Abstract
Lymphatic metastasis is an important event in the progress of metastasis in colorectal cancer (CRC). The purpose of this article is to assess the role of lymphangiogenesis on CRC. In peritumoral areas of CRC, the lymphatic microvessel density (LMVD) is higher than those in normal colorectal tissues. Morever, the high LMVD is correlated with DFS and local recurrence in CRC. The VEGF-C/VEGF-D/VEGFR-3 pathway, sonic hedgehog (Shh) signaling pathway and extracellular matrix (ECM) are involved in the regulation of lymphangiogenesis in CRC. Inhibition of the VEGF-C/VEGF-D/VEGFR-3 pathway by specific antibodies has been reported to efficiently inhibit experimental tumor lymphangiogenesis and metastasis in animal experiments. Although lymphangiogenesis has been reported to play an important role in the occurrence of colon cancer and to be associated with prognosis, it remains unclear whether it is a valid therapeutic target molecule. Further study of the potential of targeting this process for anti-lymphatic therapies is worthwhile.
Colorectal cancer (CRC), a common gastrointestinal malignancy, frequently shows malignant metastatic behavior, which affects different organs, such as the lymph nodes, liver and lungs. In the progress of CRC development, lymphatic metastasis is one of the most important metastatic routes.1,2 Clinical findings have suggested that lymphatic vessels are key for metastatic spread because they provide a pathway for tumor cell dissemination.3 For patients with early stage CRC, which presents without lymph mode metastasis, a 5-year survival rate of 80-90% has been reported.4 However, the 5-year survival rate decreased to 25-60% in patients with advanced stage CRC, which presents with regional lymph node metastasis and involvement of the bowel wall and adjacent structures.5
Lymphatic capillaries consist of a single layer of endothelium and a discontinuous basement membrane. One of the main functions of the lymphatic system is to maintain body fluid balance.6 It is also involved in many physiological and pathological activities, such as immune surveillance, fat absorption and tumor metastasis. However, the research on the relationship between lymphatic vessels and tumor formation is not much, and the results are not consistent. One major obstacle is the absence of effective bio-markers to stain lymphatic endothelium. Recently, novel antibodies, such as D2-40 (podoplanin) and LYVE-1 (lymphatic endothelial hyaluronan receptor), have emerged which significantly increased the accuracy of the identification of lymphatic vessels.6 These novel antibodies are effective bio-markers for different types of research associated with lymphatic vessel proliferation.7
Lymph node dissection is very important for CRC surgery because lymph node metastasis is an important mode of CRC metastasis. There lymph nodes adjacent to the colon and rectum are abundant, and numerous lymphatic vessels are located longitudinally beneath the colorectal mucosa. At present, the role of lymphangiogenesis in the metastasis of CRC is still controversial. The purpose of this article is to assess the role of lymphangiogenesis on CRC. We review the relationship of lymphangiogenesis with the clinicopathological parameters and survival rate of CRC, and analyze the molecular mechanisms of lymphangiogenesis in the development of CRC.
Relationship between lymphatic microvessel density (LMVD) and clinicopathological parameters of CRC
The detection of LMVD by immunohistochemical method is a reliable and acceptable method. Lymphatic vessels at tumor borders often have large, open lumina, while those centrally located in the tumor have a reticular architecture with numerous tiny and ill-defined lumina.8 Peritumoral lymphatic vessels are often expanded and functional, while intratumoral lymphatic vessels are typically collapsed and nonfunctional because of the high interstitial pressure within tumors. In the center of colorectal tumors, the lymphatic vessels have no cavities and non-functional streaks, while in the peritumoral areas, the lymphatic vessels have larger lumens with a hollow, oval shape. In the distant normal colorectal tissues, there are fewer lymphatic vessels and they have small lumens (Figure 1). The LMVD was greater at the tumor borders than in the center of the tumor and had large and open lumina in CRC specimens.9,10 Therefore, frequently, LMVD values presented by researchers are actually peritumoral LMVD values.
Figure 1.
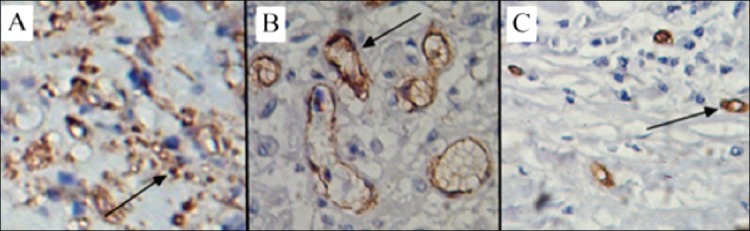
Different shapes of lymphatic vessels in tumor, peritumoral and distant normal tissues: A) center of CRC, lymphatic vessels are collapsed with no cavities and non-functional streaks; B) peritumoral area, lymphatic vessels have a larger size with a hollow, oval shape; C) distant normal tissue, lymphatic vessels are small in number and have small lumens.
Clinicopathological parameters of CRC are very important for judging disease condition and prognoses and choosing the right treatment strategies. However, the results of studies on the relationships between lymphangiogenesis and clinicopathological parameters of CRC have been inconsistent. Most studies on the relationships between LMVD and clinicopathological parameters of CRC were published from 2006-2011. The results of those studies are presented in Table 1. All of those studies reported that the number of LMVD was higher in the CRC specimens than in normal tissues. For each additional lymphatic vessel, a 1.45-fold increase in the risk of metastasis in CRC was observed.8
Table 1.
Studies of lymphangiogenesis with clinicopathological parameters of colorectal cancer (CRC).
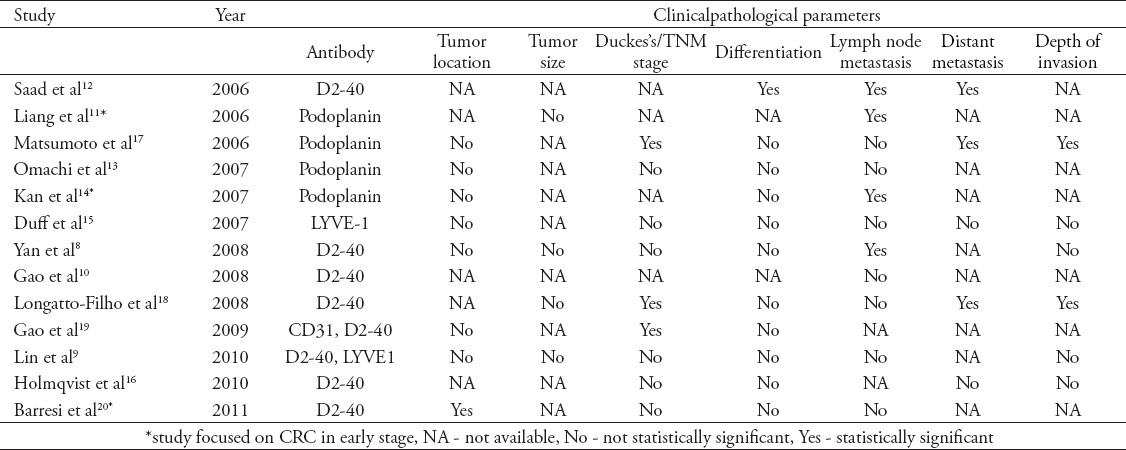
However, there are inconsistent results regarding the relationships of LMVD with the clinicopathological parameters of CRC, such as tumor stage, degree of differentiation, and distant metastasis.8-20 We speculate that these discrepancies might be due to the using different antibodies and lacking standardization for the assesment of LMVD. Currently, 3 antibodies (D2-40, LYVE-1 and CD31) are frequently used for LMVD assessment. However, the specificity of these antibodies was different. For example, D2-40 is sometimes false positive in adenomas and intramuscular carcinomas.21 Although all studies using the ‘hot spot’ method developed by Weidner et al,22 different ‘hot spots’ (0.15 mm2, 0.24 mm2, and 0.74 mm2) were selected. Moreover the LMVD counting was performed by pathologists in some studies and by software in other studies.
Lymphangiogenesis may be an early event in CRC development
Colorectal cancer is a multi-step disease, and approximately 80% CRC develop from adenomas. The adenoma-carcinoma sequence involves accumulation of numerous molecular genetic alterations. Many studies were conducted to determine whether lymphangiogenesis is associated with these genetic alterations. In 2011, Moreia et al23 performed a retrospective study including 10 colorectal non-neoplastic lesions, 30 colorectal adenomas and 60 sporadic CRC tumors and found that LMVD increased significantly and sequentially from non-neoplastic tissues to adenomas and cancerous tumors. Another study showed that LMVD was significantly higher in adenomas from patients with carcinoma than those in patients without cancer.24 However, Liang et al11 reported that LMVD was not higher in colorectal adenomas than in normal colorectal tissues and that LMVD was significantly higher in T1 carcinomas than in Tis carcinomas, colorectal adenomas and normal tissues. Additional studies with larger sample sizes are needed to determine the relationship between lymphangiogenesis and the adenoma-carcinoma sequence.
After colorectal disease progresses from adenoma to CRC, lymphangiogenesis is often observed in the early stage and not the advanced stage of CRC. In an investigation of 52 stage CRC patients, Barresi et al20 reported that a high density of LMVD was significantly associated with the progression of stage I colorectal carcinoma. Gao et al19 reported that LMVD was reversely related to Dukes’ stage of CRC, it was significantly lower in tumors of Dukes’ stage C + D than in those of Dukes’ stage A + B. Eirik et al25 suggested that lymphangiogenesis is initiated before the tumor reaches stage II by examining LMVD in 18 paired samples of CRC tissues and normal mucosa. Liang et al11 reported that lymphangiogenesis might be induced in the peritumoral areas of T1 colorectal carcinoma, and evaluation of the diameter and density of lymphatic microvessels is important for predicting lymph node metastasis in T1 colorectal carcinoma. All above studies suggest that lymphangiogenesis may be involved in the earlier stage, but not the later stage, of CRC development.
Lymphangiogenesis with anastomotic leakage of CRC
High LMVD in CRC may relate to anastomotic leakage. Anastomotic leakage is one of the factors that contribute to morbidity, mortality, local recurrence and functional deficits after CRC resection. Despite the enormous progress in surgical techniques for CRC resection, approximately 3-15% of patients experience postoperative anastomotic leakage.26 A predictive marker of the risk of anastomotic fistula would be very helpful for improving surgical outcomes. In 2011, Chen et al27 conducted a retrospective study of 750 consecutive patients who underwent anterior resection with restoration of the bowel continuity. They reported that high LMVD in the tumor margins and the distal clearance margin and lower tumor location were independent predictive markers of anastomotic leakage. Their results showed that active lymphangiogenesis and lower tumor localization were independent risk factors for anastomotic leakage. If additional clinical studies obtain similar results, high LMVD may be considered a risk factor for anastomotic leakage, and quick frozen section results of LMVD would help surgeons decide whether to create a temporary stoma.
Relationships of lymphangiogenesis with survival and local recurrence of CRC
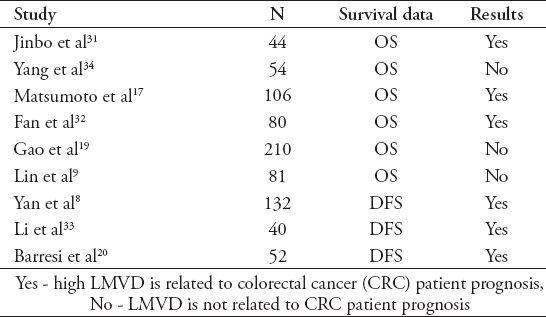
There is great interest in identifying new prognostic markers for CRC patients because they may help improve the clinical or therapeutic management of CRC.28 However, in contrast to microvessel density, the effect of lymphatic microvessel density on survival data remains a subject of intense debate. Many studies suggest that LMVD might be a valuable prognostic factor in colorectal cancer,29-33 while other surveys provide the opposite result.19,34 In 2013, we performed a meta-analysis to examine the relationship between LMVD and the overall survival (OS) and disease-free survival (DFS) of CRC.35 Nine studies including 799 patients who focused on OS or DFS were enrolled. These studies and their results are presented in Table 2. A close relationship was observed between LMVD and DFS (HR 2.29; 95% CI 1.11, 3.48), while no correlation between LMVD and OS was apparent (HR 1.02; 95% CI 0.71, 1.33). Our results suggest that LMVD may be a useful indicator of poor prognosis in CRC patients.
Table 2.
Nine studies investigated the relationship between lymphatic microvessel density (LMVD) and survival time.
Local recurrence after CRC resection is a severe postoperative complication (with a morbidity of 6-15%) and associated with a poor prognosis. Many efforts have been made to reduce the rate of local recurrence, such as improving surgical techniques and using stapling instruments, but the effects of these changes have not been satisfactory. Therefore, it is very important to identify effective molecular markers to predict and monitor the local recurrence of CRC. Chen et al36 examined LMVD in 352 primary rectal cancer cases and 34 local recurrent specimens. They reported that lymphatic vessel density was one of significant independent predictive factors of local recurrence. Noda et al37 studied lymphangiogenesis in surgical specimens of rectal carcinoma from patients with (n=26) or without (n=74) local recurrence. Their research showed correlation between local recurrence and lymphangiogenesis detected by LMVD and VEGF-C expression. Univariate and multivariate analyses showed that VEGF-C expression was an independent risk factor for the local recurrence of rectal carcinoma. A study by Fania et al28 also showed that high LMVD is an independent predictive factor of disease recurrence in node-negative CRC. The results of these studies suggest that LMVD has an important role in the pathological process of local recurrence of CRC and that markers of lymphangiogenesis may be useful predictors of CRC recurrence.
Mechanisms of lymphangiogenesis in the processes of CRC
The invasion and metastasis of cancer cells is a multistep process involving the following successive abnormal changes of lymphatic vessels: cancer cells migrate into nearby lymphatic vessels, transport of cancer cells through the lymphatic systems, escape of cancer cells from the lumina of lymphatic into the distant tissues.38 In this process, lymphatic vessels provide one of the routes for cancer cells metastasis, especially for tumors of the gastrointestinal tract, breast and lung.39 The number and diameter of lymphatic vessels is increased in peritumoral tissues, providing a larger contact area and facilitating tumor cell metastasis. Although the exact mechanism of lymphangiogenesis is unclear, studies have indicated that it may be regulated by the VEGF-C/VEGF-D/VEGFR-3 pathway, sonic hedgehog (Shh) signaling pathway and extracellular matrix (ECM). A simplified schematic is shown in Figure 2.
Figure 2.
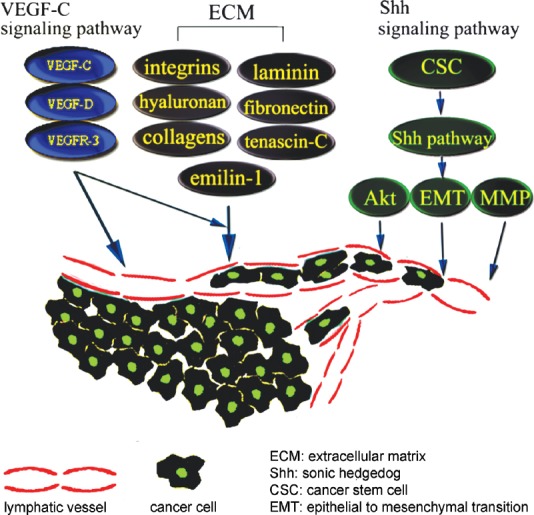
A simplified schematic of the possible mechanism of lymphangiogenesis in cancer cell metastasis. In this multi-factorial and multi-step process, the VEGF-C/VEGF-D/VEGFR-3 pathway, Shh signaling pathway and extracellular matrix have important roles in tumor-associated lymphatic sprout formation and cancer cell migration.
When considering the possible molecular mechanisms of lymphangiogenesis, major researchers pay attention to the VEGF-C/VEGF-D/VEGFR-3 pathway. This signaling pathway can drive lymphangiogenesis, with ligand-receptor binding stimulating proliferation, survival and migration of lymphatic endothelial cells.40 Prior to the initiation of lymphangiogenesis, VEGF-C can directly disrupt the endothelial lymphatic barrier to promote CRC invasion. In humans, the expression levels of VEGF-D is higher in the heart, lung, skeletal muscle, colon and small intestine than other organs.41 VEGF-C and VEGF-D not only enhance cancer cell metastasis through increasing the number of lymphatic vessels, but also increase vascular leakage and lymph flow, and the resulting increased tumor interstitial fluid pressure helps tumor cells enter the circulatory system.39 Animal studies have provided evidence that overexpression of VEGF-C or VEGF-D can increase LMVD quantity, diameter and the proliferation rates of tumor-associated lymphatic microvessels.42 After the VEGF-C/VEGFR-3 axis activated, the formation of lymphatic vessels within and around tumors were increased.43 In animal tumor models, blocking the VEGF-C/VEGF-D/VEGFR-3 signaling pathway not only inhibits the formation of new lymphatic vessels but also reduces metastasis formation.44,45
Recently, the cancer stem cell (CSC) theory has emerged as an attractive hypothesis for the processes of tumor development and progression. Cancer stem cells participate in lymphangiogenesis directly and indirectly.46 The Shh signaling pathway plays a critical role in stem cell maintenance and in specifying patterns of cell growth and differentiation.47 The Shh signaling pathway is correlated with lymphangiogenesis in many tumor types. Young et al48 reported that Shh signaling promotes metastasis and lymphangiogenesis via activation of Akt, the EMT (epithelial to mesenchymal transition), and the MMP-9 pathway in gastric cancer. A study by Bailey et al49 showed that Shh signaling regulates metastasis and lymphangiogenesis in pancreatic cancer. However, few studies focused on the relationship between the CSC and Shh signaling pathway with lymphangiogenesis in CRC. We hypothesize that Shh signaling pathway has a role in lymphangiogenesis in CRC and it is worth studying in the future. The ECM plays an important role in the generation and growth of new lymphatic microvessels. Extracellular matrix-lymphatic vessels interactions as well as biophysical characteristics of the stroma have consequences for tumor formation, growth and metastasis.50 In the ECM, anchoring filaments adhere to the outside of the lymphatic endothelial cell and connect to elastic fibers.51 These anchoring filaments allow for fluid and cells to initially enter the lymphatic system. There are many ligands in the ECM, including integrins, hyaluronan, collagens, laminin, fibronectin, tenascin-C and emilin-1.52 These extracellular matrix ligands interact with receptors on lymphatic vessels, which affect normal lymphatic function and lymphangiogenesis.53 Although the contribution of matrix metalloproteases (MMPS) to angiogenesis is well-documented, the exploration of MMP functions in lymphangiogenesis is still in its infancy.
Therapy
Many strategies have been used to prevent lymphatic metastasis, and some studies have shown potential approaches for blocking the growth of lymphatic vessels to prevent tumor metastasis.42 Inhibition of the VEGF-C/VEGF-D/VEGFR-3 pathway by specific antibodies, soluble receptor constructs and small molecule kinase inhibitors has been reported to efficiently inhibit experimental tumor lymphangiogenesis and metastasis.54 Sorafenib, a small molecule inhibitor that targets the RAF/MEK/ERK pathway, VEGFR-2, VEGFR-3 and platelet-derived growth factor receptor (PDGFR-b), has been approved by the FDA for the treatment of advanced renal cell carcinoma.55 Vatalanib, a small molecule inhibitor that blocks VEGFR-3, has been investigated for use in combination with FOLFOX-4 in phase III clinical trials for metastatic CRC, but the current results are negative.56
In addition to antibodies that VEGF inhibit, new therapeutic approaches are being tested in experimental clinical studies. AEE788, a dual-receptor tyrosine kinase inhibitor of EGFR and VEGFR-2, is currently being investigated in clinical trials. In a colon cancer orthotopic animal model, AEE788 decreased the number of peritumoral lymphatic vessels and the incidence of lymph node metastasis by decreasing the migration, proliferation and survival of lymphatic endothelial cells.57 Karaday et al58 showed that iNOS-mediated NO formation plays an important role in tumor lymphangiogenesis and the development of lymphatic metastases. Inhibition of the NO pathway may be an alternative treatment for gastric carcinoma.
The above studies suggest that lymphangiogenesis and anti-lymphangiogenesis are complex processes that involve several pathways. When one signaling pathway is blocked, others may compensate for its absence. Therefore, future anti-lymphatic treatment strategies may be complex involving inhibitors of multiple pathways.6
Summary and perspectives
Clinicopathological findings have suggested that lymphangiogenesis may be an early event in CRC development. Lymphatic microvessel density is correlated with DFS and local recurrence of CRC and, thus, may be an indicator of poor prognosis in CRC patients. However, there are no clear relationships between LMVD and some important clinicopathological parameters of CRC, such as tumor location, degree of differentiation, tumor stage, invasive depth and distant metastasis. In future clinical studies with larger sample sizes, uniform methods for the detection and counting of LMVD are required.
The VEGF-C/VEGF-D/VEGFR-3 pathway and ECM were found to play important roles in lymphangiogenesis in CRC. Although no substances that specifically interfere with tumor lymphatic vessels are in clinical use, the results of animal experiments that inhibited the VEGF-C/VEGF-D/VEGFR-3 pathway are encouraging. Whether lymphangiogenesis is a valid therapeutic target remains unclear. However, research has indicated that further study of the potential of targeting this process for anti-lymphatic therapies is worthwhile.
Related Articles.
Guraya SY. Optimum level of inferior mesenteric artery ligation for the left-sided colorectal cancer. Systematic review for high and low ligation continuum. Saudi Med J 2016; 37: 731-736. doi: 10.15537/smj.2016.7.14831.
Gazzaz F, Mosli MH, Jawa H, Sibiany A. Detection of human papillomavirus infection by molecular tests and its relation to colonic polyps and colorectal cancer. Saudi Med J 2016; 37: 256-261. doi: 10.15537/smj.2016.3.13514.
Bazarbashi SN, Alzahrani AM, Rahal MM, Al-Shehri AS, Aljubran AH, Alsanea NA, et al. Saudi Oncology Society clinical management guideline series. Colorectal cancer 2014. Saudi Med J 2014; 35: 1538-1544.
References
- 1.Ayman Eldesoky, Ashraf Shouma, Yousef Mosaad, Amira Elhawary. Clinical relevance of serum vascular endothelial growth factor and Interleukin-6 in patients with colorectal cancer. Saudi J Gastroenterol. 2011;17:170–173. doi: 10.4103/1319-3767.80378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Du B, Yang ZY, Zhong XY, Fang M, Yan YR, Qi GL, et al. Metastasis-associated protein 1 induces VEGF-C and facilitates lymphangiogenesis in colorectal cancer. World J Gastroenterol. 2011;17:1219–1226. doi: 10.3748/wjg.v17.i9.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pepper MS. Lymphangiogenesis and tumor metastasis myth or reality? Clin Cancer Res. 2001;7:462–468. [PubMed] [Google Scholar]
- 4.Swanson RS, Compton CC, Stewart AK, Bland KI. The prognosis of T3N0 colon cancer is dependent on the number of lymph nodes examined. Ann Surg Oncol. 2003;10:65–71. doi: 10.1245/aso.2003.03.058. [DOI] [PubMed] [Google Scholar]
- 5.Compton CC, Greene FL. The staging of colorectal cancer:2004 and beyond. CA Cancer J Clin. 2004;54:295–308. doi: 10.3322/canjclin.54.6.295. [DOI] [PubMed] [Google Scholar]
- 6.Sundlisæter E, Dicko A, Sakariassen PO, Sondenaa K, Enger PO, Bjerkvig R. Lymphangiogenesis in colorectal cancer-prognostic and therapeutic aspects. Int J Cancer. 2007;121:1401–1409. doi: 10.1002/ijc.22996. [DOI] [PubMed] [Google Scholar]
- 7.Chu AY, Litzky LA, Pasha TL, Acs G, Zhang PJ. Utility of D2-40, a novel mesothelial marker, in the diagnosis of malignant mesothelioma. Mod Pathol. 2005;18:105–110. doi: 10.1038/modpathol.3800259. [DOI] [PubMed] [Google Scholar]
- 8.Yan G, Zhou XY, Cai SJ, Zhang GH, Peng JJ, Du X. Lymphangiogenic and angiogenic microvessel density in human primary sporadic colorectal carcinoma. World J Gastroenterol. 2008;14:101–107. doi: 10.3748/wjg.14.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin M, Ma SP, Lin HZ, Ji P, Xie D, Yu JX. Intratumoral as well as peritumoral lymphatic vessel invasion correlates with lymph node metastasis and unfavourable outcome in colorectal cancer. Clin Exp Metastasis. 2010;27:123–132. doi: 10.1007/s10585-010-9309-0. [DOI] [PubMed] [Google Scholar]
- 10.Gao Y, Zhong WX, Mu DB, Yuan YP, Zhang YH, Yu JM, et al. Distributions of angiogenesis and lymphangiogenesis in gastrointestinal intramucosal tumors. Ann Surg Oncol. 2008;15:1117–1123. doi: 10.1245/s10434-007-9752-6. [DOI] [PubMed] [Google Scholar]
- 11.Liang P, Hong JW, Ubukata H, Liu HR, Watanabe Y, Katano M, et al. Increased density and diameter of lymphatic microvessels correlate with lymph node metastasis in early stage invasive colorectal carcinoma. Virchows Arch. 2006;448:570–575. doi: 10.1007/s00428-006-0166-9. [DOI] [PubMed] [Google Scholar]
- 12.Saad RS, Kordunsky L, Liu YL, Denning KL, Kandil HA, Silverman JF. Lymphatic microvessel density as prognostic marker in colorectal cancer. Mod Pathol. 2006;19:1317–1323. doi: 10.1038/modpathol.3800651. [DOI] [PubMed] [Google Scholar]
- 13.Omachi T, Kawai Y, Mizuno R, Nomiyama T, Miyagawa S, Ohhashi T, et al. Immunohistochemical demonstration of proliferating lymphatic vessels in colorectal carcinoma and its clinicopathological significance. Cancer Lett. 2007;246:167–172. doi: 10.1016/j.canlet.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Kaneko I, Tanaka S, Oka S, Yoshida S, Hiyama T, Arihiro K, et al. Immunohistochemical molecular markers as predictors of curability of endoscopically resected submucosal colorectal cancer. World J Gastroenterol. 2007;13:3829–3835. doi: 10.3748/wjg.v13.i28.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duff SE, Jeziorska M, Kumar S, Haboubi N, Sherlock D, O’Dwyer ST, et al. Lymphatic vessel density, microvessel density and lymphangiogenic growth factor expression in colorectal cancer. Colorectal Dis. 2007;9:793–800. doi: 10.1111/j.1463-1318.2006.01199.x. [DOI] [PubMed] [Google Scholar]
- 16.Holmqvist A, Gao J, Adell G, Carstensen J, Sun XF. The location of lymphangiogenesis is an independent prognostic factor in rectal cancers with or without preoperative radiotherapy PDF. 2010 doi: 10.1093/annonc/mdp486. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto K, Nakayama Y, Inoue Y, Minagawa N, Katsuki T, Shibao K, et al. Lymphatic microvessel density is an independent prognostic factor in colorectal cancer. Dis Colon Rectum. 2007;50:308–314. doi: 10.1007/s10350-006-0792-y. [DOI] [PubMed] [Google Scholar]
- 18.Longatto-Filho A, Pinheiro C, Ferreira L, Scapulatempo C, Alves VA, Baltazar F, et al. Peritumoural, but not intratumoural, lymphatic vessel density and invasion correlate with colorectal carcinoma poor-outcome markers. Virchows Archiv. 2008;452:133–138. doi: 10.1007/s00428-007-0550-0. [DOI] [PubMed] [Google Scholar]
- 19.Gao J, Knutsen A, Arbman G, Carstensen J, Frånlund B, Sun XF. Clinical and biological significance of angiogenesis and lymphangiogenesis in colorectal cancer. Dig Liver Dis. 2009;41:116–1122. doi: 10.1016/j.dld.2008.07.315. [DOI] [PubMed] [Google Scholar]
- 20.Barresi V, Reggiani-Bonetti L, Di Gregorio C, De Leon MP, Barresi G. Lymphatic vessel density and its prognostic value in stage I colorectal carcinoma. J Clin Pathol. 2011;64:6–12. doi: 10.1136/jcp.2010.083550. [DOI] [PubMed] [Google Scholar]
- 21.Fogt F, Zimmerman R, Ross H, Daly T, Gausas R. Identification of lymphatic vessels in malignant, adenomatous and normal colonic mucosa using the novel immunostain D2-40. Oncology Reports. 2004;11:47–50. doi: 10.3892/or.11.1.47. [DOI] [PubMed] [Google Scholar]
- 22.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis-correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 23.Moreira LR, Schenka AA, Latuf-Filho P, Penná AL, Lima CS, Soares FA, et al. Immunohistochemical analysis of vascular density and area in colorectal carcinoma using different markers and comparison with clinicopathologic prognostic factors. Tumour Biol. 2011;32:527–534. doi: 10.1007/s13277-010-0147-0. [DOI] [PubMed] [Google Scholar]
- 24.Moreira L, Schenka A, Latuf Filho P, Lima C, Trevisan M, Vassallo J. Comparison of blood neoangiogenesis and lymphatic vascularization in colorectal adenomas from patients with and without concomitant colorectal cancer. Brazilian Journal of Medical and Biological Research. 2009;42:593–598. doi: 10.1590/s0100-879x2009005000004. [DOI] [PubMed] [Google Scholar]
- 25.Sundlisaeter E, Rosland GV, Hertel JK, Sakariassen PO, Almås B, Dicko A, et al. Increased lymphatic vascular density is seen before colorectal cancers reach stage II and growth factor FGF 2 is downregulated in tumor tissue compared with normal mucosa. Apmis. 2009;117:212–221. doi: 10.1111/j.1600-0463.2008.00025.x. [DOI] [PubMed] [Google Scholar]
- 26.Kruschewski M, Rieger H, Pohlen U, Hotz HG, Buhr HJ. Risk factors for clinical anastomotic leakage and postoperative mortality in elective surgery for rectal cancer. Int J Colorectal Dis. 2007;22:919–927. doi: 10.1007/s00384-006-0260-0. [DOI] [PubMed] [Google Scholar]
- 27.Chen W, Li Y, Liao Z, Lin G, Cai G, Lin K, et al. Active lymphangiogenesis is a major risk factor for anastomotic leakage following sphincter sparing resection of rectal cancer. J Surg Oncol. 2011;104:493–498. doi: 10.1002/jso.21965. [DOI] [PubMed] [Google Scholar]
- 28.Doekhie FS, Morreau H, de Bock GH, Speetjens FM, Dekker-Ensink NG, Putter H, et al. Sialyl Lewis X expression and lymphatic microvessel density in primary tumors of node-negative colorectal cancer patients predict disease recurrence. Cancer Microenviron. 2008;1:141–151. doi: 10.1007/s12307-008-0014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levin B, Lieberman DA, McFarland B, Smith RA, Brooks D, Andrews KS, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58:130–160. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 30.Tsirlis TD, Papastratis G, Masselou K, Tsigris C, Papachristodoulou A, Kostakis A, et al. Circulating lymphangiogenic growth factors in gastrointestinal solid tumors, could they be of any clinical significance? World J Gastroenterol. 2008;14:2691–2701. doi: 10.3748/wjg.14.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jinbo J, Xuemei L, Weidong Z, Min Z, Nanhai S. Relationship of vascilar endothelial growth factor-C and lymphangiogenesi with the development and prognosis of colon cancer. Chin J Gastrointest Surg. 2006;8:516–519. [PubMed] [Google Scholar]
- 32.Fan Y, Li G, Huang G, Li X. [Clinical significance of detection on lymphatic microvessel, lymphatic microvessel density and vascular endothelial growth factor-C in patients with colorectal carcinoma] Zhonghua Wei Chang Wai Ke Za Zhi. 2006;9:477–482. Chinese. [PubMed] [Google Scholar]
- 33.Li Z, Zhang G, Hu Z, Fan Y. [Expression of lymphatic vessel endothelial hyaluronan receptor-1 in human colorectal cancer and its clinical significance] Zhonghua Wei Chang Wai Ke Za Zhi. 2009;12:511–514. Chinese. [PubMed] [Google Scholar]
- 34.Yang X, Li L, Pan Z, Zhou Z, Wan D. [Lymphatic vessel density in Dukes’ B rectal carcinoma and its correlation to prognosis] Ai Zheng. 2006;25:749–752. Chinese. [PubMed] [Google Scholar]
- 35.Chen Y, Yan J, Wang Z, Yu S, Yuan Z, Yang C, et al. A meta-analysis of the relationship between lymphatic microvessel density and the survival of patient with colorectal cancer. Lymphology. 2013;46:42–51. [PubMed] [Google Scholar]
- 36.Chen W, Chen M, Liao Z, Wang Y, Zhan Q, Cai G. Lymphatic vessel density as predictive marker for the local recurrence of rectal cancer. Dis Colon Rectum. 2009;52:513–519. doi: 10.1007/DCR.0b013e31819a2498. [DOI] [PubMed] [Google Scholar]
- 37.Noda E, Maeda K, Inoue T, Nishihara T, Nishiguchi Y, Ohira M, et al. Predictive value of vascular endothelial growth factor-C expression for local recurrence of rectal carcinoma. Oncol Rep. 2007;17:1327–1331. [PubMed] [Google Scholar]
- 38.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 39.Saharinen P, Tammela T, Karkkainen MJ, Alitalo K. Lymphatic vasculature: development, molecular regulation and role in tumor metastasis and inflammation. Trends Immunol. 2004;25:387–395. doi: 10.1016/j.it.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 40.Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R, et al. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med. 2001;7:186–191. doi: 10.1038/84635. [DOI] [PubMed] [Google Scholar]
- 41.Lohela M, Bry M, Tammela T, Alitalo K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr Opin Cell Biol. 2009;21:154–165. doi: 10.1016/j.ceb.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 42.Achen M, Mann G, Stacker S. Targeting lymphangiogenesis to prevent tumour metastasis. Br J Cancer. 2006;94:1355–1360. doi: 10.1038/sj.bjc.6603120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Su JL, Yen CJ, Chen PS, Chuang SE, Hong CC, Kuo IH, et al. The role of the VEGF-C/VEGFR-3 axis in cancer progression. Br J Cancer. 2007;96:541–545. doi: 10.1038/sj.bjc.6603487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burton JB, Priceman SJ, Sung JL, Brakenhielm E, An DS, Pytowski B, et al. Suppression of prostate cancer nodal and systemic metastasis by blockade of the lymphangiogenic axis. Cancer Res. 2008;68:7828–7837. doi: 10.1158/0008-5472.CAN-08-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krishnan J, Kirkin V, Steffen A, Hegen M, Weih D, Tomarev S, et al. Differential in vivo and in vitro expression of vascular endothelial growth factor (VEGF)-C and VEGF-D in tumors and its relationship to lymphatic metastasis in immunocompetent rats. Cancer Res. 2003;63:713–722. [PubMed] [Google Scholar]
- 46.Sun S, Qiu XS. Cancer stem cells and tumor metastasis. J Cancer Res Ther. 2013;9(Suppl):S150–S152. doi: 10.4103/0973-1482.122510. [DOI] [PubMed] [Google Scholar]
- 47.Hooper JE, Scott MP. Communicating with hedgehogs. Nat Rev Mol Cell Biol. 2005;6:306–317. doi: 10.1038/nrm1622. [DOI] [PubMed] [Google Scholar]
- 48.Yoo YA, Kang MH, Lee HJ, Kim B-h, Park JK, Kim HK, et al. Sonic hedgehog pathway promotes metastasis and lymphangiogenesis via activation of Akt, EMT, and MMP-9 pathway in gastric cancer. Cancer Res. 2011;71:7061–7070. doi: 10.1158/0008-5472.CAN-11-1338. [DOI] [PubMed] [Google Scholar]
- 49.Bailey JM, Mohr AM, Hollingsworth MA. Sonic hedgehog paracrine signaling regulates metastasis and lymphangiogenesis in pancreatic cancer. Oncogene. 2009;28:3513–3525. doi: 10.1038/onc.2009.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wiig H, Keskin D, Kalluri R. Interaction between the extracellular matrix and lymphatics: consequences for lymphangiogenesis and lymphatic function. Matrix Biol. 2010;29:645–656. doi: 10.1016/j.matbio.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gerli R, Solito R, Weber E, Agliano M. Specific adhesion molecules bind anchoring filaments and endothelial cells in human skin initial lymphatics. Lymphology. 2000;33:148–157. [PubMed] [Google Scholar]
- 52.Wiig H, Gyenge C, Iversen PO, Gullberg D, Tenstad O. The role of the extracellular matrix in tissue distribution of macromolecules in normal and pathological tissues: potential therapeutic consequences. Microcirculation. 2008;15:283–296. doi: 10.1080/10739680701671105. [DOI] [PubMed] [Google Scholar]
- 53.Tammela T, Alitalo K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell. 2010;140:460–476. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 54.Wissmann C, Detmar M. Pathways targeting tumor lymphangiogenesis. Clin Cancer Res. 2006;12:6865–6868. doi: 10.1158/1078-0432.CCR-06-1800. [DOI] [PubMed] [Google Scholar]
- 55.Kane RC, Farrell AT, Saber H, Tang S, Williams G, Jee JM, et al. Sorafenib for the treatment of advanced renal cell carcinoma. Clin Cancer Res. 2006;12:7271–7278. doi: 10.1158/1078-0432.CCR-06-1249. [DOI] [PubMed] [Google Scholar]
- 56.Los M, Roodhart JM, Voest EE. Target practice: lessons from phase III trials with bevacizumab and vatalanib in the treatment of advanced colorectal cancer. Oncologist. 2007;12:443–450. doi: 10.1634/theoncologist.12-4-443. [DOI] [PubMed] [Google Scholar]
- 57.Busby JE, Kim SJ, Yazici S, Nakamura T, Kim JS, He J, et al. Therapy of multidrug resistant human prostate tumors in the prostate of nude mice by simultaneous targeting of the epidermal growth factor receptor and vascular endothelial growth factor receptor on tumor associated endothelial cells. Prostate. 2006;66:1788–1798. doi: 10.1002/pros.20519. [DOI] [PubMed] [Google Scholar]
- 58.KaradayıN Kandemır NO, Yavuzer D, Korkmaz T, Gecmen G, Kokturk F. Inducible nitric oxide synthase expression in gastric adenocarcinoma: impact on lymphangiogenesis and lymphatic metastasis. Diagn Pathol. 2013;8:151. doi: 10.1186/1746-1596-8-151. [DOI] [PMC free article] [PubMed] [Google Scholar]


