Abstract
Objectives:
To determine the frequency of antibody seropositivity of Toxoplasma gondii infection in a cancer patient population. We also explored on association of Toxoplasma gondii seropositivity with selected variables.
Methods:
This is a prospective cross-sectional study conducted at Prince Faisal bin Bandar cancer center, Qassim, Saudi Arabia, from November 2014 to March 2015. One hundred thirty seven patients were involved in the study. Demographic data was collected using structured questionnaire, and clinical information was retrieved from the patient’s medical reports. Enzyme-linked immunosorbent assay technique was used for antibody assay.
Results:
The frequency of seropositivity for Toxoplasma gondii infection was 30.6%. The patient’s age range from 1.5-84 years with a geometric mean of 42.7 years. The seropositivity was significantly higher (p<0.05) among the 40-80 years age group (71.4%) as compared to 0-39 years one (28.6%).
Conclusion:
The prevalence of Toxoplasma gondii increases with increasing age among cancer patients in this region of central Saudi Arabia. More research is advisable for better understanding of ageing in pathogenesis of toxoplasmosis among patients with malignancies.
Toxoplasma gondii (T. gondii) is one of the most prevalent causes of human infection, and is estimated to attack 30-50% of the world population.1 It is among the leading causes of death attributed to foodborne diseases.2 The majority of infections due to T. gondii are usually mild or subclinical in individuals with normal immune system.3 However, severe and fatal infections are observed in immuno compromised individuals, such as patients suffering from cancer disease.4 Low gamma globulin levels and impaired cellular immunity have been observed in cancer patients, and are suggested, partly, as pathogenetic mechanisms for development of T. gondii infection in those patients.5 Globally, serologic evidence (IgG + IgM) of T. gondii infection was found to correlate with many cancer diseases1 such as leukemias and cancer of lung and larynx. Serologic evidence of T. gondii infection among cancer patients followed by confirmatory tests and specific treatment, usually results in improvement of life quality for the patient group with active toxoplasmosis.
Cancer patients who are seronegative for T. gondii infection could benefit from advice on preventive measures, to avoid seroconversion that may lead to active severe toxoplasmosis. Most of the published data on seroprevalence of T. gondii infection worldwide, including Kingdom of Saudi Arabia (KSA), is on women of childbearing age and / or pregnant women.6-10
The purpose of the present study was to determine the frequency of serologic evidence of T. gondii infection (immunoglobulin G [IgG] IgG + immunoglobulin M [IgM]) in a population of cancer patients from KSA. We also explored on the association of T. gondii seropositivity with some demographic data and chemotherapy.
Methods
Study design and patients involved
This study is a prospective cross-sectional study conducted at Prince Faisal bin Bandar cancer center (PFCC), Qassim region, KSA. The study was carried out for four months starting from November 2014 to March 2015. All patients who gave written consent were recruited in this study using the convenience sampling method. In-patients with confirmed cancer disease and under treatment in PFCC were included in the study. Demographic and clinical information were collected by using a standardized structured questionnaire designed by the authors. Ethical approval was obtained from Ethics Board, Ministry of Health, Qassim region, KSA.
Assay of anti-Toxoplasma IgG and IgM antibodies: Three ml of venous blood were collected from each patient, under sterile conditions in plain tube. Each blood sample was allowed to dry and then centrifuged at 1000 r.p.m. The sera were separated and stored in aliquots at -20 °C until processed at Research Laboratory 3052, College of Medicine, Qassim University. The commercial kits (VIRCELL solid-phase enzyme linked immunosorbent assay, ELISA, Parque Tecnologico de la Salud, Granada, Spain), was used for detection of anti-Toxoplasma IgG and IgM antibodies. The manufacturer instructions were followed for all samples, positive control, and negative control samples. The optical density for all samples and for the cut off sample was read. Then the antibody index (AI) was calculated using the manufacturer formula. AI of < 9 indicates Negative result, and AI of >11 indicates Positive results, for both anti-Toxoplasma IgG and IgM tests.
Statistical analysis
The data obtained was analyzed using IBM SPSS for Windows, Version 21 (IBM Corp., Armonk, New York, USA). Chi-square test for significance at 95% confidence level and p-value less than 0.05 was considered statistically significant.
Results
A total of 137 cancer patients were the study population. The age range was 1.5-84 years (mean 42.8), and 95.2% were Saudi citizens. There were68 (49.6%) males and 69 (50.4%) female patients enrolled in this study. The seropositive cases for anti-Toxoplasma IgG were 41 (29.9%), while one case (0.7%) was seropositive for anti-Toxoplasma IgM.
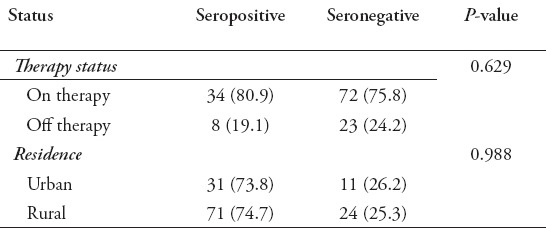
In Table 1 it is shown that seropositivity of anti-Toxoplasma antibodies (IgG + IgM) is higher (71.4%) among the age group 40-84 years, as compared to (28.6%) among the age group 0-39 years, and the difference between these two age groups is significant statistically (p<0.05). A trend of seropositivity in female patients (57.1%) versus (42.9%) in males is also shown in Table 1, but the difference between them is statistically not significant (p>0.05). The cancer patients, who are on therapy, showed seropositivity trend (80.9%) versus those who are off treatment (19.1%), and the difference between them is statistically not significant (p>0.05), Table 2. Also shown in Table 2, the seropositivity is 73.8% among cancer patients in urban residence, while it is 26.2% among patients in rural residence, and the difference between these two groups is statistically not significant (p>0.05). Patients with colo-rectal cancer showed the highest percentage of seropositivity (30.9%), followed by leukemias (26.2%), breast cancer (19.1%), Hodgkin, and non-Hodgkin lymphomas (16.7%), and other cancer diseases (7.1%).
Table 1.
Anti-toxoplasma antibody (IgG + IgM) sero-reactivity according to age and gender.
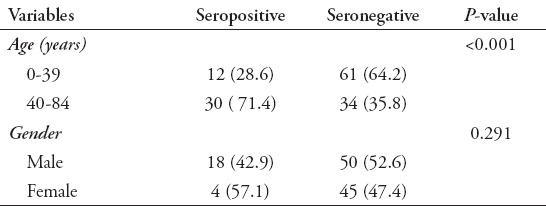
Table 2.
Anti-toxoplasma antibody (IgG + IgM) sero-reactivity according to therapy status and residence status.
Discussion
Careful repeated search in electronic databases of all Saudi medical journals, and PubMed did not recover a publication from KSA similar to the present study. The frequency of serologic evidence of T. gondii infection among the 137 cancer patient population in this work, was 30.6% seropositivity for both anti-Toxoplasma IgG + IgM. The most important finding in this study, is the statistically significant difference between seropositivity among patients >40 years, as compared to the age group 0-39 years. The exact reason for this finding is unclear. However, it seems to be a reflection of increasing exposure to the risk factors of T. gondii infection, as the human being gets older. This finding, which is in agreement with many previous studies11,12 might prove to be a clue for diagnosis of suspected toxoplasmosis secondary to reactivation of latent infection (seropositive), in an immuno compromised cancer patient. However, this is not the case in all situations. For example, an apparently seronegative 55 years old cancer patient is likely to complicate into active toxoplasmosis from his/her latent T. gondii infection. This is because his/her apparent seronegativity for T. gondii is due to inability of his/her compromised immune system to produce laboratory detectable antibodies. Similarly, a new T. gondii infection acquired from the environment in such a compromised cancer patient may progress directly to active toxoplasmosis. In support of such situations, a recent case report (2013) described disseminated toxoplasmosis after stem cell transplantation in a seronegative leukaemia patient.13 On the other hand, seropositivity is not always evidence of latent infection, as cancer patients may receive frequent blood transfusions (from seropositive donors), that could lead to passively transferred antibodies. Similarly, seropositivity is not always evidence of active infection, as shown in the present study where all our seropositive patients (30.6%) were asymptomatic. The point is, initial serologic evidence of T. gondii infection (IgG + IgM) in a cancer patient needs careful interpretation, and often requires confirmatory tests towards establishing a definitive diagnosis.
The present study does not indicate statistically significant difference between anti-Toxoplasma seropositivity in patient group on anti-cancer therapy, versus the group which is off therapy (p>0.05). Another study14 reported evidence that anti-cancer therapy suppressed circulating anti-Toxoplasma antibodies (IgG + IgM). The possible explanation for our results, is that the blood samples for serology assay were taken within few days after start of anti-cancer therapy, before it exerts its immune suppression effect. We suggest that this explanatory possibility could be utilized in diagnosis of active toxoplasmosis among cancer patients. That is, patients be tested for anti-Toxoplasma antibodies at the time cancer diagnosis is established, then repeat serology testing in 2-4 weeks be evaluated for significant rising of antibody index. Successful cure of active toxoplasmosis among cancer patients has been reported in the literature.15 The objective is to offer the cancer patients with suspected active T. gondii disease, a substantial chance of life quality improvement, in terms of decreased morbidity and increased survival rates. Although our work is novel in Qassim region and provides important information there are some limitations. The ability to generalize our findings is limited by the small cancer patient numbers. A control group was not included in this preliminary phase of the project.
In conclusion, our study shows that T. gondii seropositivity is an important feature among cancer patients from Qassim region in central Saudi Arabia. The increase in seropositivity was noted to be statistically significant in relation to increase of age. This finding deserves future research for better understanding of its role in the pathogenesis of immune suppression during cancer disease.
Acknowledgement
This study was supported by Deanship of Scientific Research, Qassim University, Qassim, Saudi Arabia. We are very thankful to the Prince Faisal bin Bandar, Cancer Center staff and their patients, for their assistance and cooperation.
Footnotes
References
- 1.Flegr J, Prandota J, Soickova M, Ispaili ZH. Toxoplasmosis: A global threat. Correlation of latent toxoplasmosis with specific disease burden in a set of 88 countries. PLoS ONE. 2014;9:e90203. doi: 10.1371/journal.pone.0090203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Havelaar AH, Haagsma JA, Manjen MJ, Kemmeren JM, Verhoef LP, Vijgen SM, et al. Disease burden of foodborne pathogens in the Netherlands. Intl J Food Microbiol. 2012;156:231–238. doi: 10.1016/j.ijfoodmicro.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 3.Coben SB, Denkers EY. The gut mucosal immune response to Toxoplasma gondii. Parasite Immunol. 2015;37:108–117. doi: 10.1111/pim.12164. [DOI] [PubMed] [Google Scholar]
- 4.Yazar S, Yaman O, Eser B, Altuntas F, Kurnaz F, Sahin E. Investigation of anti-Toxoplasma antibodies (IgG and IgM) in patients with neoplasia. J Med Microbiol. 2004;53:1183–1186. doi: 10.1099/jmm.0.45587-0. [DOI] [PubMed] [Google Scholar]
- 5.Vogel CL, Lunde MN. Toxoplasma serology in patients with malignant diseases of the reticuloendothelial system. Cancer. 1969;23:614–618. doi: 10.1002/1097-0142(196903)23:3<614::aid-cncr2820230314>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 6.Elshahawi IS, Khalil MI, Bahnass MM. Seroprevalence of Toxoplasma gondii infection in women in Najran city, Saudi Arabia. Saudi Med J. 2014;35:1143–1146. [PubMed] [Google Scholar]
- 7.Almogren A. Antenatal screening for Toxoplasma gondii infection at a tertiary care hospital in Riyadh, Saudi Arabia. Ann Saudi Med. 2011;31:569–572. doi: 10.4103/0256-4947.87090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ertug S, Okyay P, Turkmen M, Yuksel H. Seroprevalence and risk factors for Toxoplasma gondii infection among pregnant women in Aydin province, Turkey. BMC Public Health. 2005;15:66. doi: 10.1186/1471-2458-5-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imam A, Almansour MA. Serologic evidence of Toxoplasma gondii infection among pregnant women in Qassim region, Saudi Arabia. Pak J Med Res. 2015;54:84–86. [Google Scholar]
- 10.Shin DW, Cha DY, Hua QJ, Cha GH, Lee YH. Seroprevalence of Toxoplasma gondii infection and characteristics of seropositive patients in general hospitals in Daejeon, Korea. Korean J Parasitol. 2009;47:125–130. doi: 10.3347/kjp.2009.47.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nimir A, Othman A, Ese S, Musa Z, Majid IA, Kamarudin Z, et al. Latent toxoplasmosis in patients with different malignany: A hospital based study. J Clin Med Res. 2010;23:117–120. doi: 10.4021/jocmr2010.06.375w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cong W, Liu GH, Meng QF, Dong W, Qin SY, Zhang FK, et al. Toxoplasma gondii infection in cancer patients: Prevalence, risk factors, genotypes, and association with clinical diagnosis. Cancer Letters. 2015;359:307–313. doi: 10.1016/j.canlet.2015.01.036. [DOI] [PubMed] [Google Scholar]
- 13.Osthoff M, Chew E, Bajel A, Kelsey G, Hudson YP, Mason K, et al. Disseminated toxoplasmosis after allogeneic stem cell transplantation in a seronegative recipient. Transpl Infect Dis. 2013;15:e14–e19. doi: 10.1111/tid.12043. [DOI] [PubMed] [Google Scholar]
- 14.Jancalek R, Novak Z, Chrastina J, Burkon P, Slana B, Feitova V. Opportunistic infections in patients after complex therapy of cancer. Klin Onkol. 2011;24:46–49. [PubMed] [Google Scholar]
- 15.Carey RM, Kimball AC, Armstrong D, Lieberman PH. Toxoplasmosis: Clinical experience in a cancer hospital. Am J Med. 1973;54:30–38. doi: 10.1016/0002-9343(73)90080-6. [DOI] [PubMed] [Google Scholar]


