Abstract
Objective
Model the impact of targets for obesity, diabetes, raised blood pressure, tobacco use, salt intake, physical inactivity and harmful alcohol use, as outlined in the Global Non-Communicable Disease Action Plan 2013–2020, on mortality and morbidity in the UK population.
Design
Dynamic population modelling study.
Setting
UK population.
Participants
Not available.
Main outcome measures
Mortality and morbidity (years lived with disability) from non-communicable diseases (NCDs) that are averted or delayed. Probability of achieving a 25% reduction in premature mortality from NCDs by 2025 (current WHO target) and a 33% reduction by 2030 (proposed target).
Results
The largest improvements in mortality would be achieved by meeting the obesity target and the largest improvements in morbidity would be achieved by meeting the diabetes target. The UK could achieve the 2025 and 2030 targets for reducing premature mortality with only a little additional preventive effort compared with current practice. Achieving all 7 risk targets could avert a total of 300 000 deaths (95% uncertainty interval 250 000 to 350 000) and 1.3 million years lived with disability (1.2–1.4 million) from NCDs by 2025, with the majority of health gains due to reduced mortality and morbidity from heart disease and stroke, and reduced morbidity from diabetes. Potential reductions in morbidity from depression and in morbidity and mortality from dementia at older ages are also substantial.
Conclusions
The global premature mortality targets are a potentially achievable goal for countries such as the UK that can capitalise on many decades of effort in prevention and treatment. High morbidity diseases and diseases in later life are not addressed in the Global NCD Action Plan and targets, but must also be considered a priority for prevention in the UK where the population is ageing and the costs of health and social care are rising.
Keywords: PUBLIC HEALTH, PREVENTIVE MEDICINE
Strengths and limitations of this study.
The study combined functional demographic modelling of population forecasting, logistic regression modelling of ‘business-as-usual’ trends in non-communicable disease (NCD) rates and NCD risk factors, and dynamic modelling of population health outcomes.
We simulated the future changes in NCD mortality and morbidity that would occur: (1) with ‘business-as-usual’; (2) if the UK could achieve the WHO's risk factor reduction targets and (3) if all NCD burden could be addressed.
We also estimated the probability that the UK would meet the current WHO target of a 25% reduction in premature mortality by 2025 and the new target of a 30% reduction by 2030.
The modelling does not include known NCD risk factors for which there are no WHO 2025 targets (eg, intake of fruits and vegetables, red and processed meats, transfats, etc) and relies on limited evidence (eg, observational studies) to simulate the relationships between risk factors and avoid the double counting of target impacts on NCDs.
Integrating projections of risk factor and disease trends with a population demographic model that stochastically forecasts trends in mortality, fertility and migration is an important advance on existing global modelling methods.
Introduction
Non-communicable diseases (NCDs) have been recognised as a major challenge for all nations in the 21st century, affecting people of all ages, gender, race and income level.1 It is a burden that is in large part preventable; closely linked to a range of risky behaviours, including tobacco use, unhealthy diet, physical inactivity and harmful use of alcohol; risks that are themselves closely related to the societies and environments in which we live. In response to the United Nations declaration on addressing prevention and control of NCDs,1 the WHO developed a Global NCD Action Plan 2013–2020,2 which outlines global targets for improving prevalence of NCD risk factors (obesity, diabetes, raised blood pressure (BP), tobacco use, salt intake, physical inactivity and harmful use of alcohol) with an overarching goal of achieving a 25% reduction in premature mortality from the four main NCDs (cardiovascular diseases, chronic respiratory diseases, cancers and diabetes) by the year 2025. Global modelling of the risk factor impacts on NCDs shows that premature mortality from the four main NCDs could be reduced globally by 22% in men and 19% in women, between 2010 and 2025, if the targets could be achieved.3
It is the responsibility of individual countries to initiate actions to achieve the targets, and regularly measure and review national progress.4 However, it is not clear to what extent the NCD and risk factor targets should guide priorities for action or to what extent progress will reflect a nation's improvement in health. While the four main NCDs are responsible for 87% of all NCD deaths worldwide, they only contribute to 57% of the ill-health burden from NCDs.5 There are also indications that targets for reducing tobacco use could be more ambitious.3 These concerns are to some extent reflected in the new set of ‘Sustainable Development Goals’ (SDGs) and targets for 2030 that were adopted by United Nations world leaders in September 2015. The new health-related NCD goals aim to ‘promote mental health and well-being’ and to strengthen implementation of the WHO Framework Convention on Tobacco Control, alongside a revision of the premature mortality goal to a one-third reduction by 2030. In response to the SDGs, the WHO has signalled its intent to expand work on prevention of NCDs in 2016–2017.6
More information is needed about the impact of achieving the WHO risk factor targets on morbidity from NCDs and about the impact on a broad range of NCDs including mental health and neurological disorders. In a study funded by the Richmond Group of Charities,7 PROMISE, we have examined the implications of the WHO risk factor targets for priority setting in the UK. We modelled the impact of meeting the risk factor targets on morbidity and mortality from a wide range of NCDs, and examined whether the UK is likely to achieve a 25% reduction in premature mortality by 2025. In addition, in order to estimate how much of the potentially preventable portion of NCD burden could be addressed by the WHO targets, we evaluated an ‘ideal risk reduction’ scenario in which everyone in the UK achieves a normal weight, is physically active, non-smoking, without diabetes or raised BP, has an ideal salt intake and does not drink alcohol at harmful levels. In this paper, we report on the results of this study, and consider the implications for setting future targets for risk reduction.
Methods
We developed a dynamic model for simulating the effect of annual changes in risk factors on annual burden of NCDs in the UK. The modelling was carried out in two parts. First, we used historical data on risk factors in England to project trends in salt consumption, physical activity, alcohol consumption, smoking, body mass index, diabetes and BP forward to 2025, and then used these trends to estimate the proportional impact on NCDs of achieving the 25×25 targets. Second, we developed a population and mortality forecast model which estimated business-as-usual projections of NCD morbidity and mortality to 2025. This was then used as the baseline to apply the proportional changes in disease, which were calculated in the first model to estimate the impact of achieving the WHO 25×25 targets and the ideal risk reduction on NCD mortality and morbidity.
Risk factor projections model
Historical risk factor data were obtained from the Health Survey for England series.8 This survey is conducted annually and collects data on a representative sample of community-living adults in England. Annual sample sizes are ∼11 000 adults aged 18 and over. Data are collected on health behaviour (including diet, smoking, alcohol consumption and physical activity) using a standard questionnaire delivered and recorded by a trained interviewer. Anthropometric data, including body mass index and BP, are recorded by a trained nurse. For the PROMISE project, we compiled a data set (Stata V.11) with data on the seven WHO targets from all surveys conducted between 1995 and 2012.
All risk factor projections were stratified by sex and broad age group (18–35; 36–55; 56+). For each stratum, following a method used for the UK Foresight ‘Modelling future trends in obesity and their impact on health’ project,9 we modelled the relationship between prevalence of the risk factor (p) and time (t) using the following equation (where a and b are model parameters):
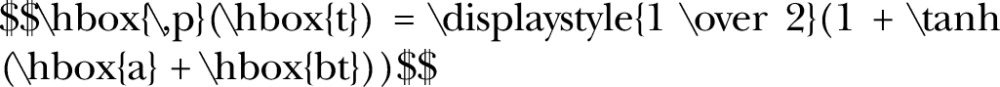 |
This relationship is convenient for modelling future projections, as it results in smooth changes over time that are constrained between minimum and maximum values of 0% and 100%. For each risk factor, we derived variables from the Health Survey for England data set to match the risk factor definitions used for the WHO targets,10 and then used logistic regression to fit the above equation, with survey year providing the time variable. We then projected prevalence forward to 2025, assuming the equations hold until this time. Table 1 provides further details regarding projections for each of the seven risk factors.
Table 1.
Methods for the projection of risk factors
| Risk factor | WHO definition | HSE years used | Comments |
|---|---|---|---|
| Overweight and obesity | Overweight: adults with BMI between 25 and 30. Obese: adults with BMI >30 | All years between 1995 and 2012 | Five BMI categories were projected: ≤20, 20 to ≤25, 25 to ≤30, 30 to ≤35, >35. The relationship between prevalence of each BMI category and survey year was modelled separately. Projections combined proportionately, in order to ensure the sum of all BMI categories was exactly 100% in any year. |
| Smoking | Prevalence of adult population currently using any tobacco product | All years between 1995 and 2012 | The prevalence of current smoking and never smoking were projected. The prevalence of former smoking was assumed to be 100%—prevalence of never-smokers and current smokers. |
| Diabetes | Prevalence of raised blood glucose, or medication for raised glucose | 1998, 2003, 2006, 2009, 2010, 2011, 2012 | We used prevalence of doctor-diagnosed diabetes. The WHO definition could not be used due to lack of representative data in England. |
| Blood pressure | Prevalence of adult population with SBP≥140 mm Hg or DBP≥90 mm Hg | All years between 1995 and 2012 | Prevalence of raised blood pressure projected. To estimate health impact of raised blood pressure, relative risks are based on median SBP of age–sex-raised blood pressure groups in HSE2012. |
| Alcohol | Prevalence of heavy episodic drinking (consuming ≥60 g alcohol on a single occasion at least monthly) | All years between 1998 and 2012 | Three alcohol categories were projected: abstainers (≤1 g alcohol per week), non-harmful drinkers, harmful drinkers. Projections were combined proportionately, in order to ensure the sum of all alcohol categories was exactly 100% in any year. Relationships between alcohol and disease outcomes were based on difference in weekly alcohol consumption between alcohol groups, estimated using the HSE2012. A dummy variable was included in regression analyses to isolate the impact of changes in alcohol measurement in the HSE in 2006. |
| Physical inactivity | Prevalence of physical inactivity (<150 min of moderate-intensity activity per week, or equivalent) | 1997, 1998, 2003, 2004, 2006, 2008, 2012 | Three physical activity categories were projected: sedentary (≤0.2 METhour/day); not sedentary, but inactive; active. Logistic regression models estimated the trend in the prevalence of first two groups combined, and we separated between these two groups based on proportion of adults in HSE2012. The prevalence of activity was assumed to be 100%—prevalence of inactivity. Data between 1997 and 2012 were available on a consistent measure of inactivity, but only HSE2012 measured inactivity equivalent to the WHO definition. Regression models were based on the consistent measure of inactivity then adjusted according to the difference in the two measures recorded in HSE2012. The risk relationship between physical activity and disease outcomes based on difference in amount of physical activity (METhour/day) between physical activity categories, estimated using HSE2012 data. |
| Salt (mediated by blood pressure) | Mean population intake of salt | 2008, 2009, 2010, 2011* | National Diet and Nutrition Survey urinary analyses data used to assess trends by age–sex groups. No trends apparent, so projections assume no change from current mean consumption levels. Mean and SDs used to generate normal distributions of salt consumption, which were converted into salt-related blood pressure using the prevalence of normotensives and hypertensives derived from the blood pressure projections, and parameters drawn from meta-analyses of salt reduction trials. |
*Note that salt estimates are taken from urinary analyses in the National Diet and Nutrition Survey.
BMI, body mass index; DBP, diastolic blood pressure; HSE, Health Survey for England; MET, metabolic equivalents; SBP, systolic blood pressure.
Relative risks for the relationship between risk factors and diseases were taken from meta-analyses of prospective studies (table 2). In most cases, these were restricted to meta-analyses of cohort studies, but in some cases meta-analyses that combined results from cohort studies and prospective case–control studies (ie, nested in cohort studies) were also included. With the exception of salt, the relationship between the risk factors and disease outcomes was modelled directly, and preference was given to relative risks that were adjusted for the most of the other risk factors. The effect of salt on BP levels was modelled using a meta-analysis of salt reduction trials,11 and then the subsequent impact of BP on disease was modelled as described above.
Table 2.
WHO risk factor definitions, target levels and modelled disease outcomes
| Risk factor | Risk definitions and targets | Modelled NCD outcomes |
|---|---|---|
| Overweight and obesity | Overweight: adults with BMI between 25 and 30. Obese: adults with BMI >30 WHO target: halt the rise in obesity. Ideal target: everyone normal weight (BMI 20–25; median 22) |
CHD;16 stroke;16 diabetes;16 cirrhosis;16 colorectal cancer;16 kidney cancer;16 breast cancer;16 pancreas cancer;17 liver cancer;16 hypertensive heart disease;16 kidney disease;16 depression;18 dementia19 |
| Smoking | Prevalence of adult population currently using any tobacco product. WHO target: 30% relative reduction. Ideal target: No current or past use of tobacco |
COPD;20 CHD;20 stroke;20 diabetes;21 lung cancer;22 oesophagus cancer;22 larynx cancer;22 stomach cancer;22 liver cancer;23 pancreas cancer;22 kidney cancer;22 cervix cancer;22 bladder cancer;22 depression;24 dementia25 |
| Diabetes | Prevalence of doctor-diagnosed diabetes WHO target: halt the rise in diabetes Ideal target: no prevalence of diabetes |
CHD;26 stroke;27 depression;28 dementia29 |
| Blood pressure | Prevalence of adult population with SBP≥140 mm Hg or DBP≥90 mm Hg WHO target: 25% relative reduction in raised blood pressure Ideal target: no prevalence of raised blood pressure |
CHD;30 stroke30 |
| Alcohol | Prevalence of heavy episodic drinking (consuming ≥60 g alcohol on a single occasion at least monthly) and per capita consumption WHO target: 10% relative reduction Ideal target: no consumption of alcohol |
CHD;31 stroke;32 hypertensive heart disease;31 diabetes;33 cirrhosis;34 liver cancer;35 mouth cancer;35 colorectal cancer;35 breast cancer;35 oesophagus cancer35 |
| Physical inactivity | Prevalence of physical inactivity (<150 min of moderate-intensity activity per week, or equivalent) WHO target: 10% relative reduction Ideal target: everyone physically active |
CHD;* stroke;* diabetes;* breast cancer;36 colorectal cancer;36 depression;37 dementia38 |
| Salt (mediated by blood pressure) | Mean population intake of salt WHO target: 30% relative reduction Ideal target: mean intake of 1000 mg/day |
CHD;11 30 stroke11 30 |
*Wahid et al.60
BMI, body mass index; CHD, coronary heart disease; COPD, chronic obstructive pulmonary disease; NCD, non-communicable disease.
The evidence relating a link between the risk factors and both dementia and depression is less established than for the other disease outcomes. In some cases the mechanisms are unclear,12 or previous results investigating the relationship have been highly heterogeneous.13 Meta-analyses of the relationship between risk factors and depression and dementia are often not based on analyses adjusted for other risk factors, increasing the risk of confounding.14 For this reason, we present two sets of modelled results, for analyses that both include and do not include depression and dementia as outcomes.
Evaluating population impact fractions
The WHO targets and the ideal risk reduction scenarios for salt consumption, physical activity, alcohol consumption, smoking, body mass index, diabetes and BP are described in table 2. Our implementation of the WHO targets depended on whether the risk reductions were relative reductions (eg, a 30% reduction in smoking) or absolute reductions (eg, halt the rise in obesity). For relative reductions in the prevalence of a risk factor (smoking, BP, alcohol, physical inactivity), the reduction was applied to the projected prevalence in every year between 2010 and 2025. For absolute reductions in the prevalence of a risk factor (obesity, diabetes), the target prevalence was assumed to be the actual prevalence in 2010. For relative reductions in a mean consumption of a risk factor (salt), the reduction was applied in every year between 2010 and 2025, and the SD of the distribution for each age–sex stratum was assumed to remain constant (ie, the entire distribution was shifted). The ideal risk reduction scenarios were based on definitions in the Global Burden of Disease study.15
Using the disease projections and the relative risk estimates, we calculated population impact fractions (PIFs) for each disease–risk factor relationship, in each age–sex stratum, in every year between 2010 and 2025. PIFs estimate the proportion of disease in a population that is removed under a counterfactual scenario (in this case, in the scenario where the WHO targets or ideal levels are achieved). We calculated PIFs using the following equation, where there are k risk factor categories, pi is the proportion of the population in risk factor category i in the estimate year based on projections, p’i is the proportion of the population in risk factor category i in the estimate year based on achieving the WHO or ideal risk reduction target, and RRi is the relative risk for the disease in risk factor category i:
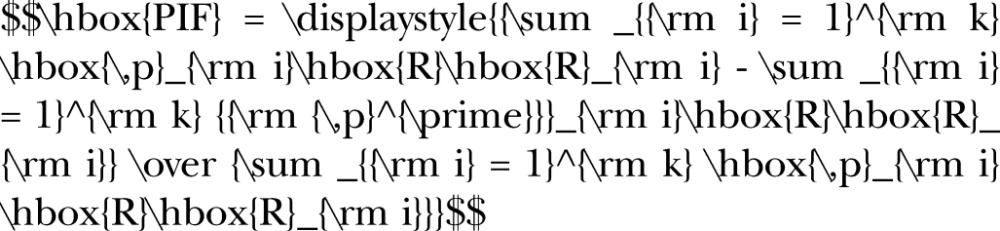 |
We present results separately for each risk factor (ie, when only the single risk factor target is achieved) and in combination (ie, if all risk factor targets are achieved). For the combined scenario, we multiplicatively combined PIFs for BP, diabetes, physical activity, body mass index (BMI, alcohol and tobacco:
 |
We adjusted for potential double counting of risk factor effects on coronary heart disease (CHD) and stroke where there are interdependencies in risks (eg, physical activity affects CHD directly and via changes in BMI; BMI affects CHD directly and via changes in BP). Figure 1 shows a conceptual model of the relationships between the risk factor targets for these diseases.
Figure 1.
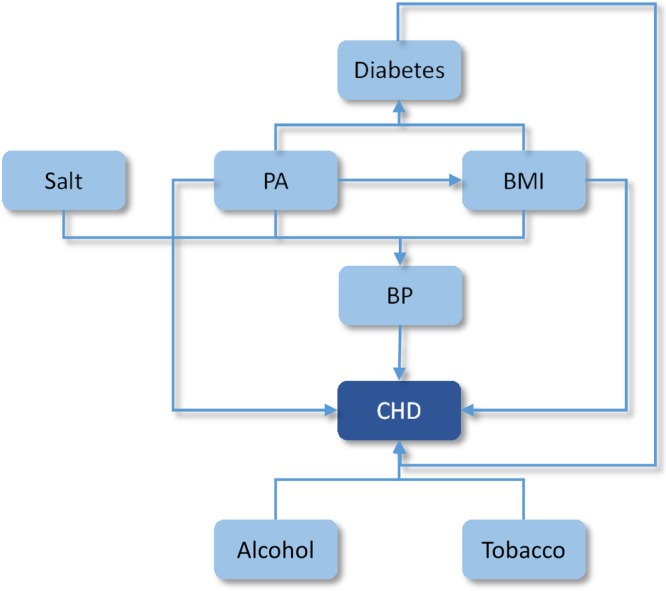
The modelled relationships between the WHO risk factor targets and CHD or stroke. BMI, body mass index; BP, blood pressure; CHD, coronary heart disease; PA, physical activity.
The effects of changes in alcohol and tobacco were assumed to be independent of each other and of all other risk factors. However, where risk factors were both mediating variables and the subject of WHO targets (BP and diabetes) we took the larger of the effects. For example, we calculated the combined effects of meeting physical activity, BMI and salt targets on BP (assuming they are independent, ie, additive), we then calculated the effect of the hypertension target on BP, and took only the larger of these two effects. Similarly, we calculated the combined effect of meeting physical activity and BMI targets on diabetes (adjusting for the effects of physical activity on BMI), then estimated the effect of the diabetes target on diabetes prevalence, and took only the larger of the two effects. Further details on the methods for avoiding double counting are provided in online supplementary appendix 1. We calculated 95% uncertainty intervals for all PIFs, based on lognormal distributions for the relative risks and normal distributions for other variables as reported in the literature (eg, effect of salt on BP), using Monte Carlo analysis.39
bmjopen-2016-012805supp_appendix1.pdf (671.4KB, pdf)
Disease projections model
The PIFs were applied to projected disease rates in a UK population and mortality forecast model in order to estimate disease outcomes of meeting WHO targets. The model was built in R (V.3.1.2) using packages demography and systemfit.
The UK population was forecast upto 2025 based on historical demographic patterns. We used data from the period 1938 to 2010, obtaining population and mortality from the Human Mortality Database,40 fertility from the Human Fertility Database,41 and deriving net migration for the same time period, from these data. These demographic rates were smoothed using penalised regression splines. We then fitted functional demographic models42 to mortality, fertility and migration rates from 1938 to 2010, and used these models to forecast future rates out to 2030. Finally, we simulated the population over time to 2030, using bootstrapping to estimate uncertainty in the population from the errors in the mortality, fertility and migration models.
For projecting disease-specific mortality, we developed regression models using methods described by Salomon and Murray43 that ensure that disease-specific mortality projections are consistent with the projected all-cause mortality envelope. We obtained cause-specific mortality data from the WHO database,44 focusing on deaths coded using the most recent version of the International Classification of Diseases (ICD-10). ICD-10 was only adopted in the UK in 2001, which limited the analyses to only 10 years of past data points. Including deaths coded using the preceding ICD-9 version increased the years of available data back to 1980, but although we applied translations from ICD-9 to ICD-10,45 there remained visible anomalies in the data before/after the change in coding for some disease. Concerned that this could lead to incorrect predictions of trends, we therefore restricted the analyses to the ICD-10 coded data, but broadened our data set to include data from all European Union (EU) countries, which have similar high-income and low mortality profile, and included gross domestic product (GDP) per capita into the model as an explanatory variable.
Deaths coded to heart failure (I50) were redistributed to the primary causes (eg, CHD, hypertensive heart disease, chronic obstructive pulmonary disease and chronic kidney disease).46 Deaths were grouped into three age groups: <35, 35–64 and 65+. For each age group, we determined country-specific mortality for each risk factor-related disease, and all other diseases, as a proportion of all-cause mortality in each country. Using methods described by Salomon and Murray,43 we specified a multivariate normal model for the log of the ratios of each cause fraction to the cause fraction for all other diseases, using the log of all-cause mortality rate and GDP per capita47 as explanatory variables. We then derived parameter values for the disease projections models, for each age group, using seemingly unrelated regression.48
To project disease rates forward in time for the UK, we applied the mortality projections from our population forecasts and GDP projections from the World Bank47 in the disease projections models, to determine the annual average change in disease rates for the UK out to 2030.
Evaluating disease burden
To determine the future changes in population if the WHO or ideal risk reduction targets are achieved, we first calculated the new population mortality, using the PIFs to determine future changes in mortality from the NCDs. We then resimulated the future population out to 2030, using the new rates of mortality, but assuming no change in forecasts of fertility and migration.
Effects on premature mortality were estimated as the change in probability of death from NCDs between the ages of 30 and 69.2 As was done in the global modelling of the WHO NCD reduction targets,3 our estimate was unconditional, in that it excluded deaths from other causes (eg, injuries), but due to the broader range of diseases of interest in the PROMISE study, we included all NCDs, rather than restricting the analysis to the four main NCD groups (cardiovascular, cancer, diabetes and chronic respiratory diseases).
We defined the morbidity burden associated with NCDs as the reduction in 1 year of life at full health. This was calculated at each age and sex, from Global Burden of Disease estimates of prevalent years lived with disability (YLDs). We included a business-as-usual trend in morbidity if there had been a significant change in Global Burden of Disease YLD estimates between 1990 and 2010. For diabetes and depression, we estimated a morbidity effect of achieving the WHO targets, which we estimated using the PIFs for these diseases in the same way that the PIFs were applied to mortality for all other NCDs.
Results
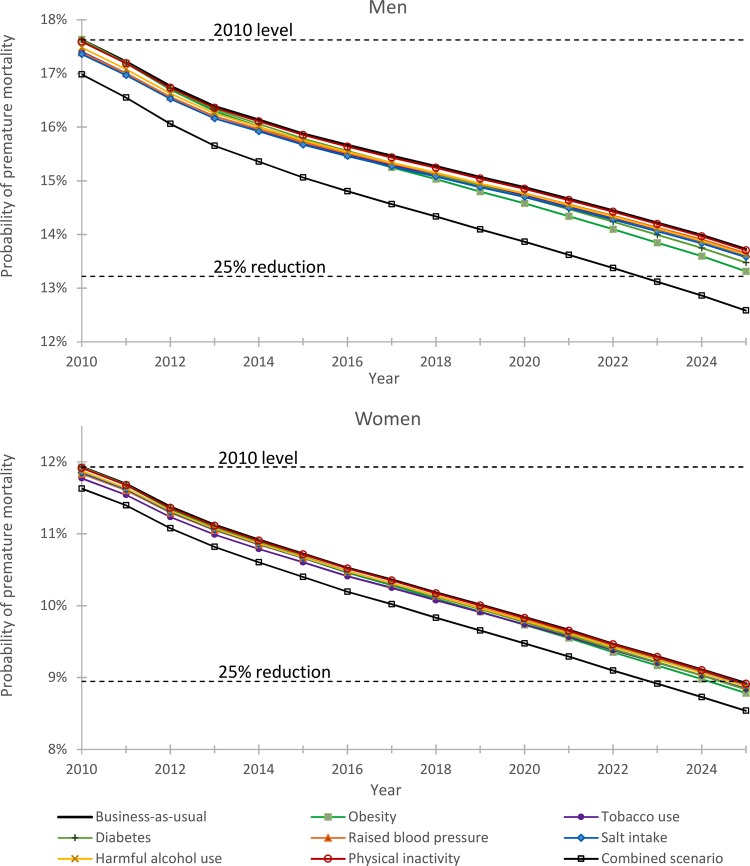
With a continuation of current trends in mortality, fertility and migration, the number of 30–69 years old in the UK population is expected to increase from around 32.5 million in 2010 to 33.4 million in 2025, with the proportion of the whole population aged over 70 rising from 12.5% in 2010 to 16.2% by 2025. With business-as-usual, the probability of premature mortality (30–69 years) from NCDs among men is expected to fall from 17.6% in 2010 to 13.7% in 2025 (figure 2). This equates to a relative reduction of 22%, which is just short of the WHO 25% reduction target. Premature mortality among women is expected to reach the WHO target, falling from 11.9% in 2010 to 8.9% in 2025 (figure 2), which equates to a relative reduction of 25%. If we assume trends in risk factors and diseases continue to 2030, the outcomes follow the same pattern, with men (30%) falling short of the 33% reduction target and women (33%) just reaching it.
Figure 2.
Projected trends in the probability of dying prematurely from non-communicable disease for the WHO risk factor target scenarios.
There is added benefit from achieving the WHO 2025 behavioural risk factor targets (table 3), which ranges from an added relative reduction of 0.1% (reduced physical inactivity) to 2.3% (rise in obesity halted). The combined effect of achieving all seven risk factor targets (relative reduction of 6.1% for men and 3.0% for women) is sufficient for men and women to reach the 25% premature mortality reduction target by 2025 (figure 2). Achieving the WHO targets would, however, achieve only a quarter of what could potentially be achieved for men and less than a fifth of what could potentially be achieved for women.
Table 3.
Relative reduction in probability of premature mortality from non-communicable diseases by 2025
| WHO risk factor targets |
Ideal risk reduction |
Proportion of ideal risk addressed by meeting WHO target |
||||
|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | |
| Business-as-usual | 22% | 25% | ||||
| Additional reduction if achieving risk factor targets or ideal risk scenario | ||||||
| Obesity | 2.3% (1.6% to 2.9%) | 1.1% (0.3% to 1.9%) | 7.9% (5.9% to 9.8%) | 4.8% (2.6% to 7.1%) | 29% | 24% |
| Tobacco use | 0.6% (0.6% to 0.7%) | 0.6% (0.6% to 0.7%) | 12.1% (10.1% to 14.0%) | 9.7% (8.2% to 11.1%) | 5% | 7% |
| Diabetes | 1.4% (1.2% to 1.7%) | 0.7% (0.6% to 0.8%) | 2.7% (2.2% to 3.2%) | 1.4% (1.2% to 1.7%) | 53% | 51% |
| Raised blood pressure | 0.4% (0.4% to 0.4%) | 0.2% (0.2% to 0.2%) | 1.6% (1.6% to 1.7%) | 0.7% (0.7% to 0.8%) | 25% | 25% |
| Salt intake | 0.8% (0.8% to 0.9%) | 0.3% (0.3% to 0.3%) | 2.1% (2.0% to 2.2%) | 0.7% (0.7% to 0.8%) | 39% | 40% |
| Harmful alcohol use* | 0.6% (0.4% to 0.8%) | 0.3% (0.22% to 0.5%) | 0.9% (−4.6% to 4.0%) | 3.2% (−1.08% to 6.9%) | 62% | 11% |
| Physical inactivity | 0.1% (0.08% to 0.1%) | 0.1% (0.0% to 0.1%) | 1.1% (0.81% to 1.4%) | 0.6% (0.4% to 0.7%) | 10% | 10% |
| Combined scenario | 6.5% (5.4% to 7.5%) | 3.2% (2.2% to 4.1%) | 26.2% (21.9% to 29.7%) | 18.4% (14.3% to 22.2%) | 25% | 17% |
Values are mean and 95% uncertainty intervals.
*Low-level consumption of alcohol is associated with a decreased risk of some diseases (eg, coronary heart disease, hypertensive heart disease and diabetes), which partly counter the modelled health benefits of abstaining from alcohol.
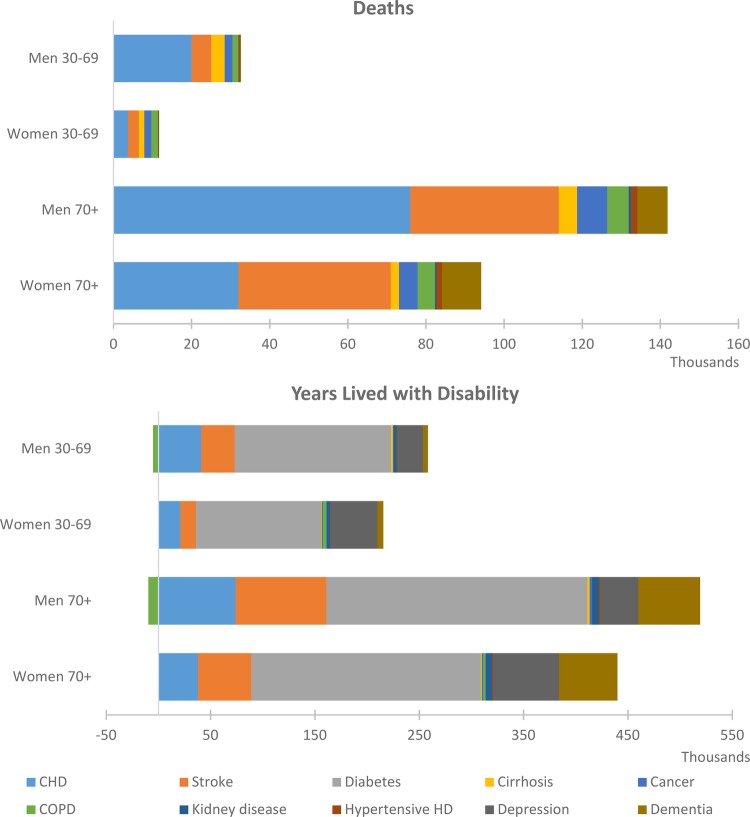
Achieving all seven behavioural risk factor targets would avert a total of 300 000 deaths (at all ages) and 1.3 million YLDs from the reductions in related NCDs (excluding effects on depression and dementia) between 2010 and 2025 (table 4). The majority of improvements in mortality are due to fewer deaths from CHD and stroke, while the majority of improvements in morbidity are due to reduced rates of diabetes, CHD and stroke (figure 3). Figure 3 also shows that there is potentially a substantial additional gain in morbidity from reduced rates of depression in the 30–69 and 70+ age groups; and substantial additional gains in morbidity and mortality from reduced rates of dementia in 70+ years old.
Table 4.
Total non-communicable disease deaths and YLDs that are averted or delayed between 2010 and 2025, for each of the risk factor target scenarios
| 30–69 years |
70+ years |
|||
|---|---|---|---|---|
| Men | Women | Men | Women | |
| Deaths | ||||
| Obesity | 13 000 (8900 to 17 000) | 4500 (500 to 8600) | 47 000 (34 000 to 60 000) | 26 000 (11 000 to 40 000) |
| Tobacco use | 5100 (4800 to 5400) | 4300 (4100 to 4400) | 14 000 (13 000 to 15 000) | 15 000 (14 000 to 15 000) |
| Diabetes | 7200 (5900 to 8600) | 2500 (2000 to 2900) | 38 000 (30 000 to 45 000) | 32 000 (26 000 to 38 000) |
| Raised blood pressure | 5700 (5400 to 6100) | 2300 (2100 to 2500) | 25 000 (24 000 to 26 000) | 23 000 (22 000 to 24 000) |
| Salt intake | 9000 (8700 to 9400) | 2500 (2400 to 2600) | 38 000 (37 000 to 39 000) | 26 000 (25 000 to 27 000) |
| Harmful alcohol use | 4900 (3000 to 6900) | 2000 (1200 to 2800) | 11 000 (7100 to 16 000) | 6100 (800 to 11 000) |
| Physical inactivity | 920 (660 to 1200) | 320 (240 to 400) | 4300 (3200 to 5400) | 3900 (2800 to 4900) |
| Combined scenario | 38 000 (32 000 to 44 000) | 13 000 (8500 to 17 000) | 150 000 (130 000 to 170 000) | 99 000 (79 000 to 120 000) |
| YLDs | ||||
| Obesity | 79 000 (66 000 to 93 000) | 50 000 (38 000 to 63 000) | 150 000 (120 000 to 170 000) | 98 000 (66 000 to 130 000) |
| Tobacco use | 20 000 (20 000 to 21 000) | 25 000 (25 000 to 26 000) | 27 000 (26 000 to 28 000) | 37 000 (36 000 to 38 000) |
| Diabetes | 190 000 (190 000 to 190 000) | 160 000 (160 000 to 160 000) | 330 000 (320 000 to 340 000) | 290 000 (290 000 to 300 000) |
| Raised blood pressure | 18 000 (17 000 to 19 000) | 12 000 (11 000 to 13 000) | 40 000 (38 000 to 42 000) | 31 000 (29 000 to 33 000) |
| Salt intake | 29 000 (28 000 to 30 000) | 14 000 (14 000 to 15 000) | 60 000 (57 000 to 62 000) | 34 000 (33 000 to 36 000) |
| Harmful alcohol use | 6200 (−18 000 to 30 000) | −280 (−8700 to 8100) | 14 000 (−25 000 to 53 000) | 3100 (−12 000 to 18 000) |
| Physical inactivity | 6200 (5300 to 7000) | 5500 (4700 to 6200) | 13 000 (11 000 to 15 000) | 13 000 (11 000 to 14 000) |
| Combined scenario | 260 000 (240 000 to 280 000) | 200 000 (190 000 to 210 000) | 480 000 (430 000 to 520 000) | 370 000 (350 000 to 400 000) |
Values are mean and 95% uncertainty intervals.
YLDs, years lived with disability.
Figure 3.
Non-communicable disease deaths and YLDs that are averted or delayed between 2010 and 2025, for the combined risk factor target scenario. (Note: the small increase in COPD YLDs is due to a shift in the age distribution of the population, primarily as a result of reductions in CHD and stroke mortality, and does not reflect an increase in COPD rates.) CHD, coronary heart disease; COPD, chronic obstructive pulmonary disease; HD, haemodialysis; YLDs, years lived with disability.
The results demonstrate that, for the UK, achieving the obesity target will result in the biggest overall impact on mortality and morbidity (figure 4). Halting the rise in diabetes and achieving a 30% reduction in salt intake would also achieve a large impact on NCD mortality, and reducing tobacco consumption by 30% would have a large impact on morbidity.
Figure 4.
DALYs averted for the WHO risk factor target scenarios. DALY, disability-adjusted life year.
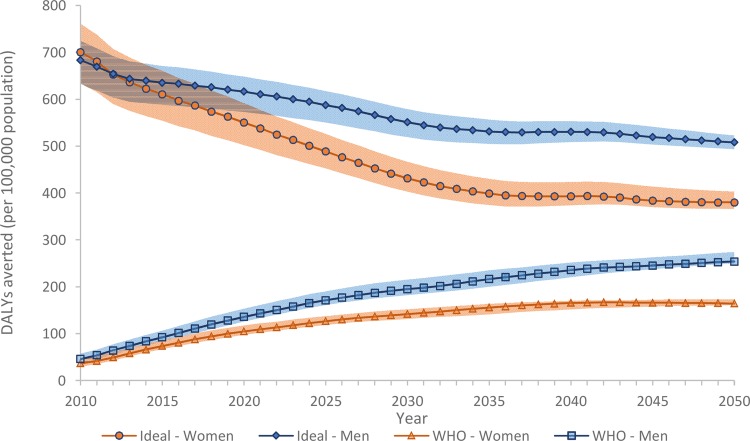
Over time, achieving the combination of WHO risk factor targets would address an increasing proportion of the health that could potentially be gained if everyone could adjust their risk behaviour to ideal levels in 2010 (figure 5 and online supplementary appendix 2 for deaths and YLDs averted under the ideal risk scenario). Taking both morbidity and mortality into account, by 2025, 29% of the health gain would be achieved for men and 26% for women. If we assume trends in risk factors and diseases continue beyond 2025, the proportions would reach 35% for men and 33% for women by 2030.
Figure 5.
DALYs averted for the combined WHO risk factor target scenario and the ideal risk reduction scenario. DALY, disability-adjusted life year.
bmjopen-2016-012805supp_appendix2.pdf (362.5KB, pdf)
Discussion
There have been considerable achievements in addressing the risks for NCD in the UK over the past three decades. Although prevalence of obesity and diabetes have risen, initiatives such as increasing access to BP-lowering drugs, reducing salt in processed foods and strengthening tobacco control have all contributed to the reduction in these risk factors over time. These changes in lifestyle behaviours, along with advances in treatment, have contributed to the reduction in NCDs that we observed. Our projections show that if these trends continue, there is likely to be a substantial further reduction in NCD burden by 2025, which will see the UK very nearly reach the current WHO target of 25% reduction in premature mortality and very likely reach the proposed 30% reduction by 2030.
It will be critical that past investments in prevention (and treatment) are sustained so that the UK does not lose the momentum built over previous decades. Preventive efforts must also be increased if the UK is to meet the 2025 target. While the targets do provide a worthwhile objective, a further two-thirds of the preventable NCD burden could potentially be reduced with an even more ambitious prevention programme in the future.
Although current WHO targets focus on premature mortality, our modelling predicted substantial added benefits from reduced NCD morbidity, particularly at older ages. Around 16% of Government spending in the UK is allocated to health, which is high compared with other European countries.49 The large reductions in NCD burden, particularly for CHD, stroke, diabetes, depression and dementia, would help reduce these costs. The social and economic burden of diseases such as depression and dementia is also high. Depression, for example, is associated with increased workplace absenteeism and reduced productivity,50 and dementia is associated with substantial social care costs.51 In 2012, the cost of health and social care services for patients with dementia in the UK was greater than the costs of CHD, stroke and cancer care combined.51
Unfortunately, the drivers of mental and neurological disorders, despite a high global burden, are poorly understood.36 Further research is needed to strengthen the epidemiological evidence of the links between lifestyle risks and prevention of diseases, such as depression and dementia, and to better understand how prevalence of these diseases may in turn increase risk of other NCDs. In the UK, funding for dementia and mental health research is low in comparison to the size of the burden.51 52 However, a review of costs and burden of mental and neurological disorders in the EU estimated that the return on investment in research would be highly favourable when taking the full cost to society into account.53
Cost-effectiveness analysis can be used to identify which interventions will provide best value-for-money. Modelling of interventions for improving diet and body mass in England suggest that fiscal measures and regulation may be more cost-effective than more individually targeted approaches such as physician counselling and worksite programmes,54 which is consistent with findings from modelling studies in comparable high-income countries such as Australia55 56 and New Zealand.57 But further work is needed to identify the most effective and cost-effective interventions for addressing a wider range of risk factor targets in the UK, including a number of dietary risks associated with a high NCD burden that are not included in the WHO targets (eg, fruits and vegetables, fats and cholesterol36). Modelling is also needed to better understand and quantify the health impacts of underlying drivers of change, such as global marketing and trade, the design of urban environments and climate change.
The WHO's inclusion of dietary risk factors as targets in the Global NCD Action Plan was minimal, given the high level of attributable NCD burden.36 For example, while a 30% reduction target was set for salt intake, no targets were defined for reducing intake of transfats, red and processed meats or sugar-sweetened beverages, or increasing intake of fibre, whole grains, fruits and vegetables. The omission of these risk factors does mean that our study and previous global modelling estimates3 are likely to have underestimated the potential for reduction in premature mortality from NCDs.
To model the impact of changing risk factors on NCDs, it is necessary to draw on a wide variety of different levels of evidence to calculate the dependencies between risk factors and avoid double counting of outcomes. For example, to calculate impacts on BMI from changes in physical activity, we relied on models of human energy balance derived by Hall et al;58 to calculate impacts on BP levels from changes in salt intake, we used measures from meta-regression of randomised controlled trials of BP reduction; and to determine dose–response relationships between risk factors and diseases we drew on a variety of meta-analyses of observational studies. While most studies adjust for potential confounding factors (eg, age, sex and smoking) it is impossible to rule out residual confounding from missing or poorly measured explanatory variables. In addition, where there are multiple pathways between a risk factor and disease (eg, there is risk of CHD from physical inactivity directly and indirectly via diabetes) we have estimated the relative risks for the two pathways from studies of the risk for the pathways combined and the prevalence of the risk factors (see Note 2 in online supplementary appendix 1). These approximations are necessary in the absence of large, long-term studies that measure and adjust for all possible risks simultaneously. Consequently, it is likely that there is greater uncertainty in our results than is reflected in the uncertainty intervals we have estimated.
This modelling study contributes to the evidence about the potential impact of changes in risk factor prevalence, but to implement change evidence is needed on interventions designed to tackle behavioural risk factors. The relative magnitude of health gain associated with the WHO risk factor targets is not necessarily a good guide for setting priorities for intervention. The apparently smaller benefits of addressing physical inactivity and harmful alcohol use, for example, are in large part because the WHO targets are relatively modest. The modelling of alcohol targets also does not capture any benefits from reduced injury rates, which fall outside of the NCD focus of the WHO targets. In addition, the modelling of some risk factors was limited by the available data (eg, self-reported physical activity) and WHO definitions (eg, prevalence of hypertension rather than the full distribution of BP). In addition, past decades of successful intervention in the UK (eg, BP-lowering drugs, tobacco taxes, smoking cessation programmes) has seen the prevalence of high BP and prevalence of smoking decline, so that the WHO targets (25% relative reduction in high BP and 30% relative reduction in smoking) are relatively less beneficial than the targets to halt the rise in prevalence of obesity and diabetes, both of which are rising steeply in the UK.
Early population health impact studies typically modelled effects on a population cohort without forward projection of disease trends or they modelled effects on a population cohort forward in time without replacement (ie, following the current adult population until death or end of projection). In evaluating global NCD impact of the WHO targets, Kontis et al3 advanced this approach by modelling the population impacts between 2010 and 2025 in three 5-year intervals, incorporating projections of disease trends and allowing for population growth and ageing between each modelled time interval. We have taken the modelling a step further by incorporating risk factor prevalence rates and model outputs of disease rates that are directly comparable to past trends, and integrating this with a population demographic model that stochastically forecasts trends in mortality, fertility and migration. This approach could facilitate future integration with other models, including economic models (eg, Agricultural Model Intercomparison and Improvement Project (AgMIP) models, which focus on food security under climate change59) allowing health outcomes to be included in broader systems modelling studies. In addition, while focused on a select range of WHO risk factor targets and NCD outcomes for these analyses, the model could potentially be adapted to address a more diverse range of risks factors and disease outcomes in the future.
This modelling study illustrates the large health gains that could be achieved by addressing unhealthy risk factors for disease. For countries such as the UK that can capitalise on many decades of effort in prevention and treatment, the WHO premature mortality target is an achievable goal. But with a further two-thirds of the NCD burden still potentially preventable, it is imperative that the UK capitalises on the momentum of past decades with further effort in prevention. For low-income and middle-income countries, the potential benefits are likely to be even greater,3 as is the need for evidence to develop cost-effective policies for prevention in these countries. Given the growing global burden of diseases such as diabetes, depression and dementia, the WHO should consider future global targets that provide incentives for addressing these diseases, which carry a high health burden for the individual and place a substantial burden on health and social care systems in society.
Acknowledgments
The authors thank Jamie Sims and Jaithri Ananthapavan for assistance with coding physical activity MET values in the Health Survey for England.
Footnotes
Contributors: LJC and PS conceived of the study, collected and analysed the data, interpreted the results and contributed to the writing and editing the manuscript.
Funding: The study was funded by a competitive grant from the Richmond Group of Charities (http://www.richmondgroupofcharities.org.uk). The researchers were independent of the funder. All authors had full access to all of the data (including statistical reports and tables) in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.United Nations. Political declaration of the high-level meeting of the General Assembly on the prevention and control of non-communicable diseases. United Nations General Assembly, Sixty-sixth session, Agenda item 117 2011.
- 2.WHO. Global action plan for the prevention and control of noncommunicable diseases 2013–2020. Geneva: World Health Organization, 2013. [Google Scholar]
- 3.Kontis V, Mathers CD, Rehm J et al. Contribution of six risk factors to achieving the 25×25 non-communicable disease mortality reduction target: a modelling study. Lancet 2014;384:427–37. 10.1016/S0140-6736(14)60616-4 [DOI] [PubMed] [Google Scholar]
- 4.Beaglehole R, Bonita R, Ezzati M et al. NCD Countdown 2025: accountability for the 25×25 NCD mortality reduction target. Lancet 2014;384:105–7. 10.1016/S0140-6736(14)61091-6 [DOI] [PubMed] [Google Scholar]
- 5.Pearce N, Ebrahim S, McKee M et al. The road to 25×25: how can the five-target strategy reach its goal? Lancet Glob Health 2014;2:e126–8. 10.1016/S2214-109X(14)70015-4 [DOI] [PubMed] [Google Scholar]
- 6.WHO. Health in 2015: from MDGs, Millennium Development Goals to SDGs, Sustainable Development Goals. Geneva: World Health Organization, 2015. [Google Scholar]
- 7.The Richmond Group of Charities. (15 December 2015). http://www.richmondgroupofcharities.org.uk/
- 8.Joint Health Surveys Unit. Health Survey for England 2013 (and previous editions). Leeds: Health and Social Care Information Centre, 2014. [Google Scholar]
- 9.McPherson K, Marsh T, Brown M. Foresight. Tackling obesities—modelling future trends in obesity and the impact on health. London: Government Office for Science, 2007. [Google Scholar]
- 10.WHO. Noncommunicable diseases global monitoring framework: indicator definitions and specifications. Geneva: World Health Organization, 2014. [Google Scholar]
- 11.He FJ, Li J, MacGregor GA. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ 2013;346:f1325 10.1136/bmj.f1325 [DOI] [PubMed] [Google Scholar]
- 12.Baumgart M, Snyder HM, Carrillo MC et al. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheimers Dement 2015;11:718–26. 10.1016/j.jalz.2015.05.016 [DOI] [PubMed] [Google Scholar]
- 13.Valkanova V, Ebmeier KP. Vascular risk factors and depression in later life: a systematic review and meta-analysis. Biol Psychiatry 2013;73:406–13. 10.1016/j.biopsych.2012.10.028 [DOI] [PubMed] [Google Scholar]
- 14.Cheng G, Huang C, Deng H et al. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J 2012;42:484–91. 10.1111/j.1445-5994.2012.02758.x [DOI] [PubMed] [Google Scholar]
- 15.Lim S, Vos T, Flaxman A et al. The burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions 1990–2010: a systematic analysis. Lancet 2013;380:2224–60. 10.1016/S0140-6736(12)61766-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prospective Studies Collaboration. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 2009;373:1083–96. 10.1016/S0140-6736(09)60318-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Norat T, Aune D, Vieira R et al. The associations between food, nutrition and physical activity and the risk of pancreatic cancer. WCRF/AICR Systematic Literature Review Continuous Update Project Report, 2011.
- 18.Luppino FS, de Wit LM, Bouvy PF et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry 2010;67:220–9. 10.1001/archgenpsychiatry.2010.2 [DOI] [PubMed] [Google Scholar]
- 19.Beydoun M, Beydoun H, Wang Y. Obesity and central obesity as risk factors for incident dementia and its subtypes: a systematic review and meta-analysis. Obes Rev 2008;9:204–18. 10.1111/j.1467-789X.2008.00473.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thun MJ, Apicella LF, Henley SJ. Smoking vs other risk factors as the cause of smoking-attributable deaths. Confounding in the courtroom. JAMA 2000;284:706–12. [DOI] [PubMed] [Google Scholar]
- 21.Willi C, Bodenmann P, Ghali WA et al. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 2007;298:2654–64. 10.1001/jama.298.22.2654 [DOI] [PubMed] [Google Scholar]
- 22.Gandini S, Botteri E, Iodice S et al. Tobacco smoking and cancer: a meta-analysis. Int J Cancer 2008;122:155–64. 10.1002/ijc.23033 [DOI] [PubMed] [Google Scholar]
- 23.Lee YCA, Cohet C, Yang YC et al. Meta-analysis of epidemiologic studies on cigarette smoking and liver cancer. Int J Epidemiol 2009;38:1497–511. 10.1093/ije/dyp280 [DOI] [PubMed] [Google Scholar]
- 24.Luger TM, Suls J, Vander Weg MW. How robust is the association between smoking and depression in adults? A meta-analysis using linear mixed-effects models. Addict Behav 2014;39:1418–29. [DOI] [PubMed] [Google Scholar]
- 25.Zhong G, Wang Y, Zhang Y et al. Smoking is associated with an increased risk of dementia: a meta-analysis of prospective cohort studies with investigation of potential effect modifiers. PLoS ONE 2015;10:e0118333 10.1371/journal.pone.0118333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peters SA, Huxley RR, Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia 2014;57:1542–51. 10.1007/s00125-014-3260-6 [DOI] [PubMed] [Google Scholar]
- 27.Peters SA, Huxley RR, Woodward M. Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 64 cohorts, including 775 385 individuals and 12 539 strokes. Lancet 2014;383:1973–80. 10.1016/S0140-6736(14)60040-4 [DOI] [PubMed] [Google Scholar]
- 28.Rotella F, Mannucci E. Diabetes mellitus as a risk factor for depression. A meta-analysis of longitudinal studies. Diabetes Res Clin Pract 2013;99:98–104. 10.1016/j.diabres.2012.11.022 [DOI] [PubMed] [Google Scholar]
- 29.Gudala K, Bansal D, Schifano F et al. Diabetes mellitus and risk of dementia: a meta-analysis of prospective observational studies. J Diabetes Investig 2013;4:640–50. 10.1111/jdi.12087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewington S, Prospective studies collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies (vol 360, pg 1903, 2002). Lancet 2003;361:1060. [DOI] [PubMed] [Google Scholar]
- 31.Shield KD, Parry C, Rehm J. Chronic diseases and conditions related to alcohol use. Alcohol Res 2014;35:155–73. [PMC free article] [PubMed] [Google Scholar]
- 32.Patra J, Taylor B, Irving H et al. Alcohol consumption and the risk of morbidity and mortality for different stroke types—a systematic review and meta-analysis. BMC Public Health 2010;10:258 10.1186/1471-2458-10-258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baliunas DM, Taylor BM, Irving HM et al. Alcohol as a risk factor for type 2 diabetes: a systematic review and meta-analysis. Diabetes Care 2009;32:2123 10.2337/dc09-0227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rehm J, Taylor B, Mohapatra S et al. Alcohol as a risk factor for liver cirrhosis: a systematic review and meta-analysis. Drug Alcohol Rev 2010;29:437–45. 10.1111/j.1465-3362.2009.00153.x [DOI] [PubMed] [Google Scholar]
- 35.Corrao G, Bagnardi V, Zambon A et al. A meta-analysis of alcohol consumption and the risk of 15 diseases. Prev Med 2004;38:613–19. 10.1016/j.ypmed.2003.11.027 [DOI] [PubMed] [Google Scholar]
- 36.Forouzanfar MH, Alexander L, Anderson HR et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;386:2287–323. 10.1016/S0140-6736(15)00128-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Report. Washington DC: U.S. Department of Health and Human Services, 2008. [Google Scholar]
- 38.Blondell SJ, Hammersley-Mather R, Veerman JL. Does physical activity prevent cognitive decline and dementia? A systematic review and meta-analysis of longitudinal studies. BMC Public Health 2014;14:510 10.1186/1471-2458-14-510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fishman G. Monte Carlo: concepts, algorithms, and applications. New York: Springer-Verlag, 1995. [Google Scholar]
- 40. University of California Berkeley (USA), Max Planck Institute for Demographic Research (Germany). Human Mortality Database (2 April 2015). http://www.mortality.org.
- 41. Max Planck Institute for Demographic Research (Germany), Vienna Institute of Demography (Austria). Human Fertility Database (29 April 2015). http://www.humanfertility.org.
- 42.Hyndman RJ, Booth H, Yasmeen F. Coherent mortality forecasting: the product-ratio method with functional time series models. Demography 2013;50:261–83. 10.1007/s13524-012-0145-5 [DOI] [PubMed] [Google Scholar]
- 43.Salomon JA, Murray CJ. The epidemiologic transition revisited: compositional models for causes of death by age and sex. Popul Dev Rev 2002;28:205–28. 10.1111/j.1728-4457.2002.00205.x [DOI] [Google Scholar]
- 44.World Health Organization. WHO Mortality Database (4 November 2014). http://www.who.int/healthinfo/statistics/mortality_rawdata/en/
- 45.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006;3:e442 10.1371/journal.pmed.0030442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Naghavi M, Makela S, Foreman K et al. Research algorithms for enhancing public health utility of national causes-of-death data. Popul Health Metr 2010;8:9 10.1186/1478-7954-8-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.The World Bank. World Development Indicators (1 May 2015). http://data.worldbank.org/data-catalog/world-development-indicators
- 48.Greene WH. Econometric analysis. 5th edn Upper Saddle River, NJ: Prentice Hall, 2003. [Google Scholar]
- 49.WHO. National health accounts: country health information. Geneva: World Health Organization, 2015. (15 December 2015). http://apps.who.int/nha/database/Country_Profile/Index/en [Google Scholar]
- 50.Donohue JM, Pincus HA. Reducing the societal burden of depression. Pharmacoeconomics 2007;25:7–24. 10.2165/00019053-200725010-00003 [DOI] [PubMed] [Google Scholar]
- 51.Luengo-Fernandez R, Leal J, Gray A. UK research spend in 2008 and 2012: comparing stroke, cancer, coronary heart disease and dementia. BMJ Open 2015;5:e006648 10.1136/bmjopen-2014-006648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kingdon D. Health research funding: mental health research continues to be underfunded. BMJ 2006;332:1510 10.1136/bmj.332.7556.1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gustavsson A, Svensson M, Jacobi F et al. Cost of disorders of the brain in Europe 2010. Eur Neuropsychopharmacol 2011;21:718–79. 10.1016/j.euroneuro.2011.08.008 [DOI] [PubMed] [Google Scholar]
- 54.Cecchini M, Sassi F, Lauer JA et al. Tackling of unhealthy diets, physical inactivity, and obesity: health effects and cost-effectiveness. Lancet 2010;376:1775–84. 10.1016/S0140-6736(10)61514-0 [DOI] [PubMed] [Google Scholar]
- 55.Cobiac LJ, Vos T, Doran C et al. Cost-effectiveness of interventions to prevent alcohol-related disease and injury in Australia. Addiction 2009;104:1646–55. 10.1111/j.1360-0443.2009.02708.x [DOI] [PubMed] [Google Scholar]
- 56.Cobiac LJ, Vos T, Veerman JL. Cost-effectiveness of interventions to reduce dietary salt intake. Heart 2010;96:1920–5. 10.1136/hrt.2010.199240 [DOI] [PubMed] [Google Scholar]
- 57.Nghiem N, Blakely T, Cobiac LJ et al. Health and economic impacts of eight different dietary salt reduction interventions. PLoS ONE 2015;10:e0123915 10.1371/journal.pone.0123915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hall KD, Sacks G, Chandramohan D et al. Quantification of the effect of energy imbalance on bodyweight. Lancet 2011;378:826–37. 10.1016/S0140-6736(11)60812-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rosenzweig C, Jones J, Hatfield J et al. The agricultural model intercomparison and improvement project (AgMIP): protocols and pilot studies. Agric For Meteorol 2013;170:166–82. 10.1016/j.agrformet.2012.09.011 [DOI] [Google Scholar]
- 60.Wahid A, Manek N, Nichols M et al. Quantifying the association between physical activity and cardiovascular disease and diabetes: a systematic review and meta-analysis, J Am Heart Assoc 2016;5:e002495. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2016-012805supp_appendix1.pdf (671.4KB, pdf)
bmjopen-2016-012805supp_appendix2.pdf (362.5KB, pdf)