletions, duplications, insertions, and inversions resulting in protein substitutions, deletions, frame shifts, nonsense, and complex variant Lmα2 chains. |
|
|
[17] |
|
| ||||
|
LAMA3 LAMB3 LAMC2 |
Frame shift, nonsense and missense mutations |
|
Junctional epidermolysis bullosa (JEB):
|
[19] |
|
| ||||
| LAMB2 | Nonsense, missense, spice site mutations, multiple sites. Missense mutations clustered in LN domain. |
|
Pierson syndrome. Congenital nephrotic syndrome with eye abnormalities, microcoria. Muscular weakness/myasthenia may be present | [20] |
|
| ||||
| COL4A1 | Frameshift | Disruption of the C-terminal NC1 domain | Hematuria, chronic kidney disease, renal cysts | [21] |
| COL4A1 | G1236R G749S |
Amino acid change in the triple-helical domain | Porencephaly, hemorrhagic strokes | [22] |
| COL4A1 | G1769A | Amino acid change in the triple-helical domain | Retinal hemorrhage, retinopathy | [23,24] |
|
COLA4A3 COL4A4 COL4A5 |
Splicing variants, missense mutations | Altered chain expression | Alport syndrome, deafness, lenticonus, cataract, maculopathy | [25,26] |
|
| ||||
| COL6A1 | Gly missense mutations | Amino acid change in the triple helix | Ulrich congenital muscular dystrophy: severe hypotonia, early childhood Bethlehem myopathy: joint laxity and hypotonia |
[27] |
|
| ||||
| COL7A1 | Amino acid mutations | Loss of α1 chain expression | Dystrophic epidermolysis bullosa | [28,29] |
|
| ||||
| COL17A1 | Missense mutations | Exon truncation | Epithelial recurrent erosion dystrophy | [30,31] |
| COL17A1 | Nonsense mutation | Premature stop codons | Junctional epidermolysis bullosa | [32] |
|
| ||||
| COL18A1 | Frame-shift mutations nonsense mutations missense mutations | Altered chain reduction Loss of chain production |
Knobloch syndrome: high myopia, vitroretinal degeneration, occipital encephalocele, fasting triglyceridemia Duplication of kidney collecting system | [33–35] |
| COL18A1 | Insertion, frame deletions splice site mutation | Change in the C-terminal chain | [36] | |
|
| ||||
| HSPG2 | Duplicated exon 34 and frameshifts | Functionally null | Dyssegmental dysplasia, Silver-Handmaker type due to altered FGF2 signaling leading to aberrant cartilage architecture | [37–39] |
|
| ||||
| HSPG2 | Missense and errors in alternative splicing | Truncated or ablated Domain V (endorepellin) | Schwartz-Jampel syndrome originating from disrupted neuromuscular junction arrangement and function | [38,40] |
|
| ||||
| AGRN | Missense and nonsense mutations at the N-terminus and LG2 domain | Failure of neuromuscular transmission, pre- and post- synaptic | Congenital myasthenic syndrome, easy fatigability and muscle weakness | [41,42] |
One set of laminin disorders are the Lmα2/merosin-deficient congenital muscular dystrophies (MDC1A and limb-girdle type) arising from mutations in the LAMA2 gene. The diseases primarily manifest in skeletal muscle but also presents in peripheral nerves and brain [17]. The dystrophies resulting from a total or near-total absence of α2-laminins are very severe and associated with failure to ambulate and early, often respiratory death. A milder limb-girdle form can be seen with a number of single amino acid substitutions or short deletions affecting the LN or other short arm domains [18]. Muscular dystrophies also arise from mutations affecting laminin receptors (DG, integrin α7β1), the DG-binding protein dystrophin, and the stromal interface protein collagen VI. These components are connected through linkage.
Another set of laminin disorders is seen in Herlitz junctional epidermolysis bullosa, a lethal blistering disease resulting from splitting of the epidermal BM and arising from mutations of the LAMA3, LAMB3 or LAMC2 [19]. Skin blistering diseases also include those resulting from ruptures at the level or keratin (EB simplex, keratin gene mutations) and anchoring fibrils (EB dystrophica, collagen VII) [28,29]. Notably, collagen VII is elevated in patients with systemic sclerosis [43]. Both MDC1A and Herlitz disease can be viewed as primarily resulting from a loss of BM integrity in which a transverse link from stroma through BM to receptor to cytoskeleton has been broken.
Mutations of the LAMB2 gene cause Pierson syndrome. Pierson syndrome is a rare form of focal segmental glomerulosclerosis in which patients develop proteinuria, eye abnormalities that include microcoria, and sometimes defects of the neuromuscular junction [20] (Table 1). Milder forms of the disease are seen with in-frame point mutations affecting the LN domain. The proteinuria occurs before onset of foot-process effacement, evidence that the glomerular BM contributes to perm-selective filtration in the kidney.
A major genetic defect in collagen IV is the cause of Alport syndrome. Most commonly, the disease is X-linked resulting from mutations of the COL4A5 gene. However, autosomal transmitted forms are seen as well resulting from mutations of COL4A4 and COL4A3 (Table 1). The disease is characterized by the nephritic syndrome (hematuria + proteinuria) and associated hearing deficits. The renal glomerular BM has a basket-weave appearance with focal attenuations at the ultrastructural level. At the light histological level, glomerulonephritis with crescent formation is present.
Glycine substitutions in COLA41, resulting in reduced secretion, can cause porencephaly (degenerative brain cavities in newborn infants) and cerebral hemorrhage [22]. Humans with COLA41 mutations were more recently found to develop small vessel disease and hemorrhagic stroke [23]. It was proposed that that mutation of COL4A1 might cause a spectrum of cerebrovascular phenotypes in which COL4A1 mutations may be predisposed to hemorrhage.
Notably, Gly missense mutations in COL6A1 gene causes two forms of congenital myopathy (Table 1): Ulrich congenital muscular dystrophy with severe hypotonia starting in early childhood, and Bethlehem myopathy, a milder form with joint laxity and hypotonia [27]. In Table 1, we have listed also genetic diseases of COL17A1, although not a strict constituent of BMs. Unlike most collagens, collagen XVII is a transmembrane protein and a structural component of hemidesmosomes. Missense and nonsense mutations in this gene are associated with epithelial recurrent erosion syndrome [30,31] and junctional epidermolysis bullosa [32], both congenital diseases that affect BM integrity.
HSPGs include collagen XVIII, perlecan and agrin. Genetic deficiencies linked to COL18A1 gene are generally due to reduced production or changes in the C-terminal region of the chain (Table 1). Knobloch syndrome is characterized by ocular defects associated with high myopia, vitreoretinal degeneration and occipital encephalocele [33–35]. Changes in the C-terminal portion of the chain result in duplication of the renal collecting system [36]. Thus, fine alterations in collagen XVIII structure and protein levels can lead to aberrant and diverse phenotypes.
Perlecan is one of the few gene products expressed by both vascular and non-vascular tissue [44], and can act as a strong elastic tether [45] as well as a modulator of cell adhesion, proliferation and growth factor signaling [46]. For example, perlecan is found as an HSPG in BMs of most if not all the vascularized organs, but is also expressed as a hybrid HS/chondroitin sulfate proteoglycan in cartilage [47,48]. Perlecan has a multi-domain protein core, including critical C-terminal LG domains with α-dystroglycan and integrin-binding activities, important for developmental and tumor angiogenesis [49–53]. Indeed, the C-terminal domain of perlecan, termed endorepellin, is processed by various proteases involved in multiple biological functions, and is anti-angiogenic [54–60] and can be pro-autophagic [61,62]. In contrast, the parent molecule perlecan can exert anti-autophagic functions [63,64].
Mutations in gene encoding perlecan (HSPG2) cause dyssegmental dysplasia Silver-Handmaker (DDSH) type. The pathogenetic mechanism of DDSH lies on genetic duplication of exon 34 and frameshifts. This leads to a functionally null protein causing altered FGF2 signaling and subsequent aberrant cartilage architecture [37–39]. Mutations in HSPG2 also cause Schwartz-Jampel syndrome (SJS), also known as chondrodystrophic myotonia, a disorder characterized by myotonia and skeletal chondrodysplasia [40,65]. The myotonia in SJS patients results from loss of the role of perlecan AChE-binding HS chains at the neuromuscular junction while the skeletal dysplasia results from loss of the CS-E chains that regulate collagen II assembly in cartilage [65].
Mutations in the gene encoding agrin (AGRN) cause congenital myasthenic syndrome [41,42]. Pathogenetically, missense and nonsense mutations at the N-terminus and LG2 domain lead to pre- and post-synaptic failure of neuromuscular transmission. Consequently, these infants exhibit muscle weakness (myasthenia) that worsens during physical exertion.
There is central and recurrent theme in BM biology, that is, the ubiquitous processing of BMs constituents by various proteases, especially those belonging to matrix metalloproteinases and bone morphogenetic proteins [66–72]. This limited proteolytic process generates fragments full of biological activity. Examples are endorepellin [73] and endostatin, derived from partial processing of the C-termini of perlecan [74] and collagen XVIII [75], respectively. Also fragments of perlecan domain IV have been recently described in advanced forms of prostate cancer [76]. Intriguingly, peptides from perlecan domain IV support cell adhesion and spreading [77], potentially acting as pro-invasive factors.
Neural and non-neural (muscle) agrins, derived from a single alternatively spliced gene, are BM HSPGs with an N-terminal laminin-binding NtA domain, intervening follistatin, S/T and SEA domains, and a C-terminal complex of 3 LG domains spaced by EGF-like modules [78]. The neural splice variant (A8B8), secreted by peripheral nerve termini, is critical for recruitment of post-synaptic acetylcholine receptors at the neuromuscular junction, whereas non-neural (“muscle”) agrin that lacks the A/B sequence inserts is widely expressed. The normal functions of non-neural agrin are less well understood. Agrin LG domains bind strongly to αDystroglycan and weakly to integrin α3β1 [79,80]. Notably, an internally shortened non-neural agrin (“miniagrin”), that consists of the laminin-binding NtA domain and the C-terminal three LG domains, partially rescues the dyW dystrophic phenotype [81]. Mechanistically, this rescue could be due to enabling the compensating Lm411 to now bind αDG. This miniagrin enables Lm111 lacking any LG domains to assemble a BM on Schwann cells [82]. This has led to the proposal that non-neural agrin acts as a “collateral linker” by binding to the coiled-coil of laminins and to αLG [12].
Agrin LG3 domain has been found, together with the LG3 domain of perlecan, in the urine [83], and is also released by neurotrypsin at the synapse [84]. Perlecan LG3 domain has been found in many biological fluids including blood, urine and cerebrovascular fluids [83,85–87]. Interestingly, LG3 could represent a biomarker for breast cancer detection as low levels of LG3 correlate with tumor progression and invasion [88].
Can we answer some of the key questions in basement membrane biology?
So far we have dwelled on demonstrating how BMs are important for the maintenance of tissue homeostasis and how they function in various diseases processes. In this special issue a number of leading investigators of various aspects of BM biology will attempt to answer some biologically-relevant questions. Compositionally, BMs are tissue and organ specific, as clearly shown by proteomic analysis of BMs isolated from various tissues (Randles MJ et al.). The demonstration that BMs are important for the maintenance of tissue homeostasis comes from the finding that mutations in BM genes lead to complex and severe phenotypes. To this end, mutations in the COL4A1 and COL4A2 chains have been associated to multisystem disorders with abnormalities in the vasculature, brain, eyes, kidneys, and muscles. Genotype phenotype correlations from more than 100 COL4A1 and COL4A2 mutations in patients and more than 15 Col4a1 and Col4a2 mutations in mice show that triple-helical glycine substitutions are the most frequent classes of mutations. Interestingly, the position of the mutation rather than the type of the substituting amino acid seems to be important in determining the severity of the disease (Jeanne M and Gould DB). Mutations in any of three collagen IV genes, COL4A3, COL4A4, or COL4A5 lead to Alport syndrome, a disease characterized by kidney disease, hearing loss, and eye abnormalities (Cosgrove D and Liu S). In addition to collagen IV, collagen XVIII is another ubiquitously expressed BM component and mutations in COL18A1 gene result in Knobloch syndrome (Table 1). Studies with gene-modified mice have elucidated some aspects of this rare disease and have highlighted the importance of collagen XVIII in the development of the eye and its function in normal situations and tissue dysregulation (Heliasvaara R et al.). Finally, mutations in collagen VII, a matrix component primarily expressed in the BMs of human skin, corneal epithelium, and gastrointestinal mucous membranes, have been associated to Dystrophic Epidermolysis Bullosa (Uitto et al.).
In addition to collagen, mutations in laminins are also associated with disease. Laminin α4, α5, and β2 chains specifically localize to the neuromuscular junction where they regulate the proper alignment of presynaptic and post-synaptic structures of motor neurons and muscle fibers. Mutations of these laminin genes lead to neuromuscular diseases including Pierson syndrome and Lambert-Eaton myasthenic syndrome as summarized in Table 1 and reviewed by (Rogers RS and Nishimune H). In vitro studies have revealed that laminin substrata “instruct” myotubes on where and how to form synapses. Interestingly, culturing myotubes on substrate bound laminin targets the formation of complex acetylcholine receptor aggregates to the lower cell surface via engagement of the receptor tyrosine kinase MuSK (Vezina-Audette et al). Although for most of these genetic BM linked diseases there are limited treatment options, cell-based therapeuthic approaches based on delivering pluripotent and/or differentiated cells are emerging as a plausible and promising treatment for these devastating pathologies (Nyström et al.).
In addition to mutations in genes encoding BM components, changes in the composition of the BMs can also lead to pathological conditions, indicating that the type of BM component is a key regulator of tissue function. In this context, while normal large arteries usually express the laminin β1 chain, in atherosclerotic plaques that form in the media of large arteries, smooth muscle cells switch from β1 to β2 chain and produce laminin α5 chain. This results in a switch of behavior with smooth muscle cells acquiring a more pronounced proliferative and migratory phenotype (Di Russo J et al.).
BM components are also involved in auto-immune diseases and tumorigenesis. To this end, epitopes on collagens and laminins are targeted by auto-antibodies and lymphocytes in Goodpasture’s disease, rheumatoid arthritis, post-lung transplant bronchiolitis obliterans syndrome, and multiple autoimmune dermatoses (Foster MH). In the context of tumorigenesis, collagen XIX, a minor collagen that belongs to the FACIT family, plays an important role in preventing tumor cell invasion by inhibiting the formation of new blood vessels (Oudart JB et al.). In order to invade and metastasize, tumor cells need to cross the BM that separates them from the vascular bed. BM invasion by malignant cells is influenced by crosstalk between three inter-related factors: extracellular matrix stiffening, epithelial cytoskeletal contractility, and growth factor/cytokine signaling (Chang TT et al.). Epithelial contractility induces greater stromal stiffening as well as formation of invadosomes, specialized regions of cells rich in proteases. These cell structures sense and transduce forces that promote extracellular matrix degradation by targeting protease expression and activity at invadosomes (Mrkonjic S et al.).
The most common defect of BMs is seen in longstanding diabetes mellitus, causing almost pathognomonic thickening of microvascular BMs in the retina, peripheral nerve and kidney glomerulus with associated blindness, neuropathy and proteinuria/chronic renal failure. Diabetic nephropathy is the leading cause of chronic kidney disease in the U.S. and is often seen in combination with cardiovascular disease. The basis for the BM thickening, which precedes proteinuria, is not well understood or studied, but may involve changes in synthesis, degradation/remodeling, and/or glycation and that may be a response to podocyte injury [89].
BM components interact with cells via specific receptors, including integrins, discoidin domain receptors (DDRs), and dystroglycan. These interactions play a key role in initiating various cell functions including extracellular matrix assembly. To this end, assembly of laminin polymers has been proposed to be dependent on the three short arms of the cross-shaped laminin heterotrimer [18,82], but is facilitated by the concentrating effect of binding to cell surface receptors.
Each short arm contains an N-terminal LN polymerization domain followed by laminin-type epidermal growth factor-like (LE) domains interspersed with globular domains. In the β1/β2 subunits, an LF domain of unknown function resides in the middle flanked on either side by LE domains. A recent high-resolution crystal structure of specific laminin β2 region LE domains interrupted by an LF domain has allowed a better understanding on how these domains interact in the short arm thus advising our understanding of laminin structure (Pulido D et al.).
In addition to matrix assembly, cell-matrix interactions are important for the regulation of cell proliferation, migration, adhesion, and extracellular matrix production. Among the matrix receptors, integrin α3β1 is a major laminin receptor. The interaction of this receptor with laminins (mainly Lm332) is essential for the initiation and the maintenance of tumor cell proliferation, while in endothelial cells laminin-integrin α3β1 interaction is associated to decreased endothelial cell proliferation and migration (Ramovs V et al.). Another laminin receptor is the integrin α6β4. This receptor is localized to hemi-desmosomes and is critical for the establishment of tight adhesions to laminin substrata. Loss of either the α6 or β4 subunit in the collecting duct of the kidney results in increased susceptibility to injury (Viquez O et al.). Besides integrins, DDRs, a class of special receptor tyrosine kinases that interact with and are activated by collagens, play a significant role. DDR1, for example, binds both fibrillar and non-fibrillar collagens (e.g. collagen IV) and this interaction triggers receptor activation and promotes collagen IV production (Borza CM et al.). A third class of BM receptors are dystroglycans that together with integrins play a key role in mediating laminin assembly and polarization of epiblasts in early embryogenesis (Li S et al.). This study suggests that integrins and dystroglycans can compensate with each other in promoting laminin assembly.
Finally, key components of BM are HSPGs. Perlecan is a large HSPG expressed by many different cell types, including endothelial cells. The C-terminal domain of perlecan, named endorepellin, plays an anti-angiogenic role by interacting with VEGFR2 and is pro-autophagic by promoting the formation of LC3-positive autophagosomes independently of nutrition deprivation (Gubbiotti MA et al.). Another beneficial effect of HSPGs is observed in the renal glomeruli where they modulate local complement activation by recruiting factor H, which in turn selectively inactivates C3b, thereby limiting complement activation. Thus, HS/factor H interaction might be beneficial in preventing and/or reducing immuno-mediated kidney diseases such as membranous nephropathy and lupus nephritis (Borza DB). HSPGs also serve as a reservoir for HS-binding growth factors, including members of the FGF family and facilitate the interaction between these growth factors and their receptors. Thus, HSPGs orchestrate growth factor availability and activity, two key important factors in organogenesis and branching morphogenesis (Patel VN et al.).
The identification of physiological and pathological roles of BM components has been made possible primarily by the generation of mutant mice lacking key BM components either globally or in selective organs. More recently, synthetic cytocompatible three-dimensional hydrogels that carry BM-like bioactive motifs have been developed and used to study specific BM-cell interactions and BM degradation (Cruz-Acuña R and García AJ). Another promising source to study BM functions is the use of decellularized organs from discarded human organs or mice. One advantage of this technique is that it preserves the three-dimensional structure of ECM matrix scaffolds. In addition, organs from healthy and diseased donors can be used thus allowing the analysis of cell function, differentiation, immune responses in health and disease (Petrosyan A et al.). Finally, these organs can offer an excellent source for the analysis of their physical and mechanical properties. These properties are influenced by many factors, including cell type, developmental stage of the organ, degree of disease, type of BM components, as well as the temporal expression of ‘BM toolkits’ such as BM binding proteins and/or enzymes able to modify and crosslink BM components (Jones-Paris CR et al.). The analysis of the physical and mechanical properties of BMs in isolated organs might enable a better understanding of the physical environment experienced by specific cell types and to selectively control cell behavior in vivo and for tissue engineering (Miller RT).
Future research questions and directions
Although we are beginning to gain a systematic understanding of BM in biology, many challenges face us going forward. For example, we have a poor understanding of BM turnover, particularly during embryogenesis and following injury. We need to better understand the reason for having different isoforms of laminins, nidogens, and collagen IV during and after embryonic development. BM proteins all have multiple domains with functions assigned to a minority of loci. Are the other domains simply spacer domains, or are there many new interactions that still need to be discovered? We are only beginning to comprehend mechano-chemical signaling as it applies to BM-cell interactions. How, for example, is viscoelasticity of the BM controlled? Is the principle function of the different integrin specificities one of controlling the ligand affinities and collective avidities that in turn affect signaling strength? We need to learn if our comprehension of BM assembly mechanisms and resulting structure can be used to intervene in those diseases that result from BM defects. Biomaterials can be altered with BM-derived constituents to alter their cell interactions. How should this be accomplished? BM components can also be used to affect differentiation of cells ex vivo for the purpose of their introduction into patients, thereby providing missing or overlooked functions. How can we collectively accomplish these tasks?
In conclusion, this special edition provides an up-to-date and hopefully informative compendium of articles focused on this interesting subject that affects all levels of biology. BMs are active participants in the regulation of cell functions in healthy and diseased organs, a sort of cliche’ statement that is true: life without basement membranes is impossible, and life with an abnormal basement membrane is quite difficult and full of obstacles and perils. Just thinking of diabetic nephropathy and lupus nephritis. A better understanding of how BMs are assembled, interact with cells, cue signals to cells, and help in the maintenance of organ shape and size might enable us to better target BM components in disease, and perhaps find new therapeutic strategies to combat genetic and acquired BM diseases.
Highlights.
Basement membranes have reached center stage due to their involvement in many physiological and pathological conditions
Genetic diseases of basement membranes are debilitating and affect many organs resulting in diverse phenotypes
We provide an overview of the field and discuss developmental, structural and biochemical aspects of basement membranes
We introduce the special issue and outline key components of basement membrane biology
Acknowledgments
The original research was supported in part by a Veteran’s Affairs Merit Awards 1I01BX002025 (to AP), the National Institutes of Health Grants, R01DK095761 (to AP), R01/R37 DK36425 (to PDY), and by the National Institutes of Health grants RO1 CA39481, RO1 CA47282, and RO1 CA164462 (to RVI). AP is the recipient of a VA Senior Research Career Scientist.
Abbreviations used
- BM
basement membrane
- ECM
extracellular matrix
- HS
heparan sulfate
- HSPG
heparan sulfate proteoglycan
- MMP
matrix metalloproteinase
- MDC1A
Lmα2/merosin-deficient congenital muscular dystrophy
- DDSH
dyssegmental dysplasia Silverman-Handmaker type
- SJS
Schwartz-Jampel syndrome
- DDR
discodin domain receptor
- AChE
acetylcholinesterase
- EB
epidermolysis bullosa
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bowman W. On the minute structure and movements of voluntary muscle. Phil Trans R Soc London Biol Sci. 1840;130:457–494. [Google Scholar]
- 2.Naba A, Clauser KR, Ding H, Whittaker CA, Carr SA, Hynes RO. The extracellular matrix: Tools and insights for the “omics” era. Matrix Biol. 2016;49:10–24. doi: 10.1016/j.matbio.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy-Ullrich JE, Sage EH. Revisiting the matricellular concept. Matrix Biol. 2014;37:1–14. doi: 10.1016/j.matbio.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nichols SA, Roberts BW, Richter DJ, Fairclough SR, King N. Origin of metazoan cadherin diversity and the antiquity of the classical cadherin/beta-catenin complex. Proc Natl Acad Sci U S A. 2012;109:13046–13051. doi: 10.1073/pnas.1120685109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fahey B, Degnan BM. Origin and evolution of laminin gene family diversity. Mol Biol Evol. 2012;29:1823–1836. doi: 10.1093/molbev/mss060. [DOI] [PubMed] [Google Scholar]
- 6.Yurchenco PD, Amenta PS, Patton BL. Basement membrane assembly, stability and activities observed through a developmental lens. Matrix Biol. 2004;22:521–538. doi: 10.1016/j.matbio.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol. 2004;20:255–284. doi: 10.1146/annurev.cellbio.20.010403.094555. [DOI] [PubMed] [Google Scholar]
- 8.Smyth N, Vatansever HS, Murray P, Meyer M, Frie C, Paulsson M, et al. Absence of basement membranes after targeting the LAMC1 gene results in embryonic lethality due to failure of endoderm differentiation. J Cell Biol. 1999;144:151–160. doi: 10.1083/jcb.144.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yurchenco PD, Patton BL. Developmental and pathogenic mechanisms of basement membrane assembly. Curr Pharm Des. 2009;15:1277–1294. doi: 10.2174/138161209787846766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khoshnoodi J, Pedchenko V, Hudson BG. Mammalian collagen IV. Microsc Res Tech. 2008;71:357–370. doi: 10.1002/jemt.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Randles MJ, Humphries MJ, Lennon R. Proteomic definitions of basement membrane composition in health and disease. Matrix Biol. 2017 doi: 10.1016/j.matbio.2016.08.006. In Press. [DOI] [PubMed] [Google Scholar]
- 12.Yurchenco PD. Basement membranes: cell scaffoldings and signaling platforms. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a004911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleger-Weckmann A, Ustun Y, Kloepper J, Paus R, Bloch W, Chen ZL, et al. Deletion of the epidermis derived laminin gamma1 chain leads to defects in the regulation of late hair morphogenesis. Matrix Biol. 2016;56:42–56. doi: 10.1016/j.matbio.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Wegner J, Loser K, Apsite G, Nischt R, Eckes B, Krieg T, et al. Laminin alpha5 in the keratinocyte basement membrane is required for epidermal-dermal intercommunication. Matrix Biol. 2016;56:24–41. doi: 10.1016/j.matbio.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Sun Z, Guo SS, Fassler R. Integrin-mediated mechanotransduction. J Cell Biol. 2016;215:445–456. doi: 10.1083/jcb.201609037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 17.Jimenez-Mallebrera C, Brown SC, Sewry CA, Muntoni F. Congenital muscular dystrophy: molecular and cellular aspects. Cell Mol Life Sci. 2005;62:809–823. doi: 10.1007/s00018-004-4510-4. [DOI] [PubMed] [Google Scholar]
- 18.Yurchenco PD. Integrating Activities of Laminins that Drive Basement Membrane Assembly and Function. Curr Top Membr. 2015;76:1–30. doi: 10.1016/bs.ctm.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Nakano A, Chao SC, Pulkkinen L, Murrell D, Bruckner-Tuderman L, Pfendner E, et al. Laminin 5 mutations in junctional epidermolysis bullosa: molecular basis of Herlitz vs. non-Herlitz phenotypes. Hum Genet. 2002;110:41–51. doi: 10.1007/s00439-001-0630-1. [DOI] [PubMed] [Google Scholar]
- 20.Matejas V, Hinkes B, Alkandari F, Al-Gazali L, Annexstad E, Aytac MB, et al. Mutations in the human laminin beta2 (LAMB2) gene and the associated phenotypic spectrum. Hum Mutat. 2010;31:992–1002. doi: 10.1002/humu.21304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gale DP, Oygar DD, Lin F, Oygar PD, Khan N, Connor TM, et al. A novel COL4A1 frameshift mutation in familial kidney disease: the importance of the C-terminal NC1 domain of type IV collagen. Nephrol Dial Transplant. 2016;31:1908–1914. doi: 10.1093/ndt/gfw051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gould DB, Phalan FC, Breedveld GJ, van Mil SE, Smith RS, Schimenti JC, et al. Mutations in Col4a1 cause perinatal cerebral hemorrhage and porencephaly. Science. 2005;308:1167–1171. doi: 10.1126/science.1109418. [DOI] [PubMed] [Google Scholar]
- 23.Gould DB, Phalan FC, van Mil SE, Sundberg JP, Vahedi K, Massin P, et al. Role of COL4A1 in small-vessel disease and hemorrhagic stroke. N Engl J Med. 2006;354:1489–1496. doi: 10.1056/NEJMoa053727. [DOI] [PubMed] [Google Scholar]
- 24.Alavi MV, Mao M, Pawlikowski BT, Kvezereli M, Duncan JL, Libby RT, et al. Col4a1 mutations cause progressive retinal neovascular defects and retinopathy. Sci Rep. 2016;6:18602. doi: 10.1038/srep18602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeanne M, Gould DB. Genotype-phenotype correlations in pathology caused by collagen type IV alpha 1 and 2 mutations. Matrix Biol. 2017 doi: 10.1016/j.matbio.2016.10.003. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cosgrove D, Liu S. Collagen IV diseases: A focus on the glomerular basement membrane in Alport syndrome. Matrix Biol. 2017 doi: 10.1016/j.matbio.2016.08.005. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Butterfield RJ, Foley AR, Dastgir J, Asman S, Dunn DM, Zou Y, et al. Position of glycine substitutions in the triple helix of COL6A1, COL6A2, and COL6A3 is correlated with severity and mode of inheritance in collagen VI myopathies. Hum Mutat. 2013;34:1558–1567. doi: 10.1002/humu.22429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryynänen M, Ryynänen J, Solberg S, Iozzo RV, Knowlton RG, Uitto J. Genetic linkage of Type VII collagen (COL7A1) to dominant dystrophic epidermolysis bullosa in families with abnormal anchoring fibrils. J Clin Invest. 1992;89:974–980. doi: 10.1172/JCI115680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vahidnezhad H, Youssefian L, Zeinali S, Saeidian AH, Sotudeh S, Mozafari N, et al. Dystrophic Epidermolysis Bullosa: COL7A1 Mutation Landscape in a Multi-Ethnic Cohort of 152 Extended Families with High Degree of Customary Consanguineous Marriages. J Invest Dermatol. 2016 doi: 10.1016/j.jid.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 30.Oliver VF, van Bysterveldt KA, Cadzow M, Steger B, Romano V, Markie D, et al. A COL17A1 Splice-Altering Mutation Is Prevalent in Inherited Recurrent Corneal Erosions. Ophthalmology. 2016;123:709–722. doi: 10.1016/j.ophtha.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 31.Jonsson F, Bystrom B, Davidson AE, Backman LJ, Kellgren TG, Tuft SJ, et al. Mutations in collagen, type XVII, alpha 1 (COL17A1) cause epithelial recurrent erosion dystrophy (ERED) Hum Mutat. 2015;36:463–473. doi: 10.1002/humu.22764. [DOI] [PubMed] [Google Scholar]
- 32.Bauer JW, Lanschuetzer C. Type XVII collagen gene mutations in junctional epidermolysis bullosa and prospects for gene therapy. Clin Exp Dermatol. 2003;28:53–60. doi: 10.1046/j.1365-2230.2003.01192.x. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki O, Kague E, Bagatini K, Tu H, Heljasvaara R, Carvalhaes L, et al. Novel pathogenic mutations and skin biopsy analysis in Knobloch syndrome. Mol Vis. 2009;15:801–809. [PMC free article] [PubMed] [Google Scholar]
- 34.Heljasvaara R, Aikio M, Ruotsalainen H, Pihlajaniemi T. Collagen XVIII in tissue homeostasis and dysregulation - Lessons learned from model organisms and human patients. Matrix Biol. 2017 doi: 10.1016/j.matbio.2016.10.002. In Press. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki OT, Sertie AL, Der KV, Kok F, Carpenter M, Murray J, et al. Molecular analysis of collagen XVIII reveals novel mutations, presence of a third isoform, and possible genetic heterogeneity in Knobloch syndrome. Am J Hum Genet. 2002;71:1320–1329. doi: 10.1086/344695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin Y, Zhang S, Rehn M, Itaranta P, Tuukkanen J, Heljasvaara R, et al. Induced repatterning of type XVIII collagen expression in ureter bud from kidney to lung type: association with sonic hedgehog and ectopic surfactant protein C. Development. 2001;128:1573–1585. doi: 10.1242/dev.128.9.1573. [DOI] [PubMed] [Google Scholar]
- 37.Arikawa-Hirasawa E, Wilcox WR, Le AH, Silverman N, Govindraj P, Hassell JR, et al. Dyssegmental dysplasia, Silverman-Handmaker type, is caused by functional null mutations of the perlecan gene. Nat Genet. 2001;27:431–434. doi: 10.1038/86941. [DOI] [PubMed] [Google Scholar]
- 38.Arikawa-Hirasawa E, Le AH, Nishino I, Nonaka I, Ho NC, Francomano CA, et al. Structural and functional mutations of the perlecan gene cause Schwartz-Jampel syndrome, with myotonic myopathy and chondrodysplasia. Am J Hum Genet. 2002;70:1368–1375. doi: 10.1086/340390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arikawa-Hirasawa E, Wilcox WR, Yamada Y. Dyssegmental dysplasia, Silverman-Handmaker type: unexpected role of perlecan in cartilage development. Am J Med Genet. 2001;106:254–257. doi: 10.1002/ajmg.10229. [DOI] [PubMed] [Google Scholar]
- 40.Nicole S, Davoine C-S, Topaloglu H, Cattolico L, Barral D, Beighton P, et al. Perlecan, the major proteoglycan of basement membranes, is altered in patients with Schwartz-Jampel syndrome (chondrodystrophic myotonia) Nat Genet. 2000;26:480–483. doi: 10.1038/82638. [DOI] [PubMed] [Google Scholar]
- 41.Huze C, Bauche S, Richard P, Chevessier F, Goillot E, Gaudon K, et al. Identification of an agrin mutation that causes congenital myasthenia and affects synapse function. Am J Hum Genet. 2009;85:155–167. doi: 10.1016/j.ajhg.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maselli RA, Fernandez JM, Arredondo J, Navarro C, Ngo M, Beeson D, et al. LG2 agrin mutation causing severe congenital myasthenic syndrome mimics functional characteristics of non-neural (z-) agrin. Hum Genet. 2012;131:1123–1135. doi: 10.1007/s00439-011-1132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rudnicka L, Varga J, Christiano AM, Iozzo RV, Jimenez SA, Uitto J. Elevated expression of type VII collagen in the skin of patients with systemic sclerosis. J Clin Invest. 1994;93:1709–1715. doi: 10.1172/JCI117154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCarthy KJ. The basement membrane proteoglycans perlecan and agrin: Something old, something new. Curr Top Membr. 2015;76:255–303. doi: 10.1016/bs.ctm.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 45.Wijeratne SS, Martinez JR, Grindel BJ, Frey EW, Li J, Wnag K, et al. Single molecule force measurements of perlecan/HSPG2: A key component of the osteocyte pericellular matrix. Matrix Biol. 2016;50:27–38. doi: 10.1016/j.matbio.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lord MS, Chuang CY, Melrose J, Davies MJ, Iozzo RV, Whitelock JM. The role of vascular-derived perlecan in modulating cell adhesion, proliferation and growth factor signaling. Matrix Biol. 2014;35:112–122. doi: 10.1016/j.matbio.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iozzo RV. Basement membrane proteoglycans: from cellar to ceiling. Nat Rev Mol Cell Biol. 2005;6:646–656. doi: 10.1038/nrm1702. [DOI] [PubMed] [Google Scholar]
- 48.Iozzo RV, Schaefer L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015;42:11–55. doi: 10.1016/j.matbio.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zoeller JJ, McQuillan A, Whitelock J, Ho S-Y, Iozzo RV. A central function for perlecan in skeletal muscle and cardiovascular development. J Cell Biol. 2008;181:381–394. doi: 10.1083/jcb.200708022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zoeller JJ, Whitelock J, Iozzo RV. Perlecan regulates developmental angiogenesis by modulating the VEGF-VEGFR2 axis. Matrix Biol. 2009;28:284–291. doi: 10.1016/j.matbio.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iozzo RV, Zoeller JJ, Nyström A. Basement membrane proteoglycans: Modulators par excellence of cancer growth and angiogenesis. Mol Cells. 2009;27:503–513. doi: 10.1007/s10059-009-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baghy K, Tatrai P, Regos E, Kovalszky I. Proteoglycans in liver cancer. World J Gastroenterol. 2016;22:379–393. doi: 10.3748/wjg.v22.i1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Farach-Carson MC, Warren CR, Harrington DA, Carson DD. Border patrol:Insights into the unique role of perlecan/heparan sulfate proteoglycan 2 at cell and tissue borders. Matrix Biol. 2014;34:64–79. doi: 10.1016/j.matbio.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bix G, Fu J, Gonzalez E, Macro L, Barker A, Campbell S, et al. Endorepellin causes endothelial cell disassembly of actin cytoskeleton and focal adhesions through the α2β1 integrin. J Cell Biol. 2004;166:97–109. doi: 10.1083/jcb.200401150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bix G, Castello R, Burrows M, Zoeller JJ, Weech M, Iozzo RA, et al. Endorepellin in vivo: targeting the tumor vasculature and retarding cancer growth and metabolism. J Natl Cancer Inst. 2006;98:1634–1646. doi: 10.1093/jnci/djj441. [DOI] [PubMed] [Google Scholar]
- 56.Bix G, Iozzo RV. Matrix revolutions: “tails” of basement-membrane components with angiostatic functions. Trends Cell Biol. 2005;15:52–60. doi: 10.1016/j.tcb.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 57.Woodall BP, Nyström A, Iozzo RA, Eble JA, Niland S, Krieg T, et al. Integrin α2β1 is the required receptor for endorepellin angiostatic activity. J Biol Chem. 2008;283:2335–2343. doi: 10.1074/jbc.M708364200. [DOI] [PubMed] [Google Scholar]
- 58.Goyal A, Pal N, Concannon M, Paulk M, Doran M, Poluzzi C, et al. Endorepellin, the angiostatic module of perlecan, interacts with both the α2β1 integrin and vascular endothelial growth factor receptor 2 (VEGFR2) J Biol Chem. 2011;286:25947–25962. doi: 10.1074/jbc.M111.243626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Douglass S, Goyal A, Iozzo RV. The role of perlecan and endorepellin in the control of tumor angiogenesis and endothelial cell autophagy. Connect Tissue Res. 2015;19:1–11. doi: 10.3109/03008207.2015.1045297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Poluzzi C, Iozzo RV, Schaefer L. Endostatin and endorepellin: A common route of action for similar angiostatic cancer avengers. Adv Drug Deliv Rev. 2016;97:156–173. doi: 10.1016/j.addr.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Poluzzi C, Casulli J, Goyal A, Mercer TJ, Neill T, Iozzo RV. Endorepellin evokes autophagy in endothelial cells. J Biol Chem. 2014;289:16114–16128. doi: 10.1074/jbc.M114.556530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goyal A, Gubbiotti MA, Chery DR, Han L, Iozzo RV. Endorepellin-evoked autophagy contributes to angiostasis. J Biol Chem. 2016;291:19245–19256. doi: 10.1074/jbc.M116.740266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ning L, Xu Z, Furuya N, Nonaka R, Yamada Y, Arikawa-Hirasawa E. Perlecan inhibits autophagy to maintain muscle homeostasis in mouse soleus muscle. Matrix Biol. 2015;48:26–35. doi: 10.1016/j.matbio.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 64.Gubbiotti MA, Iozzo RV. Proteoglycans regulate autophagy via outside-in signaling: An emerging new concept. Matrix Biol. 2015;48:6–13. doi: 10.1016/j.matbio.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kvist AJ, Johnson AE, Mörgelin M, Gustafsson E, Bengtsson E, Lindblom K, et al. Chondroitin sulfate perlecan enhances collagen fibril formation. Implications for perlecan chondrodysplasias. J Biol Chem. 2006;281:33127–33139. doi: 10.1074/jbc.M607892200. [DOI] [PubMed] [Google Scholar]
- 66.Arpino V, Brock M, Gill SE. The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol. 2015;44–46:247–254. doi: 10.1016/j.matbio.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 67.Wells JM, Gaggar A, Blalock JE. MMP generated matrikines. Matrix Biol. 2015;44–46:122–129. doi: 10.1016/j.matbio.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Deryugina EI, Quigley JP. Tumor angiogenesis: MMP-mediated induction of intravasation- and metastasis-sustaining neovasculature. Matrix Biol. 2015;44–46:94–112. doi: 10.1016/j.matbio.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rohani MG, Parks WC. Matrix remodeling by MMPs during wound repair. Matrix Biol. 2015;44–46:113–121. doi: 10.1016/j.matbio.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 70.Duarte S, Baber J, Fujii T, Coito AJ. Matrix metalloproteinases in liver injury, repair and fibrosis. Matrix Biol. 2015;44–46:147–156. doi: 10.1016/j.matbio.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eckhard U, Huesgen PF, Schilling O, Bellac CL, Butler GS, Cox JH, et al. Active site specificity profiling of the matrix metalloproteinase family: Proteomic identification of 4300 cleavage sites by nine MMPs explored with structural and synthetic peptide cleavage analyses. Matrix Biol. 2016;49:37–60. doi: 10.1016/j.matbio.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 72.Muir AM, Massoudi D, Nguyen N, Keene DR, Lee SJ, Birk DE, et al. BMP1-like proteinases are essential to the structure and wound healing of skin. Matrix Biol. 2016;56:114–131. doi: 10.1016/j.matbio.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mongiat M, Sweeney S, San Antonio JD, Fu J, Iozzo RV. Endorepellin, a novel inhibitor of angiogenesis derived from the C terminus of perlecan. J Biol Chem. 2003;278:4238–4249. doi: 10.1074/jbc.M210445200. [DOI] [PubMed] [Google Scholar]
- 74.Gonzalez EM, Reed CC, Bix G, Fu J, Zhang Y, Gopalakrishnan B, et al. BMP-1/Tolloid-like metalloproteases process endorepellin, the angiostatic C-terminal fragment of perlecan. J Biol Chem. 2005;280:7080–7087. doi: 10.1074/jbc.M409841200. [DOI] [PubMed] [Google Scholar]
- 75.O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 76.Grindel B, Li Q, Arnold R, Petros J, Zayzafoon M, Muldoon M, et al. Perlecan/HSPG2 and matrilysin/MMP-7 as indices of tissue invasion: tissue localization and circulating perlecan fragments in a cohort of 288 radical prostatectomy patients. Oncotarget. 2016;7:10433–10447. doi: 10.18632/oncotarget.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Farach-Carson MC, Brown AC, Lynam M, Safran JB, Carson DD. A novel peptide sequence in perlecan domain IV supports cell adhesion, spreading and FAK activation. Matrix Biol. 2008;27:150–160. doi: 10.1016/j.matbio.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bezakova G, Rüegg MA. New insights into the roles of agrin. Nature Rev Mol Cell Biol. 2003;4:295–308. doi: 10.1038/nrm1074. [DOI] [PubMed] [Google Scholar]
- 79.Gesemann M, Brancaccio A, Schumacher B, Ruegg MA. Agrin is a high-affinity binding protein of dystroglycan in non-muscle tissue. J Biol Chem. 1998;273:600–605. doi: 10.1074/jbc.273.1.600. [DOI] [PubMed] [Google Scholar]
- 80.McKee KK, Yang DH, Patel R, Chen ZL, Strickland S, Takagi J, et al. Schwann cell myelination requires integration of laminin activities. J Cell Sci. 2012;125:4609–4619. doi: 10.1242/jcs.107995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moll J, Barzaghi P, Lin S, Bezakova G, Lochmuller H, Engvall E, et al. An agrin minigene rescues dystrophic symptoms in a mouse model for congenital muscular dystrophy. Nature. 2001;413:302–307. doi: 10.1038/35095054. [DOI] [PubMed] [Google Scholar]
- 82.McKee KK, Capizzi S, Yurchenco PD. Scaffold-forming and adhesive contributions of synthetic laminin-binding proteins to basement membrane assembly. J Biol Chem. 2009;284:8984–8994. doi: 10.1074/jbc.M809719200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thadikkaran L, Crettaz D, Siegenthaler MA, Gallot D, Sapin V, Iozzo RV, et al. The role of proteomics in the assessment of premature rupture of fetal membranes. Clin Chim Acta. 2005;360:27–36. doi: 10.1016/j.cccn.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 84.Stephan A, Mateos JM, Kozlov SV, Cinelli P, Kistler AD, Hettwer S, et al. Neurotrypsin cleaves agrin locally at the synapse. FASEB J. 2008;22:1861–1873. doi: 10.1096/fj.07-100008. [DOI] [PubMed] [Google Scholar]
- 85.Oda O, Shinzato T, Ohbayashi K, Takai I, Kunimatsu M, Maeda K, et al. Purification and characterization of perlecan fragment in urine of end-stage renal failure patients. Clin Chim Acta. 1996;255:119–132. doi: 10.1016/0009-8981(96)06395-4. [DOI] [PubMed] [Google Scholar]
- 86.O’Riordan E, Orlova TN, Mendelev N, Patschan D, Kemp R, Chander PN, et al. Urinary proteomic analysis of chronic renal allograft nephropathy. Proteomics Clin Appl. 2008;2:1025–1035. doi: 10.1002/prca.200780137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tsangaris GT, Karamessinis P, Kolialexi A, Garbis SD, Antsaklis A, Mavrou A, et al. Proteomic analysis of amniotic fluid in pregnancies with Down syndrome. Proteomics. 2006;6:4410–4419. doi: 10.1002/pmic.200600085. [DOI] [PubMed] [Google Scholar]
- 88.Chang JW, Kang U-B, Kim DH, Yi JK, Lee JW, Noh D-Y, et al. Identification of circulating endorepellin LG3 fragment: Potential use as a serological biomarker for breast cancer. Proteomics Clin Appl. 2008;2:23–32. doi: 10.1002/prca.200780049. [DOI] [PubMed] [Google Scholar]
- 89.Marshall CB. Rethinking glomerular basement membrane thickening in diabetic nephropathy: adaptive or pathogenic? Am J Physiol Renal Physiol. 2016;311:F831–F843. doi: 10.1152/ajprenal.00313.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]