Abstract
There are few studies about systemic treatment in severe cases of alopecia areata (AA), especially in the pediatric population. Although there is more experience with systemic corticosteroids, recent reports have suggested methotrexate (MTX) as an alternative treatment, with a relatively good outcome. We describe three cases of AA in children treated with MTX, two of them with successful results.
Key words: Alopecia areata, methotrexate, treatment
INTRODUCTION
Alopecia areata (AA) in children is related to a poorer prognosis than in adults.[1] This disease, which can severely affect quality of life, lacks an optimal treatment. Recently, methotrexate (MTX) has been proposed as an alternative treatment for severe cases of AA.[2]
Our objective was to report our experience with this treatment in the pediatric population with AA.
CASE REPORT
We describe three children treated with MTX for AA [Table 1]. Two of them were managed with a dose of 15 mg/week while the third patient started with 7.5 mg/week with progressive increases according to clinical response, with a maximum dose of 17.5 mg/week. The drug was successful in two cases [Figures 1 and 2], but in one of them, it had to be stopped due to an abnormal increase in liver function enzymes.
Table 1.
Patients receiving methotrexate for alopecia areata

Figure 1.
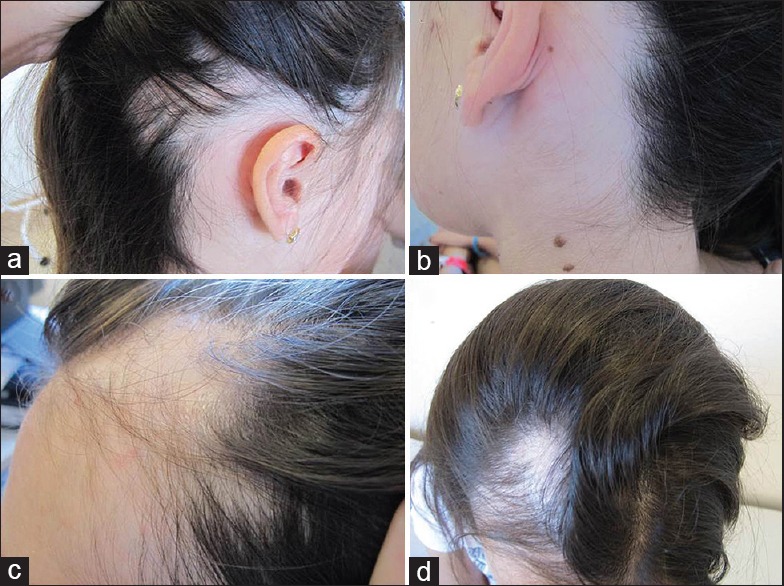
Case 1 – Progressive increase of hair density along methotrexate treatment: (a and c) 6 months after treatment; (b) 12 months after treatment; (d) 24 months after treatment
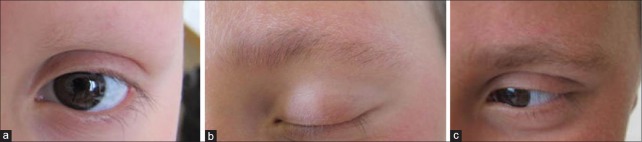
Figure 2.
Case 2 – Successful hair regrowth in the eyebrows: (a) Previous to methotrexate; (b) 2 months after treatment; (c) 4 months after treatment
DISCUSSION
AA is a difficult-to-treat condition. Available treatments could induce hair regrowth, but they do not change the course of the disease.[3]
Few studies about treatment in severe cases of AA include children among their assessed individuals. Topical immunotherapy with diphencyprone,[4] pulses of systemic corticosteroids,[5] psoralen and ultraviolet A,[6] and 308-nm excimer laser[7] have been reported as effective in pediatric patients. In addition, in the last decade, MTX has been proposed as an alternative and safe option for severe cases in AA in both adults and children.[3,8] Although the use of MTX in children is considered off-label, this drug has been widely used in this population, mainly for rheumatological diseases. The most common adverse events have been stomatitis, nausea, vomiting, and increased values of liver enzymes. They were all reversible and disappeared after discontinuation of the drug or reduction of the dose.[9]
In a retrospective study about 31 patients between 15 and 72 years under MTX treatment (mean age 40 years, dose range 10–25 mg/week), 68% had a regrowth rate of more than 50%, with a percentage of relapse of 33%. The success rate increased up to 77% in those who were receiving systemic corticosteroids in combination with MTX and decreased to 44% in those receiving MTX alone.[3] Anuset et al. developed a retrospective study of 26 patients with AA receiving MTX in combination with corticosteroids (mean dose of MTX of 10 mg/week). Among them, they included four children/adolescents (ages between 10 and 15 years). Focusing on the pediatric patients, three out of four achieved a complete regrowth, but a later relapse was observed in two of them.[10]
In the largest series of pediatric patients (n = 14), a dose between 15 and 25 mg a week reached a regrowth >50% of the hair in 38% of the cases (n = 5), without serious adverse events. It is important to note that in this series, five patients concomitantly received systemic corticosteroids at an initial stage while another three received systemic corticosteroids along with MTX.[2] On the long-term, three of these five responder patients suffered from a relapse, being MTX successful in two of them.[8]
Several authors have suggested continuing with MTX in responders for 18–24 months, with later progressive tapering of the dose.[2] However, the need for a maintenance treatment with MTX after achieving a successful hair growth rate has also been proposed.[6,10] If there is no response after 6–9 months, MTX should be stopped.[2]
Finally, and even though uncommon in severe, extensive, or long-term disease, the possibility of spontaneous hair regrowth in AA should be taken into account when interpreting the results of therapeutic intervention studies.[10]
In conclusion, we have reported three pediatric cases of AA treated with MTX with a successful response in two cases. An increase in liver function enzymes led to stopping the drug in one of the good responders. We agree with the above-discussed studies that MTX could be an alternative treatment for severe cases of AA in children.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Tosti A, Bellavista S, Iorizzo M. Alopecia areata: A long term follow-up study of 191 patients. J Am Acad Dermatol. 2006;55:438–41. doi: 10.1016/j.jaad.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Royer M, Bodemer C, Vabres P, Pajot C, Barbarot S, Paul C, et al. Efficacy and tolerability of methotrexate in severe childhood alopecia areata. Br J Dermatol. 2011;165:407–10. doi: 10.1111/j.1365-2133.2011.10383.x. [DOI] [PubMed] [Google Scholar]
- 3.Hammerschmidt M, Mulinari Brenner F. Efficacy and safety of methotrexate in alopecia areata. An Bras Dermatol. 2014;89:729–34. doi: 10.1590/abd1806-4841.20142869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El-Zawahry BM, Bassiouny DA, Khella A, Zaki NS. Five-year experience in the treatment of alopecia areata with DPC. J Eur Acad Dermatol Venereol. 2010;24:264–9. doi: 10.1111/j.1468-3083.2009.03401.x. [DOI] [PubMed] [Google Scholar]
- 5.Friedland R, Tal R, Lapidoth M, Zvulunov A, Ben Amitai D. Pulse corticosteroid therapy for alopecia areata in children: A retrospective study. Dermatology. 2013;227:37–44. doi: 10.1159/000351559. [DOI] [PubMed] [Google Scholar]
- 6.Mohamed Z, Bhouri A, Jallouli A, Fazaa B, Kamoun MR, Mokhtar I. Alopecia areata treatment with a phototoxic dose of UVA and topical 8-methoxypsoralen. J Eur Acad Dermatol Venereol. 2005;19:552–5. doi: 10.1111/j.1468-3083.2005.01226.x. [DOI] [PubMed] [Google Scholar]
- 7.Al-Mutairi N. 308-nm excimer laser for the treatment of alopecia areata in children. Pediatr Dermatol. 2009;26:547–50. doi: 10.1111/j.1525-1470.2009.00980.x. [DOI] [PubMed] [Google Scholar]
- 8.Lucas P, Bodemer C, Barbarot S, Vabres P, Royer M, Mazereeuw-Hautier J. Methotrexate in severe childhood alopecia areata: Long-term follow-up. Acta Derm Venereol. 2016;96:102–3. doi: 10.2340/00015555-2173. [DOI] [PubMed] [Google Scholar]
- 9.Dadlani C, Orlow SJ. Treatment of children and adolescents with methotrexate, cyclosporine, and etanercept: Review of the dermatologic and rheumatologic literature. J Am Acad Dermatol. 2005;52:316–40. doi: 10.1016/j.jaad.2004.07.043. [DOI] [PubMed] [Google Scholar]
- 10.Anuset D, Perceau G, Bernard P, Reguiai Z. Efficacy and safety of methotrexate combined with low- to moderate-dose corticosteroids for severe alopecia areata. Dermatology. 2016;232:242–8. doi: 10.1159/000441250. [DOI] [PubMed] [Google Scholar]