Abstract
New antibiotic options are needed for the treatment of multidrug-resistant (MDR) Pseudomonas infections. We present a case of a man aged 64 years with a bladder fistula due to radiation, ultimately causing osteomyelitis of the pubic symphysis. Repeated antibiotic courses, without correcting the fistula, resulted in infection with MDR Pseudomonas aeruginosa. He was successfully treated for his osteomyelitis through cystectomy, aggressive debridement and a prolonged course of antimicrobials directed at the MDR Pseudomonas isolate.
Background
Osteomyelitis of the pubic symphysis most commonly arises from vaginal delivery and pelvic surgery. We encountered a patient who developed pubic symphysis osteomyelitis as a consequence of a bladder fistula stemming from radiation therapy for prostate cancer. While prostate cancer is a common malignancy, pubic symphysis osteomyelitis after treatment is exceedingly rare. We also offer our experience to alert clinicians to the presence of this entity and to distinguish associated urinary tract infections (UTIs) from those due to more common causes. Our experience further highlights the dangers of selection of multidrug-resistant (MDR) organisms with recurrent antibiotic use and the scarcity of appropriate antimicrobial agents for these infections.
Case presentation
The patient is a man aged 64 years with a history of coronary artery disease, hypertension, dyslipidaemia, rheumatoid arthritis, gastro-oesophageal reflux disorder, bilateral cataracts and prostate cancer. Prostate cancer was treated with radical prostatectomy and later external beam radiotherapy for recurrence. Subsequently, he developed urinary incontinence and a bladder neck stricture treated with transurethral incision and stent placement. The patient developed haematuria, clot formation and urinary retention. He underwent a second bladder neck incision for urinary retention. Symptoms improved but 1 month later, urinary retention again developed, this time accompanied by intractable suprapubic pain, radiating bilaterally to his hips and associated with nausea. The patient was admitted and empirically received 3 days of ceftriaxone 1 g intravenous daily and 4 days of cefdinir 300 mg orally two times per day.
Investigations
At the time of his admission, abdominal CT showed no acute pelvic or abdominal abnormalities. A pelvic MRI performed 2–3 weeks later demonstrated a fistula between the bladder neck and pubic symphysis with erosive changes of the pubic symphysis (figures 1 and 2). Urine cultures grew >100 000 colony forming units (CFU) of Group B β haemolytic streptococcus (GBS). Urinalysis included high-grade pyuria (>100 white cell count/high powered field). Evidence supported pubic symphysis osteomyelitis arising from infected urine being diverted through the bladder neck fistula. The patient was started on ampicillin 2 g intravenous every 6 hours, directed at GBS, for a planned duration of 8 weeks. Minimum inhibitory concentrations (MICs) for GBS were: penicillin 0.094 µg/mL (S); moxifloxacin 0.19 µg/mL (S) and ertapenem 0.032 µg/mL (S).
Figure 1.
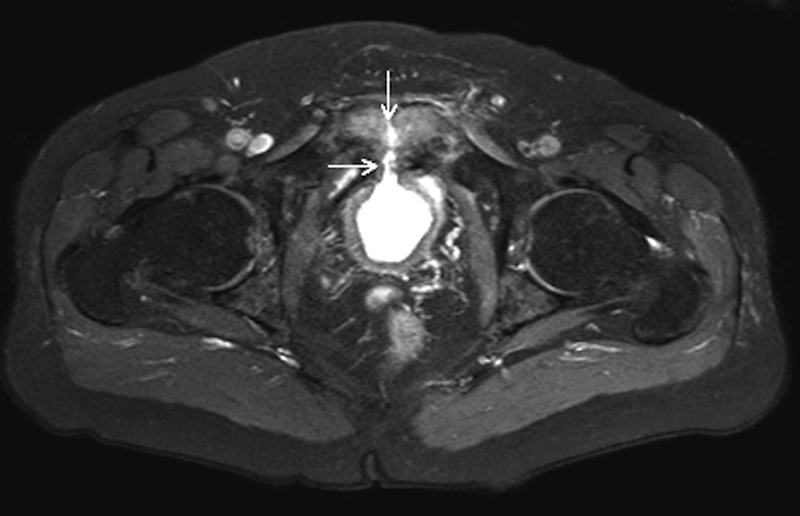
MRI of the lower pelvis (T2-weighted, fat-suppressed) demonstrated an anterior bladder defect with a fluid signal from the bladder to the symphysis pubis (arrow).
Figure 2.
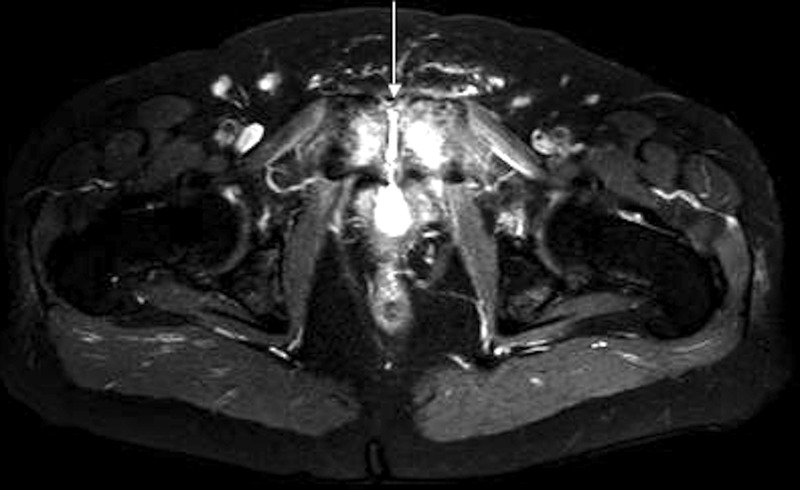
MRI of the lower pelvis (T2-weighted, fat-suppressed) demonstrated oedema at the symphysis pubis (arrow), consistent with osteomyelitis. Circumferential thickening of the bladder wall related to chronic inflammation was also present.
Ampicillin was changed to daptomycin 6 days later, due to development of acute interstitial nephritis (AIN). Clostridium difficile colitis occurred 11 days into therapy, and was treated with oral vancomycin. His course was further complicated by development of repeated UTIs, the first of which was due to extended-spectrum β-lactamase (ESBL)-producing Klebsiella pneumoniae (>100 000 CFU), sensitive to ampicillin/sulbactam, gentamicin, piperacillin/tazobactam, tobramycin, amikacin, imipenem, meropenem and levofloxacin. An abbreviated course of levofloxacin was administered. A subsequent UTI developed 4 days after completion of levofloxacin. Urine culture grew two strains of >100 000 CFU Pseudomonas aeruginosa. Both isolates were sensitive to gentamicin, piperacillin/tazobactam, tobramycin, amikacin, aztreonam and meropenem and our patient was treated with aztreonam 2 g intravenous every 12 hours for 2 weeks. A third UTI occurred, again due to ESBL K. pneumoniae (>100 000 CFU), but now resistant to levofloxacin, and P. aeruginosa (31 000 CFU), now resistant to meropenem and of intermediate sensitivity to aztreonam. Gentamicin 80 mg intravenous two times per day for 7 days was started and he was referred to an outside facility for surgical intervention.
Treatment
The patient underwent radical cystectomy with the creation of an ileal loop and pubic bone debridement. Bone cultures grew MDR P. aeruginosa and diphtheroids. MICs to P. aeruginosa were: amikacin 16 µg/mL (S), aztreonan 32 µg/mL (R), cefepime 16 µg/mL (I), ciprofloxacin 2 µg/mL (I), colistin 1 µg/mL (S), gentamicin 8 µg/mL (I), meropenem ≥16 µg/mL (R), piperacillin/tazobactam >256 µg/mL (R), tobramycin ≤1 µg/mL (S) and ceftolozane/tazobactam 2 µg/mL (S). It was apparent that emergence of MDR P. aeruginosa was triggered by serial treatment of bacteria causing recurrent UTIs, without correcting the underlying cause. To treat the P. aeruginosa and diphtheroid osteomyelitis, vancomycin and ceftolozane/tazobactam were used for a planned 6-week course. The vancomycin dose was adjusted to achieve trough levels between 15 and 20 µg/mL. Ceftolozane/tazobactam was dosed at 1.5 g intravenous every 8 hours.
Outcome and follow-up
Over the course of treatment, the patient was admitted for two separate occurrences of partial small bowel obstructions (SBO). Each resolved after 48 hours without oral intake. Through the course of ceftolozane/tazobactam and vancomycin, erythrocyte sedimentation rate declined from 69 to 18 mm/hour and C reactive protein declined from 2.1 to <0.3 mg/dL. Eighteen months postcystectomy, pubic bone debridement and completion of antibiotics, the patient remains well with no pain or other evidence of recurrence of osteomyelitis. He has been able to resume his normal activities of daily living.
Discussion
Pubic symphysis osteomyelitis is exceedingly rare. While most often caused by vaginal delivery and pelvic surgery, it can arise from bladder fistulisation, which is an exceptionally infrequent complication of radiation treatment for prostate cancer.1–3 Of a reported series of 12 patients who developed this complication, 8 underwent external beam radiation therapy; all developed bladder neck contractures; and cystectomy was ultimately performed in 10.4 Thus, despite the rarity of this entity, our patient exhibited relatively common features.
The prevalence of prostate cancer in men underscores the need for awareness of pubic symphysis osteomyelitis as a serious consequence of treatment.5 Distinguishing UTIs associated with this entity from those with more common causes is critical. Our patient's bladder fistula formation and urinary diversion led to development of osteomyelitis. He was subsequently treated with serial courses of antibiotics for UTIs, which contributed to the selection of MDR P. aeruginosa.
Infections with MDR P. aeruginosa have become an increasing concern in the USA accounting for 13% of the yearly Pseudomonas infections and leading to 440 deaths a year.6 Strains of P. aeruginosa, resistant to aminoglycosides, cephalosporins, fluoroquinolones and carbapenems, offer limited available antibiotic options. The treatment choices for these strains frequently include the use of polymyxins and often require combination therapy due to multiple resistance mechanisms. Our patient's strain of P. aeruginosa was susceptible to colistin, amikacin and tobramycin; however, their toxicities and poor outcomes for osteomyelitis made them poor treatment options.7 8 Ceftolozane/tazobactam was an attractive choice since cephalosporins are well tolerated and have been successful in the treatment of osteomyelitis.8 Ceftolozane/tazobactam comprises a novel cephalosporin combined with a β-lactamase inhibitor. This combination has potent activity against Pseudomonas, which appears to be unaltered by loss of porin channels or upregulation of efflux pumps.9
This is the first reported case of the successful use of ceftolozane/tazobactam in the treatment of MDR P. aeruginosa osteomyelitis. While ceftolozane/tazobactam is not yet FDA approved for the treatment of osteomyelitis, it has been used to treat MDR Pseudomonas sternal osteomyelitis. However, this report focused on serum kinetics and did not comment on cure.10 A second case report describes successful treatment of Stenotrophomonas maltophilia osteomyelitis with ceftolozane/tazobactam.11 While there are currently no published data on bone penetration of ceftolozane/tazobactam, historically cephalosporins have achieved adequate bone concentrations to successfully treat osteomyelitis.8 Bone (mg/kg) to serum (mg/L) concentration ratios 0.5–2 hours postdose for ceftriaxone, ceftazidime and cefepime have been reported as 0.156, 0.54 and 0.76, respectively.8 Pharmacokinetic studies of ceftolozane/tazobactam 1.5 g intravenous every 8 hours have reported serum concentrations of 74.4 µg/mL.12 The MIC of ceftolozane/tazobactam for P. aeruginosa of ≤4 µg/mL (FDA-approved package insert) should readily be achievable in bone.13
A second challenge that arose with the use of ceftolozane/tazobactam was the patient's history of AIN due to ampicillin. AIN is an antibody-mediated reaction that occurs an average of 10 days after the start of a therapy.14 Early studies reporting occurrence of AIN with the use of penicillins and cephalosporins recommended avoidance of all β-lactams and cephalosporins in future treatment.15 However, a recent study examined safety and tolerability of switching patients from nafcillin to cefazolin due to antibody-mediated nafcillin hypersensitivity. Nafcillin therapy was converted to cefazolin in 28.3% (n=17) of the patients and all but one patient tolerated cefazolin and had no occurrence of immune-mediated hypersensitivity.16
Ileus and abdominal distension are listed as side effects of ceftolozane/tazobactam in <1% of patients.13 It is unclear if our patient's SBO was a side effect of the antibiotic or a sequela of surgery. Regardless, our patient's two partial SBOs each responded successfully to 48 hours of no oral intake.
We offer our experience to alert clinicians to the presence of postradiation bladder fistulisation as a cause of pubic symphysis osteomyelitis. This report further highlights the dangers of indiscriminate use of antibiotics in this setting and the value of ceftolozane/tazobactam for MDR Pseudomonas infections.
Learning points.
Our experience highlights the pathogenesis of a multidrug-resistant (MDR) Pseudomonas infection triggered by repeated treatment of urinary pathogens without correcting the underlying cause.
Radiation-induced bladder fistulisation is an exceedingly rare cause of pubic symphysis osteomyelitis that requires prompt recognition and multidisciplinary efforts to correct.
Ceftolozane–tazobactam, directed by in vitro sensitivity testing, provides one means of treating serious MDR Pseudomonas infections, in conjunction with surgical revisions.
Footnotes
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Gupta S, Zura RD, Hendershot EF et al. Pubic symphysis osteomyelitis in the prostate cancer survivor: clinical presentation, evaluation, and management. Urology 2015;85:684–90. 10.1016/j.urology.2014.11.020 [DOI] [PubMed] [Google Scholar]
- 2.Robison CM, Gor RA, Metro MJ. Pubic bone osteomyelitis after salvage high-intensity focused ultrasound for prostate cancer. Curr Urol 2013;7:149–51. 10.1159/000356268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore DC, Keegan KA, Resnick MJ et al. A 57-year-old man with a history of prostatectomy and pelvic irradiation presents with recurrent urinary tract infections, hematuria, and pelvic pain. Urology 2013;81:221–5. 10.1016/j.urology.2012.10.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsushita K, Ginsburg L, Mian BM et al. Pubovesical fistula: a rare complication after treatment of prostate cancer. Urology 2010;80:446–51. 10.1016/j.urology.2012.04.036 [DOI] [PubMed] [Google Scholar]
- 5.Mohler JL, Armstrong AJ, Bahnson RR et al. Prostate cancer, version 1.2016. J Natl Compr Canc Netw 2016;14:19–30. [DOI] [PubMed] [Google Scholar]
- 6.Frieden T. Antibiotic resistant threats in the United States, 2013. Atlanta: Centers for Disease Control and Department of Health and Human Services, 2013. [Google Scholar]
- 7.Ortwine JK, Kaye KS, Li J et al. Colistin: understanding and applying recent pharmacokinetic advances. Pharmacotherapy 2015;35:11–16. 10.1002/phar.1484 [DOI] [PubMed] [Google Scholar]
- 8.Landersdorfer CB, Bulitta JB, Kinzig M et al. Penetration of antibacterials into bone: pharmacokinetic, pharmacodynamic and bioanalytical considerations. Clin Pharmacokinet 2009;48:89–124. 10.2165/00003088-200948020-00002 [DOI] [PubMed] [Google Scholar]
- 9.Hong MC, Hsu DI, Bounthavong M. Ceftolozane/tazobactam: a novel antipseudomonal cephalosporin and beta-lactamase-inhibitor combination. Infect Drug Resist 2013;6:215–23. 10.2147/IDR.S36140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bremmer DN, Nicolau DP, Burcham P et al. Ceftolozane/tazobactam pharmacokinetics in a critically ill adult receiving continuous renal replacement therapy. Pharmacotherapy 2016;36:e30–3. 10.1002/phar.1744 [DOI] [PubMed] [Google Scholar]
- 11.Jolliff JC, Ho J, Joson J et al. Treatment of polymicrobial osteomyelitis with ceftolozane-tazobactam: case report and sensitivity testing of isolates. Case Rep Infect Dis 2016;2016:1628932 Epub 2016 Jun 29; doi:10.1155/2016/1628932 10.1155/2016/1628932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller B, Hershberger E, Benziger D et al. Pharmacokinetics and safety of intravenous ceftolozane-tazobactam in healthy adult subjects following single and multiple ascending doses. Antimicrob Agents Chemother 2012;56:3086–91. 10.1128/AAC.06349-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merck Sharp & Dohme Corp. Zerbaxa Package Insert. July 2015 (updated Oct 2016).
- 14.Robinson JL, Hameed T, Carr S. Practical aspects of choosing an antibiotic for patients with a reported allergy to an antibiotic. Clin Infect Dis 2002;35:26–31. 10.1086/340740 [DOI] [PubMed] [Google Scholar]
- 15.Kleinknecht D, Kanfer A, Morel-Maroger L et al. Immunologically mediated drug-induced acute renal failure. Contrib Nephrol 1978;10:42–52. 10.1159/000401522 [DOI] [PubMed] [Google Scholar]
- 16.Blumenthal KG, Youngster I, Shenoy ES et al. Tolerability of cefazolin after immune-mediated hypersensitivity reactions to nafcillin in the outpatient setting. Antimicrob Agents Chemother 2014;58:3137–43. 10.1128/AAC.02504-13 [DOI] [PMC free article] [PubMed] [Google Scholar]


