Abstract
Expression of the PML-retinoic acid receptor α (PML-RARα) fusion protein is the initiating genetic event for acute promyelocytic leukemia (APL), but the molecular mechanisms responsible for disease initiation are not yet clear. Several observations have suggested that early myeloid cells are uniquely susceptible to transformation by PML-RARα. Recently, we have shown that the early myeloid-specific protease neutrophil elastase is important for APL development in the mouse. To better understand the role of neutrophil elastase for the pathogenesis of APL, we examined the consequences of PML-RARα expression in early myeloid cells with or without neutrophil elastase. We found that high-level PML-RARα expression was associated with cellular toxicity that was dependent on the expression of neutrophil elastase; a mutant form of PML-RARα that resisted neutrophil elastase cleavage was not toxic. When PML-RARα was expressed at very low levels in the early myeloid cells of mice, it induced myeloid expansion and delayed myeloid maturation; neutrophil elastase was also required for these activities. The activities of PML-RARα in early myeloid cells are therefore strongly influenced by the presence of neutrophil elastase. To assure physiologic relevance, PML-RARα functions should be evaluated in neutrophil elastase-expressing early myeloid cells.
Almost all patients with acute promyelocytic leukemia (APL) have transformed promyelocytes that carry the t(15;17)(q22;q11.2) translocation, which generates the PML-retinoic acid receptor α (PML-RARα) fusion gene product. The requirement of PML-RARα for APL initiation was established by expressing PML-RARα in the early myeloid compartment of mice; some of these animals develop APL after a long latent period. However, the mechanisms by which PML-RARα predisposes early myeloid cells to eventual leukemic transformation are not yet completely understood.
The cellular compartment in which PML-RARα is expressed is important for its ability to cause leukemia. Transgenic and knock-in models expressing PML-RARα in early myeloid cells (by using human cathepsin G [CG] [9, 10, 31] or MRP8 [3] regulatory sequences) developed APL, but when PML-RARα was expressed in late myeloid cells (by using CD11b regulatory sequences), they did not (6). A retroviral model directed PML-RARα expression to all early hematopoietic cells, but the only malignancy that developed was APL (23). Together, these observations suggested that the early myeloid compartment is uniquely susceptible to transformation as a result of PML-RARα expression; the interaction of PML-RARα with one or more unique components of early myeloid cells may therefore be relevant for APL pathogenesis (32). We recently found that neutrophil elastase (NE), an early myeloid-specific serine protease, is important for the development of APL in mice (16); NE expression may therefore help to define the susceptible hematopoietic cell for PML-RARα actions.
PML-RARα has been proposed to act as a dominant negative inhibitor of endogenous PML (5) and RARα (13) function, causing resistance to apoptosis and a myeloid differentiation block (8). However, data from two APL models generated in our laboratory (using the same human PML-RARα cDNA under the control of different CG regulatory sequences) has argued against a simple dominant negative model. A high-penetrance knock-in model expressed significantly less PML-RARα mRNA than a low-penetrance transgenic model (31). A simple dominant negative model would have predicted a direct relationship between expression level and disease severity. Instead, our results suggested that the selection of cells with a low level of PML-RARα expression may be optimal for APL development. While these results suggest that PML-RARα possesses gain-of-function properties that are relevant for APL pathogenesis, they do not rule out a contribution from dominant negative effects, since the level of expression in APL-initiating cells is not yet known.
In this study, we have addressed some of these issues by determining the consequences of direct PML-RARα expression in early myeloid cells that express or do not express neutrophil elastase. We found that PML-RARα-mediated disruption of PML oncogenic domains (PODs) is not dependent on neutrophil elastase activity. However, high-level expression of PML-RARα in early myeloid cells results in toxicity and cell death that is neutrophil elastase dependent. Low-level expression of PML-RARα caused increased myeloid proliferation and delayed maturation, which was also dependent on the expression of neutrophil elastase. These findings suggest that the functions of PML-RARα are strongly influenced by its level of expression and the context of the cells in which it is expressed. PML-RARα is expressed only in committed myeloid progenitors and transformed promyelocytes in human APL patients (30), and these primary APL cells contain abundant NE activity (16). Therefore, the most relevant actions of PML-RARα occur at “physiologically appropriate” doses (defined by regulatory sequences in the translocated locus) in APL blasts that express NE. Study of the actions of PML-RARα should therefore be performed with NE-expressing early myeloid cells to assure physiologic relevance.
MATERIALS AND METHODS
Mice.
hCG-PML-RARα transgenic mice (9), mCGPML-RARα knock-in mice (31), and mCG+/PR NE−/− mice (16) have been previously described. Wild-type C3H × C57BL/6 F1 mice were obtained from Taconic. Animals expressing PML-RARα in the context of NE deficiency were generated by intercrossing mCGPR/+ NE+/− animals to obtain littermate progeny of all indicated genotypes. For competitive repopulation experiments, recipient mice were Ly5.1-expressing C57BL/6 congenic mice (B6.SJL-Ptprc*Pep3bBoyJ; Jackson Laboratory, Bar Harbor, Maine). Donor mice were wild-type C57BL/6 congenic (Ly5.1/Ly5.2 heterozygous) and NE-deficient C57BL/6 animals (Ly5.2).
Transient transfection and clonogenic assays.
Cells (107) were electroporated with 50 μg of supercoiled plasmid DNA in RPMI medium and immediately centrifuged at 300 × g for 2 min. The cell pellet was incubated at 37°C for 15 min. The resuspended cells were plated in RPMI medium containing 10% fetal calf serum, l-glutamine, nonessential amino acids, sodium pyruvate, 50 μM β-mercaptoethanol, and penicillin-streptomycin at 37°C in a humidified, 5% CO2 incubator. After 4 h, cells were washed with phosphate-buffered saline and individual green fluorescent protein (GFP)-positive, 7-actinomycin D (7-AAD)-negative cells were collected with a MoFlo (Cytomation, Inc., Fort Collins, Colo.) high-speed cell sorter and plated directly into 96-well plates containing growth medium. Fourteen days after sorting, plates were scored for the number of colonies present. Data represent means and standard deviations from three independent electroporations of each construct.
TUNEL staining.
At 8 h after transfection, terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) staining was performed with the TMR Red in situ death detection kit (Roche, Indianapolis, Ind.) according to the manufacturer's instructions. TUNEL positivity was scored in each transfected population as the number of TUNEL-positive nuclei in one microscopic field, with respect to the total number of nuclei present. Three fields were scored for each of two separate electroporations; data represent means and standard deviations.
In vitro myeloid differentiation.
Eight- to 12-week-old C3H × C57Bl/6 F1, C57BL/6 × 129/SvJ F1, hCG-PML-RARα, mCGPML-RARα, mCG+/+ NE−/−, or mCG+/PR NE−/− mice were treated with 150 mg of 5-fluorouracil per kg intraperitoneally. Forty-eight hours later, marrow was harvested and plated in Dulbecco's modified Eagle's medium containing 20% fetal calf serum, 100 ng of murine stem cell factor (SCF) (R&D Systems, Minneapolis, Minn.) per ml, 6 ng of murine interleukin-3 (R&D Systems) per ml, 50 ng of murine FLT3 ligand (R&D Systems) per ml, and 10 ng of human thrombopoietin (PeproTech Inc., Rocky Hill, N.J.) per ml. After 72 h, mononuclear cells were purified by centrifugation on Histopaque-1077 (Sigma, St. Louis, Mo.), and 100,000 Lineage− Sca+ light-density cells were collected on a MoFlo high-speed cell sorter. Those cells were plated in Dulbecco's modified Eagle's medium with 20% fetal calf serum containing 100 ng of SCF per ml and 100 ng of human granulocyte colony-stimulating factor (G-CSF) (Amgen, Thousand Oaks, Calif.) per ml. Cells were harvested daily for total RNA collected by using RNeasy (Qiagen, Valencia, Calif.), manual cell counts, May-Grunwald-Giemsa staining (Sigma), and immunofluorescence. Data are representative of those from three separate pools of animals (n = 5 per pool) of each genotype assayed in duplicate cultures in three independent experiments (n = 15 mice total per genotype).
Real-time quantitative reverse transcription-PCR (qRT-PCR).
Real-time PCR for PML-RARα and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) mRNAs was performed as previously described (31), with the exception that RNA was subjected to one round of linear amplification by the Siteman Cancer Center Multiplexed Gene Analysis Core prior to PCR, as described previously (http://pathology.wustl.edu/∼mgacore/protocols.htm), to compensate for low RNA abundance. For CG and NE, RT-PCR was performed with primers 5′-AGTCCAGAAGGGCTGAGTGC-3′ (forward) and 5′-ATCAGGATGGCGGATGGCTC-3′ (reverse) for CG and with primers 5′-CCTTCTCTGTGCAGCGGATCTTC-3′ (forward) and 5′-ACATGGAGTTCTGTCACCCAC-3′ (reverse) for NE. Fluorescence ΔCT values were used to calculate mRNA levels relative to GAPDH mRNA; data represent means and standard deviations for triplicate samples.
Immunofluorescence microscopy.
Staining for human PML was performed by fixing GFP fusion transfected cells on glass slides by incubation in methanol for 10 min at −20°C. Slides were blocked by incubation in Tris-buffered saline-Tween 20 with 2% goat serum. The primary antibody was anti-human PML (PG-M3; Santa Cruz Biotechnology, Santa Cruz, Calif.), and the secondary antibody was Texas Red-conjugated anti-mouse immunoglobulin (Vector Labs, Burlingame, Calif.), each diluted 1:200 in blocking buffer. Staining for murine PML was performed with the Mouse on Mouse kit (Vector) with a monoclonal antibody raised against murine PML (a generous gift of Scott Lowe, Cold Spring Harbor, N.Y.). Fluorescein isothiocyanate-conjugated streptavidin was used for fluorescence visualization. Cells were overlaid with fluorescence mounting medium containing DAPI (4′,6′-diamidino-2-phenylindole) (Vector) for microscopy.
Western blotting.
Western blot analysis for PML-RARα protein with an anti-human RARα antibody (C-20; Santa Cruz) was performed as previously described (16).
Neutrophil elastase protease activity.
Quantitation of functional neutrophil elastase activity by using a synthetic peptide substrate was performed as previously described (19). Cleavage activity in cell extracts was normalized to a standard curve of purified human neutrophil elastase (Elastin Products, Owensville, Mo.).
In vitro PML-RARα cleavage assay.
Cleavage of in vitro-translated PML-RARα by cell extracts was performed as previously described (16).
Competitive repopulation assay.
NE−/− (Ly5.2) and NE+/+ (Ly5.1/Ly5.2) bone marrow samples were harvested from four congenic C57BL/6 age- and sex-matched donor mice of each genotype. Total bone marrow cells (5 × 106) were injected at ratios of 1:1, 9:1, and 1:9 (NE−/− to NE+/+ cells) via the tail vein into lethally irradiated (900 cGy) 8-week-old wild-type C57BL/6 (Ly5.1) mice. Peripheral blood cells from transplant recipients (n = 5 per group; n = 15 total recipients) were collected at 3 and 12 weeks posttransplant via the retroorbital plexus, at which time fluorescent-antibody-mediated Ly5.1/Ly5.2 cell surface staining and analysis of the relative contribution of each donor genotype to various hematopoietic lineages were performed as previously described (27). Antibodies and their appropriate isotype controls were obtained from BD Biosciences PharMingen (San Diego, Calif.) and included CD45.1-fluorescein isothiocyanate, CD45.2-PerCP, and phycoerythrin-conjugated B220, CD3ɛ, and Gr-1.
RESULTS
Transient PML-RARα overexpression is associated with toxicity in early myeloid cell lines.
To assess the effects of de novo PML-RARα expression in cells that had not previously been exposed to this protein, PML-RARα was transiently expressed in several myeloid and nonmyeloid human cell lines. To define the transfected population, we created N-terminal GFP fusions with PML-RARα or with the PML or RARα portion of PML-RARα. Expression was detected by GFP fluorescence in transfected cells (Fig. 1A). Several independent studies have found that GFP fusions to PML-RARα (22) and to full-length PML (14, 25) or RARα (20) behave similarly to the corresponding wild-type proteins for all functions assayed. Here, in all cell lines tested, the transiently expressed fusion proteins localized to the expected cellular compartments. GFP-RARα was localized to the nucleus, GFP-PML was detected in a small number of discrete subnuclear structures (PODs), and GFP-PML-RARα was distributed in a microspeckled subnuclear pattern, characteristic of disrupted PODs. Using an anti-human PML antibody that recognizes both the transfected PML protein and endogenous PML, we found that both GFP-PML and GFP-PML-RARα colocalized with endogenous PML, either in intact PODs or in the microspeckled distribution. U937 (myeloid) and K562 (nonmyeloid) cells demonstrated a similar localization of all GFP fusion proteins.
FIG. 1.
Cell death induced by transient expression of PML-RARα. (A) GFP fusion proteins were visualized by fluorescence microscopy 8 h after transfection in U937 (top) or K562 (bottom) cells. Staining with an antibody recognizing human PML is also shown, as indicated (green, GFP; red, PML; blue, DAPI; yellow, colocalization of GFP and PML signals). (B) TUNEL staining for U937 and K562 cells expressing the indicated GFP fusion proteins. (C) TUNEL staining data for multiple cell lines expressing GFP or GFP-PML-RARα. Data are expressed as means and standard deviations of the percentage of TUNEL-positive nuclei; significance was calculated by Student's t test (**, P < 0.01). (D) Experimental procedure for the clonogenic assay. The flow cytometry plot shows the GFP-positive, 7-AAD-negative sorted population. (E) Clonogenic data for K562 and U937 cells transfected with the indicated GFP fusion constructs. The data are expressed as the number of positive wells relative to the number of cells plated, normalized to the number of positive wells per cell plated for GFP-alone transfectants. Data are represented as means and standard deviations for three independent electroporations; significance was calculated by Student's t test (**, P < 0.01).
Using flow cytometry, we consistently observed a high percentage of 7-AAD-positive cells in bulk populations of U937 myeloid cells transfected with GFP-PML-RARα, suggestive of cell death (data not shown). To investigate the nature of this death phenotype, cells were incubated in the presence of terminal deoxynucleotidyl transferase, which labels free 3′-OH termini of DNA after single- or double-strand breakage (TUNEL). U937 cells (and another early myeloid line, PLB-985) demonstrated significantly increased TUNEL positivity (and nuclear fragmentation) 8 h after electroporation with GFP-PML-RARα versus GFP alone (5.4- ± 1.1-fold-increased TUNEL positivity for PLB-985 and 7.0- ± 0.4-fold-increased positivity for U937; P < 0.01). HL60 cells (which are also myeloid, but which express less NE activity [see Fig. 4]) and nonmyeloid K562 and RAJI cells did not display a significant difference in TUNEL positivity or nuclear fragmentation after GFP-PML-RARα expression (Fig. 1B and C).
FIG. 4.
Relationship between PML-RARα toxicity and its cleavage by neutrophil elastase. (A) Clonogenic data for U937 cells transfected with the indicated GFP fusion cDNAs. WT, wild-type PML-RARα; 2VR, = PML-RARα containing the double NE cleavage-site mutant; N-term and C-term, predicted N-terminal and C-terminal NE cleavage products of PML-RARα, respectively; N+C-term, cotransfection of both cleavage fragments. The data are represented as in Fig. 1D (**, P < 0.01). (B) Clonogenic data for U937 or U937-PR9 cells transfected with GFP or GFP-PML-RARα. The data are represented as in Fig. 1D (**, P < 0.01).
To directly examine the survival of single cells transiently overexpressing PML-RARα, we developed a clonogenic assay to assess cell death. Transfected cells were sorted on the basis of GFP positivity 4 h after electroporation (Fig. 1D). At this time point, we detected no difference in cell viability among any of the constructs, as measured by 7-AAD, propidium iodide, or TUNEL staining (data not shown). Individual GFP-positive cells were sorted, at dilutions of several cells per well, into 96-well plates containing normal growth medium and were cultured for 14 days, at which time the number of colonies was scored relative to the number of cells plated. U937 cells that experienced transient expression of GFP-PML-RARα had a significantly reduced clonogenic potential relative to cells expressing GFP alone (78% ± 5% reduction; P < 0.01). This effect was specific for the GFP-PML-RARα cDNA, since GFP fusions containing only the PML or RARα domain of PML-RARα were no more toxic than GFP alone. K562 cells displayed no change in clonogenic potential after transfection with GFP-PML-RARα (Fig. 1E).
PML-RARα expression in primary murine early myeloid cells results in toxicity or proliferation, depending on the transgene dose.
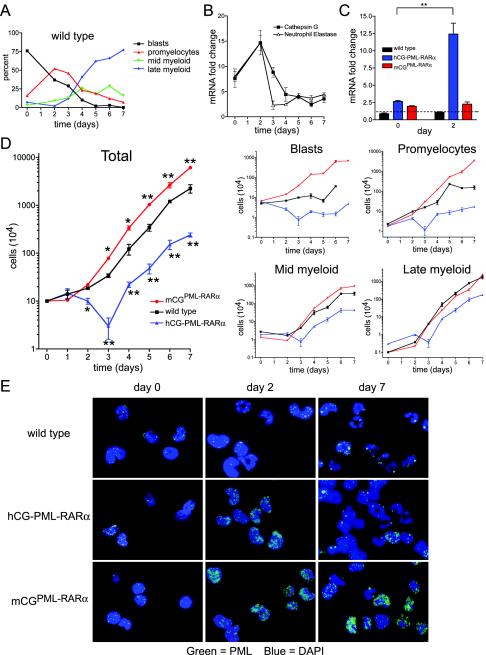
In humans with APL, PML-RARα is normally expressed in a committed myeloid progenitor compartment and in transformed promyelocytes, not in the earliest stem or progenitor cells (2, 30). We decided to study the de novo expression of PML-RARα in early myeloid cells to better understand the relevant effects of PML-RARα in primary cells. In our murine APL models, PML-RARα expression is driven exclusively in early myeloid cells under the control of cathepsin G regulatory sequences. Transgenic hCG-PML-RARα mice express significantly more PML-RARα mRNA than the knock-in mCGPML-RARα mouse. However, the mCGPML-RARα model demonstrated greater than 90% penetrance of APL, while the hCG-PML-RARα mouse demonstrated 15 to 20% penetrance (31).
We hypothesized that early myeloid toxicity might be occurring with the relatively high level of PML-RARα expression in the hCG-PML-RARα mouse model. We therefore examined bone marrow and spleen samples derived from wild-type 8- to 12-week-old mice, or from the hCG-PML-RARα and mCGPML-RARα strains, for evidence of cell death (determined by cellular morphology and annexin V, 7-AAD, and TUNEL staining). We did not observe increased numbers of apoptotic cells in any sample (data not shown). However, apoptotic cells can be difficult to detect in vivo, especially in tissues where resident macrophages rapidly engulf dying cells (28). Furthermore, since promyelocytes represent only a very small fraction of total bone marrow cells, a dying population of these cells would be difficult to detect.
We therefore decided to examine the effects of PML-RARα expression in primary cells in vitro, using a system where early myeloid cell growth properties could be more easily detected and quantified. We employed a modified version of a G-CSF-dependent myeloid differentiation system developed by McLemore et al. (21). Hematopoietic progenitors were purified from murine bone marrow and treated for 7 days with G-CSF and SCF, which induces a coordinate wave of myeloid maturation (Fig. 2A). Real-time qRT-PCR of RNA derived from wild-type cultures demonstrated that mRNA produced by the primary granule protease cathepsin G and neutrophil elastase genes peaked in abundance on day 2 of culture, coincident with the peak of promyelocyte accumulation (Fig. 2B). As expected, PML-RARα mRNA levels increased from day 0 to 2 in cells derived from hCG-PML-RARα mice (4.7- ± 0.6-fold; P < 0.01). PML-RARα from mCGPML-RARα cells was much less abundant, and it was difficult to measure induction since the measured mRNA levels were so close to that of background (Fig. 2C).
FIG. 2.
In vitro granulocytic differentiation of myeloid progenitors from two murine models of APL. (A) Manual myeloid differential counts from wild-type cultures over 7 days. Black, myeloblasts and undifferentiated progenitors; red, promyelocytes; green, myelocytes and metamyelocytes; blue, bands and mature neutrophils. (B) Real-time qRT-PCR on wild-type cells for the primary granule proteases cathepsin G and neutrophil elastase. Data are expressed as mean mRNA fold change for each gene relative to GAPDH mRNA abundance and standard deviation, each performed in triplicate. (C) Real-time qRT-PCR on cells from wild-type, hCG-PML-RARα, and mCGPML-RARα cells at days 0 and 2 of culture. Data are expressed as in panel B. The dotted line represents the maximum background signal from wild-type cultures. Significance was calculated by Student's t test (**, P < 0.01). (D) (Left) Mean cell numbers and standard deviations over time in culture; data represent those for one of three independent experiments, each plated in duplicate (*, P < 0.05; **, = P < 0.01 [for hCG-PML-RARα or mCGPML-RARα versus wild-type cultures]). (Right) Manual differentials were scored on each day in culture by counting 100 May-Grunwald-Giemsa-stained cells. Absolute numbers of the indicated myeloid subsets were calculated by multiplying the fraction of each by the total number of cells. (E) Immunofluorescence microscopy with an antibody recognizing mouse PML.
Cell counts performed on these cultures over 7 days revealed that the transgenic hCG-PML-RARα cells experienced a significant loss of cell numbers on day 3 of culture, 1 day after the induction of PML-RARα mRNA (the total cell number was 8.8% ± 4.1% of that of the wild type on day 3 in the experiment shown). The major cell losses occurred in the blast-promyelocyte compartment. Mature myeloid cells developed normally, but their accumulation was delayed (Fig. 2D). Knock-in mCGPML-RARα cells demonstrated consistently higher cell numbers than wild-type cultures (2.61- ± 0.34-fold over wild type from day 3 to 7), with the excess cells consisting primarily of blasts and promyelocytes (Fig. 2D). Variability did occur among experiments in terms of fold expansion and the magnitude of the cell loss in hCG-PML-RARα cultures on days 2 to 3, but several observations were consistent: hCG-PML-RARα cells expanded at a lower rate than wild-type cells, and mCGPML-RARα cells expanded more rapidly than wild-type cells and displayed a more immature phenotype.
Cells were examined on days 0, 2, and 7 for endogenous mouse PML localization as a functional assay for the presence of PML-RARα protein; cells expressing PML-RARα should display a microspeckled distribution of endogenous PML (5). Nearly all progenitor cells displayed normal POD structures on day 0 of culture, suggesting that these early progenitors had not yet reached the stage of transgene activation (Fig. 2E). On day 2, the majority of cells in both hCG-PML-RARα and mCGPML-RARα cultures displayed a microspeckled distribution of PML. By day 7, most hCG-PML-RARα cells displayed a normal POD-like distribution of PML, although rare cells still demonstrated a microspeckled pattern. In contrast, the majority of mCGPML-RARα cells continued to display a microspeckled PML distribution from day 3 to 7.
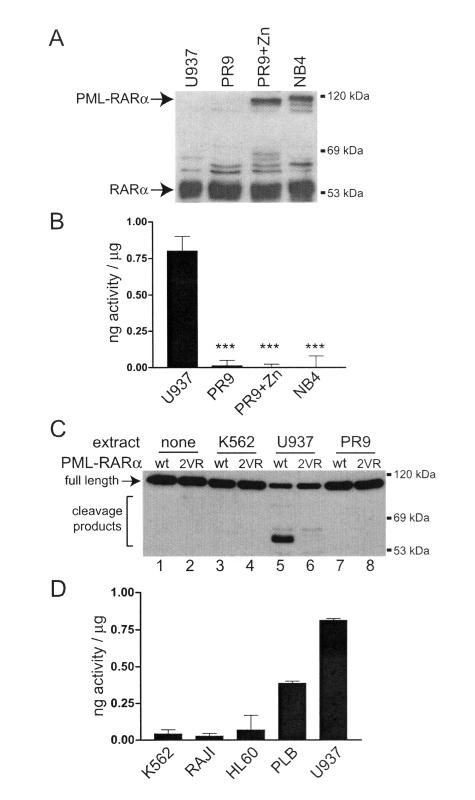
Myeloid cell lines containing full-length PML-RARα express no neutrophil elastase activity.
Two frequently used cell line models of APL are U937-PR9, a U937 subclone carrying a zinc-inducible PML-RARα cDNA (8), and NB4, a cell line cloned from an APL patient with t(15;17) (17). Induced PR9 cells and NB4 cells express abundant full-length bcr-1-derived PML-RARα protein, as determined by Western blotting against RARα (Fig. 3A). The ability of these cells to express full-length PML-RARα is paradoxical, in light of the demonstrated toxicity of PML-RARα in unmanipulated early myeloid cells. We previously showed that PML-RARα is cleaved by NE in early myeloid cells and cell lines (including U937) (16); we therefore measured the activity of NE in these cell lines by using a specific, peptide-based cleavage assay. PR9 and NB4 cells contained essentially no measurable NE activity (Fig. 3B). This data are supported by a recent report by Park et al. (26), who found that steady-state levels of NE mRNA were 4,000-fold lower in U937-PR9 cells than in U937 cells.
FIG. 3.
Neutrophil elastase levels and PML-RARα protein expression in cell line models of APL. (A) Western blotting with a human RARα-specific antibody for U937 cells, untreated U937-PR9 cells, U937-PR9 cells induced with 100 μM ZnSO4 for 16 h, and NB4 cells. (B) Quantitation of NE protease activity in the indicated cell lines. The data are shown as the means and standard deviations from two experiments, each performed in triplicate, of nanograms of equivalent activity per microgram of extract, normalized to a dilution curve of purified human NE (***, P < 0.0001). (C) Western blotting against RARα for the cleavage products of in vitro-translated wild-type PML-RARα (wt) or the double-cleavage-site mutant of PML-RARα (2VR). In vitro-translated PML-RARα was incubated with 10 μg of the indicated cell extracts for 15 min at 37°C. (D) Quantitation of NE protease activity in the indicated cell lines. The data are expressed as in panel B.
We next examined the cleavage of PML-RARα by using extracts from these cell lines. While extracts from U937 cells efficiently cleaved PML-RARα (Fig. 3C, lane 5), extracts from K562 (lane 3) and PR9 (lane 7) cells did not. Mutations of the dominant NE cleavage sites of PML-RARα (16) resulted in a near-complete loss of cleavage by U937 extracts (Fig. 3C, lane 6). We also correlated neutrophil elastase activity and the sensitivity of human hematopoietic cells to PML-RARα-induced toxicity (Fig. 1C). U937 and PLB-985 cells contained the highest levels of NE activity and were the most susceptible; K562, RAJI, and HL60 cells contained lower levels of NE activity and were not killed by PML-RARα (Fig. 3D).
PML-RARα toxicity in early myeloid cell lines is associated with its cleavage by neutrophil elastase.
In light of the findings described above, we reasoned that the toxicity of PML-RARα in early myeloid cells might be dependent on its cleavage by NE. We found that the toxicity of PML-RARα in U937 cells was lost when the two primary NE cleavage sites (V420 and V432) were mutated to arginines (2VR) (Fig. 4A). The 2VR PML-RARα protein was expressed at levels comparable to those of wild-type PML-RARα, and it caused a similar microspeckled nuclear distribution of PML (as expected, since NE is not required for POD disruption [Fig. 1 and data not shown]). Cotransfection and expression of either the N-terminal or C-terminal dominant cleavage fragments alone (or in combination) did not result in any loss of clonogenic potential compared to GFP alone (Fig. 4A), suggesting that expression of the intact protein, followed by NE-dependent cleavage, is required to cause toxicity. This observation is in agreement with immunofluorescence localization studies of the cleaved fragments of PML-RARα, where we observed differential localization of the fragments depending on whether they were cleaved in the cell or expressed exogenously as cleavage fragments (16). Finally, we transiently expressed PML-RARα in U937-PR9 cells, which possess minimal NE activity compared with parental U937 cells. Overexpression of wild-type PML-RARα induced no measurable toxicity in these cells, as predicted (Fig. 4B).
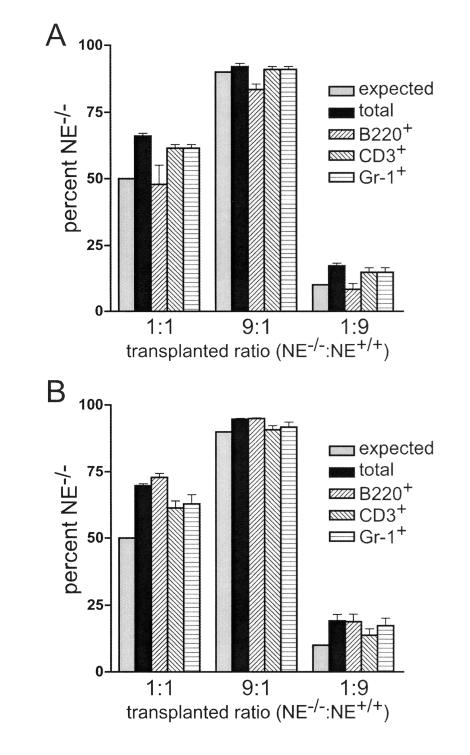
Neutrophil elastase deficiency does not alter the repopulating activity of hematopoietic progenitor cells.
We previously demonstrated that in the absence of NE, the penetrance of APL in mCGPML-RARα mice is reduced from 100 to 45% at 300 days (16). One potential explanation for those results is that NE deficiency alters the size or differentiation capacity of the “transformable pool” of myeloid progenitors. To address this issue, we performed a competitive repopulation assay to assess potentially subtle defects in NE-deficient myeloid progenitor cells. NE−/− and NE+/+ bone marrow cells were combined at various ratios to reconstitute the hematopoietic systems of congenic, lethally irradiated, wild-type recipient mice. Ly5 cell surface markers differed between the two donors and the recipient animals, allowing the measurement of the relative contribution of each population to multilineage hematopoiesis. NE−/− and NE+/+ bone marrow cells reconstituted the B-cell, T-cell, and myeloid lineages with equal efficiency at 3 and 12 weeks posttransplant (Fig. 5). These data suggest that NE deficiency per se does not cause a cell autonomous growth or differentiation defect in hematopoietic progenitors.
FIG. 5.
Competitive repopulation assay with NE−/− and NE+/+ hematopoietic progenitor cells. The percentages of NE−/− cells in various peripheral blood hematopoietic subsets at 3 weeks (A) and 12 weeks (B) after bone marrow transplantation are shown. The expected percentage of NE−/− cells, based on the transplanted input ratio, is 50% (for a 1:1 transplant, NE−/−/NE+/+), 90% (9:1, NE−/−/NE+/+), or 10% (1:9, NE−/−/NE+/+). The percentages of NE−/− cells of donor origin in total peripheral blood leukocytes, or within the B220+, CD3+, or Gr-1+ subset, are shown. The data are expressed as means and standard deviations for n = 5 mice at each ratio.
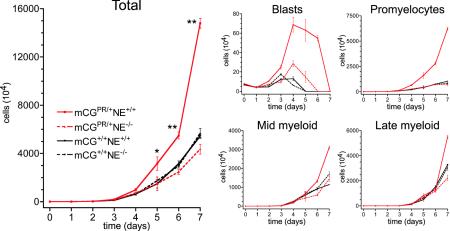
Neutrophil elastase influences the proliferation and differentiation of primary early myeloid cells expressing low levels of PML-RARα.
We decided to determine whether NE influences the phenotype of early myeloid cells expressing low levels of PML-RARα. We therefore performed in vitro myeloid differentiation of cells from wild-type (mCG+/+ NE+/+) mCGPML-RARα mice, NE-deficient (mCG+/+ NE−/−) mCGPML-RARα mice, or knock-in mCGPML-RARα mice with (mCGPR/+ NE+/+) or without (mCGPR/+ NE−/−) NE. First, it was important to determine whether NE deficiency alone alters myeloid development in our in vitro granulocytic differentiation assay. In agreement with the results of the competitive repopulation assay described above, we observed no measurable differences in the growth of wild-type versus NE-deficient myeloid progenitors in vitro (Fig. 6). Together, these data suggest that a cell-intrinsic interaction between PML-RARα and NE or a combined effect of their expression in early myeloid cells is important for APL in vivo. To test this hypothesis, we compared the expansion and development of myeloid progenitors derived from mCGPR/+ mice with or without NE. The myeloproliferative phenotype and immaturity observed with mCGPR/+-derived progenitors was not detected in the NE-deficient background (Fig. 6).
FIG. 6.
In vitro differentiation of myeloid progenitors from mCGPML-RARα cells with or without neutrophil elastase. (Left) Mean cell numbers and standard deviations over time in culture. Data represent cultures of bone marrow from one of two independent experiments with five mice of each genotype, each plated in duplicate (*, P < 0.05; **, P < 0.01 [for mCG+/PR NE+/+ versus wild-type cultures]). Cell counts are presented on a linear scale because no toxicity occurred in this experiment, unlike for Fig. 2, where a log scale was used to show cell loss on days 2 to 3 and expansion at later time points. (Right) Manual differentials were scored on each day in culture by counting 100 May-Grunwald-Giemsa-stained cells. Absolute numbers of the indicated myeloid subsets were calculated by multiplying the fraction of each by the total number of cells.
DISCUSSION
In this report, we have shown that several of the measurable activities of PML-RARα in early myeloid cells depend on neutrophil elastase, a myeloid-specific serine protease that is maximally expressed in promyelocytes. NE is not important for the ability of PML-RARα to disrupt PODs, since this activity occurs in K562 cells, which do not express NE. When expressed at high levels, PML-RARα is toxic to human myeloid cell lines that express NE. Conversely, two commonly used myeloid cell lines (NB4 and U937-PR9) that express high levels of full-length PML-RARα protein completely lack NE activity. Primary murine bone marrow progenitors that expressed relatively high levels of PML-RARα in promyelocytes demonstrated significant cell loss at the peak of expression. However, with low-level expression of PML-RARα in primary early myeloid cells, the cells proliferated more rapidly and differentiated with delayed kinetics. Both of these effects were NE dependent, although NE deficiency alone did not affect myeloid development. In sum, the data presented here clearly show that NE is relevant for many of the activities of PML-RARα in early myeloid cells, and this may help to explain why promyelocytes are uniquely susceptible to the transforming activity of PML-RARα.
The t(15;17) translocation is not associated with any malignancy other than APL. Mouse models have shown that PML-RARα must be targeted to the early myeloid compartment to initiate APL and have suggested that other hematopoietic cells are not susceptible to transformation by this fusion protein. Promyelocytes are the cells that accumulate in APL, suggesting that a unique interaction occurs between the t(15;17) fusion protein(s) and the promyelocytic environment. We previously showed that neutrophil elastase was important (but not required) for the development of PML-RARα-initiated APL in the mouse. In this report, we have shown that NE is important for several of the measurable effects of PML-RARα in early myeloid cells, including toxicity (at high doses) and excessive proliferation and altered development (at low doses). However, it is also clear that NE-deficient mice can develop APL that is phenotypically identical to APL in wild-type animals; the main differences are that latency is prolonged and penetrance is reduced in the NE-deficient mice. These data strongly suggest that other promyelocyte-specific factors may also be involved in APL pathogenesis.
How does neutrophil elastase affect the activities of PML-RARα? The answer is not yet clear. We previously showed that PML-RARα is cleaved by NE in early myeloid cells and identified the dominant cleavage sites within the carboxy-terminal domain of PML in the fusion protein. However, alternative minor cleavage sites also exist, and with prolonged exposure to NE, proteolysis progresses to yield additional cleavage fragments and ultimate destruction of the protein. Alternative early myeloid-specific proteases (cathepsin G and proteinase 3) also have the ability to cleave PML-RARα in vitro, but with reduced efficiency and in different sites (16). Thus, it is entirely possible that NE may not create PML-RARα fragments with novel functions but may instead initiate a generalized turnover mechanism for PML-RARα by first cleaving the two prominent sites in the PML domain. Accordingly, neither of the large NE cleavage products of PML-RARα was properly trafficked in U937 cells, and neither caused toxicity on its own (Fig. 4) (16).
Is it possible that cleavage of PML-RARα is simply a marker for NE activity and that the effects of NE on myeloid growth and development are not mediated by a direct interaction with the PML-RARα protein itself? This line of reasoning has been suggested by the study of severe congenital neutropenia, a syndrome associated with heterozygous mutations the Ela2 gene (encoding NE); these mutant NE molecules may contribute to the altered myeloid development and excess AML that is seen in these patients (11, 18). However, two lines of evidence suggest that this does not explain our results. First, NE deficiency per se does not appear to alter myeloid development in vitro or in vivo. Second, the NE-resistant form of PML-RARα has a phenotype: it does not cause toxicity in U937 cells that express NE. Regardless of the precise molecular mechanism, our findings strongly suggest that NE directly alters PML-RARα functions and that it does not influence APL development by altering the myeloid progenitor pool. However, to directly assess the importance of NE-induced PML-RARα cleavage for leukemogenesis, it will be important to express the NE-resistant form of PML-RARα in the early myeloid cells of transgenic mice. This experiment is in progress, but it will require 2 years to complete.
PML-RARα-induced toxicity has previously been described. In an attempt to generate stable, PML-RARα-expressing subclones of hematopoietic cell lines by using a retroviral vector, Grignani et al. (8) found that certain cell lines were “permissive” for PML-RARα expression. Interestingly, U937 cells were one of the cell types that could stably express PML-RARα, but only a fraction of the total stable integrants expressed detectable amounts of protein. In several “nonpermissive” fibroblast cell lines, PML-RARα expressed under the control of the retroviral vector induced toxicity and apoptotic cell death (7). One study, using a human fibroblast cell line, suggested that PML-RARα could induce toxicity by causing the unfolded protein response and endoplasmic reticulum stress (15). Finally, when PML-RARα was expressed in transgenic mice under the control of actin regulatory sequences, no transgene-positive mice were born, suggesting that high levels of PML-RARα in nonhematopoietic tissues may be toxic and incompatible with embryonic development (10). Similarly, when transgenic mice carrying a PLZF-RARα cDNA under control of the strong, ubiquitous cytomegalovirus or simian virus 40 promoter were created, no expression of the transgene could be detected, nor did any of the mice develop myeloproliferation or leukemia (4). However, since many of these studies were performed by expressing PML-RARα in nonhematopoietic cells that do not express NE, it is hard to assess their physiologic relevance, in light of the data presented here.
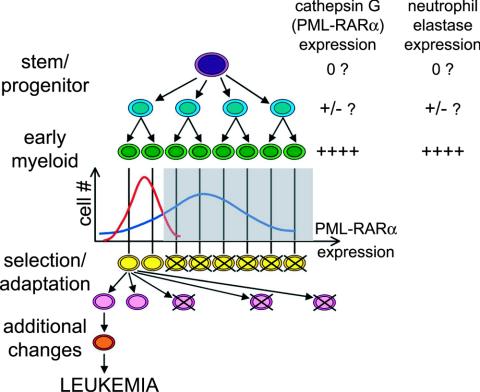
In our transgenic mouse model of APL, a relatively high level of PML-RARα expression at the promyelocyte stage is associated with cellular loss; these mice have a relatively low penetrance of APL. In an attempt to explain these findings, we have developed the model shown in Fig. 7. The majority of early myeloid cells in the high-expressing hCG-PML-RARα transgenic model may cross a “toxic threshold” of PML-RARα expression, resulting in their deletion and removal from the pool of transformable cells (Fig. 7). In contrast, in the low-expression mCGPML-RARα knock-in model, many fewer cells would express toxic amounts of PML-RARα. At these low doses, PML-RARα may expand the pool of myeloid progenitors, increasing the number of cells that could potentially acquire the “hits” required for progression to acute leukemia. How could NE play a role in the low-dose, proliferative phenotype? It is possible that NE and CG are both expressed at very low levels before they are fully activated at the promyelocyte stage (Fig. 7); recent studies have indeed suggested that “late” myeloid genes can be expressed at low levels in hematopoietic stem and myeloid progenitor cells (12, 24, 33). In early myeloid progenitors, our data suggest that low levels of NE and PML-RARα may cooperate to cause the proliferation and altered differentiation detected in the knock-in model.
FIG. 7.
Model for the roles of PML-RARα dose and NE expression in APL penetrance. At the time when differentiating myeloid cells reach the stage of peak cathepsin G transgene activation (green cells), the population experiences a normal (stochastic) distribution of PML-RARα expression on a per-cell basis. Some earlier progenitor cells may also express very low levels of cathepsin G (i.e., PML-RARα) that may actually increase progenitor proliferation (see Discussion). Since the cathepsin G and neutrophil elastase genes are regulated in a similar fashion, NE expression should also occur in cells expressing PML-RARα. The low-expressing mCGPML-RARα model is represented by the red histogram, and the high-expressing hCG-PML-RARα transgenic model is represented by the blue histogram. In this model, cells expressing PML-RARα above a threshold level (gray box) experience toxicity, while the low expressers survive and expand to support the myeloid compartment. More cells in the mCGPML-RARα model fall within the survival window. Since the transformable progenitor pool is larger in these mice, they may be more likely to acquire the necessary adaptations or changes that predispose early myeloid cells to leukemic transformation, which is detected as a higher penetrance of disease.
The NB4 cell line and induced U937-PR9 cells contain abundant full-length PML-RARα protein, and they can grow in culture, even though PML-RARα is known to be toxic to early myeloid cells. This paradox may be explained by the fact that both of these cell lines completely lack NE activity, which we have shown to be necessary for PML-RARα-induced toxicity. This suggests that these two cell lines may have lost NE expression as a required adaptation for the growth of PML-RARα-expressing cells in vitro. In support of this hypothesis, preliminary data from our lab have suggested that murine APL blasts lose NE activity when they are established as long-term in vitro cultures (A. A. Lane and T. J. Ley, unpublished data). Are NB4 cells true “APL blasts,” or are they in fact a selected, adapted population of cells that express high levels of PML-RARα? NB4 cells are cytogenetically complex, with a hypotetraploid karyotype and multiple chromosomal alterations, and they have very few (if any) azurophil granules and no NE (reference 17 and data not shown). In contrast, the majority of primary human APL blasts demonstrate t(15;17) as their sole cytogenetic abnormality, they contain abundant azurophil granules, and they express very large amounts of NE (1, 16, 29). Together, these observations imply that NB4 and PR9 cells represent a strongly selected, adapted subpopulation of early myeloid cells that do not contain many of the key features of primary APL blasts. We are currently studying the adaptations that occur in early myeloid cells that allow for PML-RARα expression, to better understand their biological relevance in vitro and, potentially, in vivo.
Taken together, the data reported here show that the interaction of PML-RARα with neutrophil elastase can cause a toxic insult to early myeloid cells, or it can confer a proliferative advantage, depending on the dose of PML-RARα that is expressed. These data underscore the importance of understanding oncogene expression levels, and the cellular compartments in which they act, as additional models of cancer are created and analyzed in mice. These observations have suggested a mechanism that can explain the paradoxical features of several mouse models of APL, and they highlight the importance of studying PML-RARα functions in a physiologic context (i.e., early myeloid cells that contain NE activity). These findings also implicate NE as a potential therapeutic target in APL. We do not yet know whether NE activity is important only at the initiation stage of APL or whether it is also important for the growth properties of fully transformed APL cells. Regardless, human and murine APL cells contain abundant NE activity; assessing the effects of NE inhibition in primary APL cells is a logical next step and is under investigation.
Acknowledgments
We thank Dan Link, Matt Walter, and Michael Tomasson for helpful discussions and reagents. The Siteman Cancer Center High Speed Cell Sorter Core provided invaluable expertise. Mieke Hoock and Kelly Schrimpf provided excellent animal husbandry. Nancy Reidelberger provided expert editorial help.
This work was supported by grant T32 HLO 7088 (to A.A.L.) and grant CA83962, The Buder Charitable Foundation, and the Edward J. Bakewell, Jr., Trust (to T.J.L.).
REFERENCES
- 1.Berger, R., M. Le Coniat, J. Derre, D. Vecchione, and P. Jonveaux. 1991. Cytogenetic studies in acute promyelocytic leukemia: a survey of secondary chromosomal abnormalities. Genes Chromosomes Cancer 3:332-337. [DOI] [PubMed] [Google Scholar]
- 2.Bonnet, D., and J. E. Dick. 1997. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 3:730-737. [DOI] [PubMed] [Google Scholar]
- 3.Brown, D., S. Kogan, E. Lagasse, I. Weissman, M. Alcalay, P. G. Pelicci, S. Atwater, and J. M. Bishop. 1997. A PMLRARα transgene initiates murine acute promyelocytic leukemia. Proc. Natl. Acad. Sci. USA 94:2551-2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng, G.-X., X.-H. Zhu, X.-Q. Men, L. Wang, Q.-H. Huang, X. L. Jin, S. M. Xiong, J. Zhu, W.-M. Guo, J.-Q. Chen, S.-F. Xu, E. So, L.-C. Chan, S. Waxman, A. Zelent, G.-Q. Chen, S. Dong, J.-X. Liu, and S.-J. Chen. 1999. Distinct leukemia phenotypes in transgenic mice and different corepressor interactions generated by promyelocytic leukemia variant fusion genes PLZF-RARα and NPM-RARα. Proc. Natl. Acad. Sci. USA 96:6318-6323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dyck, J. A., G. G. Maul, J. Miller, W. H., J. D. Chen, A. Kakizuka, and R. M. Evans. 1994. A novel macromolecular structure is a target of the promyelocytic-retinoic acid receptor. Cell 76:333-343. [DOI] [PubMed] [Google Scholar]
- 6.Early, E., M. A. Moore, A. Kakizuka, K. Nason-Burchenal, P. Martin, R. M. Evans, and E. Dmitrovsky. 1996. Transgenic expression of PML/RARα impairs myelopoiesis. Proc. Natl. Acad. Sci. USA 93:7900-7904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrucci, P. F., F. Grignani, M. Pearson, M. Fagioli, I. Nicoletti, and P. G. Pelicci. 1997. Cell death induction by the acute promyelocytic leukemia-specific PML/RARα fusion protein. Proc. Natl. Acad. Sci. USA 94:10901-10906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grignani, F., P. F. Ferrucci, U. Testa, G. Talamo, M. Fagioli, M. Alcalay, A. Mencarelli, F. Grignani, C. Peschle, I. Nicoletti, and P. G. Pelicci. 1993. The acute promyelocytic leukemia-specific PML-RARα fusion protein inhibits differentiation and promotes survival of myeloid precursor cells. Cell 74:423-431. [DOI] [PubMed] [Google Scholar]
- 9.Grisolano, J. L., R. L. Wesselschmidt, P. G. Pelicci, and T. J. Ley. 1997. Altered myeloid development and acute leukemia in transgenic mice expressing PML-RARα under control of cathepsin G regulatory sequences. Blood 89:376-387. [PubMed] [Google Scholar]
- 10.He, L.-Z., C. Tribioli, R. Rivi, D. Peruzzi, P. G. Pelicci, V. Soares, G. Cattoretti, and P. P. Pandolfi. 1997. Acute leukemia with promyelocytic features in PML/RARα transgenic mice. Proc. Natl. Acad. Sci. USA 94:5302-5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horwitz, M., F. Q. Li, D. Albani, Z. Duan, R. E. Person, K. Meade-White, and K. F. Benson. 2003. Leukemia in severe congenital neutropenia: defective proteolysis suggests new pathways to malignancy and opportunities for therapy. Cancer Investig. 21:579-587. [DOI] [PubMed] [Google Scholar]
- 12.Hu, M., D. Krause, M. Greaves, S. Sharkis, M. Dexter, C. Heyworth, and T. Enver. 1997. Multilineage gene expression precedes commitment in the hemopoietic system. Genes Dev. 11:774-785. [DOI] [PubMed] [Google Scholar]
- 13.Kastner, P., A. Perez, Y. Lutz, C. Rochette-Egly, M. P. Gaub, B. Durand, M. Lanotte, R. Berger, and P. Chambon. 1992. Structure, localization and transcriptional properties of two classes of retinoic acid receptor alpha fusion proteins in acute promyelocytic leukemia (APL): structural similarities with a new family of oncoproteins. EMBO J. 11:629-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katano, H., K. Ogawa-Goto, H. Hasegawa, T. Kurata, and T. Sata. 2001. Human-herpesvirus-8-encoded K8 protein colocalizes with the promyelocytic leukemia protein (PML) bodies and recruits p53 to the PML bodies. Virology 286:446-455. [DOI] [PubMed] [Google Scholar]
- 15.Khan, M. M., T. Nomura, T. Chiba, K. Tanaka, H. Yoshida, K. Mori, and S. Ishii. 2004. The fusion oncoprotein PML-RARalpha induces endoplasmic reticulum (ER)-associated degradation of N-CoR and ER stress. J. Biol. Chem. 279:11814-11824. [DOI] [PubMed] [Google Scholar]
- 16.Lane, A. A., and T. J. Ley. 2003. Neutrophil elastase cleaves PML-RARα and is important for the development of acute promyelocytic leukemia in mice. Cell 115:305-318. [DOI] [PubMed] [Google Scholar]
- 17.Lanotte, M., V. Martin-Thouvenin, S. Najman, P. Balerini, F. Valensi, and R. Berger. 1991. NB4, a maturation inducible cell line with t(15;17) marker isolated from a human acute promyelocytic leukemia (M3). Blood 77:1080-1086. [PubMed] [Google Scholar]
- 18.Li, F.-Q., and M. Horwitz. 2001. Characterization of mutant neutrophil elastase in severe congenital neutropenia. J. Biol. Chem. 276:14230-14241. [DOI] [PubMed] [Google Scholar]
- 19.MacIvor, D. M., S. D. Shapiro, C. T. Pham, A. Belaaouaj, S. N. Abraham, and T. J. Ley. 1999. Normal neutrophil function in cathepsin G-deficient mice. Blood 94:4282-4293. [PubMed] [Google Scholar]
- 20.Maruvada, P., C. T. Baumann, G. L. Hager, and P. M. Yen. 2003. Dynamic shuttling and intranuclear mobility of nuclear hormone receptors. J. Biol. Chem. 278:12425-12432. [DOI] [PubMed] [Google Scholar]
- 21.McLemore, M. L., S. Grewal, F. Liu, A. Archambault, J. Poursine-Laurent, J. Haug, and D. C. Link. 2001. STAT-3 activation is required for normal G-CSF-dependent proliferation and granulocytic differentiation. Immunity 14:193-204. [DOI] [PubMed] [Google Scholar]
- 22.Minucci, S., M. Maccarana, M. Cioce, P. De Luca, V. Gelmetti, S. Segalla, L. Di Croce, S. Giavara, C. Matteucci, A. Gobbi, A. Bianchini, E. Colombo, I. Schiavoni, G. Badaracco, X. Hu, M. A. Lazar, N. Landsberger, C. Nervi, and P. G. Pelicci. 2000. Oligomerization of RAR and AML1 transcription factors as a novel mechanism of oncogenic activation. Mol. Cell 5:811-820. [DOI] [PubMed] [Google Scholar]
- 23.Minucci, S., S. Monestiroli, S. Giavara, S. Ronzoni, F. Marchesi, A. Insigna, D. Diverio, P. Gasparini, M. Capillo, E. Colombo, C. Matteucci, F. Contegno, F. Lo-Coco, E. Scanziani, A. Gobbi, and P. G. Pelicci. 2002. PML-RAR induces promyelocytic leukemias with high efficiency following retroviral gene transfer into purified murine hematopoietic progenitors. Blood 100:2989-2995. [DOI] [PubMed] [Google Scholar]
- 24.Miyamoto, T., H. Iwasaki, B. Reizis, M. Ye, T. Graf, I. L. Weissman, and K. Akashi. 2002. Myeloid or lymphoid promiscuity as a critical step in hematopoietic lineage commitment. Dev. Cell 3:137-147. [DOI] [PubMed] [Google Scholar]
- 25.Ogawa, H., S. Inouye, F. I. Tsuji, K. Yasuda, and K. Umesono. 1995. Localization, trafficking, and temperature-dependence of the Aequorea green fluorescent protein in cultured vertebrate cells. Proc. Natl. Acad. Sci. USA 92:11899-118903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park, D. J., P. T. Vuong, S. de Vos, D. Douer, and H. P. Koeffler. 2003. Comparative analysis of genes regulated by PML/RARα and PLZF/RARα in response to retinoic acid using oligonucleotide arrays. Blood 102:3727-3736. [DOI] [PubMed] [Google Scholar]
- 27.Richards, M. K., F. Liu, H. Iwasaki, K. Akashi, and D. C. Link. 2003. Pivotal role of granulocyte colony-stimulating factor in the development of progenitors in the common myeloid pathway. Blood 102:3562-3568. [DOI] [PubMed] [Google Scholar]
- 28.Rudolph, B., A. O. Hueber, and G. I. Evan. 2000. Reversible activation of c-Myc in thymocytes enhances positive selection and induces proliferation and apoptosis in vitro. Oncogene 19:1891-1900. [DOI] [PubMed] [Google Scholar]
- 29.Slack, J. L., D. C. Arthur, D. Lawrence, K. Mrozek, R. J. Mayer, F. R. Davey, R. Tantravahi, M. J. Pettenati, S. Bigner, A. J. Carroll, K. W. Rao, C. A. Schiffer, and C. D. Bloomfield. 1997. Secondary cytogenetic changes in acute promyelocytic leukemia—prognostic importance in patients treated with chemotherapy alone and association with the intron 3 breakpoint of the PML gene: a cancer and leukemia group B study. J. Clin. Oncol. 15:1786-1795. [DOI] [PubMed] [Google Scholar]
- 30.Turhan, A. G., F. M. Lemoine, C. Debert, M. L. Bonnet, C. Baillou, F. Picard, E. A. Macintyre, and B. Varet. 1995. Highly purified primitive hematopoietic stem cells are PML-RARα negative and generate nonclonal progenitors in acute promyelocytic leukemia. Blood 85:2154-2161. [PubMed] [Google Scholar]
- 31.Westervelt, P., A. A. Lane, J. L. Pollock, K. Oldfather, M. S. Holt, D. B. Zimonjic, N. C. Popescu, J. F. DiPersio, and T. J. Ley. 2003. High-penetrance mouse model of acute promyelocytic leukemia with very low levels of PML-RARα expression. Blood 102:1857-1865. [DOI] [PubMed] [Google Scholar]
- 32.Westervelt, P., and T. J. Ley. 1999. Seed versus soil: the importance of the target cell for transgenic models of human leukemias. Blood 93:2143-2148. [PubMed] [Google Scholar]
- 33.Ye, M., H. Iwasaki, C. V. Laiosa, M. Stadtfeld, H. Xie, S. Heck, B. Clausen, K. Akashi, and T. Graf. 2003. Hematopoietic stem cells expressing the myeloid lysozyme gene retain long-term, multilineage repopulation potential. Immunity 19:689-699. [DOI] [PubMed] [Google Scholar]