Summary
Every year 15 million preterm infants are born, and most spend their first weeks in neonatal intensive care units (NICUs)[1]. Although essential for the support and survival of these infants, NICU sensory environments are dramatically different from those in which full-term infants mature, and, thus, likely impact the development of functional brain organization[2]. Yet, the integrity of sensory systems determines effective perception and behaviour[3,4]. In neonates, touch is a cornerstone of interpersonal interactions and sensory-cognitive development[5–7]. NICU treatments used to improve neurodevelopmental outcomes rely heavily on touch[8]. Yet, we understand little of how brain maturation at birth (i.e. prematurity) and quality of early-life experiences (e.g. supportive vs. painful touch) interact to shape the development of the somatosensory system[9]. Here, we identified the spatial, temporal and amplitude characteristics of cortical responses to light touch differentiating them from sham stimuli in full-term infants. We then utilized this data-driven analytical framework to show that the degree of prematurity at birth determines the extent to which brain responses to light touch (but not sham) are attenuated at the time of discharge from the hospital. Building on these results, we showed that when controlling for prematurity and analgesics, supportive experiences (e.g. breastfeeding, skin-to-skin care) are associated with stronger brain responses, whereas painful experiences (e.g. skin punctures, tube insertions) are associated with reduced brain responses to the same touch stimuli. Our results shed crucial insights into the mechanisms through which common early perinatal experiences may shape the somatosensory scaffolding of later perceptual, cognitive and social development.
Keywords: tactile, pain, infant, preterm, sensory, development
eTOC blurb
Maitre et al. show that the degree of prematurity at birth determines the extent of attenuated brain responses to touch. This directly depended on the quality of touch experiences, even when controlling for prematurity and analgesics. Perinatal experiences shape the somatosensory scaffolding of later perceptual, cognitive and social development.
Results and Discussion
In a large cohort of 125 preterm (24–36 weeks’ gestational age, GA) and full-term infants (38–42 weeks GA) before discharge from the hospital, we recorded high-density 128-channel EEG and event-related potentials (ERPs) to calibrated light touch (see Supplemental Experimental Procedures for full exclusion criteria and Supplemental Table S1 for subject characteristics). Parents consented prior to testing using Vanderbilt IRB-approved protocols. Participant data on hospitalizations and experiences were extracted from medical and nursing records. Cumulative nociceptive exposure was measured as in seminal work in this field “in the absence of an empirical basis for assigning weights to every procedure” [10,11]. All surgical procedures involved the use of opioids, whereas non-surgical procedures involved oral sucrose administration, per unit protocols. The cumulative number of positive tactile experiences was measured in the absence of empirical evidence that one type of stimulus is more positive than another or that one duration is more optimal than another [10,11]. Only touch with the purpose of providing supportive and positive tactile experience for the infant beyond usual nursing care was recorded.
Characterization of full-term responses to light touch
We first objectively characterized full-term infants’ brain responses to light touch (Figure 1a) compared to the sham stimulus (see Supplemental Experimental Procedures). Fifty-five full-term infants (median GA: 39.5 weeks; 49% female) were tested at a median of 2 post-natal days (range 1–3 days). To characterize temporal and topographic responses, we applied a normative approach wherein brain activity of full-term infants was presumed to reflect typical tactile processing. These analyses were implemented with Cartool freeware[12]. We first compared ERPs as a function of time across the entire electrode montage. We only considered as reliably “tactile” those time intervals when >10% of the electrode montage exhibited a significantly different response from that to the sham stimulus for a minimal duration of 40ms (non-parametric randomization test at each time sample of the ERP; p<0.05; temporal extent >40ms)[13]. This analysis identified the 184–500ms post-stimulus interval as showing differential responses to touch vs. sham in full-term infants (Figure 1b–c).
Figure 1.
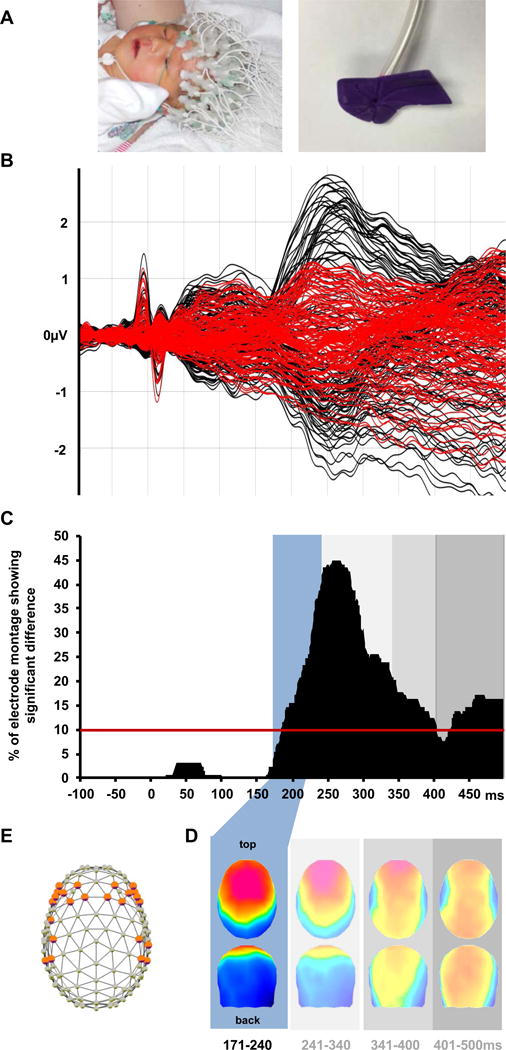
Normative analysis of ERPs from full-term infants. A. Photos of a full-term infant undergoing EEG recording (left) and the tubing and nozzle for delivering calibrated light touch to the hand (right). B. Superimposed ERPs to touch and sham stimuli (black and red traces, respectively). C. Significant ERP differences began at 184ms post-stimulus onset (percentage of significant electrodes across time shown). D. Hierarchical topographic clustering identified a series of touch-related ERP components (shaded boxes); the earliest, 171–240ms, was the focus of the present analyses. e. 24 bilateral electrodes were at the maxima/minima of the blue ERP topography, and measures from these were used in subsequent analyses. For term patient characteristics see Table S1.
Because it cannot be assumed that this entire period reflects a singular brain process that is stable over time, we next submitted the group-averaged ERP data from full-term infants in response to touch and sham stimuli to a hierarchical topographic cluster analysis[14]. This process identified time intervals of stable ERP topography, a data-driven manner to identify the series of ERP components. This data-driven approach identified four time windows comprising distinct ERP topographies in response to touch: (1) 171–240 ms, (2) 241–340 ms, (3) 341–400 ms, and (4) 401–500 ms (Figure 1d). Using the time intervals of these components and the loci of topographic maxima (fronto-central scalp sites F3/F4, C3/C4, an F7/F8; Figure 1e), we derived mean amplitudes at a subset of electrodes used in all subsequent analyses. This provided an objective validation of the choice of electrodes used in our (and others’) prior studies of tactile processing[4,15–17], allowing the convergence of our data-driven approach with existing methodological knowledge.
Characterization of preterm responses to light touch
The above results obtained from full-term infants served as a normative framework within which to compare the brain responses to touch by preterm infants (N= 61; median GA = 31 weeks, range 24–36; 51% female; median age at time of EEG recording = 28 post-natal days, range 2–103, or 36 weeks’ post-menstrual age, range 35–43). The mean amplitude values for each time interval of stable ERP topography described above were compared between full-term and preterm infants using Wilcoxon rank sum tests. Two time-windows demonstrated differences (Table 1; Figure 2), as measured using the selected subset of electrodes identified objectively above. Full-term infants’ cortical responses to light touch were of higher amplitude in the 171–240ms window than those of preterm infants (0.67 μV difference; CI 0.45–1.09 μV; p <0.001). Next, we compared the topographic distribution of the ERPs from full-term and preterm infants, to determine whether differences were due to changes in the active configuration of brain circuitry[14]. This was achieved by quantifying and analyzing global dissimilarity between the topography of ERPs, independent of their strength. Global dissimilarity equals the square root of the mean of the squared differences between the potentials measured at each electrode (versus the average reference), each of which is first scaled to unitary strength by dividing by the instantaneous global field power (i.e. the standard deviation across the electrode montage). This measure can range from 0 to 2, with 0 meaning topographies are identical and 2 meaning that topographies are inverted. It is therefore directly converted to correlation; i.e. spatial correlation equals 1 minus the squared value of global dissimilarity divided by 2). Global dissimilarity was statistically tested here using non-parametric randomization. Effects were considered reliable for p≤0.05 and if also temporally sustained for ≥40ms.
Table 1. Immaturity at birth alters amplitudes of ERP touch responses.
Differences in ERP amplitudes between full-term and preterm infants: Comparison of responses to touch between term and preterm infants in four time windows of significance.
| Tactile processing mean amplitude in full-term and preterm infants in identified time intervals post-stimulus (μV; 95% confidence interval indicated)
| ||||
|---|---|---|---|---|
| Stimulus | Time (ms) | Full-term | Preterm | p |
| Touch | 171–240 | 0.33 (−0.09,1.16) | −0.24 (−0.60,0.26) | <0.001* |
| Touch | 241–340 | 0.75 (0.19,1.37) | −0.10 (−0.53,0.56) | <0.001* |
| Touch | 341–400 | 0.27 (−0.40,1.14) | −0.17 (−0.73,0.70) | 0.051* |
| Touch | 401–500 | 0.07 (−0.71,0.92) | −0.18 (−0.93,0.62) | 0.212 |
|
| ||||
| Sham | 171–240 | 0.32 (−0.35,0.73) | 0.12 (−0.24,0.26) | 0.291 |
| Sham | 241–340 | 0.14 (−0.31,0.81) | 0.05 (−.37,0.41) | 0.319 |
| Sham | 341–400 | 0.09 (−0.46,0.66) | −0.11 (−0.56,0.51) | 0.603 |
| Sham | 401–500 | 0.24 (−0.61,1.05) | −0.07 (−0.72,0.52) | 0.212 |
Wilcoxon Rank Sum test. All results expressed as median amplitude (interquartile range).
Increase in microvolts ERP response per 1week unit in the predictor
Figure 2.
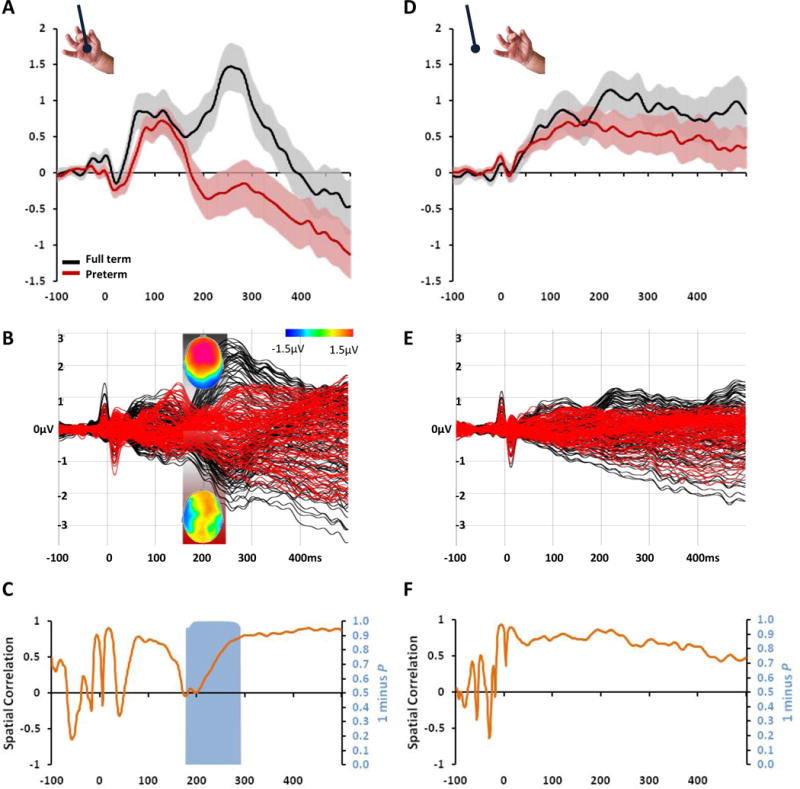
Impaired ERP responses to light touch in preterm infants. A. Group-averaged ERPs from full-term and preterm infants (black and red traces, respectively; s.e.m. shown) at a left frontal scalp site. B. Overlay of ERPs from the entire electrode montage. Insets show mean ERP topographies over the 171–240ms period (top view) when significant differences were observed (Table 1). C. The orange curve displays the spatial correlation between ERPs from full-term vs. preterm infants. The blue area displays statistically significant differences in ERP topography, indicative of differences in the active brain circuits in responses from full-term vs. preterm infants. Corresponding data and analyses in response to sham stimuli are shown in d–f. No statistically reliable differences were observed. For preterm patient characteristics see Table S1.
Combining these topographical and temporal amplitude analyses, we confirmed the presence of differential responses to touch (but not sham) between the two groups as early as the P2 component (171–240 and 241–340ms) (Table 1 and Figure 2). Infants’ responses to median nerve stimulation over this period have been previously linked with their neuro-developmental outcomes at 18 months [18]. Our analytical framework revealed that brain responses to touch in our cohort of preterm infants prior to discharge from the NICU not only were of significantly lower amplitude, but also differed in their topographic distribution relative to full-term infants (Figure 2a–c). No attenuation of the sham response was seen between preterm infants and no correlation was present between amplitude/response to sham and degree of prematurity, underlining the possible specificity of our results to touch.
Prematurity can contribute to these differences in somatosensory functional activity via altered post-natal experiences, interruptions in the normal sequence of brain maturation by preterm birth itself, or interactions between the two [9,12]. In animal models, somatosensory map formation via thalamo-cortical afferent axon organization[19] is initiated at both full-term or preterm birth and appears experience independent. Studies of human infants’ auditory system however show that postnatal experience does not appear to compensate for immaturity at birth during the first months of life[20]. In our current cohort of preterm infants, the amplitude of responses to touch in the 171–240 ms time window increased by 0.08 μV (p<0.001) for each week of GA (sham responses did not). GA and post-natal days were strongly correlated (r2(59)=−0.757, p<0.001). Therefore, we used the Akaike Information Criterion (AIC) and likelihood ratio tests to summarize overall model fits and identify independent effects[21]. A model with both GA and post-natal days explained significantly more variance in the amplitude of light touch responses than post-natal days alone (AIC 323 vs. 329; p =0.004). Conversely, including the number of post-natal days did not enhance the fit of the model with GA alone (AIC 323 vs. 321, p = 0.58). Thus, preterm infants cared for in NICUs exhibit decreased touch responses when they are discharged home compared to full-term infants, and these decreases are proportional to their degree of immaturity at birth. This finding may superficially contrast observations [22] that somatosensory responses to a non-noxious stimulus appeared equivalent between term and term-equivalent preterm infants. However, Slater et al. used stimuli ~29–145 times stronger than ours, which in all likelihood activated deep pressure receptors (Pacinian corpuscles). While processing of deep pressure as measured in central locations may be typically developed at term equivalent in preterm infants, more complex processing of light touch in frontal and central locations appears to still be attenuated.
Associations between painful and supportive experiences in the NICU and touch response in preterm infants
While the number of post-natal days was not strongly associated with amplitude of touch response, length of stay is an imprecise surrogate for multiple components of intensive care, which could potentially impact somatosensory development. In particular, painful experiences are associated with childhood problems in somatosensory function and socio-emotional development[9]. Conversely, developmental care approaches (e.g. Newborn Individualized Developmental Care and Assessment Program) often include varied tactile components purported to improve neuro-developmental outcomes[8], but rarely studied in association with quantitative changes in somatosensory processing.
Therefore, we next determined dose-response associations between both positive tactile experiences (beyond usual nursing care) as well as painful procedures and the tactile ERP amplitudes in preterm infants at the time of discharge from the NICU (related to Supplemental Experimental Procedures and Figure S1). All analyses were performed with and without the inclusion of minor surgical procedures without a change in conclusions. Supportive tactile experiences were associated with increased amplitude of cortical responses to light touch, even when controlling for GA and postnatal days (r2(110) = 0.177; p <0.001). Additionally, we provide the first demonstration that nociceptive exposures are associated with decreased amplitude of cortical responses to light touch after controlling for variations in GA and PND (r2(110) = 0.153; p=0.002). In preterm infants, painful exposures may have a negative impact on typical processing of non-painful tactile stimuli. Importantly, the preterm cohort had no known conditions associated with severe illness (necrotizing enterocolitis, sepsis, severe bronchopulmonary dysplasia, abnormalities on cranial imaging). Between birth and time of EEG recording, the preterm infants had a median 32 (range 10–103) painful procedures and a median 4 (range 0–46) supportive tactile experiences, with no discernible associations between these two types of experiences. This cross-modal association between exposure to pain and attenuated light touch response supports previous findings showing that repeated exposure to painful procedures is associated with decreased responses across multiple other modalities (e.g. temperature[23]) and systems (autonomic nervous system[24]).
Sensory processing throughout infancy and early childhood enables learning from experiences, and constitutes a foundation for the construction of higher-level perceptual and cognitive representations. Among the sensory systems, the somatosensory system is the earliest to develop, with physiological responses first observable at 14 weeks of gestational age (GA) and detectable cortical responses at 24 weeks[3,4]. The somatosensory system mediates biological and social interactions with the mother[7] during early life and, thus, scaffolds the development of other sensory systems (e.g., vision, hearing). Yet, objective and quantitative metrics of the consequences of an early-life NICU experience on light touch – including those associated with medical procedures and even potentially therapeutic interventions – were previously missing.
Our collective results now extend current understanding of the development of the somatosensory system and its susceptibility to the quality of early-life experiences. The emergence of evoked brain activity alongside the disappearance of unstructured and spontaneous “neural bursts” is considered an index of the maturation of neural circuits across sensory systems[25,26]. In the case of the human somatosensory system, this shift occurs at 35–37 weeks GA[11] with discrimination of different types of somatosensory inputs (e.g. nociceptive vs. touch) perhaps maturing along a similar trajectory[11]. The case could have been made that greater amount of any somatosensory experience would have resulted in an enhanced response to somatosensory stimuli, thus compensating for immaturity at birth through amount of experience. This appeared to be the case for deep pressure processing[22]. However, in the case of complex processing of light touch, our results suggest that repeated painful experiences in early life attenuate the formation of later typical responses perhaps through cross-modal inhibition established from non-specific neuronal bursts to both light touch and to nociception. Previous work has also demonstrated that nociceptive experiences can alter the perception of multiple somatosensory modalities (pain, touch, temperature) at the site of a procedure or in other parts of the body[23]. These findings, along with observed long-term dysesthesias of preterm infants undergoing surgical procedures[23] also argue in favor of cross-modal interactions at both peripheral and central levels. Individual modalities within the somatosensory system may be differentially affected by maturation, experience, and the complexity of connections between modalities.
One limitation of this study was that controlling for opiate use was infeasible, as all infants undergoing painful procedures received some form of analgesia. Recent evidence supports conventional anesthetics and sucrose as both contributing to altered brain maturation[27–29]. Additional analyses were conducted in the preterm infants that considered cumulative total opiate exposure. In particular, we examined the robustness of the significant associations between GA and ERP amplitude of touch response (Table 2) and associations of pain and touch with ERP amplitudes, controlling for postnatal days and GA (Figure S1). Results of these analyses are consistent with pain being significantly associated with ERP response to light touch after accounting for opiate exposure. The reported association with total supportive touch exposure was unchanged when controlling for cumulative opiate exposure (see Supplemental Experimental Procedures). Together, our results support the hypothesis that exposure to painful procedures in preterm infants, even when analgesics are used to mitigate pain and when sucrose is used as a sedative to mask the behavioral expression of pain, may contribute to attenuated responses to non-noxious tactile stimuli at discharge to home.
Table 2. Improved tactile processing amplitude is associated with increasing gestational age at birth in preterm infants.
Associations between ERP amplitudes to touch, gestational age at birth (GA) and postnatal days (PND)
| Univariable models | ||||
|---|---|---|---|---|
| Predictor | Stimulus | Slope** | Confidence Interval | p |
| GA | Touch | 0.08 | (0.05, 0.12) | <0.001* |
| PND | Touch | −0.02 | (0.07, 0.04) | 0.524 |
| GA | Sham | 0.01 | (−0.02, 0.05) | 0.530 [−0.684] |
| PND | Sham | 0.01 | (0.04, 0.06) | 0.684 |
|
| ||||
| Multivariable model for tactile stimulus | ||||
| Value | Standard Error | t-value | p | |
|
| ||||
| Intercept | −3.19 | 1.31 | −2.43 | 0.02 |
| GA | 0.10 | 0.03 | 2.85 | 0.01 |
| PND | 0.02 | 0.04 | 0.54 | 0.59 |
Wilcoxon Rank Sum test. All results expressed as median amplitude (interquartile range).
Increase in microvolts ERP response per 1week unit in the predictor
Table 2 is related to Figure S1
With respect to painful procedures themselves, our study faced the same challenges as groundbreaking work in the field: we could not quantify the intensity of each painful stimulus: EEG data on each experience throughout the entire hospital stay for each infant would have been infeasible due to methodological (EEG nets are not designed for infants <30 weeks PMA) and time limitations (interrupting care to measure intensity of stimulus). Behavioral manifestations of pain would have been difficult to prospectively collect on an hourly basis, and are not always reliable in preterm infants. Furthermore, our study was limited in its focus on the processing of pain in the brain, and did not address the subjective and emotional experience of pain. Similarly, our analysis of supportive tactile experiences was limited by feasibility considerations, such as controlling for individual variations in nursing handling and parent education; we relied instead on the consistent and extensive training – disseminating knowledge-base and following protocols -implemented by nursing leaders and the intent to provide support, rather than the behaviors elicited in response to support.
The results of our study have important clinical implications for infants cared for in NICUs and for those aiming to improve their neurodevelopmental outcomes. Current efforts aim to minimize the number and intensity of painful procedures, especially through non-pharmacological pain management[30]. Concurrently, family-centered initiatives and therapeutic interventions may remedy the relative paucity of supportive tactile experiences; a common problem in referral center NICUs, where geography, socioeconomic conditions and support systems impact parents’ direct involvement with their infants[31,32]. Simultaneously, our study raises concerns with regards to subjectively inferring positive or negative experiences for infants hospitalized in the neonatal period without first examining their impact on brain processing. However, regardless of these concerns, a greater one remains: infants currently discharged to their homes from NICUs have decreased cortical processing of touch compared to their full-term counterparts. This creates an altered learning scaffold for motor, tactile and multisensory exploration of the environment and self, as well as for social-emotional interactions. Abnormal tactile processing and neurological thresholds are associated with worse cognitive, motor and language outcomes in preterm infants[5,6]. Promoting optimal development and function in newborns hospitalized in NICUs may help establish the sensory building blocks of cognition, behavior and communication[33].
Supplementary Material
Highlights.
Preterm infants’ first weeks can be in neonatal intensive care units (NICUs).
NICU treatments for improving neurodevelopmental outcomes rely heavily on touch.
Prematurity and touch quality determine the extent of attenuated brain responses.
Perinatal somatosensory experiences may scaffold later development.
Acknowledgments
We would like to thank Dr. Paul Yoder for his guidance and revisions of the final draft, and Ms. Ellyn Hamm and Dorita Jones for their help in ERP data acquisition. This study was supported by 1 K23 HD074736-01A1 and 1 R01 HD081120-01A1 to NLM and UL1 TR000445 from NCATS/NIH to Vanderbilt. MMM receives support from the Swiss National Science Foundation (grants 320030-149982 and 320030-169206 as well as National Centre of Competence in Research project “SYNAPSY, The Synaptic Bases of Mental Disease” [project 51AU40-125759]) and Carigest. PJM receives support from the Pierre Mercier Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We have no conflicts of interest to report.
Author Contributions: NLM identified the link between sensory dysfunction and prematurity, directed intensive care research methods, performed preliminary analyses and proposed connections between tactile processing and nociceptive and positive experiences, was the primary author of the text and created all tables. AK directed ERP acquisition and pre-processing and ensured paradigm integrity. OC directed all intensive care protocols, data acquisition and tested all subjects. JCS conceived and performed all biostatistics data analyses. MMM directed all ERP post-processing and analyses, interpreted the results, generated all figures, and was involved in all stages of the writing and editing of the text. PJM interpreted the results, and was involved in all stages of the writing and editing of the text, as well as in editing the figures MTW was involved in the early conceptualization of the study and provided input during the writing and editing of the manuscript.
References
- 1.Lawn JE, Davidge R, Paul V, Xylander Von C. Born Too Soon: The Global Action Report on Preterm Birth. World Health Organization; 2012. http://www.who.int/pmnch/media/news/2012/20120522_joylawn_presentation.pdf?ua=1. [Google Scholar]
- 2.Pineda RG, Neil J, Dierker D, Smyser CD, Wallendorf M, Kidokoro H, Reynolds LC, Walker S, Rogers C, Mathur AM, et al. Alterations in brain structure and neurodevelopmental outcome in preterm infants hospitalized in different neonatal intensive care unit environments. J Pediatr. 2014;164:52–60.e52. doi: 10.1016/j.jpeds.2013.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hrbek A, Karlberg P, Olsson T. Development of visual and somatosensory evoked responses in pre-term newborn infants. Electroencephalography and Clinical Neurophysiology. 1973;34:225–32. doi: 10.1016/0013-4694(73)90249-6. 1973. [DOI] [PubMed] [Google Scholar]
- 4.Vanhatalo S, Lauronen L. Neonatal SEP – back to bedside with basic science. Semin Fetal Neonatal Med. 2006;11:464–470. doi: 10.1016/j.siny.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Spittle AJ, Walsh J, Olsen JE, McInnes E, Eeles AL, Brown NC, Anderson PJ, Doyle LW, Cheong JLY. Neurobehaviour and neurological development in the first month after birth for infants born between 32–42 weeks’ gestation. Early Hum Dev. 2016;96:7–14. doi: 10.1016/j.earlhumdev.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Chorna O, Solomon JE, Slaughter JC, Stark AR, Maitre NL. Abnormal sensory reactivity in preterm infants during the first year correlates with adverse neurodevelopmental outcomes at 2 years of age. Arch Dis Child Fetal Neonatal Ed. 2014;99:F475–F479. doi: 10.1136/archdischild-2014-306486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brauer J, Xiao Y, Poulain T, Friederici AD, Schirmer A. Frequency of maternal touch predicts resting activity and connectivity of the developing social brain. Cereb Cortex. 2016;26:3544–3552. doi: 10.1093/cercor/bhw137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohlsson A, Jacobs SE. NIDCAP: a systematic review and meta-analyses of randomized controlled trials. Pediatrics. 2013;131:e881–e893. doi: 10.1542/peds.2012-2121. [DOI] [PubMed] [Google Scholar]
- 9.Grunau RE. Neonatal pain in very preterm infants: long-term effects on brain, neurodevelopment and pain reactivity. Rambam Maimonides Medical Journal. 2013;4:e0025. doi: 10.5041/RMMJ.10132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grunau RE, Whitfield MF, Petrie-Thomas J, Synnes AR, Cepeda IL, Keidar A, Rogers M, MacKay M, Hubber-Richard P, Johannesen D. Neonatal pain, parenting stress and interaction, in relation to cognitive and motor development at 8 and 18 months in preterm infants. Pain. 2009;143:138–146. doi: 10.1016/j.pain.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fabrizi FL, Slater R, Worley A, Meek J, Boyd S, Olhede S, Fitzgerald M. A shift in sensory processing that enables the developing human brain to discriminate touch from pain. Current Biology. 2011;21:1552–1558. doi: 10.1016/j.cub.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brunet D, Murray MM, Michel CM. Spatiotemporal analysis of multichannel EEG: Cartool. Computational Intelligence and Neuroscience. 2011;2011:2–15. doi: 10.1155/2011/813870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guthrie D, Buchwald JS. Significance testing of difference potentials. Psychophysiol. 1991;28:240–4. doi: 10.1111/j.1469-8986.1991.tb00417.x. [DOI] [PubMed] [Google Scholar]
- 14.Murray MM, Brunet D, Michel CM. Topographic ERP analyses: a step-by-step tutorial review. Brain Topogr. 2008;20:249–264. doi: 10.1007/s10548-008-0054-5. [DOI] [PubMed] [Google Scholar]
- 15.Rahkonen P, Nevalainen P, Lauronen L, Pihko E, Lano A, Vanhatalo S, Pesonen AK, Heinonen K, Räikkönen K, Valanne L, et al. Cortical somatosensory processing measured by magnetoencephalography predicts neurodevelopment in extremely low-gestational-age infants. Pediatr Res. 2013;73:763–771. doi: 10.1038/pr.2013.46. [DOI] [PubMed] [Google Scholar]
- 16.Vanhatalo S, Kaila K. The Newborn Brain: Neuroscience & Clinical Applications. Cambridge UK: University Press; 2009. Spontaneous and evoked activity in the early human brain; pp. 229–243. [Google Scholar]
- 17.Maitre NL, Henderson G, Gogliotti S, Pearson J, Simmons A, Wang L, Slaughter JC, Key AP. Feasibility of event-related potential methodology to evaluate changes in cortical processing after rehabilitation in children with cerebral palsy: A pilot study. Journal of Clinical and Experimental Neuropsychology. 2014;36:669–679. doi: 10.1080/13803395.2014.925094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Vries LS, Pierrat V, Eken P, Minami T, Daniels H, Casaer P. Prognostic value of early somatosensory evoked potentials for adverse outcome in full-term infants with birth asphyxia. Brain Dev. 1991;13:320–5. doi: 10.1016/s0387-7604(12)80126-4. [DOI] [PubMed] [Google Scholar]
- 19.Toda T, Homma D, Tokuoka H, Hayakawa I, Sugimoto Y, Ichinose H, et al. Birth regulates the initiation of sensory map formation through serotonin signaling. Dev Cell. 2013;27:32–46. doi: 10.1016/j.devcel.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Maitre NL, Key AP. Quantitative Assessment of Cortical Auditory-tactile Processing in Children with Disabilities. Journal of Visualized Experiments. 2014;83:e51054–e51054. doi: 10.3791/51054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akaike H. A Bayesian extension of the minimum AIC procedure of autoregressive model fitting. Biometrika. 1979;66:237–242. [Google Scholar]
- 22.Slater R, Fabrizi L, Worley A, Meek J, Boyd S, Fitzgerald M. Premature infants display increased noxious-evoked neuronal activity in the brain compared to healthy age-matched term-born infants. NeuroImage. 2010;52:583–9. doi: 10.1016/j.neuroimage.2010.04.253. [DOI] [PubMed] [Google Scholar]
- 23.Walker SM, Franck LS, Fitzgerald M, Myles J, Stocks J, Marlow N. Long-term impact of neonatal intensive care and surgery on somatosensory perception in children born extremely preterm. Pain. 2009;141:79–87. doi: 10.1016/j.pain.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 24.Grunau RE, Oberlander TF, Whitfield MF, Fitzgerald C, Lee SK. Demographic and Therapeutic Determinants of Pain Reactivity in Very Low Birth Weight Neonates at 32 Weeks’ Postconceptional Age. Pediatrics. 2001;107:105–12. doi: 10.1542/peds.107.1.105. [DOI] [PubMed] [Google Scholar]
- 25.Colonnese MT, Kaminska A, Minlebaev M, Milh M, Bloem B, Lescure S, Moriette G, Chiron C, Ben-Ari Y, Khazipov R. A conserved switch in sensory processing prepares developing neocortex for vision. Neuron. 2010;67:480–498. doi: 10.1016/j.neuron.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tritsch NX, Yi E, Gale JE, Glowatzki E, Bergles DE. The origin of spontaneous activity in the developing auditory system. Nature. 2007;450:50–55. doi: 10.1038/nature06233. [DOI] [PubMed] [Google Scholar]
- 27.Backeljauw B, Holland SK, Altaye M, Loepke AW. Cognition and brain structure following early childhood surgery with anesthesia. Pediatrics. 2015;136:e1–12. doi: 10.1542/peds.2014-3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spann MN, Serino D, Bansal R, Hao X, Nati G, Toth Z, Walsh K, Chiang IC, Sanchez-Peña J, Liu J, et al. Morphological features of the neonatal brain following exposure to regional anesthesia during labor and delivery. Magn Reson Imaging. 2015;33:213–21. doi: 10.1016/j.mri.2014.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneider J, Duerden EG, Guo T, Hagmann P, Grunau RE, Chakravarty MM, et al. Neonatal pain management with glucose and the impact on thalamus and basal ganglia development: sex-specific effects. Pediatric Academic Societies. 2016 [Google Scholar]
- 30.Keels E, Sethna N, Watterberg KL, Cummings JJ, Benitz WE, Eichenwald EC, Poindexter BB, Stewart DL, Aucott SW, Goldsmith JP, et al. Prevention and management of procedural pain in the neonate: an update. Pediatrics. 2016;137:e20154271–e20154271. doi: 10.1542/peds.2015-4271. [DOI] [PubMed] [Google Scholar]
- 31.Lester BM, Hawes K, Abar B, Sullivan M, Miller R, Bigsby R, Laptook A, Salisbury A, Taub M, Lagasse LL, et al. Single-family room care and neurobehavioral and medical outcomes in preterm infants. Pediatrics. 2014;134:754–760. doi: 10.1542/peds.2013-4252. [DOI] [PubMed] [Google Scholar]
- 32.Anderzén-Carlsson A, Lamy ZC, Tingvall M, Eriksson M. Parental experiences of providing skin-to-skin care to their newborn infant–part 2: a qualitative meta-synthesis. Int J Qual Stud Health Well-Being. 2014;9:24907. doi: 10.3402/qhw.v9.24907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maitre NL. Neurorehabilitation after neonatal intensive care: evidence and challenges. Arch Dis Child Fetal Neonatal Ed. 2015;100:F534–F540. doi: 10.1136/archdischild-2013-305920. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


