Abstract
The oncogenic transcription factor v-Myb disrupts myelomonocytic differentiation and transforms myelomonocytic cells by deregulating the expression of specific target genes. One of these genes, the chicken mim-1 gene, is activated by Myb exclusively in myelomonocytic cells and, therefore, has been an interesting model system to study how Myb activates a target in a lineage-specific manner. Previous work has suggested that Myb activates mim-1 by cooperating with CCAAT box/enhancer binding protein beta (C/EBPβ) or other C/EBP transcription factors at the mim-1 promoter. We have now identified and characterized a powerful Myb-dependent enhancer located 2 kb upstream of the mim-1 promoter. The enhancer is preferentially active in myelomonocytic cells, confers Myb responsiveness onto a heterologous promoter, and dramatically increases Myb responsiveness of the mim-1 promoter. Chromatin immunoprecipitation demonstrates that v-Myb and C/EBPβ are bound to the enhancer in v-Myb-transformed cells; furthermore, cooperation of the enhancer with the mim-1 promoter is greatly stimulated by C/EBPβ and p300. Taken together, our results show that the regulation of mim-1 expression by v-Myb is more complex than previously assumed and involves two distinct regions of the mim-1 gene. A major function of v-Myb (in addition to its role at the mim-1 promoter) apparently is to activate the mim-1 enhancer and, together with C/EBPβ and p300, facilitate its cooperation with the promoter. Interestingly, our work also shows that the v-Myb protein encoded by avian myeloblastosis virus is defective in this function, suggesting an explanation for why primary avian myeloblastosis virus-transformed myeloblasts do not express the mim-1 gene.
The oncogene v-myb of Avian myeloblastosis virus (AMV) and avian leukemia virus E26 encodes a transcription factor which enables these viruses to transform cells of the myelomonocytic lineage (12, 26, 32, 39). The v-myb genes of AMV and E26 are mutated forms of the chicken c-myb gene, which itself plays a central role in the development of the hematopoietic system. c-myb is expressed in the immature cells of all hematopoietic lineages and is turned off during their terminal differentiation. c-myb is thought to act as part of a genetic switch that directs hematopoietic progenitor cells to alternative fates, such as proliferation, differentiation, and apoptosis (39, 47).
The proteins encoded by v-myb and c-myb (v-Myb and c-Myb) bind to the sequence motif PyAAC(G/T)G (1) and activate promoters containing this sequence (17, 24, 33, 48). So far, a number of genes have been identified as targets of Myb, including mim-1 (33), the lysozyme gene (18), bcl-2 (10, 46), tom-1 (7), c-kit (16), GBX2 (25), Pdcd4 (42), the genes for neutrophil elastase (37), and the A2B adenosine receptor (49), among others. Myb binding sites in the promoters of most of these genes have been identified; however, detailed studies on how Myb activates the expression of its target genes have been performed only in a few cases.
One of the most thoroughly studied Myb target genes is the chicken mim-1 gene. Within the hematopoietic system, mim-1 is expressed only in the myelomonocytic lineage and its expression reaches very high levels in those cells (33). Although c-Myb is expressed in all hematopoietic lineages, it activates the mim-1 gene only in myelomonocytic cells. The mim-1 promoter contains several Myb binding sites as well as binding sites for CCAAT box/enhancer binding proteins (C/EBPs) and is activated synergistically by Myb and C/EBP family members (6, 34). Myb, together with a C/EBP factor, such as C/EBPβ, has been shown to activate the endogenous mim-1 gene even in nonmyelomonocytic cells, such as fibroblasts or erythroid cells, which normally do not express this gene (6, 34). This observation has suggested that Myb, together with C/EBP family members, is also responsible for the cell-type-specific expression of mim-1 in myelomonocytic cells (6, 34).
Although the promoters of genes whose expression is restricted to particular cell types often display cell-type-specific activity, there are many examples showing that the expression of such genes is rarely controlled only by their promoters. In most cases, the cell-type specificity is achieved by the combined action of promoters and other cis-acting regulatory elements, such as cell-type-specific enhancers or silencers. Such elements usually coincide with so-called DNase I-hypersensitive sites (DHS) in the chromatin; mapping of DHS is therefore a straightforward strategy to localize cis-acting regulatory elements in a gene of interest. We were interested in investigating whether mim-1, as a model of a cell-type-specific Myb target gene, is activated by Myb only through its promoter or whether other regulatory elements are involved. The work described here identifies a powerful cell-specific enhancer in the mim-1 upstream region and shows that this enhancer plays a major role in the regulation of mim-1 expression by Myb.
MATERIALS AND METHODS
Cells.
HD11 is a line of MC29-transformed chicken macrophages. 10.4 is a subclone of the HD11 line expressing a v-Myb/estrogen receptor (ER) fusion protein (5). The ER domain of the fusion protein contains a functional transcriptional activation domain. Both lines were grown in basal Iscove's medium supplemented with 8% fetal calf serum and 2% chicken serum. BM2 is a line of AMV-transformed chicken myeloblasts (31) and was grown in RPMI 1640 medium supplemented with 5% fetal calf serum, 5% chicken serum, and 10% tryptone phosphate broth (Gibco). HD3 is a line of avian erythroblastosis virus-transformed chicken erythroblasts grown in RPMI 1640 medium supplemented with 8% fetal calf serum and 2% chicken serum. DF-1 is a chicken fibroblast line (15) and was grown in Dulbecco modified Eagle medium supplemented with 10% fetal calf serum. DT40 is a chicken pre-B-cell line (4) and was grown in basal Iscove's medium supplemented with 10% fetal calf serum and 0.001% β-mercaptoethanol. QT6 is a line of chemically transformed Japanese quail fibroblasts and was grown in basal Iscove's medium supplemented with 8% fetal calf serum and 2% chicken serum (30).
Mapping of DNaseI-hypersensitive sites (DHS).
DHS were mapped according to Sippel et al. (43) as described in detail by Braas et al. (2). Genomic DNA isolated from DNase I-treated nuclei was digested with HindIII and analyzed by Southern blotting. Blots were probed with a 0.6-kb BglII/HindIII DNA fragment from the mim-1 upstream region.
Reporter genes and transfections.
Luciferase reporter genes were constructed by inserting genomic DNA fragments from the mim-1 gene upstream of the herpes simplex virus thymidine kinase (tk) promoter into the luciferase vector ptk81-Luc (2). ptk81-Luc was generated by cloning a 150-bp SmaI/BglII fragment from plasmid p-81Tk-Luc containing tk promoter sequences from −81 to +53 bp (35) between the SmaI and BglII sites of pGL3-Basic. ptk81-Luc contains unique SmaI and KpnI restriction sites immediately upstream of the tk promoter, which were used to insert genomic mim-1 DNA fragments. These fragments were obtained by PCR, subcloned, sequenced, and excised with the appropriate restriction enzymes before they were cloned into the reporter plasmid. The plasmids encoding the following reporter genes were generated (numbers in parentheses refer to the sequence of the mim-1 enhancer region shown in Fig. 2): pGL3-mim3tk81-Luc (nucleotides 1 to 795), pGL3-mim4tk81-Luc (795 to 1), and pGL3-tk81-mimwt (182 to 560). All mim-1 enhancer mutants are derivatives of pGL3-tk81-mimwt and carry mutations of one or several Myb binding sites (182 to 560). The Myb binding sites were mutated as follows. The sequence of MBS1 was changed from CCAACGTTT to CCAAATTTT, the sequence of MBS2 was changed from GCAACTGCA to GCAAATTCA, the sequence of MBS3 was changed from CCAACTGTT to CCAAATTTT, and the sequence of MBS4 was changed from TGAACTGAG to TGAATTTAG. All constructs were generated by PCR with appropriate primers, and the mutations were verified by sequencing. Constructs containing multiple mutations were made by successive rounds of mutagenesis. Some reporter genes contained the mim-1 promoter instead of the tk promoter. First, the mim-1 promoter region (−240 to +150 bp) was cloned as a XmaI/BglII fragment between the XmaI and BglII sites of pGL3-Basic (Promega). The resulting plasmid (pGL3-240-Luc) was then used to insert the mim-1 enhancer fragment upstream of the promoter in both orientations, resulting in plasmids pGL3-mim3mim-Luc (1 to 795) and pGL3-mim4mim-Luc (795 to 1). The numbers in parentheses refer to the sequence of the mim-1 enhancer region shown in Fig. 2. pCMVβ, the plasmid encoding the β-galactosidase reporter gene, was obtained from Clontech. DNA transfection of adherent cells (HD11, 10.4, and QT6) was performed by calcium-phosphate coprecipitation as described previously (6). In case of nonadherent cells (BM2 and HD3), DNA transfection was performed by electroporation. Cells were suspended in phosphate-buffered saline at a concentration of 6 × 106 cells per 300 μl. Electroporation was carried out at 300 V and 900 μF. Cells were then transferred to fresh growth medium and harvested after 24 h. The preparation of cell extracts, luciferase, and β-galactosidase assays was performed as described previously (6).
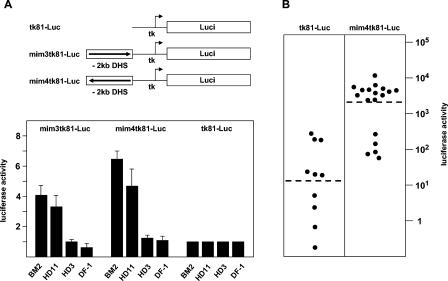
FIG. 2.
Activity of mim-1 reporter genes in different cell lines. (A) Luciferase reporter genes are illustrated schematically at the top. The arrows indicate the orientation of the insert fragments relative to the tk promoter. Cell lines were cotransfected by calcium-phosphate coprecipitation (HD11, DF-1) using 2 μg of the indicated reporter genes and 0.2 μg of the β-galactosidase plasmid pCMVβ or by electroporation (BM2, HD3) using 5 μg of luciferase reporter gene and 5 μg of CMVβ. Cells were harvested 24 h after transfection and analyzed for luciferase and β-galactosidase activity. The columns show the average luciferase activity normalized with respect to the activity of the cotransfected β-galactosidase plasmid. The T bars show standard deviations. The activity of the reporter gene containing only the thymidine kinase promoter (tk81-Luc) was designated 1. (B) BM2 cells were electroporated with the indicated reporter genes and a plasmid containing the neomycin resistance gene under the control of the actin promoter. Clones of stable transfectants were then analyzed for luciferase activity. Each point represents an individual clone. Luciferase activities (shown on a logarithmic scale) were determined from similar numbers of cells for each clone and were normalized with respect to the amount of integrated luciferase construct, determined by Southern blotting. The average activity of all clones of the same type is indicated by the broken lines.
To select stable transfectants of the BM2 line 30 μg of reporter gene and 4 μg of a plasmid containing the neomycin resistance gene fused to the β-actin promoter were electroporated into BM2 cells, as described above. The cells were then seeded into microtiter wells and selected in the presence of 1 mg of G418/ml. G418-resistant clones were expanded and analyzed for luciferase activity as described above. In addition, genomic DNA isolated from individual clones was digested with appropriate restriction enzymes and analyzed by Southern blotting by using probes specific for the transfected luciferase gene and the endogenous C/EBPβ gene.
Expression vectors.
pCDAMVv-myb is an expression vector for AMV v-Myb. To generate this vector, the v-myb coding region of AMV was isolated from a cDNA clone of subgenomic AMV v-myb mRNA and inserted as a 1.2-kb EcoRI/XbaI fragment between the EcoRI and XbaI sites of pCDNA3 (Invitrogen). pCDE26v-myb is a derivative of pCDAMVv-myb obtained by replacing the v-myb coding region between the NcoI and SalI sites of v-myb with the corresponding region from c-myb. pCDE/Av-myb is a derivative of pCDAMVv-myb that was generated by replacing the v-myb coding region between the NcoI and EcoRI sites of v-myb with the corresponding sequence from c-myb. pCDE26v-mybN186A is a point-mutated derivative of pCDE26v-myb carrying a substitution of alanine for Asp186 (numbering refers to c-Myb) and was obtained by PCR mutagenesis with appropriate primers. pCDNA3-CCR encodes the full-length chicken C/EBPβ and has been described previously (28). pCMV-p300-CHA encodes full-length human p300 containing a C-terminal hemagglutinin tag and was a gift from R. Eckner (8). The p300 histone acetyltransferase (HAT) mutant p300HA-DI1485AL was obtained from A. Hecht (13).
Electrophoretic mobility shift assays.
Pairs of complementary single-stranded oligonucleotides containing wild-type or mutated Myb binding sites were annealed and used for gel retardation assays. Oligonucleotides included MBS1wt, 5′CCATCCTTCTCCAACGTTTGTAGCTATGAGCAT3′ and 5′GATGCTCATAGCTACAAACGTTGGAGAAGGATGG3′; MBS1mut, 5′CCATCCTTCTCCAAATTTTGTAGCTATGAGCAT3′ and 5′GATGCTCATAGCTACAAAATTTGGAGAAGGATGG3′; MBS2wt, 5′GGGAAGTGGTGCAACTGCAGCTCTGTGCAACTC3′ and 5′GGAGTTGCACAGAGCTGCAGTTGCACCACTTCCC3′; MBS2mut, 5′GGGAAGTGGTGCAAATTCAGCTCTGTGCAACTC3′ and 5′GGAGTTGCACAGAGCTGAATTTGCACCACTTCCC3′; MBS3wt, 5′ACCACACATCCCAACTGTTGGATTAATGCCT3′ and 5′GAGGCATTAATCCAACAGTTGGGATGTGTGGT3′; MBS3mut, 5′ACCACACATCCCAAATTTTGGATTAATGCCT3′ and 5′GAGGCATTAATCCAAAATTTGGGATGTGTGGT3′; and mimA, 5′GCTCTAAAAAACCGTTATAATGTACAGATATCTT3′ and 5′AAGATATCTGTACATTATAACGGTTTTTTAGAG3′.
After annealing, oligonucleotides were radiolabeled by filling in the ends with [α-32P]dCTP. Bacterial v-Myb was prepared by using the expression vector pVM2060, and binding experiments were performed as described previously (36).
Chromatin immunoprecipitation.
Chromatin immunoprecipitation assays were performed as follows. Approximately 108 cells were incubated at room temperature with 1/10 cross-linking solution (11% formaldehyde; 50 mM Tris-HCl, pH 8.0; 0.1 M NaC1; 1 mM EDTA; and 0.5 mM EGTA) for 1 h and quenched for 5 min with 125 mM glycine. After being washed twice with ice-cold phosphate-buffered saline, cells were treated for 20 min (each) with washing solution A (0.25% Triton X-100; 10 mM Tris-HCl, pH 8.0; 10 mM EDTA; and 0.5 mM EGTA) and B (200 mM NaCl; 50 mM Tris-HCl, pH 8.0; 1 mM EDTA; and 0.5 mM EGTA). Nuclei were sonicated on ice (4 times for 20 s each in 2-min intervals) in egg lysis buffer (120 mM NaCl; 50 mM Tris-HCl, pH 7.5; 20 mM NaF; 1 mM EDTA; 6 mM EGTA; 15 mM sodium pyrophosphate; 1 mM phenylmethylsulfonyl fluoride; and 0.1% NP-40). The DNA-protein complexes were preincubated with protein A-Sepharose for 1 h at 4°C on a rotating wheel. After centrifugation, the supernatant was incubated with Myb-specific monoclonal antibody 5E11 (44), polyclonal antiserum raised against the DNA-binding domain of v-Myb (23), polyclonal antiserum raised against C/EBPβ (27), normal rabbit serum, or water overnight at 4°C on a rotating wheel. Samples were then incubated with protein A-Sepharose for 1 h and washed eight times in egg lysis buffer. The immunoprecipitates were eluted first with elution buffer 1 (10 mM Tris-HCl, pH 7.5; 0.5% sodium dodecyl sulfate [SDS]; and 10 mM dithiothreitol) and then with elution buffer 2 (10 mM Tris-HCl, pH 7.5, and 1% SDS). Eluates 1 and 2 were combined, and the DNA was recovered by reverse cross-linking for 6 h (each), first at 37 and then 65°C, in a buffer containing 0.5% SDS, 10 mM dithiothreitol, and 100 μg of proteinase K. Finally, the immunoprecipitated DNA was extracted by phenol-chloroform, ethanol precipitated, resuspended in 150 μl of 10 mM Tris-HCl (pH 7.5), and stored at −20°C. PCR was performed with the following primers: 5′-CAGACTGATGTTGGAGGCAC-3′ and 5′-TGTGGTGGTTGAGGCTTCTC-3′. PCR products were resolved on 2% agarose gels and stained with ethidium bromide.
RESULTS
Mapping of DNase I-hypersensitive sites in the chromatin of the chicken mim-1 gene.
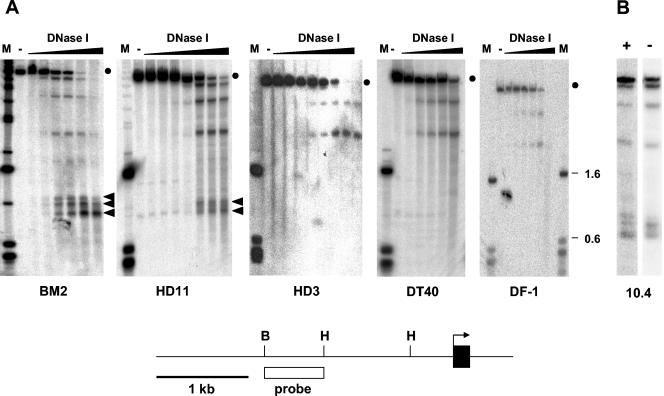
DHS in the chromatin surrounding a gene often mark positions of cis-regulatory sequences that contribute to the expression of the corresponding gene. To search for cis-acting sequences involved in the regulation of mim-1 expression, we have used different chicken cell lines to map DNase I-hypersensitive sites in the region upstream of the mim-1 gene. BM2 is a line of v-myb-transformed myeloblasts which expresses high levels of mim-1 RNA. HD11 is a macrophage line expressing neither v-myb nor c-myb nor mim-1. HD3 and DT40 are erythroid and B-lymphoid lines, respectively. They express the c-myb gene but not mim-1. DF-1 cells are fibroblasts expressing neither c-myb nor mim-1. Nuclei prepared from these cells were treated with increasing amounts of DNase I, followed by isolation of the DNA, digestion with HindIII, and Southern blotting. Upon hybridization with a radiolabeled probe, we detected a prominent group of DNase I-hypersensitive sites approximately 2 kb upstream of the mim-1 promoter in BM2 and HD11 cells, but not in HD3, DF-1, and DT40 cells (Fig. 1A). Two additional DNase I-hypersensitive sites located further upstream were detected in all of the cells. Finally, a DHS located very far upstream of the mim-1 promoter was detected only in BM2 and HD11 cells. The pattern of those DNase I-hypersensitive sites that mapped around 2 kb upstream of the mim-1 promoter showed some differences between BM2 and HD11 cells. While three bands could be discerned in BM2 cells, only two major bands could be seen in HD11 cells.
FIG. 1.
Analysis of DNase I-hypersensitive sites upstream of the mim-1 gene. The strategy used for mapping DNase I-hypersensitive sites in the chromatin of the chicken mim-1 gene is illustrated schematically at the bottom of the figure. The first exon is depicted as a black rectangle, and the arrow points in the direction of transcription. Relevant restriction sites are marked as follows: H, HindIII; B, BglII. The white rectangle marks the region that was used as the hybridization probe. (A) Nuclei from BM2, HD11, HD3, DT40, and DF-1 cell lines were treated without (lanes −) or with increasing concentrations of DNase I, as indicated. DNA isolated from the nuclei was then digested with HindIII and analyzed by Southern blotting by using the probe shown at the bottom. Lanes M contain size markers. The lengths (in kbp) of some of the fragments are indicated at the right. The full-length genomic fragments detected by the probe are marked by dots. Bands which are not present in lanes lacking DNase I and whose intensity increases with increasing DNase I concentration represent DNase I-hypersensitive sites. Bands corresponding to the mim-1 enhancer are marked by arrowheads. (B) Nuclei from 10.4 cells grown in the presence (+) or absence (−) of 2 μM β-estradiol were analyzed as described for panel A. Only one lane of the DNase I digestion kinetics is shown in each case.
To investigate whether the appearance of an additional band in BM2 cells was related to the v-Myb protein present in these cells, we also analyzed the pattern of DNase I-hypersensitive sites in 10.4 cells, a subclone of the HD11 line which stably expresses a v-Myb/estrogen receptor fusion protein. Treatment of 10.4 cells with β-estradiol activates the v-Myb/ER protein and induces mim-1 expression (5). As shown in Fig. 1B, in the absence of β-estradiol the pattern was similar to that seen with HD11 cells, whereas the pattern resembled that of BM2 in the presence of β-estradiol. This finding suggests that v-Myb either directly or indirectly affects the region upstream of the mim-1 gene.
Transcriptional activity of the −2-kb DNase I-hypersensitive region.
To investigate whether the −2-kb DHS acts as a cis-regulatory element, we analyzed its effect on the activity of the basal promoter of the herpes simplex virus thymidine kinase (tk) gene. The region encompassing the DNase I-hypersensitive sites was cloned in both orientations upstream of the tk promoter/luciferase reporter gene, and the resulting reporter genes were transfected into different chicken cell lines. Figure 2A shows that in both myelomonocytic cell lines (BM2 and HD11), the activity of the reporter genes containing the mim-1 upstream region was increased compared to the basal promoter construct. By contrast, in erythroid cells (HD3) and fibroblasts (DF-1) these reporter genes had only background activities. These results suggest that the region identified by mapping of DNase I-hypersensitive sites contains an enhancer element that is preferentially active in myelomonocytic cells. We will therefore refer to this region as the mim-1 enhancer.
To determine whether the mim-1 enhancer is also active when stably integrated into the genome of a myelomonocytic cell, we selected stable transfectants of the BM2 line carrying the mim4tk81-Luc reporter gene. For control purposes, we also generated BM2 clones carrying the tk81-Luc reporter gene, which lacks the enhancer. Individual clones were assayed for luciferase activity, which was then normalized with respect to the amount of luciferase construct integrated into each clone, as determined by Southern blotting (data not shown). Figure 2B shows that the luciferase activity of the clones containing only the tk promoter was relatively low and varied over a wide range, most likely because of position effects exerted by the genomic regions into which the reporter genes had been integrated. The average luciferase activity of the clones containing the mim-1 enhancer was about 100 times higher than that of the clones containing only the tk promoter. This result clearly shows that the enhancer also has an extremely strong stimulatory effect on an adjoining promoter when integrated into the cellular genome. The variation among the clones containing the tk promoter fused to the mim-1 enhancer was somewhat less than that of the clones with the enhancerless constructs, suggesting that the enhancer partially overrides position effects.
mim-1 enhancer is stimulated by v-Myb.
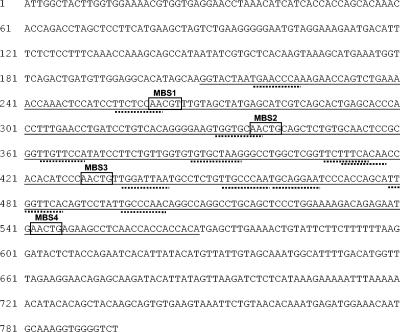
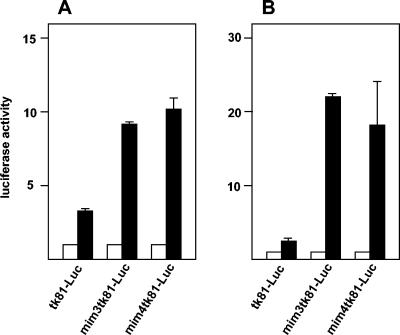
We determined the nucleotide sequence of the mim-1 upstream region by using a genomic chicken mim-1 clone kindly provided by S. Ness (Fig. 3). We noted that there are several potential Myb binding sites (designated MBS1 to MBS4) in the region corresponding to the cell-specific −2-kb DHS described above, suggesting that this region might be involved in the Myb-dependent regulation of the mim-1 gene. To explore whether Myb plays a direct role at the enhancer, we cotransfected the reporter genes containing the mim-1 enhancer into 10.4 cells. As shown in Fig. 4A, the luciferase activity of reporter genes containing the enhancer was strongly increased after the addition of β-estradiol. The construct containing only the tk promoter was also affected by β-estradiol; however, the stimulation was much less in this case. To further demonstrate that v-Myb activates the enhancer, we cotransfected the reporter genes containing or lacking the enhancer with a v-Myb expression vector (or an empty vector as the control) into the HD11 cell line and determined the luciferase activity levels (Fig. 4B). The expression of v-Myb strongly increased the activity of the reporter genes containing the mim-1 enhancer, whereas the reporter lacking the enhancer was stimulated only poorly by v-Myb. Thus, both experiments clearly demonstrate that v-Myb is involved in controlling the activity of the enhancer. Cotransfection of an expression vector for c-Myb also increased the activity of reporter genes containing the mim-1 enhancer in HD11 cells, demonstrating that the enhancer responds to v-Myb and c-Myb (data not shown).
FIG. 3.
Nucleotide sequence of the mim-1 enhancer region. The region corresponding to the DNase I-hypersensitive sites is underlined. Potential Myb (consensus: AACG/T) binding sites MBS1 to MBS4 within the region of DNase I hypersensitivity are marked by boxes. Potential C/EBP binding sites (consensus: TT/GNNNNAA) are marked by broken underlining.
FIG. 4.
Stimulation of the mim-1 enhancer by v-Myb. (A) 10.4 cells were transfected with 5 μg of the indicated luciferase reporter genes and 0.2 μg of the β-galactosidase plasmid pCMVβ. The cells were then grown for 24 h without (white bars) or with (black bars) 2 μM β-estradiol, and the luciferase and β-galactosidase activities were analyzed. The average luciferase activity was normalized with respect to the β-galactosidase activity and is expressed in arbitrary units. The T bars show standard deviations. (B) HD11 cells were transfected as described above except that the v-Myb expression vector (pCDE26v-myb; 5 μg) was included (black bars). Controls contained an equivalent amount of empty expression vector (white bars). Luciferase and β-galactosidase activities were analyzed as for panel A.
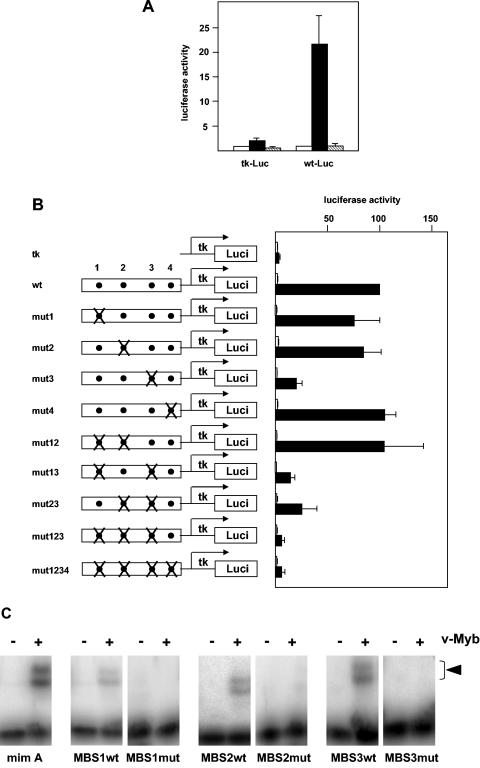
To determine whether the Myb-dependent stimulation of the enhancer activity is mediated by the Myb binding sites shown in Fig. 3, we initially performed a transactivation experiment using a v-Myb mutant that lacks DNA binding activity. In this mutant, a single amino acid residue (Asp186), which is directly involved in contacts with specific bases of the Myb recognition motif and has been shown to be crucial for the specific DNA-binding activity of Myb (11, 38), has been mutated to alanine. As shown in Fig. 5A, the mutant v-Myb was unable to stimulate the enhancer, suggesting that one or several of the Myb binding sites mediate the effect of v-Myb. To identify which of the sites are required for the stimulation of the enhancer, we generated a set of point mutants in which one or several of the Myb binding sites had been destroyed. The mutants were constructed by using a slightly truncated version of the enhancer that more closely corresponds to the region of DNase I hypersensitivity (nucleotides 182 to 560 of the sequence shown in Fig. 3). Cotransfection of the resulting reporter genes with v-Myb expression vector showed that mutation of binding site MBS3 had the strongest effect on Myb-inducibility, whereas mutation of sites MBS1 and MBS2 had little effect (Fig. 5B). Mutation of MBS1, MBS2, and MBS3 together almost completely abolished the Myb inducibility of the enhancer. MBS4 appeared not to be functional. Thus, we concluded that v-Myb stimulates the enhancer activity mainly through binding site MBS3 and to lesser extent through binding sites MBS1 and MBS2. Electrophoretic mobility shift experiments (Fig. 5C) using bacterially expressed v-Myb protein confirmed that v-Myb binds to these sites and that binding was abolished by the mutations.
FIG. 5.
Mutation analysis of the mim-1 enhancer region. (A) HD11 cells were transfected with 10 μg (each) of luciferase reporter genes containing only the tk promoter (tk-Luc) or the tk promoter fused to the mim-1 enhancer (wt-Luc), 3 μg of the β-galactosidase reference plasmid pCMVβ, and 5 μg (each) of empty expression vector (pCDNA3; white bars), expression vector for wild-type v-Myb (pCDE26v-myb; black bars), or the N186A mutation of v-Myb (pCDE26v-mybN186A; hatched bars). Luciferase and β-galactosidase activities were determined 24 h after transfection. The luciferase activity was normalized with respect to the β-galactosidase activity and is expressed in arbitrary units. The activity of each reporter gene in the absence of v-Myb was designated 1. The T bars show standard deviations. (B) Reporter genes containing the wild-type (wt) or different mutants of the mim-1 enhancer fused to the tk promoter are illustrated schematically on the left. Myb binding sites 1 to 4 are represented as dots, and mutants are marked with Xs. HD11 cells were transfected with 5 μg of the indicated reporter genes, 0.2 μg of theβ-galactosidase reference plasmid pCMVβ, and 5 μg of v-Myb expression vector Myb (pCDE26v-myb; black bars) or empty expression vector (pCDNA3; white bars). Luciferase and β-galactosidase activities were analyzed as for panel A. The luciferase activity of the wild-type construct in the presence of v-Myb was designated 100. (C) Electrophoretic mobility shift experiments using bacterially expressed v-Myb bound to radiolabeled oligonucleotides corresponding to unmutated (wt) or mutated (mut) Myb binding sites MBS1 to MBS3. For comparison, an oligonucleotide containing Myb binding site A (mim A) from the mim-1 promoter was also used. Binding reactions contained (+) or lacked (−) v-Myb protein. Complexes of v-Myb and the oligonucleotides are marked by an arrowhead. The two bands of retarded complexes are present due to partial proteolysis of the v-Myb protein preparation used. The intense bands at the bottom correspond to unbound oligonucleotides.
mim-1 enhancer and mim-1 promoter respond synergistically to activation by Myb.
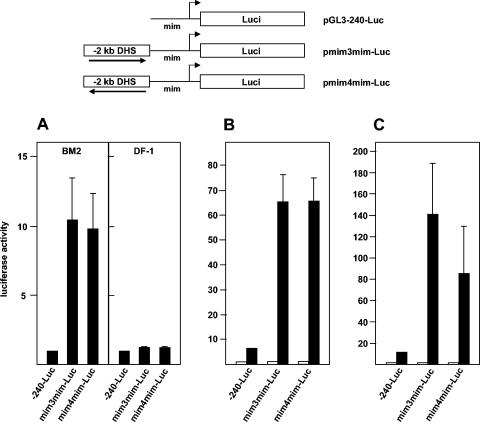
Because previous work had shown that v-Myb binds to and activates the promoter of the mim-1 gene (33), our results raised the interesting question of how strongly the mim-1 promoter and mim-1 enhancer contribute to the overall stimulation of mim-1 expression by v-Myb. To address this question, we constructed reporter genes containing the mim-1 enhancer and the mim-1 promoter. First, we introduced these constructs into BM2 cells. Figure 6A clearly demonstrates that the −20-kb DHS also acts as an enhancer in the context of the mim-1 promoter. A comparison of the data shown in Fig. 6A and Fig. 2A shows that the stimulatory effect of the enhancer on the mim-1 promoter was even higher than its effect on the tk promoter (10-fold stimulation of the mim-1 promoter versus 4-fold stimulation of the tk promoter). As in the case of the reporter genes containing the tk promoter, the enhancer was not active in nonmyelomonocytic cells, such as DF1 fibroblasts (Fig. 6A). This result suggests that the combination of mim-1 enhancer and mim-1 promoter constitutes a very potent expression system that is preferentially active in myelomonocytic cells.
FIG. 6.
mim-1 enhancer and mim-1 promoter cooperate in the activation of the mim-1 gene by Myb. Reporter genes containing only the mim-1 promoter or the mim-1 promoter and mim-1 enhancer are shown schematically at the top. The arrows indicate the orientation of the insert fragments relative to the mim-1 promoter. (A) The luciferase reporter plasmids indicated at the bottom were electroporated together with the β-galactosidase plasmid pCMVβ into BM2 cells or transfected into DF-1 cells. The cells were harvested 24 h later and analyzed for luciferase and β-galactosidase activities. Bars show the average luciferase activity normalized with respect to the β-galactosidase activity. The activity of the plasmid containing only the mim-1 promoter (pGL3-240-Luc) was designated 1. The T bars show standard deviations. (B) 10.4 cells were transfected with the indicated luciferase reporter genes (5 μg) and 0.2 μg of the β-galactosidase plasmid pCMVβ. The cells were then grown for 24 h without (white bars) or with (black bars) 2 μM β-estradiol, and the luciferase and β-galactosidase activities were analyzed as described for panel A except that the activity of each reporter gene in the absence of β-estradiol was designated 1. (C) HD11 cells were transfected as described for panel B except that v-Myb expression vector (pCDE26v-myb; 5 μg) was included (black bars). Controls contained an equivalent amount of empty expression vector (white bars). Luciferase and β-galactosidase activities were analyzed as for panel A. The activity of each reporter gene in the absence of v-Myb was designated 1.
To assess the role of the mim-1 promoter and mim-1 enhancer in the Myb inducibility of the mim-1 gene, we compared the effects of v-Myb on reporter genes containing only the mim-1 promoter or the mim-1 enhancer coupled to the mim-1 promoter. Figure 6B shows the results of introducing these reporter genes into the 10.4 cell line and growing the cells in the absence or presence of estrogen. Figure 6C shows a similar experiment, in which the reporter genes were cotransfected with a v-Myb expression vector into HD11 cells. In both systems, the effect of v-Myb on the activity of reporter genes containing the mim-1 enhancer and mim-1 promoter was extremely high and exceeded by far the effect of v-Myb exerted on the mim-1 promoter alone. Thus, these experiments clearly show that mim-1 promoter and mim-1 enhancer cooperate in the activation of mim-1 expression by v-Myb.
Synergistic activation of the mim-1 gene by Myb and C/EBPβ is mediated by the mim-1 promoter and mim-1 enhancer.
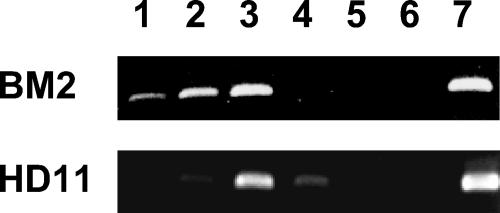
The data described so far provide strong evidence that v-Myb acts on the promoter as well as on the −2-kb enhancer of the mim-1 gene. To confirm that v-Myb is bound to the enhancer in vivo, we employed chromatin immunoprecipitation (ChIP) with BM2 and HD11 cells. BM2 cells express v-Myb, C/EBPβ, and mim-1, whereas HD11 cells express similar amounts of C/EBPβ but neither v-Myb nor mim-1. Consistent with the results of our cotransfection experiments, the ChIP analysis (Fig. 7) revealed that v-Myb is present at the mim-1 enhancer in BM2 cells. In HD11 cells, ChIP experiments with myb-specific antibodies were negative, as expected, confirming the specificity of the immunoprecipitations.
FIG. 7.
Chromatin immunoprecipitation of the mim-1 enhancer region. Chromatin fragments prepared from BM2 cells or HD11 cells were subjected to immunoprecipitation using Myb-specific monoclonal antibody 5E11 (lanes 1), polyclonal antiserum against v-Myb (lanes 2), or polyclonal antiserum against C/EBPβ (lanes 3). Control precipitations were performed with normal rabbit serum (lanes 4) or without antibody (lanes 5). DNA isolated from the immunoprecipitates (lanes 1 to 6) or from the total chromatin preparation before immunoprecipitation (lanes 7) was analyzed by PCR using primers specific for the mim-1 enhancer. Lanes 6 show a PCR control to which no target DNA was added.
Previous work has demonstrated that Myb activates the mim-1 gene by cooperating with C/EBPβ and, furthermore, that the mim-1 promoter contains Myb and C/EBP binding sites that permit the cooperation of both factors (6, 34). The identification of the −2-kb mim-1 enhancer therefore raised the question of whether the synergy of Myb and C/EBPβ is mediated only by the mim-1 promoter or whether the enhancer is also involved in the cooperation of both factors. As a first step to address this question, C/EBPβ-specific antibodies were included in the ChIP experiment shown in Fig. 7. It is evident that these antibodies also precipitated the enhancer region by using either BM2 or HD11 cells, both of which express C/EBPβ. We therefore concluded that C/EBPβ is bound to the mim-1 enhancer in vivo in both cell lines.
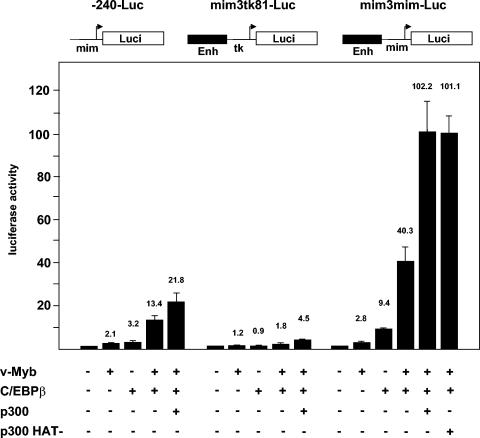
To further explore the role of C/EBPβ at the mim-1 enhancer, we systematically compared the effects of v-Myb and C/EBPβ on reporter genes containing only the mim-1 promoter, the mim-1 enhancer linked to the tk promoter, or the mim-1 enhancer linked to the mim-1 promoter. We performed these experiments with QT6 fibroblasts, which do not express v-Myb or c-Myb and have only low levels of endogenous C/EBP factors; these cells have been instrumental for studying the cooperation of Myb and C/EBP at the mim-1 promoter (6, 7, 34). Since our previous work has shown that the coactivator p300 enhances the synergy of Myb and C/EBPβ (28), we also included an expression vector for p300 in this analysis. Figure 8 shows the results of the effects of v-Myb, C/EBPβ, and p300 on the different reporter genes. In agreement with previous work, v-Myb, and C/EBPβ synergistically activate the mim-1 promoter and p300 increases the activity of the promoter even further. When the mim-1 enhancer is linked to the tk promoter, v-Myb and C/EBPβ on their own have virtually no effect in QT6 cells. The two factors together are weakly synergistic, particularly in the presence of p300. When the mim-1 enhancer is linked to the mim-1 promoter, v-Myb and C/EBPβ are strongly synergistic and the stimulation of this reporter gene is much higher than that of the reporter gene containing only the mim-1 promoter. Finally, p300 caused an extremely strong activation that was much higher than that of the reporter gene containing only the mim-1 promoter. These results strongly suggest that the synergistic effect of Myb and C/EBPβ on mim-1 expression is not only mediated by the mim-1 promoter but that mim-1 promoter and mim-1 enhancer together are responsible for the dramatic activation of the mim-1 gene by both transcription factors.
FIG. 8.
Transactivation of the mim-1 enhancer by v-Myb, C/EBPβ, and p300. Reporter genes containing only the mim-1 promoter (p-240-Luc), the mim-1 enhancer (Enh) upstream of the tk promoter (mim3tk81-Luc), or the mim-1 enhancer upstream of the mim-1 promoter (mim3mim-Luc) are shown schematically at the top. QT6 cells were transfected with 2 μg of the indicated reporter genes and different combinations of expression vectors for v-Myb (pCDE26v-myb; 1 μg), C/EBPβ (pCDNA3-CCR; 1 μg), p300 (pCMVβ-p300CHA; 5 μg), or a HAT-deficient mutant of p300 (p300HA-DI1485AL; 5 μg), as indicated at the bottom. Controls contained the equivalent amount of empty expression vector. pCMVβ (0.2 μg) was included in all transfections to control the transfection efficiencies. Luciferase and β-galactosidase activities were determined 24 h after transfection. Bars show the average luciferase activity normalized with respect to the β-galactosidase activity. The activity of each reporter gene in the absence of any exogenous factor was designated 1. The T bars show standard deviations.
We also performed cotransfections by using an expression vector for a p300 mutant that lacks acetyltransferase activity. Interestingly, this mutant stimulated the activity of the reporter gene containing mim-1 enhancer and mim-1 promoter to the same extent as wild-type p300 (see Fig. 8, last bar). Thus, p300 is able to support the cooperation of v-Myb and C/EBPβ in the absence of its intrinsic acetyltransferase activity.
The AMV version of v-Myb does not support synergy between mim-1 promoter and mim-1 enhancer and cooperates with p300 less efficiently than the E26 version of v-Myb.
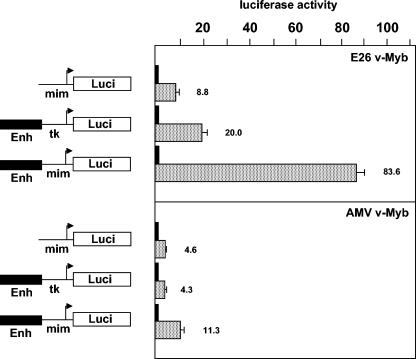
It has previously been shown that the AMV version of v-Myb does not activate certain Myb target genes, such as mim-1 or the lysozyme gene in primary transformed myeloblasts, whereas the E26 version of v-Myb does (18). The v-Myb proteins of AMV and E26 differ by several amino acid substitutions, which are located in the Myb DNA-binding domain and have been implicated in the inability of AMV v-Myb to activate the mim-1 gene. Alteration of the AMV v-Myb DNA-binding domain such that its sequence is identical to the DNA-binding domain of E26 v-Myb restores the ability to activate the mim-1 gene (18). Since C/EBPβ interacts with the Myb DNA-binding domain (27, 45), it has been suggested that the lack of activation of the mim-1 gene by AMV v-Myb is due to an inability of this version of v-Myb to cooperate with C/EBPβ (45). Because our work has identified the mim-1 enhancer as an important element in the activation of mim-1 expression by v-Myb, we reinvestigated the activity of both forms of v-Myb in the context of the mim-1 enhancer. Figure 9 compares the effects of the E26 and AMV v-Myb versions on the activity of reporter genes containing only the mim-1 promoter, the mim-1 enhancer linked to the tk promoter, or the mim-1 enhancer linked to the mim-1 promoter. The activities of the two versions of v-Myb (which were expressed in similar amounts) (Fig. 10A) differed by a factor of only 2 when assayed on a reporter gene containing only the mim-1 promoter (8.8-fold versus 4.6-fold). The differences between the two v-Myb versions were more pronounced when a reporter gene containing the mim-1 enhancer linked to the tk promoter was used (20-fold versus 4.3-fold). Interestingly, the differences between the two forms of v-Myb were very drastic when the reporter gene containing the mim-1 promoter and mim-1 enhancer was used (83-fold versus 11.3-fold). A comparison of the effects observed with the different reporter genes shows that the stimulation of the reporter gene containing mim-1 enhancer and mim-1 promoter by AMV v-Myb did not significantly exceed the sum of the effects observed for the enhancer and the promoter alone. Thus, in the presence of AMV v-Myb, mim-1 enhancer and mim-1 promoter appear to contribute additively to the activity of the construct containing both elements. By contrast, the stimulation of this construct by E26 v-Myb was much higher than the sum of the activation factors observed when promoter and enhancer were tested individually. These observations suggest that mim-1 enhancer and mim-1 promoter act synergistically in the presence of E26 v-Myb but not in the presence of AMV v-Myb. Taken together, these observations raise the intriguing possibility that the failure of AMV v-Myb to activate the mim-1 gene might be due primarily to an inability to fully support synergy between mim-1 enhancer and mim-1 promoter.
FIG. 9.
Transactivation of mim-1 reporter genes by different variants of v-Myb. HD11 cells were transfected with 5 μg of the reporter genes shown on the left, pCMVβ (0.2 μg), and 5 μg of expression vectors for E26 v-Myb (top panel) or AMV v-Myb (bottom panel). Controls lacking v-Myb (black columns) were transfected with 5 μg of empty expression vector. Luciferase and β-galactosidase activities were determined 24 h after transfection. Bars show the average luciferase activity normalized with respect to the β-galactosidase activity and expressed in arbitrary units. The luciferase activity in the absence of v-Myb was designated 1 in each case. The T bars show standard deviations. Enh, enhancer.
FIG. 10.
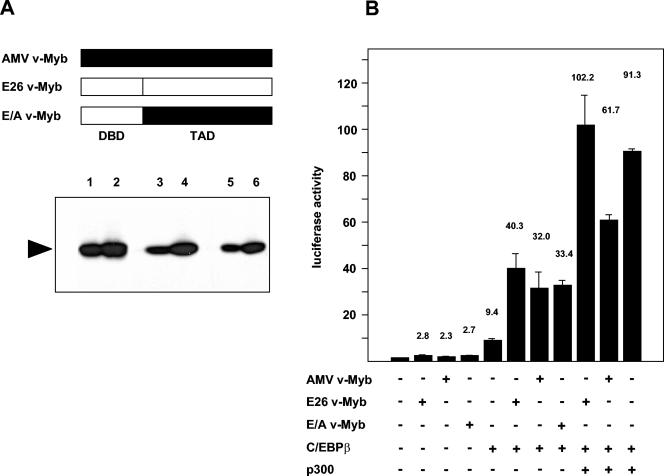
Cooperation of C/EBPβ and p300 with different v-Myb variants. (A) A schematic illustration of different forms of v-Myb is shown at the top. The bottom shows a Western blot of QT6 cells transfected with 3 (lanes 1, 3, and 5) or 5 (lanes 2, 4, and 6) μg of the indicated expression vector stained with Myb-specific antibodies. DBD, DNA-binding domain; TAD, transcription activation domain. (B) QT6 cells were transfected with 2 μg of the reporter gene containing mim-1 enhancer and mim-1 promoter (mim3mim-Luc), pCMVβ (0.2 μg), and expression vectors for C/EBPβ (0.2 μg), p300 (5 μg), E26 v-Myb (1 μg), AMV v-Myb (1 μg), or E26 v-Myb and AMV v-Myb (E/A v-Myb) (1 μg), as indicated at the bottom. Controls contained equivalent amounts of empty expression vector. Luciferase and β-galactosidase activities were determined 24 h after transfection. Bars show the average luciferase activity normalized with respect to the β-galactosidase activity and expressed in arbitrary units. The T bars show standard deviations. The luciferase activity in the absence of exogenous factors was designated 1.
The data presented in Fig. 8 have implicated p300 in the cooperation of mim-1 enhancer and mim-1 promoter. We were therefore interested to see whether the failure of AMV v-Myb to fully support synergy between mim-1 enhancer and mim-1 promoter is somehow mirrored by a lack of cooperation with p300. We therefore compared the ability of E26 and AMV v-Myb as well as a version of AMV v-Myb whose DNA-binding domain has been replaced by that of E26 to cooperate with C/EBPβ and p300, using the reporter gene containing mim-1 enhancer and mim-1 promoter. The results of these experiments (shown in Fig. 10) permit several conclusions to be drawn. The three variants of v-Myb cooperated with C/EBPβ to similar extent. In contrast to previous claims, AMV v-Myb and C/EBPβ clearly acted synergistically on the reporter gene. However, the ability of AMV v-Myb to cooperate with p300 was significantly reduced compared with that of E26 v-Myb. Interestingly, the replacement of the DNA-binding domain of AMV v-Myb by that of E26 (in the construct E/A v-Myb) to a large extent restored the ability of the protein to cooperate with p300. We note that the difference in activation of the reporter gene between the E26 and AMV versions of v-Myb is more pronounced in HD11 cells (Fig. 9) than in QT6 cells (Fig. 10). This difference may be due to the overexpression of p300 in the latter case, which also might enforce some degree of cooperation of p300 with AMV v-Myb. Taken together, our results suggest that the lack of activation of the mim-1 gene by AMV v-Myb is probably due to a problem of the protein in cooperating with p300 rather than with C/EBPβ, leading to a loss of synergy between mim-1 enhancer and mim-1 promoter.
DISCUSSION
Identification of a Myb-responsive enhancer in the mim-1 upstream region.
A large body of evidence has shown that c-Myb plays an essential role during hematopoietic proliferation and differentiation and that oncogenic variants of Myb disturb these processes, presumably by deregulating certain target genes. Although there is a growing list of known or suspected Myb targets, how Myb proteins regulate these genes has been studied in detail in only a few cases. mim-1 is the prototypical Myb-regulated gene which has played a pivotal role in exploring how Myb proteins activate downstream targets (33). Several features make the mim-1 gene an extremely interesting Myb target. v-Myb and c-Myb are extremely potent activators of mim-1 expression. Furthermore, although c-Myb is expressed in all hematopoietic lineages, it activates mim-1 only in myelomonocytic cells (33). Lineage-restricted activation of mim-1 has been attributed to the synergistic cooperation of Myb with C/EBP family members, particularly C/EBPβ, which are highly expressed in myelomonocytic cells (6, 20, 34).
Previous studies on the regulation of mim-1 expression have focused exclusively on the mim-1 promoter. In the work reported here, we have employed mapping of DNase I-hypersensitive sites in the chromatin to identify a very potent and apparently myelomonocytic-specific enhancer upstream of the mim-1 gene. Several lines of evidence suggest that this enhancer, like the promoter, is a direct target of v-Myb. The enhancer contains several Myb consensus binding sites, some of which are bound by v-Myb in vitro. Chromatin immunoprecipitation experiments have shown that v-Myb is bound to the enhancer in vivo. Furthermore, the enhancer is strongly activated by v-Myb and dramatically increases the Myb responsiveness of the mim-1 promoter or of a heterologous promoter. Thus, together with previous studies of the mim-1 promoter (6, 27, 34), our data show that v-Myb activates the mim-1 gene by acting simultaneously on two distinct regulatory regions of the gene.
Like the activity of mim-1 promoter, that of the mim-1 enhancer is controlled cooperatively by v-Myb and C/EBPβ. Several potential binding sites for C/EBP are present in the enhancer sequence, and chromatin immunoprecipitation has confirmed that C/EBPβ is bound to the enhancer in myelomonocytic cells. However, even with coexpression of Myb and C/EBPβ, the activity of the enhancer is lower in QT6 cells than in the myelomonocytic HD11 line, suggesting that other transcription factors present in myelomonocytic cells contribute to its activity in addition to Myb and C/EBPβ. Our work also provides strong evidence for a role of p300 in the activation of mim-1 by v-Myb. Figure 8 shows that p300 exerts a much stronger stimulatory effect on a reporter gene containing the mim-1 enhancer and mim-1 promoter than on reporter genes containing only the mim-1 promoter or the mim-1 enhancer and the tk promoter. Thus, p300 might be especially important for the communication between mim-1 enhancer and mim-1 promoter. Interestingly, the HAT activity of p300 apparently is not required for this activity, suggesting that p300 mainly acts as a bridging factor.
Our work also has implications for Myb-regulated genes in general. The regulation of the chicken C/EBPβ gene, another Myb target, has recently been analyzed (29, 41), and it was found that v-Myb acts on the C/EBPβ promoter as well as on a cell-specific enhancer located downstream of the gene (21). The chicken adenosine receptor 2B gene, another Myb target (19, 49), also has a Myb-responsive promoter and, additionally, a Myb-responsive enhancer (unpublished observations). Thus, that v-Myb activates downstream targets by acting on two separate regions of these genes may be a more common phenomenon. Most Myb target genes that have been identified so far have been analyzed only with respect to their promoters. Only in a few cases, such as the myeloperoxidase gene (3), the T-cell receptor δ and pre-T cell receptor α genes (14, 40), and the adenosine deaminase gene (9), have enhancers been implicated in gene activation by Myb. It will therefore be interesting to see if additional Myb-dependent cis-regulatory elements are also present in other Myb-regulated genes.
Functional differences between E26 and AMV versions of v-Myb affect the cooperation of the mim-1 enhancer and mim-1 promoter.
Finally, our work sheds new light on the differences between the v-Myb proteins encoded by the retroviruses AMV and E26. The DNA-binding domain of AMV v-Myb differs by several amino acid substitutions from that of E26 v-Myb or c-Myb. These substitutions have been implicated in the lack of activation of the mim-1 gene by AMV v-Myb (18). As the DNA-binding domain of v-Myb participates in direct protein-protein interaction with C/EBPβ (27, 45), it has been argued that these substitutions have weakened the Myb-C/EBPβ interaction, thereby preventing the cooperation of AMV v-Myb and C/EBPβ (45). Our data are not really consistent with this picture. First, our results suggest that the lack of activation of mim-1 by AMV v-Myb is due mainly to the inability of AMV v-Myb to support the cooperation of mim-1 enhancer and mim-1 promoter rather than to the activation of the mim-1 promoter per se. This is evident from the data shown in Fig. 9, which demonstrate that the E26 and AMV versions differ much less in activity if assayed on the mim-1 promoter (8.8-fold versus 4.6-fold activation) than if assayed on a reporter gene containing mim-1 promoter and mim-1 enhancer (83.6-fold versus 11.3-fold activation). Second, the results shown in Fig. 10 demonstrate that AMV v-Myb cooperates with C/EBPβ almost as efficiently as E26 v-Myb (compare 32-fold activation by AMV v-Myb and C/EBPβ to 40-fold activation by E26 v-Myb and C/EBPβ). Furthermore, an interaction between C/EBPβ and AMV v-Myb was previously demonstrated (27). However, the two v-Myb versions respond differently to p300 (see Fig. 10). Interestingly, reversion of the amino acid substitutions in the DNA-binding domain of AMV v-Myb increases the ability of the protein to cooperate with p300. Thus, the picture that emerges from these observations is that the AMV version of v-Myb cooperates with p300 less well than E26 v-Myb. Since p300 appears to be involved in the Myb-mediated cooperation of mim-1 enhancer and mim-1 promoter, our results may explain why AMV v-Myb does not stimulate mim-1 expression. We do not yet understand how the amino acid substitutions in the DNA-binding domain of AMV v-Myb affect its cooperation with p300. The structure of a complex of the CBP KIX domain and the c-Myb transactivation domain has recently been reported (50). Although AMV v-Myb carries a few additional amino acid substitutions C-terminal to the DNA-binding domain, they do not map to the region which contacts p300, making it unlikely that AMV v-Myb binds less well to p300. Indeed, in vitro binding experiments using a GST-p300 fusion protein did not show differences in binding to the AMV or E26 version of v-Myb (data not shown). Thus, there is at present no evidence that the poor cooperation of AMV v-Myb with p300 is due to a lack of binding to p300, suggesting that it is caused by indirect mechanisms.
It is surprising that the AMV-transformed BM2 line shows mim-1 expression. The v-myb gene, cloned from the proviral copy of AMV present in BM2 cells, does not carry additional mutations (22). BM2 is an immortalized cell line which was generated from a culture of AMV-transformed primary bone marrow cells by in vitro and subsequent in vivo selection (31). Most likely, the cells have accumulated unknown mutations in cellular genes which have restored the ability of AMV v-Myb to activate the mim-1 gene.
Acknowledgments
We thank A. Brehmer-Fastnacht and L. Hübers for expert technical assistance, J. Miethe for help in the mapping of DHS, and the members of our group for discussions.
This work was supported by grants from the DFG (SFB 291/A13 and Kl 461/10-1) and by the Fonds der chemischen Industrie. O.C. holds a fellowship from the Graduate School in Chemistry (GSC-MS) at the University of Münster. D.B. is supported by the Studienstiftung des deutschen Volkes and the Fonds der chemischen Industrie.
REFERENCES
- 1.Biedenkapp, H., U. Borgmeyer, A. E. Sippel, and K.-H. Klempnauer. 1988. Viral myb oncogene encodes a sequence-specific DNA-binding activity. Nature 335:835-837. [DOI] [PubMed] [Google Scholar]
- 2.Braas, D., D. Kattmann, J. Miethe, and K.-H. Klempnauer. 2003. Analysis of DNase I-hypersensitive sites in the chromatin of the chicken adenosine receptor 2B gene reveals multiple cell-type-specific cis-regulatory elements. Gene 303:157-164. [DOI] [PubMed] [Google Scholar]
- 3.Britos-Bray, M., and A. D. Friedman. 1997. Core binding factor cannot synergistically activate the myeloperoxidase proximal enhancer in immature myeloid cells without c-Myb. Mol. Cell. Biol. 17:5127-5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buerstedde, J. M., and S. Takeda. 1991. Increased ratio of targeted to random integration after transfection of chicken B cell lines. Cell 67:179-188. [DOI] [PubMed] [Google Scholar]
- 5.Burk, O., and K.-H. Klempnauer. 1991. Estrogen-dependent alterations in differentiation state of myeloid cells caused by a v-myb/estrogen receptor fusion protein. EMBO J. 10:3713-3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burk, O., S. Mink, M. Ringwald, and K.-H. Klempnauer. 1993. Synergistic activation of the chicken mim-1 gene by v-myb and C/EBP transcription factors. EMBO J. 12:2027-2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burk, O., S. Worpenberg, B. Haenig, and K.-H. Klempnauer. 1997. tom-1, a novel v-Myb target gene expressed in AMV- and E26-transformed myelomonocytic cells. EMBO J. 16:1371-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckner, R., M. E. Ewen, D. Newsome, M. Gerdes, J. A. DeCaprio, J. Bentley-Lawrence, and D. M. Livingston. 1994. Molecular cloning and functional analysis of the adenovirus E1A-associated 300-kD protein (p300) reveals a protein with properties of a transcriptional adaptor. Genes Dev. 8:869-884. [DOI] [PubMed] [Google Scholar]
- 9.Ess, K. C., T. L. Whitaker, G. J. Cost, D. P. Witte, J. J. Hutton, and B. J. Aronow. 1995. A central role for a single c-Myb binding site in a thymic locus control region. Mol. Cell. Biol. 15:5707-5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frampton, J., T. Ramqvist, and T. Graf T. 1996. v-Myb of E26 leukemia virus up-regulates bcl-2 and suppresses apoptosis in myeloid cells. Genes Dev. 10:2720-2731. [DOI] [PubMed] [Google Scholar]
- 11.Gabrielsen, O. S., A. Sentenac, and P. Fromageot. 1991. Specific DNA binding by c-Myb: evidence for a double helix-turn-helix-related motif. Science 253:1140-1143. [DOI] [PubMed] [Google Scholar]
- 12.Ganter, B., and J. S. Lipsick. 1999. Myb and oncogenesis. Adv. Cancer Res. 76:21-60. [DOI] [PubMed] [Google Scholar]
- 13.Hecht, A., K. Vleminckx, M. P. Stemmler, F. van Roy, and R. Kemler. 2000. The p300/CBP acetyltransferases function as transcriptional coactivators of beta-catenin in vertebrates. EMBO J. 19:1839-1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hernandez-Munain, C., and M. S. Krangel. 2002. Distinct roles for c-Myb and core binding factor/polyoma enhancer-binding protein 2 in the assembly and function of a multiprotein complex on the TCR delta enhancer in vivo. J. Immunol. 169:4362-4369. [DOI] [PubMed] [Google Scholar]
- 15.Himly, M., D. N. Foster, I. Bottoli, J. S. Iacovoni, and P. K. Vogt. 1998. The DF-1 chicken fibroblast cell line: transformation induced by diverse oncogenes and cell death resulting from infection by avian leukosis viruses. Virology 248:295-304. [DOI] [PubMed] [Google Scholar]
- 16.Hogg, A., S. Schirm, H. Nakagoshi, P. Bartley, S. Ishii, J. M. Bishop, and T. J. Gonda. 1997. Inactivation of a c-Myb/estrogen receptor fusion protein in transformed primary cells leads to granulocyte/macrophage differentiation and down regulation of c-kit but not c-myc or cdc2. Oncogene 15:2885-2898. [DOI] [PubMed] [Google Scholar]
- 17.Ibanez, C. E., and J. S. Lipsick. 1990. trans activation of gene expression by v-myb. Mol. Cell. Biol. 10:2285-2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Introna, M., J. Golay, J. Frampton, T. Nakano, S. A. Ness, and T. Graf. 1990. Mutations in v-myb alter the differentiation of myelomonocytic cells transformed by the oncogene. Cell 63:1289-1297. [DOI] [PubMed] [Google Scholar]
- 19.Kattmann, D., and K.-H. Klempnauer. 2002. Identification and characterization of the Myb-inducible promoter of the chicken adenosine receptor 2B gene. Oncogene 21:4663-4673. [DOI] [PubMed] [Google Scholar]
- 20.Katz, S., L. E. Kowenz, C. Müller, K. Meese, S. A. Ness, and A. Leutz. 1993. The NF-M transcription factor is related to C/EBPβ and plays a role in signal transduction, differentiation and leukemogenesis of avian myelomonocytic cells. EMBO J. 12:1321-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kintscher, J., V. Yamkamon, D. Braas, and K.-H. Klempnauer. 2004. Identification of a Myb-responsive enhancer of the chicken C/EBPβ gene. Oncogene 23:5807-5814. [DOI] [PubMed] [Google Scholar]
- 22.Klempnauer, K.-H., T. J. Gonda, and J. M. Bishop. 1982. Nucleotide sequence of the retroviral leukemia gene v-myb and its cellular progenitor c-myb: the architecture of a transduced oncogene. Cell 31:453-463. [DOI] [PubMed] [Google Scholar]
- 23.Klempnauer, K.-H., C. Bonifer, and A. E. Sippel. 1986. Identification and characterization of the protein encoded by the human c-myb proto-oncogene. EMBO J. 5:1903-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klempnauer, K.-H., H. Arnold, and H. Biedenkapp. 1989. Activation of transcription by v-myb: evidence for two different mechanisms. Genes Dev. 3:1582-1589. [DOI] [PubMed] [Google Scholar]
- 25.Kowenz-Leutz, E., P. Herr, K. Niss, and A. Leutz. 1997. The homeobox gene GBX2, a target of the myb oncogene, mediates autocrine growth and monocyte differentiation. Cell 91:185-195. [DOI] [PubMed] [Google Scholar]
- 26.Lipsick, J. S., and D. M. Wang. 1999. Transformation by v-Myb. Oncogene 18:3047-3055. [DOI] [PubMed] [Google Scholar]
- 27.Mink, S., U. Kerber, and K.-H. Klempnauer. 1996. Interaction of C/EBPβ and v-Myb is required for synergistic activation of the mim-1 gene. Mol. Cell. Biol. 16:1316-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mink, S., B. Haenig, and K.-H. Klempnauer. 1997. Interaction and functional collaboration of p300 and C/EBPβ. Mol. Cell. Biol. 17:6609-6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mink, S., S. Jaswal, O. Burk, and K.-H. Klempnauer. 1999. The v-Myb oncoprotein activates C/EBPβ expression by stimulating an autoregulatory loop at the C/EBPβ promoter. Biochim. Biophys. Acta 1447:175-184. [DOI] [PubMed] [Google Scholar]
- 30.Moscovici, C., M. G. Moscovici, H. Jimenez, M. M. Lai, M. J. Hayman, and P. K. Vogt. 1977. Continuous tissue culture cell lines derived from chemically induced tumors of Japanese quail. Cell 11:95-103. [DOI] [PubMed] [Google Scholar]
- 31.Moscovici, C., N. Zeller, and M. G. Moscovici. 1982. Continuous lines of AMV-transformed non-producer cells: growth and oncogenic potential in the chick embryo, p. 435-449. In R. F. Revoltella et al. (ed.), Expression of differentiated functions in cancer cells. Raven Press, New York, N.Y.
- 32.Ness, S. A. 1996. The Myb oncoprotein: regulating a regulator. Biochim. Biophys. Acta 1288:F123-F139. [DOI] [PubMed] [Google Scholar]
- 33.Ness, S. A., A. Marknell, and T. Graf. 1989. The v-myb oncogene product binds to and activates the promyelocyte-specific mim-1 gene. Cell 59:1115-1125. [DOI] [PubMed] [Google Scholar]
- 34.Ness, S. A., E. Kowentz-Leutz, T. Casini, T. Graf, and A. Leutz. 1993. Myb and NF-M: combinatorial activators of myeloid genes in heterologous cell types. Genes Dev. 7:749-759. [DOI] [PubMed] [Google Scholar]
- 35.Nordeen, S. K. 1988. Luciferase reporter gene vectors for analysis of promoters and enhancers. BioTechniques 6:454-458. [PubMed] [Google Scholar]
- 36.Oehler, T., H. Arnold, H. Biedenkapp, and K.-H. Klempnauer. 1990. Characterization of the v-myb DNA binding domain. Nucleic Acids Res. 18:1703-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oelgeschläger, M., I. Nuchprayoon, B. Lüscher, and A. D. Friedman. 1996. C/EBP, c-Myb, and PU.1 cooperate to regulate the neutrophil elastase promoter. Mol. Cell. Biol. 16:4717-4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ogata, K., S. Morikawa, H. Nakamura, A. Sekikawa, T. Inoue, H. Kanai, A. Sarai, S. Ishii, and Y. Nishimura. 1994. Solution structure of a specific DNA complex of the Myb DNA-binding domain with cooperative recognition helices. Cell 79:639-648. [DOI] [PubMed] [Google Scholar]
- 39.Oh, I.-H., and E. P. Reddy. 1999. The myb gene family in cell growth, differentiation and apoptosis. Oncogene 18:3017-3033. [DOI] [PubMed] [Google Scholar]
- 40.Reizis, B., and P. Leder. 2001. The upstream enhancer is necessary and sufficient for the expression of the pre-T cell receptor alpha gene in immature T lymphocytes. J. Exp. Med. 194:979-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rushton, J. J., L. M. Davis, W. Lei, X. Mo, A. Leutz, and S. A. Ness. 2003. Distinct changes in gene expression induced by A-Myb, B-Myb and c-Myb proteins. Oncogene 22:308-313. [DOI] [PubMed] [Google Scholar]
- 42.Schlichter, U., O. Burk, S. Worpenberg, and K.-H. Klempnauer. 2001. The chicken Pdcd4 gene is regulated by v-Myb. Oncogene 20:231. [DOI] [PubMed] [Google Scholar]
- 43.Sippel, A. E., H. Saueressig, M. C. Huber, H. C. Hoefer, A. Stief, U. Borgmeyer, and C. Bonifer. 1996. Identification of cis-acting elements as DNase I hypersensitive sites in lysozyme gene chromatin. Methods Enzymol. 274:233-246. [DOI] [PubMed] [Google Scholar]
- 44.Sleeman, J. P. 1993. Xenopus A-myb is expressed during early spermatogenesis. Oncogene 8:1931-1941. [PubMed] [Google Scholar]
- 45.Tahirov, T. H., K. Sato, E. Ichikawa-Iwata, M. Sasaki, T. Inoue-Bungo, M. Shiina, K. Kimura, S. Takata, A. Fujikawa, H. Morii, T. Kumasaka, M. Yamamoto, S. Ishii, and K. Ogata. 2002. Mechanism of c-Myb-C/EBPβ cooperation from separate sites on a promoter. Cell 108:57-70. [DOI] [PubMed] [Google Scholar]
- 46.Taylor, D., P. Badiani, and K. Weston. 1996. A dominant interfering Myb mutant causes apoptosis in T cells. Genes Dev. 10:2732-2744. [DOI] [PubMed] [Google Scholar]
- 47.Weston, K. 1998. Myb proteins in life, death and differentiation. Curr. Opin. Genet. Dev. 8:76-81. [DOI] [PubMed] [Google Scholar]
- 48.Weston, K., and J. M. Bishop. 1989. Transcriptional activation by the v-myb oncogene and its cellular progenitor, c-myb. Cell 58:85-93. [DOI] [PubMed] [Google Scholar]
- 49.Worpenberg, S., O. Burk, and K.-H. Klempnauer. 1997. The chicken adenosine receptor 2B gene is regulated by v-myb. Oncogene 15:213-221. [DOI] [PubMed] [Google Scholar]
- 50.Zor, T., R. N. De Guzman, H. J. Dyson, and P. E. Wright. 2004. Solution structure of the KIX domain of CBP bound to the transactivation domain of c-Myb. J. Mol. Biol. 337:521-534. [DOI] [PubMed] [Google Scholar]