Abstract
Objective
Tacrolimus, an immunosuppressant widely used in solid organ transplantation, is available as a prolonged-release capsule for once-daily oral administration. In the immediate postsurgical period, if patients cannot take intact capsules orally, tacrolimus therapy is often initiated as a suspension of the capsule contents, delivered orally or via a nasogastric tube. This study evaluated the relative bioavailability of prolonged-release tacrolimus suspension versus intact capsules in healthy participants.
Design
A phase 1, open-label, single-dose, cross-over study.
Setting
A single clinical research unit.
Participants
In total, 20 male participants, 18–55 years old, entered and completed the study.
Interventions
All participants received nasogastric administration of tacrolimus 10 mg suspension in treatment period 1, with randomisation to oral administration of suspension or intact capsules in periods 2 and 3. Blood concentration–time profile over 144 hours was used to estimate pharmacokinetic parameters.
Primary and secondary outcome measures
Primary end point: relative bioavailability of prolonged-release intact capsule versus oral or nasogastric administration of prolonged-release tacrolimus suspension (area under the concentration–time curve (AUC) from time 0 to infinity post-tacrolimus dose (AUC0–∞); AUC measured until the last quantifiable concentration (AUC0–tz); maximum observed concentration (Cmax); time to Cmax (Tmax)). Tolerability was assessed throughout the study.
Results
Relative bioavailability of prolonged-release tacrolimus suspension administered orally was similar to intact capsules, with a ratio of least-square means for AUC0–tz and AUC0–∞ of 1.05 (90% CI 0.96 to 1.14). Bioavailability was lower with suspension administered via a nasogastric tube versus intact capsules (17%; ratio 0.83; CI 0.76 to 0.92). Cmax was higher for oral and nasogastric suspension (30% and 28%, respectively), and median Tmax was shorter (difference 1.0 and 1.5 hours postdose, respectively) versus intact capsules (2.0 hours). Single 10 mg doses of tacrolimus were well tolerated.
Conclusions
Compared with intact capsules, the rate of absorption of prolonged-release tacrolimus from suspension was faster, leading to higher peak blood concentrations and shorter time to peak; relative bioavailability was similar with suspension administered orally.
Keywords: intact capsule, healthy subjects, nasogastric tube, prolonged-release tacrolimus
Strengths and limitations of this study.
This study is the first to report relative bioavailability of suspension prepared from contents of prolonged-release tacrolimus capsules administered orally and via a nasogastric tube, compared with intact capsules.
The study was conducted in healthy participants and used a three-way cross-over design to evaluate characteristics of a single dose of prolonged-release tacrolimus (10 mg) by administration method.
Limitations of this study include the potential treatment effect (as randomisation did not occur for the nasogastric tube administration) and that no formal statistical assessment of sample size was performed.
Introduction
Tacrolimus is an immunosuppressant drug widely used in solid organ transplantation for the prevention and treatment of allograft rejection. Tacrolimus is a medication with a narrow therapeutic index and large interpatient and intrapatient pharmacokinetic variability. High intrapatient variability in systemic exposure to tacrolimus and non-adherence to medication are two of the major causes of poor graft survival.1–3 Tacrolimus is available as immediate-release formulations administered twice daily and prolonged-release formulations administered once daily. Prolonged-release tacrolimus was developed to improve adherence and reduce intrapatient variability of tacrolimus exposure.
As a narrow therapeutic index drug, tacrolimus therapy is optimised on an individual patient basis using therapeutic drug monitoring (TDM). Trough levels of whole blood tacrolimus concentrations (C0) are used as a surrogate marker of area under the concentration–time curve (AUC).4 Comparative pharmacokinetic studies in de novo and in stable patient populations have shown that the relationship between C0 and AUC is similar for evaluated formulations of prolonged-release and immediate-release tacrolimus.5–7 As such, the same target C0 is used for TDM for both such formulations. Clinical studies have confirmed similar efficacy and tolerability of the two formulations in adult kidney and liver transplantation during the first year of treatment.8–11
In the immediate postsurgical period when many patients cannot swallow intact capsules, tacrolimus therapy is often initiated by administering the contents of tacrolimus capsules as a suspension orally or via a nasogastric tube. Although immediate-release tacrolimus is routinely administered as a suspension in clinical practice, the pharmacokinetic profile of a suspension prepared from prolonged-release tacrolimus capsules has not been established. This was the first study to compare the relative bioavailability of a suspension prepared from the contents of prolonged-release tacrolimus capsules administered orally or via a nasogastric tube, with oral administration of intact capsules, in healthy participants. Tolerability was also assessed throughout the study.
Materials and methods
Study design
This was a phase 1, open-label, partially randomised, single-dose, three-period cross-over study in healthy participants. The study was conducted in accordance with the Declaration of Helsinki and the International Council for Harmonisation Good Clinical Practice consolidated guidelines. The protocol was approved by a local independent ethics committee. Participants provided written consent for prestudy screening procedures and signed a study-specific consent form prior to the start of the study.
Participants
Eligible participants were male, 18–55 years old and had a body mass index of 19–29 kg/m2. All participants were in good health according to predefined criteria assessed during a physical examination, a 12-lead ECG, clinical laboratory evaluations and a medical history review. Exclusion criteria included prescribed topical/systemic medication taken within 14 days of first tacrolimus administration, or non-prescribed topical/systemic medication within 7 days, or any medications taken within 30 days that were known to have a chronic impact on drug absorption or elimination processes. Participants who had been enrolled in a clinical study of an investigational drug in the previous 4 months or a marketed drug within the past 3 months were also excluded. Participants who had participated in a clinical study involving single-dose administration of prolonged-release tacrolimus in the previous 12 months, multiple-dose administration of tacrolimus or any other immunosuppressants preceding the first dose in this study were also excluded.
Preparation of suspension
Tacrolimus suspension was prepared in the pharmacy on the day before dosing. Two 5 mg prolonged-release tacrolimus (Advagraf; Astellas Pharma Europe BV, The Netherlands, hereafter referred to as prolonged-release tacrolimus) capsules were opened and the contents (powder) were mixed in a brown glass bottle with 50 mL of tap water to produce a suspension. The suspension was transferred to the clinic on the day of dosing.
Study treatment
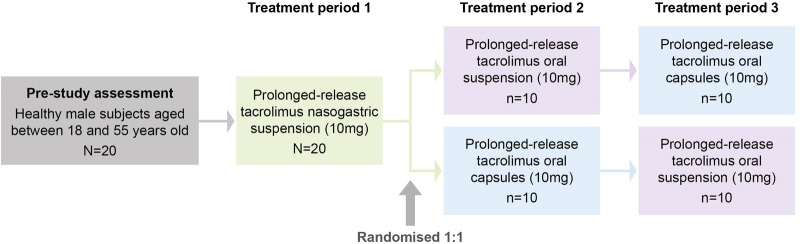
During the course of this study, all participants received the following three treatments on separate occasions, with an interval of at least 14 days between each treatment period. All dosing occurred in the morning following an overnight fast (figure 1):
Suspension via a nasogastric tube: participants were intubated with a polyethylene nasogastric tube (size 10 or 12) ∼1 hour prior to dosing and the position of the nasogastric tube was confirmed by a water recovery test. The total administration of water via the nasogastric tube was 100 mL; the suspension (50 mL containing 10 mg of prolonged-release tacrolimus) was drawn into a glass syringe and administered via the nasogastric tube, and a further 50 mL of tap water was used to rinse the syringe and the nasogastric tube. The nasogastric tube was spigotted for 45–60 min postdose and the tube was then removed ∼1 hour prior to the lunchtime meal.
Suspension administered orally: participants drank the 50 mL suspension containing 10 mg of prolonged-release tacrolimus, followed by an additional 50 mL of tap water, which was at room temperature, giving a total volume administered of 100 mL.
Intact capsules administered orally: participants swallowed two intact 5 mg capsules of prolonged-release tacrolimus (10 mg) with 100 mL of tap water, which was at room temperature.
Figure 1.
Study design. Interval of at least 14 days between each treatment period.
Since nasogastric tubes can be difficult to swallow, all participants received tacrolimus via a nasogastric tube during treatment period 1; participants unable to tolerate the nasogastric tube were discontinued from the study. Participants were randomised 1:1 for treatment period 2 to receive prolonged-release tacrolimus by intact capsules or by oral suspension, and then received prolonged-release tacrolimus via the remaining route of administration during treatment period 3. Each participant participated in all three treatment periods and remained resident in the clinical research unit from ∼16:00 on day 0 (the day before dosing) until day 3 (48 hours postdose) in each period. Dosing occurred on day 1 and each participant returned for outpatient visits in the mornings of days 4–7 of each period.
To prevent variation between treatment periods, participants were asked to adhere to the following dietary restrictions and requirements during the study: no grapefruit or grapefruit juice from 7 days prior to dosing in treatment period 1 until completion of treatment period 3, no caffeine-containing food and beverages for 48 hours prior to dosing in each treatment period until discharge on day 7, no alcohol for 48 hours prior to the screening visit and from 48 hours before dosing in each treatment period until discharge on day 7 (up to two units of alcohol per day were allowed from discharge on day 7 to 48 hours prior to the next treatment period), no smoking on the morning of dosing until 4 hours postdose in each treatment period, and then for 1 hour prior to each blood pressure and pulse rate measurement. Participants were instructed to refrain from any vigorous exercise during the 7-day period prior to the initial screening visit, and from 7 days before the first dose administration until after the final assessments in treatment period 3. Participants were instructed to fast from food and fluids, with the exception of water, for at least 6 hours prior to the screening visit. Standard meals, which were identical in each treatment period, were provided for all participants while they were resident in the clinical research unit.
Pharmacokinetic profiles and assay
Blood samples to define concentration–time profiles were collected predose (at time point 0) and at 0.5, 1, 2, 3, 4, 6, 8, 10, 12, 18, 24, 36, 48, 72, 96, 120 and 144 hours postdose. At each time point, 1×3 mL aliquot whole blood samples were collected into 3 mL ethylenediaminetetraacetic acid tubes/vacutainers and placed in a cool box containing crushed ice/water. Each blood sample was then transferred into two 5 mL polypropylene tubes and stored at ∼−20°C within 2 hours of collection. Blood samples were transported on dry ice to be analysed at a central laboratory (Covance Laboratories Europe, Harrogate, UK).
The blood samples were prepared using protein precipitation followed by solid phase extraction. Tacrolimus in the centrifuged eluates was quantified by the liquid chromatography with tandem mass spectrometric detection method.12 The lower limit of quantification of tacrolimus in whole blood was 0.10 ng/mL. The interassay precision of quality control samples analysed throughout the study was 4.2% at 0.25 ng/mL, 3.6% at 9.00 ng/mL and 4.0% at 22.50 ng/mL. Interassay accuracy varied between 96.0% and 105.5%. The pharmacokinetic analysis was performed using WinNonlin Enterprise V.3.2 (Pharsight Corporation, Mountain View, California, USA); parameters were determined from the whole blood concentrations of tacrolimus using non-compartmental procedures. The analysis was performed by Covance Clinical Research Unit, Leeds, UK.
End points
The primary end point of the study was to compare the relative bioavailability of prolonged-release tacrolimus suspension versus intact prolonged-release tacrolimus capsules. Parameters examined included the AUC from time 0 to infinity post-tacrolimus dose (AUC0–∞), AUC measured until the last quantifiable concentration (AUC0–tz), maximum observed concentration (Cmax) and time to Cmax (Tmax). The secondary end point was the elimination half-life of tacrolimus (T½).
Safety and tolerability were assessed at initial screening and throughout the study. Participants were questioned about adverse events during each treatment period predose and then postdose at 3, 12, 24 and 48 hours, and then daily from days 4–7. Prior to discharge in each treatment period, each participant was assessed by the study investigator and, if necessary, remained at the clinical research unit until any adverse events causing concern had resolved. A poststudy assessment was performed prior to discharge on day 7 of treatment period 3.
Vital signs were assessed at screening, predose and 48 hours postdose for each treatment period, with a further assessment at 144 hours postdose in treatment period 3. A 12-lead ECG was performed at screening and predose in treatment period 1 and 144 hours postdose in treatment period 3. Physical examinations took place at initial screening and 144 hours postdose in treatment period 3. Clinical laboratory evaluation occurred at screening, day 1 and 144 hours postdose in each treatment period.
Statistical analyses
Using the pharmacokinetic parameters, AUC0–∞, AUC0–tz and Cmax, the bioavailability of each suspension regimen was compared with intact capsules using Schuirmann's two one-sided tests procedure with 90% CIs for the differences in least-squares means obtained from a mixed-effects model; T½ was similarly compared. The median (range) was reported for Tmax and compared using non-parametric CIs (Wilcoxon signed-rank method assuming no period effects). Intraparticipant and interparticipant variability was assessed using the geometric coefficient of variation (CV). All tests were two-sided and used a 5% level of significance. The mean data reported are the geometric mean, unless indicated otherwise. Descriptive statistics for the pharmacokinetic data were determined using SAS V.8.2 (SAS Institute, Cary, North Carolina, USA).
Results
The study was conducted from 3 January 2003 to 17 March 2003 at a single study site (Covance Clinical Research Unit, Leeds, UK). In total, 20 participants were included, all of whom received three single 10 mg doses of prolonged-release tacrolimus (one in each treatment period) and completed the study. All participants were male with a mean age of 34 years (range 20–54 years). Mean weight was 75.2 kg (range 53.5–96.9 kg) and mean body mass index was 23.7 kg/m2 (range 19.7–28.6 kg/m2). All participants were Caucasian, with the exception of one participant who was of mixed race (Caucasian/Afro-Caribbean).
Pharmacokinetic parameters
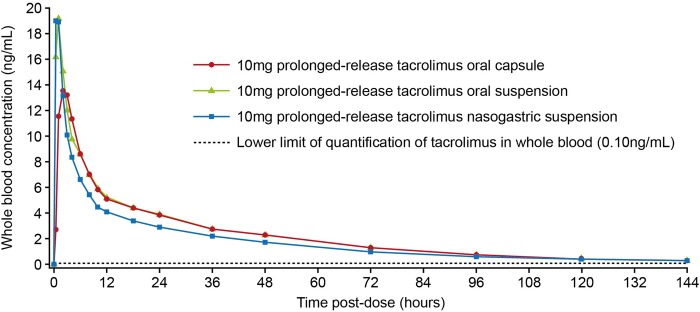
The relative bioavailability of prolonged-release tacrolimus suspension administered orally was similar to that of intact prolonged-release tacrolimus capsules, with a ratio of least-square means (90% CI) between the two administrations for AUC0–tz and AUC0–∞ of 1.05 (0.96 to 1.14). However, when prolonged-release tacrolimus suspension administered via a nasogastric tube was compared with intact prolonged-release tacrolimus capsules, the bioavailability was lower (17%), with a ratio of least-square means (90% CI) between administrations for AUC0–tz and AUC0–∞ of 0.83 (0.76 to 0.92). Cmax was higher for suspension administered both orally and via a nasogastric tube (30% and 28%, respectively). The median difference (90% CI) in Tmax for suspension and nasogastric tube administration was −1.00 (−1.25 to −0.75) and −1.50 (−1.50 to −0.75) versus intact prolonged-release tacrolimus capsules, respectively (table 1, figure 2).
Table 1.
Summary of pharmacokinetic parameters of prolonged-release tacrolimus administered as a suspension orally and via nasogastric tube compared with oral administration of intact capsules
| Mode of prolonged-release tacrolimus administration (10 mg)* |
Ratio of least-squares means (90% CI) |
||||
|---|---|---|---|---|---|
| Intact capsule (oral) (n=20) | Suspension (oral) (n=20) | Suspension (nasogastric tube) (n=20) | Suspension (oral)— intact capsule |
Suspension (nasogastric tube)—intact capsule | |
| AUC0–∞ (ng.h/mL) | 334 (28.0) | 350 (29.6) | 277 (31.4) | 1.05 (0.96 to 1.14) | 0.83 (0.76 to 0.92) |
| AUC0–tz (ng.h/mL) | 318 (27.0) | 333 (29.4) | 263 (31.1) | 1.05 (0.96 to 1.14) | 0.83 (0.76 to 0.92) |
| Cmax (ng/mL) | 15.7 (28.4) | 20.4 (31.3) | 20.9 (22.6) | 1.30 (1.16 to 1.45) | 1.28 (1.13 to 1.45) |
| Tmax (h)† | 2.0 (1.0–3.0) | 1.0 (0.5–1.0) | 0.5 (0.5–1.0) | −1.0 (−1.25 to −0.75) | −1.5 (−1.50 to −0.75) |
| T½ (h) | 33.2 (13.4) | 33.2 (14.6) | 34.0 (12.2) | 1.00 (0.97 to 1.03) | 1.01 (0.98 to 1.05) |
All tests were two-sided and used the 5% level of significance.
*Geometric mean (CV%) are presented.
†Tmax is presented as median (range).
AUC0–∞, area under the concentration–time curve from time 0 to infinity post-tacrolimus dose; AUC0–tz, AUC measured up to the last quantifiable concentration of tacrolimus; Cmax, maximum observed concentration; CV, coefficient of variation; Tmax, time to maximum concentration; T½, elimination half-life of tacrolimus.
Figure 2.
Geometric mean of whole blood concentrations of tacrolimus following a single 10 mg dose of prolonged-release tacrolimus suspension administered orally and via a nasogastric tube compared with oral administration of intact capsules.
Variability in tacrolimus exposure was similar when administered as a suspension (orally and via a nasogastric tube) and as intact prolonged-release tacrolimus capsules. Interparticipant variability, as determined by CV%, was 28–31% for AUC0–∞ and 23–31% for Cmax. The intraparticipant variability was 16.2% for AUC0–∞ and 20.9% for Cmax. Mean T½ of tacrolimus was similar (∼33 hours) for each administration (intact capsule: range 24.7–45.8 hours; oral suspension: range 22.8–44.2 hours; nasogastric suspension: range 26.0–41.8 hours).
Tolerability analyses
A total of 28 adverse events were reported by 18 participants. Thirteen participants reported 17 treatment-emergent adverse events that were considered possibly related to the administration of tacrolimus (intact capsule: 5; oral suspension: 3; nasogastric suspension: 9), all of which were mild or moderate in severity (table 2). With the exception of four events that required concomitant medication (Strepsils (Reckitt Benckiser, Nottingham, UK), aciclovir or paracetamol), all other drug-related adverse events resolved without intervention. There were no deaths, serious adverse events or significant adverse events during the study. There were also no clinically important findings in physical examinations at screening or in vital signs, ECG parameters or clinical laboratory evaluations for any participant.
Table 2.
Summary of treatment-emergent adverse events considered possibly related to study treatment with prolonged-release tacrolimus
| Number of adverse events |
|||
|---|---|---|---|
| Adverse event* | Intact capsule (oral) (n=20) | Suspension (oral) (n=20) | Suspension (nasogastric tube) (n=20) |
| Participants, n (%) | 4 (20) | 2 (10) | 7 (35) |
| Events, n | 5 | 3 | 9 |
| Mild | 4 | 3 | 5 |
| Moderate | 1 | 0 | 4 |
| Severe | 0 | 0 | 0 |
| Eye disorders | |||
| Eye pain | 0 (0) | 0 (0) | 1 (1) |
| Gastrointestinal disorders | |||
| Diarrhoea not otherwise specified | 0 (0) | 0 (0) | 1 (1) |
| Nausea | 1 (1) | 0 (0) | 0 (0) |
| Toothache | 0 (0) | 0 (0) | 1 (1) |
| General disorders and administration site conditions | |||
| Fatigue | 0 (0) | 0 (0) | 1 (1) |
| Infections and infestations | |||
| Herpes simplex | 0 (0) | 0 (0) | 1 (1) |
| Upper respiratory tract infection | 0 (0) | 1 (1) | 0 (0) |
| Musculoskeletal and connective tissue disorders | |||
| Back pain | 0 (0) | 0 (0) | 1 (1) |
| Nervous system disorders | |||
| Headache | 1 (1) | 0 (0) | 1 (1) |
| Dizziness | 1 (1) | 0 (0) | 0 (0) |
| Dysgeusia | 0 (0) | 1 (1) | 0 (0) |
| Paraesthesia | 1 (1) | 0 (0) | 0 (0) |
| Respiratory, thoracic and mediastinal disorders | |||
| Throat irritation | 0 (0) | 1 (1) | 1 (1) |
| Nasopharyngitis | 1 (1) | 0 (0) | 0 (0) |
| Skin and subcutaneous tissue disorders | |||
| Rash maculopapular | 0 (0) | 0 (0) | 1 (1) |
*Adverse event with possible, probable or definite relationship to study drug. Adverse events presented as number of adverse events (number of participants with adverse event) unless otherwise specified.
Discussion
This phase I study is the first to report relative bioavailability of suspension prepared from contents of prolonged-release tacrolimus capsules administered orally and via a nasogastric tube, compared with intact capsules. The overall systemic exposure to tacrolimus, in terms of AUC0–∞, was similar when single doses of prolonged-release tacrolimus were given orally as suspension and as intact capsules. The geometric mean ratio for this parameter was close to unity and the 90% CIs around the ratio were contained within the limits of 0.8 to 1.25, indicating similar relative bioavailability. However, the rate of absorption of prolonged-release tacrolimus was faster when administered as an oral suspension compared with administration by intact capsules, resulting in a higher mean Cmax and a shorter median Tmax.
Tacrolimus as a compound has poor water solubility; however, for oral dosing, the solubility was improved by developing a solid dispersion formulation using hydroxypropylmethylcellulose as an excipient in the immediate-release formulation of tacrolimus and prolonged-release formulation.13 Prolonged-release tacrolimus has been formulated to release a portion of tacrolimus at a rate similar to that of the immediate-release formulation and the remaining proportion of the tacrolimus over an extended time. This allows the tacrolimus to be delivered over a larger part of the gastrointestinal tract. These two components can be seen in the absorption profile as a rapid increase in mean whole blood tacrolimus concentration within the first hour postadministration due to the immediate-release component, followed by a steady decline in tacrolimus levels.14 In this study, the contents of prolonged-release tacrolimus capsules were prepared as a suspension in the pharmacy and then transferred to the clinic for dosing. We believe that the more immediate-release component was already dissolved in the suspension before administration. This would have resulted in a more rapid absorption rate compared with that of intact capsules and could subsequently explain the faster absorption rate with suspension administered orally and via the nasogastric route. However, the T½ of tacrolimus was not affected, being ∼33 hours irrespective of the route of administration, and the interparticipant variability of prolonged-release tacrolimus pharmacokinetics was similar for both oral administrations (suspension and intact capsules).
Nasogastric dosing of the prolonged-release tacrolimus suspension had a lower AUC0–tz and AUC0–∞ compared with oral suspension or intact capsules. While it would be tempting to suggest that part of the tacrolimus dose may have adhered to tubing and syringes in the cohorts receiving the nasogastric suspension, the probability of such a phenomenon occurring is, at best, highly unlikely, as all materials used to administer such doses were free of polyvinylchloride to minimise the potential for adsorption. It is also important to realise that the Cmax of tacrolimus administered via nasogastric dosing occurred at the first sampling time point (0.5 hours postdose) for the majority of participants, so the absorption phase was not fully defined. Taken in conjunction with the fact that all participants in treatment period 1 received the suspension via a nasogastric tube, a period effect cannot be ruled out. For this reason, AUC0–∞ may have been underestimated during nasogastric administration. The pharmacokinetic profiles obtained in this study following administration of intact prolonged-release tacrolimus capsules were consistent with previously reported studies in healthy participants.15
The recommended range of tacrolimus dose for the prophylaxis of liver transplant rejection is 6.5–13.0 mg, based on a body weight of 65 kg. Therefore, the mid-range of 10 mg was selected for dosing in this study. Furthermore, 10 mg was the maximum dose for healthy volunteers permitted by the ethics committee. Single doses of prolonged-release tacrolimus were well tolerated when administered as oral capsules, oral suspension and a suspension administered via a nasogastric tube. The incidence of adverse events for oral administration (capsules and suspension) was low and similar, with the majority of the events being mild in severity. The incidence of adverse events for the nasogastric suspension was higher, with both mild and moderate events being reported. However, with the exception of three events, all adverse events that were possibly related to tacrolimus administration resolved without the need for treatment. Adverse events possibly related to prolonged-release tacrolimus administration occurred only with the oral suspension and nasogastric tube suspension. Toothache, upper respiratory tract infection and throat infection could have been as a direct result of nasogastric tube use, due to bias in the open-label setting or as a result of the period effect. Previous studies in healthy participants showed that oral administration of intact prolonged-release tacrolimus capsules was well tolerated, with a safety profile consistent with immediate-release tacrolimus.15
Limitations of this study include the potential treatment effect, as randomisation did not occur for the nasogastric tube administration. This design was chosen to ensure that all participants included in the study were able to swallow a nasogastric tube. Additionally, the absorption phase of the study was not fully defined, as Cmax was achieved at the first sampling time point for the majority of participants receiving nasogastric administration of prolonged-release tacrolimus. Earlier blood sampling points would therefore be required in order to fully define the absorption phase. Finally, no formal statistical assessment of sample size was performed. However, the number of participants included in this study is typical of clinical pharmacology studies, and was considered sufficient to achieve the primary objectives of the study.
This is the first clinical study to assess the absorption and pharmacokinetic profile of prolonged-release tacrolimus when administered by a nasogastric tube. These data will facilitate a better understanding of future postmarketing pharmacokinetic data, as well as previously conducted phase 2 and 3 studies. As such, this phase 1 study demonstrated that a suspension of prolonged-release tacrolimus results in a faster rate of absorption compared with intact prolonged-release tacrolimus capsules, resulting in a higher Cmax and a shorter Tmax for the suspension. While total systemic exposure to tacrolimus was ∼17% lower with nasogastric compared with oral administration, this finding should be interpreted with caution due to the potential treatment effect and not fully defined absorption phase of nasogastric administration. However, single 10 mg doses of tacrolimus were well tolerated in all three administrations, with similar elimination half-lives and no marked differences in the interparticipant variability of systemic exposure. Owing to demonstrated dose proportionality14 and lack of therapeutic effect of the tacrolimus excipients, similar results can be expected with other prolonged-release tacrolimus capsule strengths.
Acknowledgments
The authors would like to thank Amy MacLucas and James Wallis from iS LifeScience for drafting the initial version of the manuscript and providing editorial support throughout the development of the manuscript.
Footnotes
Contributors: NU was involved in the conception and design of the study. JD was involved in project management of the study. Both authors were involved in the writing of the paper and approved the final version of the manuscript for submission.
Funding: The study was funded by Fujisawa GmbH. Astellas Pharma was formed following the merger of Yamanouchi Pharmaceutical and Fujisawa Pharmaceutical in 2005. Astellas Pharma Europe BV is the manufacturer of prolonged-release tacrolimus (Advagraf). Editorial support was funded by Astellas Pharma Europe.
Competing interests: The authors of this manuscript have the following disclosures: NU is employed by Astellas.
Ethics approval: Local independent ethics committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Borra LCP, Roodnat JI, Kal JA et al. High within-patient variability in the clearance of tacrolimus is a risk factor for poor long-term outcome after kidney transplantation. Nephrol Dial Transplant 2010;25:2757–63. 10.1093/ndt/gfq096 [DOI] [PubMed] [Google Scholar]
- 2.Sapir-Pichhadze R, Wang Y, Famure O et al. Time-dependent variability in tacrolimus trough blood levels is a risk factor for late kidney transplant failure. Kidney Int 2014;85:1404–11. 10.1038/ki.2013.465 [DOI] [PubMed] [Google Scholar]
- 3.Sellarés J, de Freitas DG, Mengel M et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant 2012;12:388–99. 10.1111/j.1600-6143.2011.03840.x [DOI] [PubMed] [Google Scholar]
- 4.Scott LJ, McKeage K, Keam SJ et al. Tacrolimus: a further update of its use in the management of organ transplantation. Drugs 2003;63:1247–97. 10.2165/00003495-200363120-00006 [DOI] [PubMed] [Google Scholar]
- 5.Van Hooff J, Van der Walt I, Kallmeyer J et al. Pharmacokinetics in stable kidney transplant recipients after conversion from twice-daily to once-daily tacrolimus formulations. Ther Drug Monit 2012;34:46–52. 10.1097/FTD.0b013e318244a7fd [DOI] [PubMed] [Google Scholar]
- 6.Wlodarczyk Z, Squifflet J-P, Ostrowski M et al. Pharmacokinetics for once- versus twice-daily tacrolimus formulations in de novo kidney transplantation: a randomized, open-label trial. Am J Transplant 2009;9:2505–13. 10.1111/j.1600-6143.2009.02794.x [DOI] [PubMed] [Google Scholar]
- 7.Alloway R, Vanhaecke J, Yonan N et al. Pharmacokinetics in stable heart transplant recipients after conversion from twice-daily to once-daily tacrolimus formulations. J Heart Lung Transplant 2011;30:1003–10. 10.1016/j.healun.2011.02.008 [DOI] [PubMed] [Google Scholar]
- 8.Silva HT, Yang HC, Abouljoud M et al. One-year results with extended-release tacrolimus/MMF, tacrolimus/MMF and cyclosporine/MMF in de novo kidney transplant recipients. Am J Transplant 2007;7:595–608. 10.1111/j.1600-6143.2007.01661.x [DOI] [PubMed] [Google Scholar]
- 9.Krämer BK, Charpentier B, Bäckman L et al. Tacrolimus once daily (ADVAGRAF) versus twice daily (PROGRAF) in de novo renal transplantation: a randomized phase III study. Am J Transplant 2010;10:2632–43. 10.1111/j.1600-6143.2010.03256.x [DOI] [PubMed] [Google Scholar]
- 10.Trunečka P, Boillot O, Seehofer D et al. Once-daily prolonged-release tacrolimus (ADVAGRAF) versus twice-daily tacrolimus (PROGRAF) in liver transplantation. Am J Transplant 2010;10:2313–23. 10.1111/j.1600-6143.2010.03255.x [DOI] [PubMed] [Google Scholar]
- 11.Albano L, Banas B, Klempnauer JJL et al. OSAKA trial: a randomized, controlled trial comparing tacrolimus QD and BD in kidney transplantation. Transplantation 2013;96:897–903. 10.1097/TP.0b013e3182a203bd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alak A M, Moy S, Cook M et al. An HPLC/MS/MS assay for tacrolimus in patient blood samples. Correlation with results of an ELISA assay. J Pharm Biomed Anal 1997;16:7–13. [DOI] [PubMed] [Google Scholar]
- 13.Tsunashima D, Yamashita K, Ogawara K-I et al. Preparation of extended release solid dispersion formulations of tacrolimus using ethylcellulose and hydroxypropylmethylcellulose by solvent evaporation method. J Pharm Pharmacol 2016;68:316–23. 10.1111/jphp.12515 [DOI] [PubMed] [Google Scholar]
- 14.Tanzi MG, Undre N, Keirns J et al. Pharmacokinetics of prolonged-release tacrolimus and implications for use in solid organ transplant recipients. Clin Transplant 2016;30:901–11. 10.1111/ctr.12763 [DOI] [PubMed] [Google Scholar]
- 15.First MR, Fitzsimmons WE. Modified release tacrolimus. Yonsei Med J 2004;45:1127–31. 10.3349/ymj.2004.45.6.1127 [DOI] [PubMed] [Google Scholar]