Abstract
A 50-year-old woman presented with chest tenderness. On examination, both breasts were lumpy. Bilateral mammography showed heterogeneously dense parenchyma, with possible stromal distortion laterally on the right at the 0900 position. On ultrasound (US), a corresponding 13×9×10 mm irregular hypoechoic mass with internal vascularity was noted and both breasts had a complex heterogeneous fibroglandular background pattern. US-guided core biopsy with marker clip insertion was performed with the diagnosis of a grade 2 invasive ductal carcinoma (IDC). In view of the parenchymal pattern on mammography and US, contrast-enhanced spectral mammography (CESM) was performed for local staging. Mild background enhancement was noted, but there was no enhancement at the lesion site. The patient elected to have bilateral mastectomies and sentinel node biopsies. Final histopathology showed a node negative 11 mm grade 2 oestrogen and progesterone receptor positive, IDC.
Background
Contrast-enhanced spectral mammography (CESM) is a new digital imaging modality in which dual-energy full-field digital mammography is performed following IV contrast administration. This provides high-resolution morphological information and functional information by revealing areas of neovascularity within the breast parenchyma.1 Studies to date show that CESM has similar sensitivity to contrast-enhanced MRI (CEMRI) for the detection of breast cancer but with higher specificity.2 3 CESM is less expensive, faster and easier to perform than MRI, and is preferred by patients.4
In a multireader study of 199 women who underwent CESM during workup an abnormality detected on screening mammography, Lalji et al5 noted an improvement in the diagnostic parameters of all 10 readers. Mean sensitivity increased from 93% to 96.9% and specificity from 35.9% to 69.7%. For all readers combined, the area under the receiver operating characteristic curve increased from 0.645 to 0.833 (p<0.0001). With all diagnostic tests however, false negatives can occur and a negative result on one test should not be used to overrule the presence of concerning findings on another. This case emphasises the importance of the ‘triple test’ (physical examination, imaging and pathology) and the use of more than one imaging modality in the assessment of patients with breast symptoms.
Case presentation
A 50-year-old perimenopausal woman with no family history of breast cancer presented to her family doctor with chest tenderness and on examination, both breasts were ‘lumpy’. She was not on any medications. There was a previous history of endometrial ablation with a Mirena intrauterine device (IUD) currently in situ. Mammography showed the presence of heterogeneously dense fibroglandular tissue with possible architectural distortion at the 0900 position, 10 cm from the nipple (figure 1A, B). On ultrasound (US), a 13×9×10 mm irregular hypoechoic mass (figure 2A) with prominent internal vascularity on colour Doppler was seen at the lesion site (figure 2B). A 6 mm hypoechoic focus with no internal vascularity on colour Doppler was also noted in the left breast at 0300, 6 cm from the nipple, thought most likely to represent fibrocystic change.
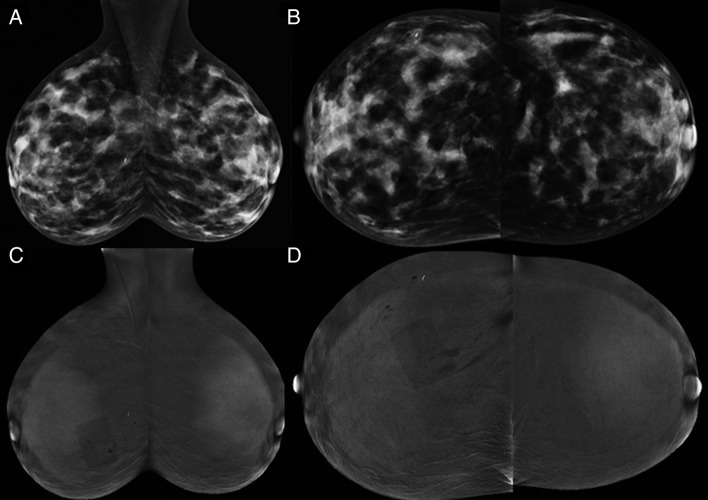
Figure 1.
Contrast-enhanced spectral mammography (CESM) images: (A,B) low energy and (C,D) recombined iodine-enhanced oblique and craniocaudal (CC) views of both breasts. The metallic marker placed at time of ultrasound-guided core biopsy marks the site of the grade 2 invasive ductal carcinoma (IDC). The low-energy images are equivalent to a standard full-field digital mammogram. The rectangular artefact projected over right breast is due to overlying dressing at site of core needle insertion.
Figure 2.
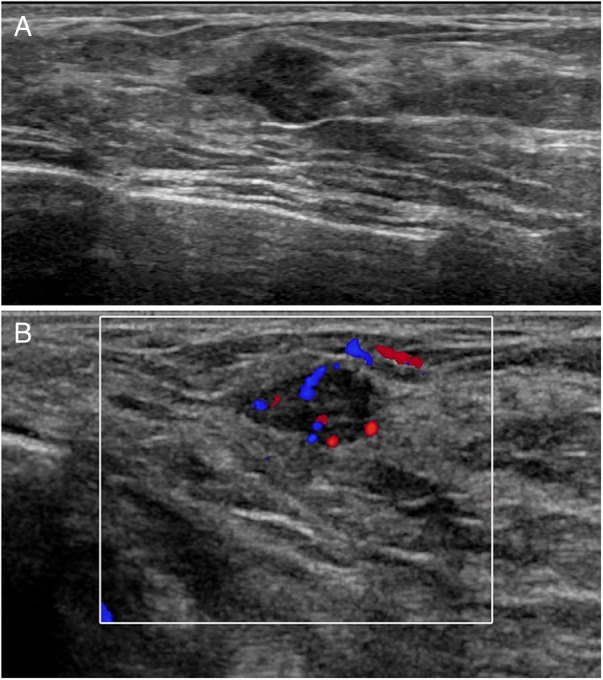
Ultrasound images: (A) there is an irregularly shaped, hypoechoic mass with indistinct margins (B) colour Doppler shows prominent internal vascularity (Doppler settings: 77% saturation, 487 Hz, wall filter 26 Hz, pulse repetition frequency (PRF)±2.5 cm/s).
US-guided fine needle aspiration (FNA) of the lesion in each breast was performed. The cytological findings on the left side were of benign apocrine and ductal cells consistent with fibrocystic change, and this was considered concordant with the imaging findings.
On the right side however, the smear was cellular containing epithelial cells in crowded sheets and cohesive groups. Some cells showed columnar cell change. Many of the cells were small; however, some variation in nuclear size and minor nuclear irregularity was noted. There were very occasional bare oval nuclei and small fragments of stroma. These features were considered suspicious for malignancy, and the patient referred for core biopsy.
A repeat US was performed in our clinic 2 weeks later. The left breast lesion was no longer evident and the right breast lesion appeared unchanged. US-guided 14G core biopsy was performed, and a marker clip inserted. The histopathology (figure 3A) demonstrated a grade 2 invasive ductal carcinoma (IDC). Stains for CD 31, an endothelial cell marker (figure 3B), showed that the degree of vascularity in the lesion was no less than expected for a tumour of this size and grade.
Figure 3.
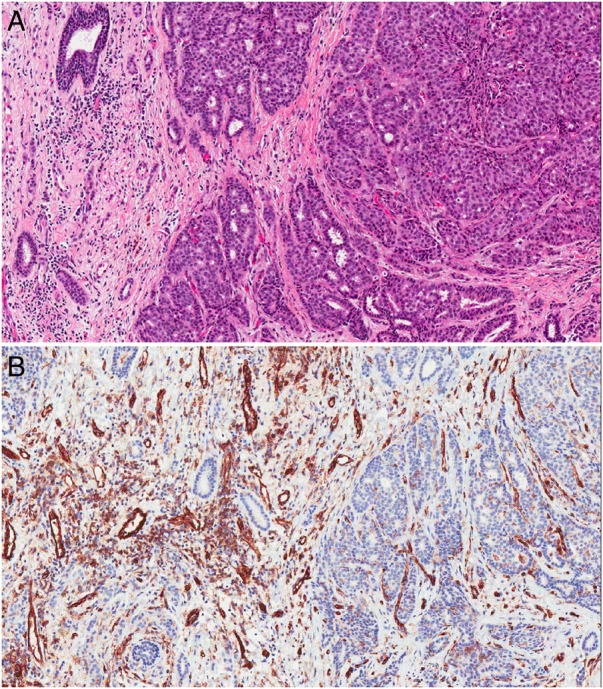
Histology images: (A) There is a grade 2 invasive duct carcinoma (H&E stain, original magnification ×100) (B) The endothelial cells of the small vessels are highlighted by CD 31. The degree of vascularity of the lesion is no less than expected for a tumour of this type (original magnification ×100).
In view of the difficulties associated with interpreting the dense parenchymal pattern on mammography and US, CESM was undertaken to help assess the remainder of the breasts to help exclude disease elsewhere. After obtaining written informed consent and confirming presence of normal renal function, an IV cannula was inserted and 90 mL of iohexol 350 mg iodine/mL (1.5 cc/kg) administered using power injector at 3 cc/s with the patient seated. Approximately 2 min later with the patient erect, bilateral craniocaudal (CC) and mediolateral oblique (MLO) mammograms were performed using dual-energy acquisition and standard compression as outlined by Dromain et al6 (figure 3A–D). Although the study was technically adequate, with mild bilateral background parenchymal enhancement (BPE), no detectable enhancement was visible at the lesion site as denoted by the biopsy marker.
Treatment
After consultation with her surgeon, the patient chose to undergo bilateral mastectomies and sentinel node biopsies.
Outcome and follow-up
The final pathology report was of an 11 mm grade 2 IDC, oestrogen and progesterone receptor positive. Apart from fibrocystic changes in both breasts, there were no other abnormalities. The sentinel nodes in both axillae were negative.
No other treatment was required and the patient remains well on routine clinical surveillance.
Discussion
It is important to remember that all diagnostic tests including breast imaging have a false-negative rate. For digital mammography, this varies from 20% to 30%,7 8 and is higher for women with dense breasts.9 Techniques that are able to show areas of neoangiogenesis like CEMRI are reported to have significantly lower rates: 3.2–8.4% for invasive disease, although some have reported a rate of 9.8–65% for DCIS lesions.10 11
During CESM, a low-energy and high-energy image is obtained for each view. Logarithmic subtraction of the high-energy and low-energy images reveals areas of increased iodine uptake corresponding to sites of neovascularity as shown using CEMRI. Like CEMRI, the sensitivity of CESM for detection of malignancy is usually unaffected by presence of dense parenchyma.12 13 Review of 22 published CESM studies (many with very small numbers of patients) shows a false-negative rate of between 0% and 20%, with a median of 4%. Lack of enhancement has been reported for tumours ranging between 4mm to 26 m and for all tumour types (including invasive ductal and lobular carcinomas, mucinous carcinoma and DCIS).12–27
While the contrast resolution of CESM is inferior to that of CEMRI, spatial resolution is higher, as the low-energy image is equivalent to a standard mammogram.28 29 Even where enhancement may not be detectable, lesion morphology can be assessed on the low-energy images, and unlike MRI, microcalcifications associated with DCIS may also be visible.30 31 Lack of contrast enhancement should not be used to downgrade a lesion with concerning morphology.
The reported causes for false-negative CESM examinations include technical difficulties (lesions situated in the posterior or peripheral aspects of the breast not included on the images) or masking of tumour uptake by surrounding BPE, although the latter appears to be less of an issue for CESM than CEMRI.18
The reason for the lack of enhancement in our case is uncertain. Mild BPE was present in our case, insufficient to mask tumour uptake but sufficient to confirm satisfactory contrast injection. Neoangiogenesis is thought to be the basis for contrast enhancement of breast cancers32 and aggressive tumours with a diameter exceeding 3 mm are said to need neoangiogenesis for further growth.33 Our patient's lesion was a 13 mm grade 2 IDC (figure 3A). Immunohistological staining with CD 31 (figure 3B) showed no lack of tumour vessels, and prominent intralesional blood flow was present on evaluation with colour Doppler. Although the breast is compressed during mammography, IV contrast is injected at least 2 min before compression is applied, which usually gives sufficient time for contrast to enter areas of neovascularity.
As postulated by Dromain et al,1 it is likely that the contrast enhancement that occurs in breast cancer is related exclusively to the number of vessels and functional parameters such as vessel permeability, particularly when a contrast agent that migrates into the extracellular fluid space is used. Perhaps the blood vessels in our patient's tumour were not abnormally permeable.
This case study emphasises the importance of using the triple test (physical examination, imaging and pathology) and not relying on any one imaging modality for diagnosis, particularly in the presence of dense fibroglandular tissue. The complementary nature of mammography, US and MRI for breast cancer detection is illustrated in articles by Shimauchi et al11 and Ghai et al,34 in which all non-enhancing malignant lesions on MRI were visible with either mammography or US. In the case of our patient, it was the presence of symptoms and dense parenchyma on mammography that prompted further investigation with US-guided core biopsy providing the diagnosis of breast cancer.
Learning points.
To avoid a ‘missed diagnosis’ of breast cancer in a symptomatic woman, the triple test (physical examination, breast imaging and pathology) should be applied.
Breast ultrasound (US) is a valuable supplementary examination particularly in women with dense breasts.
The possibility of false-negative results exists for all breast imaging tests.
As with contrast-enhanced MRI (CEMRI), the absence of contrast enhancement on a contrast-enhanced spectral mammography (CESM) study does not exclude the presence of breast cancer.
Concerning features on any component of the triple test should prompt further investigation.
Footnotes
Contributors: DT, SO'H and BL collected the data. DT, SO'H, BL prepared the manuscript. DT, SO'H, BL contributed in manuscript editing and proofing.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Dromain C, Balleyguier C, Muller S et al. Evaluation of tumor angiogenesis of breast carcinoma using contrast-enhanced digital mammography. Am J Roentgenol 2006;187:W528–37. 10.2214/AJR.05.1944 [DOI] [PubMed] [Google Scholar]
- 2.Jochelson MS, Dershaw DD, Sung JS et al. Bilateral contrast-enhanced dual-energy digital mammography: feasibility and comparison with conventional digital mammography and MR imaging in women with known breast carcinoma. Radiology 2013;266:743–51. 10.1148/radiol.12121084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fallenberg EM, Dromain C, Diekmann F et al. Contrast-enhanced spectral mammography versus MRI: initial results in the detection of breast cancer and assessment of tumour size. Eur Radiol 2014;24:256–64. 10.1007/s00330-013-3007-7 [DOI] [PubMed] [Google Scholar]
- 4.Hobbs MM, Taylor DB, Buzynski S et al. Contrast-enhanced spectral mammography (CESM) and contrast enhanced MRI (CEMRI): patient preferences and tolerance. J Med Imaging Radiat Oncol 2015;59:300–5. [DOI] [PubMed] [Google Scholar]
- 5.Lalji UC, Houben IPL, Prevos R et al. Contrast-enhanced spectral mammography in recalls from the Dutch breast cancer screening program: validation of results in a large multireader, multicase study. Eur Radiol 2016;26:4371–9. 10.1007/s00330-016-4336-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dromain C, Balleyguier C, Adler G et al. Contrast-enhanced digital mammography. Eur J Radiol 2009;69:34–42. 10.1016/j.ejrad.2008.07.035 [DOI] [PubMed] [Google Scholar]
- 7.Holland R, Mravunac M, Hendriks JH et al. So-called interval cancers of the breast: pathologic and radiologic analysis of sixty-four cases. Cancer 1982;49:2527–33. [DOI] [PubMed] [Google Scholar]
- 8.Pisano ED, Gatsonis C, Hendrick E et al. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med 2005;353:1773–83. 10.1056/NEJMoa052911 [DOI] [PubMed] [Google Scholar]
- 9.Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast us and evaluation of factors that influence them: an analysis of 27,825 patient evaluations 1. Radiology 2002;225:165–75. 10.1148/radiol.2251011667 [DOI] [PubMed] [Google Scholar]
- 10.Teifke A, Hlawatsch A, Beier T et al. Undetected malignancies of the breast: dynamic contrast-enhanced MR imaging at 1.0T 1. Radiology. 2002;224:881–8. 10.1148/radiol.2243010547 [DOI] [PubMed] [Google Scholar]
- 11.Shimauchi A, Jansen SA, Abe H et al. Breast cancers not detected at MRI: review of false-negative lesions. Am J Roentgenol 2010;194:1674–9. [DOI] [PubMed] [Google Scholar]
- 12.Cheung YC, Lin YC, Wan YL et al. Diagnostic performance of dual-energy contrast-enhanced subtracted mammography in dense breasts compared to mammography alone: interobserver blind-reading analysis. Eur Radiol 2014;24:2394–403. 10.1007/s00330-014-3271-1 [DOI] [PubMed] [Google Scholar]
- 13.Mokhtar O, Mahmoud S. Can contrast enhanced mammography solve the problem of dense breast lesions? Egyptian J Radiol Nuclear Med 2014;45:1043–52. [Google Scholar]
- 14.Jong RA, Yaffe MJ, Skarpathiotakis M et al. Contrast-enhanced digital mammography: initial clinical experience. Radiology 2003;228:842–50. 10.1148/radiol.2283020961 [DOI] [PubMed] [Google Scholar]
- 15.Lewin JM, Isaacs PK, Vance V et al. Dual-energy contrast-enhanced digital subtraction mammography: feasibility. Radiology 2003;229:261–8. 10.1148/radiol.2291021276 [DOI] [PubMed] [Google Scholar]
- 16.Thibault F, Balleyguier C, Tardivon A et al. Contrast enhanced spectral mammography: better than MRI? Eur J Radiol 2012;81 Suppl 1:S162–S4. 10.1016/S0720-048X(12)70068-2 [DOI] [PubMed] [Google Scholar]
- 17.Dromain C, Thibault F, Muller S et al. Dual-energy contrast-enhanced digital mammography: initial clinical results. Eur Radiol 2011;21:565–74. 10.1007/s00330-010-1944-y [DOI] [PubMed] [Google Scholar]
- 18.Tardivel AM, Balleyguier C, Dunant A et al. Added value of contrast-enhanced spectral mammography in postscreening assessment. Breast J 2016;22:520–8. 10.1111/tbj.12627 [DOI] [PubMed] [Google Scholar]
- 19.Diekmann F, Diekmann S, Jeunehomme F et al. Digital mammography using iodine-based contrast media: initial clinical experience with dynamic contrast medium enhancement. Invest Radiol 2005;40:397–404. [DOI] [PubMed] [Google Scholar]
- 20.Diekmann F, Freyer M, Diekmann S et al. Evaluation of contrast-enhanced digital mammography. Eur J Radiol 2011;78:112–21. 10.1016/j.ejrad.2009.10.002 [DOI] [PubMed] [Google Scholar]
- 21.Fallenberg E, Dromain C, Diekmann F et al. Contrast-enhanced spectral mammography versus MRI: Initial results in the detection of breast cancer and assessment of tumour size. Eur Radiol. 2013;23:1–9. 10.1007/s00330-012-2713-x [DOI] [PubMed] [Google Scholar]
- 22.Cheung YC, Tsai HP, Lo YF et al. Clinical utility of dual-energy contrast-enhanced spectral mammography for breast microcalcifications without associated mass: a preliminary analysis. Breast 2015;1:3. [DOI] [PubMed] [Google Scholar]
- 23.Lalji UC, Jeukens CRLPN, Houben I et al. Evaluation of low-energy contrast-enhanced spectral mammography images by comparing them to full-field digital mammography using EUREF image quality criteria. Eur Radiol 2015;25:2813–20. 10.1007/s00330-015-3695-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Łuczyńska E, Heinze-Paluchowska S, Hendrick E et al. Comparison between breast MRI and contrast-enhanced spectral mammography. Med Sci Monit 2015;21:1358–67. 10.12659/MSM.893018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blum KS, Rubbert C, Mathys B et al. Use of contrast-enhanced spectral mammography for intramammary cancer staging: preliminary results. Acad Radiol 2014;21:1363–9. 10.1016/j.acra.2014.06.012 [DOI] [PubMed] [Google Scholar]
- 26.Mori M, Akashi-Tanaka S, Suzuki S et al. Diagnostic accuracy of contrast-enhanced spectral mammography in comparison to conventional full-field digital mammography in a population of women with dense breasts. Breast Cancer 2016:1–7. [DOI] [PubMed] [Google Scholar]
- 27.Badr S, Laurent N, Regis C et al. Dual-energy contrast-enhanced digital mammography in routine clinical practice in 2013. Diagn Interv Imaging 2014;95:245–58. 10.1016/j.diii.2013.10.002 [DOI] [PubMed] [Google Scholar]
- 28.Francescone MA, Jochelson MS, Dershaw DD et al. Low energy mammogram obtained in contrast-enhanced digital mammography (CEDM) is comparable to routine full-field digital mammography (FFDM). Eur J Radiol 2014;83:1350–5. 10.1016/j.ejrad.2014.05.015 [DOI] [PubMed] [Google Scholar]
- 29.Fallenberg EM, Dromain C, Diekmann F et al. Contrast-enhanced spectral mammography: does mammography provide additional clinical benefits or can some radiation exposure be avoided? Breast Cancer Res Treat 2014;146:371–81. 10.1007/s10549-014-3023-6 [DOI] [PubMed] [Google Scholar]
- 30.Cheung YC, Tsai HP, Lo YF et al. Clinical utility of dual-energy contrast-enhanced spectral mammography for breast microcalcifications without associated mass: a preliminary analysis. Eur Radiol 2016;26:1082–9. 10.1007/s00330-015-3904-z [DOI] [PubMed] [Google Scholar]
- 31.Kamal RM, Helal MH, Wessan R et al. Contrast-enhanced spectral mammography: Impact of the qualitative morphology descriptors on the diagnosis of breast lesions. European Journal of Radiology 2015;84:1049–55. [DOI] [PubMed] [Google Scholar]
- 32.Kuhl C. The current status of breast MR imaging part I. Choice of technique, image interpretation, diagnostic accuracy, and transfer to clinical practice 1. Radiology 2007;244:356–78. 10.1148/radiol.2442051620 [DOI] [PubMed] [Google Scholar]
- 33.Folkman J. Clinical applications of research on angiogenesis. N Engl J Med 1995;333:1757–63. 10.1056/NEJM199512283332608 [DOI] [PubMed] [Google Scholar]
- 34.Ghai S, Muradali D, Bukhanov K et al. Nonenhancing breast malignancies on MRI: sonographic and pathologic correlation. AJR Am J Roentgenol 2005;185:481–7. 10.2214/ajr.185.2.01850481 [DOI] [PubMed] [Google Scholar]