Abstract
Castleman's disease is a benign lymphoproliferative disorder in which interleukin-6 (IL-6), a pleiotropic proinflammatory cytokine, is thought to play a pathogenetic role. Presented is the case of a 72-year-old man with Castleman's disease who exhibited progressive renal dysfunction with proteinuria. Renal biopsy revealed mesangial hypercellularity and matrix expansion in most glomeruli and peritubular inflammatory cell infiltration. Immunofluorescence studies showed intense deposition of IgG in a granular pattern along the glomerular basement membrane. Histological features were compatible with membranoproliferative glomerulonephritis accompanied by interstitial inflammatory cell infiltration. Immunohistological analysis showed that IL-6 was abundantly expressed by tubular cells and interstitial macrophages, suggesting involvement of IL-6 in the renal injury. As a result of administration of tocilizumab, a humanized anti-IL-6 receptor antibody, the patient experienced clinical and biochemical improvement of Castleman's disease, including marked reduction of proteinuria and stabilization of renal function. These findings suggest the efficacy of tocilizumab against Castleman's disease and its renal complications.
Keywords: Castleman's disease, Interleukin-6, Tocilizumab, Membranoproliferative glomerulonephritis
Introduction
Castleman's disease is a lymphoproliferative disorder with benign hyperplastic lymph nodes characterized by follicular hyperplasia and capillary proliferation with endothelial hyperplasia [1]. Histologically, Castleman's disease has been classified into two categories: the hyaline-vascular type and plasma cell type. Patients with plasma cell-type Castleman's disease frequently have systemic manifestations such as fever, anemia, leukocytosis, hypergammaglobulinemia, and hypoalbuminemia [2]. The etiology of Castleman's disease is currently unknown, but interleukin (IL)-6, a pleiotropic proinflammatory cytokine secreted by lymphoid and nonlymphoid cells, is thought to be implicated in its pathogenesis [3]. Large quantities of IL-6 have been demonstrated to be generated from germinal centers of hyperplastic lymph nodes of patients with Castleman's disease. Furthermore, a significant decrease in IL-6 levels has been found after surgical lymph node excision in such patients [4]. In animal models, retroviral transduction of the gene coding for IL-6 has resulted in a syndrome similar to multicentric Castleman's disease, which includes the development of peripheral lymphadenopathy, marked splenomegaly, and diffuse hypergammaglobulinemia [5]. On the basis of these findings, a humanized anti-IL-6 receptor antibody, tocilizumab, has recently been shown to be therapeutically effective for treatment of Castleman's diseases [6].
Renal complications associated with Castleman's disease are heterogeneous [7, 8]. Histological assessment by El Karoui et al. [8] demonstrated that small-vessel lesions with endotheliosis, glomerular double contours, glomerular/arteriolar thrombi, and mesangiolysis are observed in most patients with Castleman's disease with renal involvement. Xu et al. [7] recently reported that thrombotic microangiopathy-like lesions are the most common pathological characteristics of Castleman's disease-associated renal injury. Other groups have reported cases of Castleman's disease with mesangial proliferative glomerulonephritis [9], renal amyloidosis [10], membranous glomerulonephritis [11], membranoproliferative glomerulonephritis (MPGN) [12, 13], crescentic glomerulonephritis [14], thrombotic microangiopathy [15], and interstitial nephritis [16].
Described herein is a case of Castleman's disease with proteinuria and progressive renal dysfunction. Kidney biopsy revealed MPGN accompanied by interstitial nephritis. Tocilizumab treatment improved both clinical and biochemical parameters of Castleman's disease, and led to a reduction of proteinuria and stabilization of renal dysfunction. Immunostaining revealed abundant production of IL-6 in tubular cells and interstitial macrophages. These findings suggest a central role of IL-6 in the development of renal injury found in the present case of Castleman's disease.
Case report
In March 1993, the patient was found to have an elevated white blood cell count and C-reactive protein, polyclonal hypergammaglobulinemia, facial erythema, and swelling of the cervical lymph nodes. Biopsy of facial erythema and lymph nodes showed reactive lymphocytosis with no evidence of monoclonal B- or T-cell populations. Pathological examination was compatible with plasma cell type Castleman's disease. He received no medication because he did not have constitutional symptoms. In April 2004, he was given a diagnosis of diabetes mellitus and was treated with an oral ant-hyperglycemic drug. In May 2006, he was noted to have proteinuria and hematuria. In February 2007, he presented with hypertension and received an angiotensin-converting enzyme inhibitor, angiotensin II receptor blocker, and furosemide.
On admission to our hospital in September 2007, physical examination showed conjunctival anemia, bilateral pedal pitting edema, and nontender bilateral cervical and inguinal lymph nodes. Blood pressure was 165/83 mmHg. Laboratory data included marked anemia (hemoglobin 8.3 g/dl), a normal white blood cell count (5.8 × 103/μl), thrombocytopenia (130 × 103/μl), increased inflammatory indices [C-reactive protein; 2.2 mg/dl, and erythrocyte sedimentation rate; 46 mm/h, and IL-6; 7.1 pg/ml (normal <5.0 pg/ml)], hypergammaglobulinemia with no monoclonal bands (total protein 8.8 g/dl, IgG 4,260 mg/dl), hypoalbuminemia (2.6 g/dl), and renal dysfunction [blood urea nitrogen 36 mg/dl, serum creatinine 2.5 mg/dl (221 μmol/l), and creatinine clearance 29 ml/min]. Liver enzymes and electrolytes were normal. Hematuria and proteinuria were 10.6 g/day. Serological tests showed total hemolytic complement activity (CH50) of 9.7 U/ml, C3 of 19.9 mg/dl, C4 of 19.1 mg/dl, and serum amyloid A protein of 46.5 μg/ml (normal <8.0 μg/ml). Anti-nuclear antibodies, anti-neutrophil cytoplasmic antibodies (ANCA), and anti-glomerular basement membrane (GBM) antibodies were all negative. Hepatitis B surface antigen, hepatitis C antibody, and cryoglobulins were not detected. Chest X-ray and ECG were normal. Computed tomography of the whole body showed cervical, mediastinal, and inguinal multiple lymphadenopathy and several renal cysts in both kidneys.
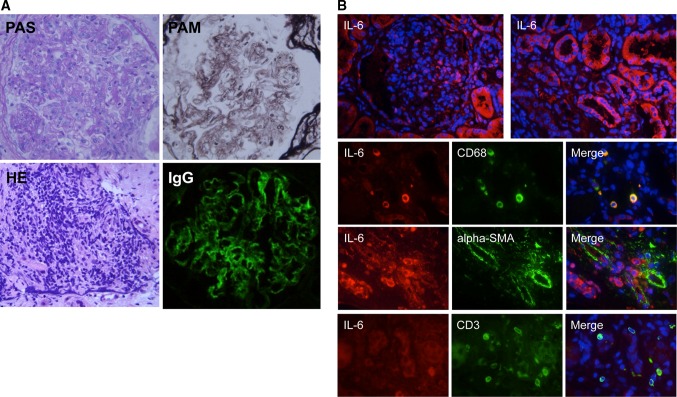
Open renal biopsy demonstrated mesangial hypercellularity and matrix expansion with a lobular appearance (PAS staining) (Fig. 1a). GBMs showed segmental double contouring and mesangial interposition (PAM staining). Infiltration of atypical lymphocytes was observed in peritubular capillaries (H&E staining). Mild interstitial fibrosis and tubular atrophy were present (Masson-trichrome staining, data not shown). Congo-red staining revealed no evidence of amyloid deposition. Immunofluorescence showed IgG deposition in a granular pattern along the GBM. There was no staining for IgM, IgA, or C3. Immunostaining was performed as described previously [17] using antibodies as follows: rabbit polyclonal anti-human IL-6 and anti-alpha-smooth muscle actin (SMA) antibodies, mouse monoclonal anti-human CD68 and anti-human CD3 antibodies (Abcam, Cambridge, MA, USA). Production of IL-6 was abundantly observed in tubular cells and interstitial CD68-positive macrophages, but not in alpha-SMA-positive vessels and CD3-positive T-lymphocytes (Fig. 1b). Electron microscopy was not performed.
Fig. 1.
a Increased mesangial cellularity and a lobular appearance (PAS staining ×1,000). Glomerular basement membranes showed segmental double contouring and mesangial interposition (PAM staining ×1,000). Atypical lymphocytes infiltrates were observed in peritubular capillaries (H&E staining ×1,000). IgG was positive (×1,000). b Immunostaining of IL-6 in renal specimens showed localization of IL-6 (red) in tubular cells and infiltrating CD68-positive macrophages (green), but not in glomeruli, alpha-SMA-positive vessels (green), or CD3-positive T lymphocytes (green). Nuclei (blue)
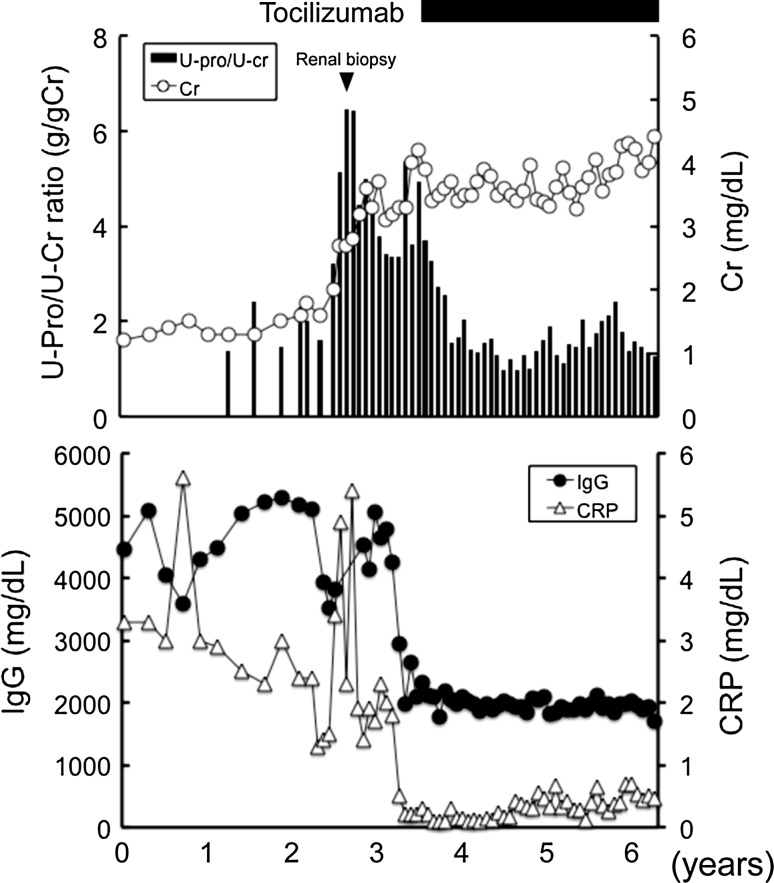
In March 2008, since proteinuria and serum creatinine progressively increased, he was treated intravenously with 8 mg/kg tocilizumab every 2 weeks without concomitant immunosuppressive drugs (Fig. 2). Upon induction of tocilizumab treatment, anemia, hypoalbuminemia, elevation of CRP, and polyclonal gammopathy were improved. Furthermore, proteinuria significantly decreased, and renal dysfunction was stabilized. Consistent with previous report [18], the IL-6 level was markedly elevated after tocilizumab treatment by a negative feedback mechanism (466 pg/ml, February 2011). At the last available follow-up (July 2011), the serum creatinine level was 4.5 mg/dl (397.8 μmol/l), and the urinary protein-to-creatinine ratio was 1.2 (g/gCr).
Fig. 2.
Time course of the urinary protein-to-creatinine (U-Pro/U-Cr) ratio, serum creatinine, IgG, and CRP. Conversion factors for units: serum creatinine in mg/dl to mol/l, ×88.4. No conversion necessary for serum IgG or CRP
Discussion
The etiology of Castleman's disease is currently unknown, but IL-6 is thought to be implicated in its pathogenesis [3]. Large quantities of IL-6 have been demonstrated to be generated from hyperplastic lymph nodes of patients with Castleman's disease. Furthermore, a significant decrease in IL-6 levels has been found after surgical lymph node excision in these patients [4]. In animal models, retroviral transduction of the IL-6 gene has resulted in a syndrome similar to Castleman's disease that presents with peripheral lymphadenopathy, marked splenomegaly, and diffuse hypergammaglobulinemia [5]. IL-6 is also involved in various renal diseases. IL-6, an autocrine growth factor for mesangial cells [19], has been reported to be involved in mesangial proliferative glomerulonephritis [20]. IL-6 transgenic mice have been shown to develop a syndrome similar to Castleman's disease with mesangial proliferative glomerulonephritis [21]. Constitutive expression of IL-6 in the liver resulted in the development of membranous glomerulonephritis, focal glomerulosclerosis, and extensive tubular damage [22]. In contrast, IL-6-deficient mice were resistant to acute kidney injury induced by HgCl2 [23] or ischemia [24] compared with wild-type mice. These data suggest that overproduction of IL-6 contributes to development of renal complications in Castleman's disease. Consistent with this idea, treatments for Castleman's disease, such as surgical excision of localized Castleman's disease, combination therapy of surgery with rituximab, or treatment with corticosteroids, have been reported to improve renal injury [7, 8]. In the present case, clinical improvement of Castleman's disease after tocilizumab treatment resulted in the reduction of proteinuria and stabilization of renal dysfunction. This raises the possibility that sustained production of circulating IL-6 by hyperplastic lymph nodes is involved in the onset of renal injury in Castleman's disease.
It has been reported that local production of IL-6 by tubular cells is involved in tubulointerstitial injury. In humans, IL-6 was detected in atrophic tubules of diseased kidneys and its expression seen to be correlated with the extent of tubular atrophy [25]. IL-6 was detected in tubular cells and interstitial monocytes/macrophages in the kidney during acute rejection after renal transplantation, while IL-6 staining of tubular cells was scarcely present in control biopsies [26]. Human proximal tubular epithelial cells have been reported to produce IL-6 in response to IL-1 [27]. In the present case, renal biopsy revealed MPGN accompanied by atypical lymphocyte infiltrates in peritubular capillaries and tubular atrophy. Furthermore, immunostaining demonstrated that IL-6 was abundantly expressed by tubular cells and interstitial macrophages. These findings suggest that local IL-6 produced by tubular cells and interstitial macrophages is implicated in renal injury as an exacerbating factor.
Three cases of Castleman's disease with renal injury treated with tocilizumab were recently reported by Komaba et al. [28]. Renal histological diagnoses in those cases were membranous nephropathy with renal plasma cell infiltration, interstitial nephritis, and amyloidosis. These patients had proteinuria (1.0–2.6 g/day, 24-h excretion) with normal renal function or mild renal insufficiency (serum creatinine 0.65–1.47 mg/dl). After treatment with tocilizumab, proteinuria was improved with no worsening of renal dysfunction during 6-month follow-up. In the present case, renal dysfunction was more severe (serum creatinine 4.6 mg/dl) when tocilizumab treatment was initiated. Nonetheless, its therapeutic efficacy was apparent. During 3-year follow-up, proteinuria was reduced, and renal dysfunction was stabilized with no adverse effects. Castleman's disease with renal involvement, in which tubulointerstitial inflammation dominates and IL-6 possibly plays a central role, might exhibit a good response to tocilizumab treatment even in the presence of advanced renal dysfunction.
The therapeutic effect on renal injury of a blockade of IL-6 action has been demonstrated using various animal models. Amelioration of renal ischemia-reperfusion injury by inhibition of IL-6 production has been observed in a mouse model of hyperlipidemia [29]. An IL-6 receptor blockade has been shown to attenuate renal injury in apolipoprotein E-deficient mice [30] and prevent cyclosporine-induced nephrotoxicity in mice [31]. In humans, Kato et al. [32] reported the efficacy of tocilizumab against renal dysfunction associated with active rheumatoid arthritis. It is therefore feasible that tocilizumab is a therapeutic option for renal diseases, especially in diseases associated with IL-6 overproduction. Further studies are needed to address this issue.
Conflict of interest
All the authors have declared no competing interest.
References
- 1.Castleman B, Iverson L, Menendez VP. Localized mediastinal lymph-node hyperplasia resembling thymoma. Cancer. 1956;9:822–830. doi: 10.1002/1097-0142(195607/08)9:4<822::AID-CNCR2820090430>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 2.Dham A, Peterson BA. Castleman disease. Curr Opin Hematol. 2007;14:354–359. doi: 10.1097/MOH.0b013e328186ffab. [DOI] [PubMed] [Google Scholar]
- 3.Casper C. The aetiology and management of Castleman disease at 50 years: translating pathophysiology to patient care. Br J Haematol. 2005;129:3–17. doi: 10.1111/j.1365-2141.2004.05311.x. [DOI] [PubMed] [Google Scholar]
- 4.Yoshizaki K, Matsuda T, Nishimoto N, Kuritani T, Taeho L, Aozasa K, et al. Pathogenic significance of interleukin-6 (IL-6/BSF-2) in Castleman’s disease. Blood. 1989;74:1360–1367. [PubMed] [Google Scholar]
- 5.Brandt SJ, Bodine DM, Dunbar CE, Nienhuis AW. Dysregulated interleukin 6 expression produces a syndrome resembling Castleman’s disease in mice. J Clin Invest. 1990;86:592–599. doi: 10.1172/JCI114749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishimoto N, Kanakura Y, Aozasa K, Johkoh T, Nakamura M, Nakano S, et al. Humanized anti-interleukin-6 receptor antibody treatment of multicentric Castleman disease. Blood. 2005;106:2627–2632. doi: 10.1182/blood-2004-12-4602. [DOI] [PubMed] [Google Scholar]
- 7.Xu D, Lv J, Dong Y, Wang S, Su T, Zhou F, et al. Renal involvement in a large cohort of Chinese patients with Castleman disease. Nephrol Dial Transplant. 2011. [DOI] [PubMed]
- 8.El Karoui K, Vuiblet V, Dion D, Izzedine H, Guitard J, Frimat L, et al. Renal involvement in Castleman disease. Nephrol Dial Transplant. 2011;26:599–609. doi: 10.1093/ndt/gfq427. [DOI] [PubMed] [Google Scholar]
- 9.Lui SL, Chan KW, Li FK, Cheng IK, Chan TM. Castleman’s disease and mesangial proliferative glomerulonephritis: the role of interleukin-6. Nephron. 1998;78:323–327. doi: 10.1159/000044943. [DOI] [PubMed] [Google Scholar]
- 10.Montoli A, Minola E, Stabile F, Grillo C, Radaelli L, Spanti D, et al. End-stage renal failure from renal amyloidosis of the AA type associated with giant lymph node hyperplasia (Castleman’s disease) Am J Nephrol. 1995;15:142–146. doi: 10.1159/000168819. [DOI] [PubMed] [Google Scholar]
- 11.Ruggieri G, Barsotti P, Coppola G, Spinelli C, Balducci A, Ventola FR, et al. Membranous nephropathy associated with giant lymph node hyperplasia. A case report with histological and ultrastructural studies. Am J Nephrol. 1990;10:323–328. doi: 10.1159/000168127. [DOI] [PubMed] [Google Scholar]
- 12.Seida A, Wada J, Morita Y, Baba M, Eguchi J, Nishimoto N, et al. Multicentric Castleman’s disease associated with glomerular microangiopathy and MPGN-like lesion: does vascular endothelial cell-derived growth factor play causative or protective roles in renal injury? Am J Kidney Dis. 2004;43:E3–E9. doi: 10.1053/j.ajkd.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 13.Nagai K, Usui J, Noguchi K, Unai K, Hiwatashi A, Arakawa Y, et al. A case of multicentric Castleman’s disease with membranoproliferative glomerulonephritis type 3-like lesion. Pathol Int. 2011;61:686–690. doi: 10.1111/j.1440-1827.2011.02727.x. [DOI] [PubMed] [Google Scholar]
- 14.Tsukamoto Y, Hanada N, Nomura Y, Hiki Y, Kasai K, Shigematsu H, et al. Rapidly progressive renal failure associated with angiofollicular lymph node hyperplasia. Am J Nephrol. 1991;11:430–436. doi: 10.1159/000168351. [DOI] [PubMed] [Google Scholar]
- 15.Suneja S, Chidambaram M, Herzenberg AM, Bargman JM. Kidney involvement in multicentric Castleman disease. Am J Kidney Dis. 2009;53:550–554. doi: 10.1053/j.ajkd.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 16.Summerfield GP, Taylor W, Bellingham AJ, Goldsmith HJ. Hyaline-vascular variant of angiofollicular lymph node hyperplasia with systemic manifestations and response to corticosteroids. J Clin Pathol. 1983;36:1005–1011. doi: 10.1136/jcp.36.9.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maeshima A, Zhang YQ, Nojima Y, Naruse T, Kojima I. Involvement of the activin-follistatin system in tubular regeneration after renal ischemia in rats. J Am Soc Nephrol. 2001;12:1685–1695. doi: 10.1681/ASN.V1281685. [DOI] [PubMed] [Google Scholar]
- 18.Nishimoto N, Terao K, Mima T, Nakahara H, Takagi N, Kakehi T. Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood. 2008;112:3959–3964. doi: 10.1182/blood-2008-05-155846. [DOI] [PubMed] [Google Scholar]
- 19.Ruef C, Budde K, Lacy J, Northemann W, Baumann M, Sterzel RB, et al. Interleukin 6 is an autocrine growth factor for mesangial cells. Kidney Int. 1990;38:249–257. doi: 10.1038/ki.1990.193. [DOI] [PubMed] [Google Scholar]
- 20.Horii Y, Muraguchi A, Iwano M, Matsuda T, Hirayama T, Yamada H, et al. Involvement of IL-6 in mesangial proliferative glomerulonephritis. J Immunol. 1989;143:3949–3955. [PubMed] [Google Scholar]
- 21.Suematsu S, Matsuda T, Aozasa K, Akira S, Nakano N, Ohno S, et al. IgG1 plasmacytosis in interleukin 6 transgenic mice. Proc Natl Acad Sci USA. 1989;86:7547–7551. doi: 10.1073/pnas.86.19.7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fattori E, Della Rocca C, Costa P, Giorgio M, Dente B, Pozzi L, et al. Development of progressive kidney damage and myeloma kidney in interleukin-6 transgenic mice. Blood. 1994;83:2570–2579. [PubMed] [Google Scholar]
- 23.Nechemia-Arbely Y, Barkan D, Pizov G, Shriki A, Rose-John S, Galun E, et al. IL-6/IL-6R axis plays a critical role in acute kidney injury. J Am Soc Nephrol. 2008;19:1106–1115. doi: 10.1681/ASN.2007070744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel NS, Chatterjee PK, Di Paola R, Mazzon E, Britti D, De Sarro A, et al. Endogenous interleukin-6 enhances the renal injury, dysfunction, and inflammation caused by ischemia/reperfusion. J Pharmacol Exp Ther. 2005;312:1170–1178. doi: 10.1124/jpet.104.078659. [DOI] [PubMed] [Google Scholar]
- 25.Fukatsu A, Matsuo S, Tamai H, Sakamoto N, Matsuda T, Hirano T. Distribution of interleukin-6 in normal and diseased human kidney. Lab Invest. 1991;65:61–66. [PubMed] [Google Scholar]
- 26.Raasveld MH, Weening JJ, Kerst JM, Surachno S, ten Berge RJ. Local production of interleukin-6 during acute rejection in human renal allografts. Nephrol Dial Transplant. 1993;8:75–78. doi: 10.1093/oxfordjournals.ndt.a092278. [DOI] [PubMed] [Google Scholar]
- 27.Boswell RN, Yard BA, Schrama E, van Es LA, Daha MR, van der Woude FJ. Interleukin 6 production by human proximal tubular epithelial cells in vitro: analysis of the effects of interleukin-1 alpha (IL-1 alpha) and other cytokines. Nephrol Dial Transplant. 1994;9:599–606. doi: 10.1093/ndt/9.6.599. [DOI] [PubMed] [Google Scholar]
- 28.Komaba H, Nakazawa T, Yamaguchi Y, Kumagai S, Fukagawa M. Interleukin-6 receptor inhibition with tocilizumab in various renal involvements associated with multicentric Castleman’s disease: a report of three cases. NDT Plus. 2008;1:423–426. doi: 10.1093/ndtplus/sfn157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharyo S, Kumagai K, Yokota-Ikeda N, Ito K, Ikeda M. Amelioration of renal ischemia-reperfusion injury by inhibition of IL-6 production in the poloxamer 407-induced mouse model of hyperlipidemia. J Pharmacol Sci. 2009;110:47–54. doi: 10.1254/jphs.08283FP. [DOI] [PubMed] [Google Scholar]
- 30.Tomiyama-Hanayama M, Rakugi H, Kohara M, Mima T, Adachi Y, Ohishi M, et al. Effect of interleukin-6 receptor blockage on renal injury in apolipoprotein E-deficient mice. Am J Physiol Ren Physiol. 2009;297:F679–F684. doi: 10.1152/ajprenal.90680.2008. [DOI] [PubMed] [Google Scholar]
- 31.LaSpina M, Tripathi S, Gatto LA, Bruch D, Maier KG, Kittur DS. An interleukin-6-neutralizing antibody prevents cyclosporine-induced nephrotoxicity in mice. J Surg Res. 2008;148:121–125. doi: 10.1016/j.jss.2007.12.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kato T, Koni I, Inoue R, Kitajima S, Kawano M, Yamagishi M. A case of active rheumatoid arthritis with renal dysfunction treated effectively with tocilizumab monotherapy. Mod Rheumatol. 2009. [DOI] [PubMed]