Through a prospective, randomized, controlled single-center trial, we compare the efficacy and recurrence rates of intravitreal injection of ranibizumab (IVR) versus laser therapy for the Zone II treatment-requiring retinopathy of prematurity and found IVR might not be suitable as a single-dose monotherapy for this disease.
Key words: retinopathy of prematurity, ranibizumab, laser therapy, recurrence
Abstract
Purpose:
To compare the efficacy of intravitreal injection of ranibizumab (IVR) monotherapy and laser therapy for treatment-requiring retinopathy of prematurity (ROP) in Zone II.
Methods:
A prospective, randomized, controlled single-center trial was applied from January 2014 to December 2014; infants who were diagnosed as Zone II treatment-requiring ROP (i.e., Zone II Stage 2 or 3 ROP with plus disease) were randomly assigned to receive IVR monotherapy or laser therapy, and the follow-up interval was at least 6 months. Any eyes that developed recurrence of ROP underwent crossover re-treatment.
Results:
A total of 100 eyes of 50 ethnic Han Chinese infants were enrolled. At the last follow-up, 26 eyes of 13 infants developed recurrence of ROP in the IVR group and 2 eyes of 1 infant developed recurrence of ROP in the laser therapy group. There was a significant statistical difference in the rate of ROP recurrence between IVR and laser therapy to treat Zone II treatment-requiring ROP (P = 0.001).
Conclusion:
Although IVR appears to regress ROP to certain levels and continue to promote the vascularization of peripheral retinal vessels, a substantial proportion of infants developed recurrence of ROP after a single-dose IVR. Therefore, IVR is not recommended as a single-dose monotherapy for Zone II treatment-requiring ROP.
Retinopathy of prematurity (ROP) still remains a leading cause of childhood blindness worldwide.1 Especially in developing countries, because of often inappropriate neonatal care and lack of local ROP screening programs, ROP still occurs in larger premature infants and leads to relatively higher incidence of childhood blindness. Although the pathogenesis of ROP is not completely understood, vascular endothelial growth factor (VEGF) plays an important role in the development of ROP.2 It was generally thought that dysregulation of VEGF leads to abnormal vasculogenesis and neovascularization.3 Conventional laser photocoagulation can reduce the overproduction of VEGF in the retina and induce the regression of new vessels by ablating peripheral retina ischemic areas.4,5 The BEAT-ROP (Bevacizumab Eliminates the Angiogenic Threat of Retinopathy of Prematurity) study6 showed improved outcomes with intravitreal injection of bevacizumab (IVB) compared with conventional laser photocoagulation for Zone I ROP. However, more recently Sato et al7 showed that bevacizumab escapes from the vitreous into the general circulation and could reduce systemic VEGF levels after IVB in premature infants, whereas Carneiro et al8 have proved that ranibizumab does not alter systemic VEGF levels in adults. Moreover, studies reported that IVB had a risk of late recurrence and development of retinal detachment.9,10 Considering ranibizumab might have a better safety profile for preterm infants, some authors shifted IVB to intravitreal injection of ranibizumab (IVR) to treat ROP.11,12 The aim of this study was to compare the efficacy as well as ROP recurrence rates of IVR monotherapy and laser therapy for Zone II treatment-requiring ROP.
Methods
Screening and Enrollment
All the hospitals from Shenzhen Screening for ROP Cooperative Group participated the screening from January 2014 to December 2014. ROP screening was performed with binocular indirect ophthalmoscope and wide-field pediatric retinal imaging system (RetCam; Clarity Medical Systems, Pleasanton, CA) at Shenzhen Eye Hospital, whereas at the neonate intensive care units of other participated hospitals, screening was conducted with binocular indirect ophthalmoscope. With reference to ROP screening criteria,13 which was published by Ministry of Health of PRC in 2004, preterm infants who receive ROP screening should meet one of the following criteria: 1) birth weight <2,000 g and 2) preterm infants with birth weight ≧2,000 g but having severe systemic disorders (according to pediatricians' requirement). The diagnosis and treatment standard were in accordance with the international classification of ROP revisited14 and early treatment for ROP.5 Inclusion criterion was infants with binocular Zone II treatment-requiring ROP (i.e., ROP with Stage 2+ or 3+ in Zone II); exclusion criterion was infants with ROP in Zone I, Stage 4 or Stage 5 ROP, and aggressive posterior ROP in either eye. Each infant was examined by 2 experienced retina specialists (with ROP screening experience for more than 10 years and each year screen more than 1,000 infants) independently, and eligibility was confirmed by both the specialists. Digital fundus images of all enrolled infants were documented by RetCam before randomization.
Study Design
The study protocol was approved by the Institutional Review Board of Shenzhen Eye Hospital and other participating hospitals from Shenzhen Screening for ROP Cooperative Group. The participants' parents were informed about the severity of disease, treatment options, and complications, and then written informed consent was signed. All enrolled participants fulfilled the criteria of the Declaration of Helsinki. Based on a computer-generated randomization schedule, all eligible infants were randomly divided into 2 groups at a 1:1 proportion. Both eyes of each infant were then treated by either conventional laser therapy or a single-dose IVR.
Treatment
Treatment was performed within 72 hours once Zone II treatment-requiring ROP was detected. Intravitreal injection of ranibizumab was performed under topical anesthesia in standard ophthalmic operating room. A dose of 0.3 mg in 0.03 mL ranibizumab (Lucentis; Novartis, Basel, Switzerland) was injected into the vitreous cavity with a 30-gauge needle, aiming the needle directly toward the optic nerve in direction of visual axis 1.0 mm posterior to the corneoscleral junction. An ophthalmic antibiotic eye drop was prescribed for the treated eye to begin immediately and be continued 4 times a day for 7 days. Laser photocoagulation was performed under sedation in neonate intensive care unit. An indirect infrared diode laser (Iridis; Quantel-Medical, Cournon d'Auvergne Cedex, France) (810 nm) was used to apply photocoagulation through a 20 diopter condensing lens. Initial laser settings were set at a power of 150 mW for 0.2 seconds, with the goal of a threshold burn. Confluent laser treatment was applied to the avascular retina between the fibrovascular ridge and the ora serrata for 360°. Photocoagulation for peripheral retina was performed under sclera indentation. Topical steroid and cycloplegicmydriatic were administrated for 1 week after photocoagulation.
Follow-up
All infants were reexamined by binocular indirect ophthalmoscope and digital fundus images recorded by Retcam 1 week after treatment, 4 weeks after treatment, and then monthly. The follow-up was at least 6 months. The main outcome measurements included regression of plus disease, resolution of neovascularization, disappearance of ridge, ROP recurrences, and any complications. Retinopathy of prematurity recurrence was defined as any of the following: recurrent plus disease, recurrent neovascularization, or reformation of ridge despite treatment. Once recurrence was determined, the same protocol was applied for crossover re-treatment within 72 hours.
Statistical Analyses
Statistical analysis was performed by SPSS software version 17.0. Descriptive analysis was represented in the form of mean ± SD for normally distributed variables. Normally distributed variables were compared by t-test, nonparametric variables by Mann–Whitney U test, and categorical variables by chi-square test. A P value of less than 0.05 was considered statistically significant for the purposes of this study.
Results
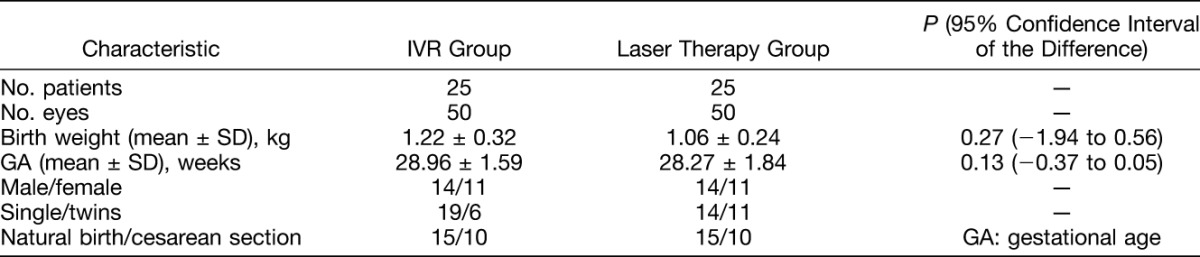
A total of 50 eyes of 25 infants were treated with IVR monotherapy, and 50 eyes of 25 infants were treated with laser photocoagulation. All infants and both their parents were ethnic Han Chinese. Table 1 shows baseline characteristics of the 2 groups. There was no statistically significant difference between gestational age, birth weight, sex ratio, the proportion of single or twin births, and delivery methods in the IVR and laser therapy groups.
Table 1.
Baseline Characteristics of Infants Treated With IVR and Laser Therapy
Main Outcomes
The mean follow-up after the first procedure was 49.94 ± 14.67 weeks (ranged from 27.71 to 78.71 weeks) in the IVR group and was 54.03 ± 12.40 weeks (ranged from 23.86 to 77.86 weeks) in the laser therapy group. There was no statistically significant difference in follow-up time between the 2 groups (P = 0.37). At the last follow-up, no infant had anterior segment ischemia, pupillary membrane, lens opacity, vitreous hemorrhage, endophthalmitis, or retinal detachment.
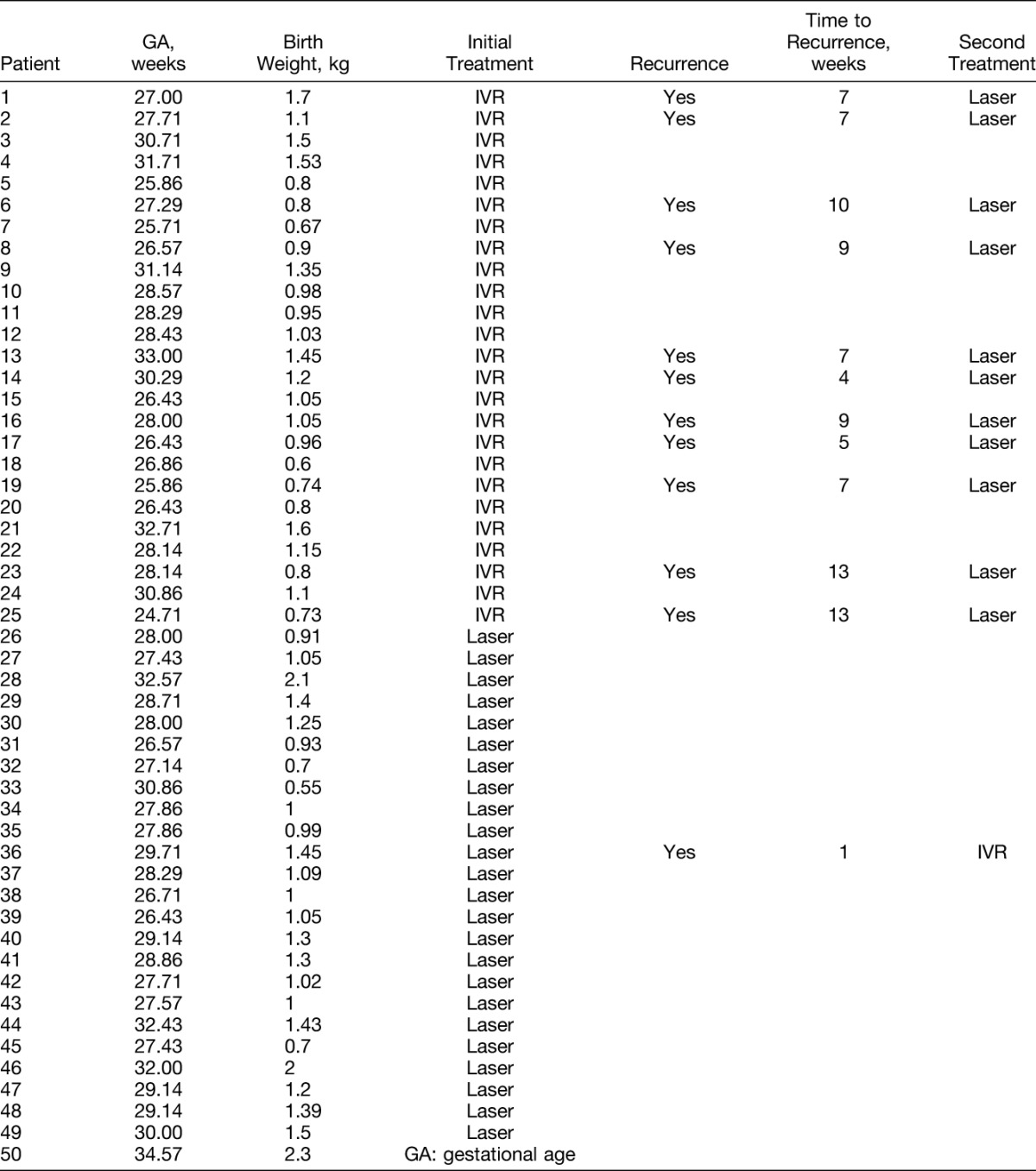
A total of 26 eyes of 13 infants (52%) developed ROP recurrence after a single-dose injection in the IVR group. Meanwhile, 2 eyes of 1 infant (4%) developed ROP recurrence after laser photocoagulation in the laser therapy group. Table 2 lists the treatment assignments and outcomes of 50 infants. A significant statistical difference was found in the ROP recurrence rates between IVR and laser therapy groups (χ2 = 12.004, P = 0.001).
Table 2.
Characteristics of Infants Treated With IVR and Laser Therapy
In IVR group, the regression of neovascularization and plus disease was found in all infants within 1 week after the initial injection. By the last follow-up, all infants have retinal vessels advanced to Zone III areas; however, no infant exhibited completely vascularized retina up to ora serrata, and 13 infants developed ROP recurrence. Recurrent ROP was then treated by laser photocoagulation inducing regression in all subjects (Figure 1, A–F). The interval from initial IVR to laser therapy was 12.62 ± 7.93 weeks.
Fig. 1.
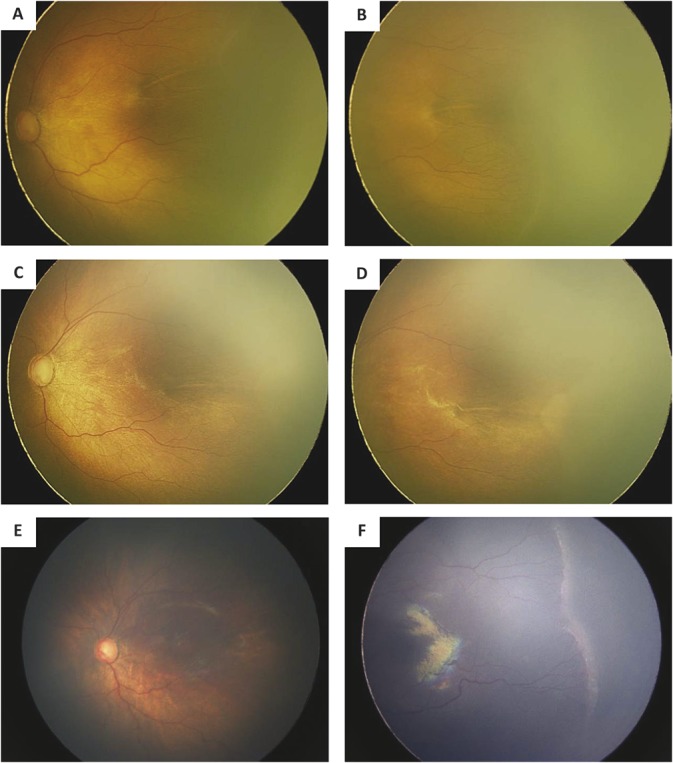
Fundus photographs of the left eye of one infant who failed IVR monotherapy and received laser therapy. A. The posterior fundus photograph before IVR shows plus disease. B. The temporal fundus photograph before IVR shows ridge and retinal neovascularization. C. The posterior fundus photograph obtained after IVR shows regression of plus disease. D. The temporal fundus photograph obtained after IVR shows regression of ridge and retinal neovascularization. E. The posterior fundus photograph obtained 13 weeks after IVR shows aggravated plus disease. F. The temporal fundus photograph obtained after IVR shows aggravated ridge and retinal neovascularization.
In laser therapy group, regressions of ROP were found in all infants except 2 eyes of 1 infant that showed aggravated plus disease and worst neovascularization with vitreous and retinal hemorrhage around the ridge 1 week after the initial laser photocoagulation (Figure 2, A–D). Intravitreal injection of ranibizumab was performed for the infant, and then vitreous hemorrhage was gradually resolved and ROP regressed.
Fig. 2.
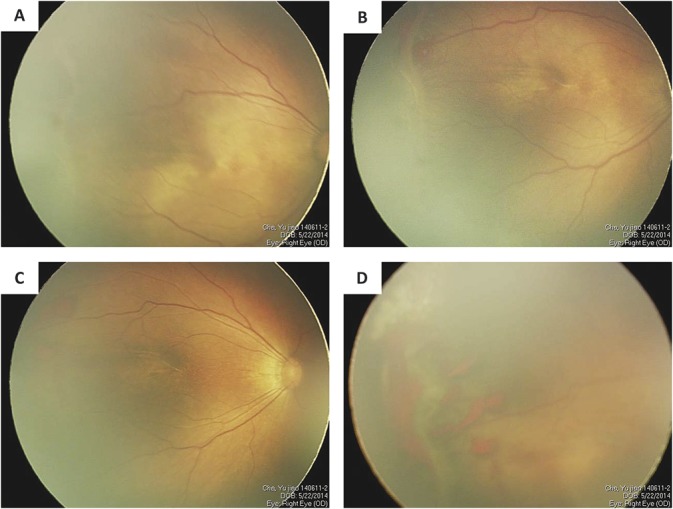
Fundus photographs of the right eye of one infant who failed laser monotherapy and received IVR. A. The posterior fundus photograph before treatment shows plus disease. B. The temporal fundus photograph before treatment shows ridge and retinal neovascularization. C. The posterior fundus photograph obtained 1 week after laser treatment shows aggravated plus disease. D. The temporal fundus photograph obtained 1 week after laser treatment shows aggravated ridge, retinal neovascularization, and hemorrhage around ridge.
Discussion
Peripheral retinal laser photocoagulation remains the current standard of care for treatment-requiring ROP in Zone II.15 The aim of this treatment is destruction of avascular retina, leading in turn to a reduction in VEGF levels and regression of neovascularization. More recently, IVB was used for Zone I ROP, aggressive posterior ROP, and failed ROP after laser treatment as primary monotherapy.16 As ranibizumab has a shorter half-life in human nonvitrectomized eyes and less measured penetration into the systemic circulation, some authors recommended IVR instead of IVB to treat severe ROP.17,18 This was further supported by several recent reports,19–21 which found IVR a more effective and well-tolerated method of treating Zone I and Zone II ROPs though recurrence is much more common; hence, long-term follow-up may be needed. Furthermore, evidence remains unclear whether IVR, IVB, or laser therapy is a better treatment option for ROP.22,23 Our study found that infants with Zone II treatment-requiring ROP had a higher recurrence rate after treatment with IVR monotherapy than laser therapy, although had similar low complication rates by 6 months follow-up.
Intravitreal injection of bevacizumab monotherapy as compared with laser showed significant lower recurrence rate for Zone I Stage 3+ ROP in BEAT-ROP study. However, our results showed that IVR monotherapy has a significant higher recurrence rate than laser for Zone II Stage 2+ or 3+ ROP. Recent advances of our understanding of the vasculogenesis and angiogenesis cascade indicate two distinct mechanisms and pathophysiologic processes that underline Zone I and Zone II ROP disease. Zone I and aggressive posterior ROP may be more related with the vasculogenesis stage, deeming treatment with cryotherapy or laser photocoagulation less effective.24 Therefore, it is reasonable to hypothesize that Zone II treatment-requiring ROP is more related to angiogenesis; thus, laser treatment might be a more durable option compared with anti-VEGF monotherapy. Hwang et al supported this in their study of VEGF-A, soluble VEGF receptor 2, and soluble Tie2. They suggested that plasma levels of the aforementioned decreased significantly after laser treatment for prethreshold Type 1 ROP.22 The hypothetical mechanism is that laser photocoagulation destroys the retina anterior to the fibrovascular ridge hence lowering VEGF levels. This eliminates the angiogenic signals by destroying distressed cells that produce both VEGF-inducing cytokines and VEGF itself. This in comparison with IVR monotherapy is favorable; hence, the latter are not definitive treatments for Zone II treatment-requiring ROP.
Intravitreal injection of ranibizumab and IVB are the two most universally applied anti-VEGF drugs for the treatment of ROP. Two reports described IVR for the treatment of ROP with both Castellanos et al25 and Hoerster et al26 not reporting reactivation of disease after IVR. However, recently, Wong et al27 in a study of IVR for Zone I or posterior Zone II treatment-requiring ROP reported a significant number (5/6 [83%]) of eyes that had reactivation of ROP after the initial response. Whereas in our study, we applied IVR to Zone II treatment-requiring ROP and more than one half (26/50 [52%]) of eyes after IVR developed recurrent ROP and underwent supplemental laser therapy. In comparison, other studies reported less recurrent ROP after IVB; for instance, in the study by Wong et al, there were no cases of reactivation after treatment with bevacizumab. In the BEAT-ROP study, ROP recurrence rates after IVB for Zone I and posterior Zone II diseases were 6% (2 of 31 infants) and 5% (2 of 39 infants), respectively. Moreover, when ROP recurrence rates after monotherapy of IVB are relatively lower in comparison with IVR, the interval from anti-VEGF injection to recurrence of IVB was also slightly longer than of IVR. The interval for posterior Zone II ROP in BEAT-ROP study after IVB was 14.4 ± 0.8 weeks, whereas in our study for Zone II posterior and anterior ROPs after IVR was 12.62 ± 7.93 weeks. The difference in the recurrence rate and recurrence interval of ROP after different anti-VEGF monotherapies can be as a result of the different molecular weights, structures, binding affinities, and half-lives of ranibizumab in comparison with bevacizumab.
Our study has a number of limitations. First, this study has a relatively small sample size and short follow-up that would not be powerful enough to assess the safety of intravitreal ranibizumab for treatment of ROP. Only two eyes of one infant with recurrent ROP in laser therapy group might have affected our statistical analysis. Second, it appears to be genetic components that attribute to the mechanism of ROP and its differential treatment responses. The infants in our study were all of Han nationality; however, this report used the same method and dose of ranibizumab as Caucasoid studies to treat Zone II treatment-requiring ROP. The differences in the results may be influenced by different racial backgrounds. Moreover, anterior and posterior Zone II treatment-requiring ROPs have a striking difference in their behaviors and presumably also in their treatment responses; however, we did not stratify them in the first instance. Nevertheless, further studies with larger sample sizes and longer follow-up are needed for the establishment of ideal dosing of IVR for Zone II treatment-requiring ROP in different ethnic groups. Safety of IVR in the treatment of ROP should be evaluated as well as anterior and posterior Zone II ROP should be stratified in future study.
In summary, intravitreal injection of anti-VEGF agents offers a promising option in our treatment armamentarium with advantages of being less time consuming than laser, potentially less risky by avoiding general anesthesia, and allowing further retinal vascularization in treatment-requiring cases. However, treatment still remains controversial as ideal dosing and long-term systemic safety have not been examined yet. The majority of data thus far come from reports and studies on bevacizumab. The shorter half-life of ranibizumab makes it an attractive treatment option when concerned about systemic absorption; yet, it may also translate into higher chance of reactivation when compared with infants treated with bevacizumab. Intravitreal ranibizumab leads to prompt regression of neovascularization and promotes peripheral retinal vascularization. It can theoretically decrease the supplementary laser spots needed and the subsequent destruction of peripheral visual fields, which might offer potential vision benefits. However, the effect of intravitreal ranibizumab injection for Zone II ROP is not as durable as conventional laser therapy according to our results. Therefore, intravitreal ranibizumab is not recommended for Zone II treatment-requiring ROP with obvious neovascularization as a single-dose monotherapy. Considering affordability of average Chinese families, conventional laser therapy is preferable to intravitreal ranibizumab for the treatment of Zone II ROP.
Acknowledgments
The authors acknowledge the dedication from Shenzhen Retinopathy of prematurity Screening Cooperative Group. They are neonate intensive care units of the following hospitals: Shenzhen People's Hospital, The Second People's Hospital of Shenzhen, PeKing University Shenzhen Hospital, The University of Hongkong-Shenzhen Hospital, Shenzhen Children's Hospital, Shenzhen Maternal and Child Health Hospital, Shenzhen Luohu District People's Hospital, Shen Zhen Luohu Maternal and Child Health Hospital, Shenzhen Nanshan Hospital, Nanshan Maternity & Child Healthcare Hospital of Shenzhen, The People's Hospital of Baoan Shenzhen, Shenzhen Baoan Maternal and Child Health Hospital, Shenzhen Songgang People's Hospital, Shenzhen Baoan Shajing People's Hospital, Longgang District People's Hospital of Shenzhen, Shenzhen Longgang District Maternity & Child Healthcare Hospital, People's Hospital of New District Longhua Shenzhen, Shenzhen Gongming People's Hospital, and Shenzhen Guangming New District People's Hospital.
Footnotes
Supported by two grants from Shenzhen Science and Technology Innovation Committee, P.R.China (Grant No. JCYJ20140415174819511/CXZZ2013051616181591).
None of the authors have any financial/conflicting interests to disclose.
References
- 1.Gillbert C. Retinopathy of prematurity: a global perspective of the epidemics, population of babies at risk and implications for control. Early Hum Dev 2008;84:77–82. [DOI] [PubMed] [Google Scholar]
- 2.Smith LE. Through the eyes of a child: understanding retinopathy through ROP: the Friedenwald lecture. Invest Ophthalmol Vis Sci 2008;49:5177–5182. [DOI] [PubMed] [Google Scholar]
- 3.Alon T, Hemo I, Itin A, et al. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med 1995;1:1024–1028. [DOI] [PubMed] [Google Scholar]
- 4.Uparkar M, Sen P, Rawal A, et al. Laser photocoagulation (810 nm diode) for threshold retinopathy of prematurity: a prospective randomized pilot study of treatment to ridge and a vascular retina versus vascular retina alone. Int Ophthalmol 2011;31:3–8. [DOI] [PubMed] [Google Scholar]
- 5.Early Treatment for Retinopathy of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol 2003;121:1684–1694. [DOI] [PubMed] [Google Scholar]
- 6.Mintz-Hittner HA, Kennedy KA, Chuang AZ. Efficacy of intravitrealbevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med 2011;364:603–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sato T, Wada K, Arahori H, et al. Serumconcentrations of bevacizumab (avastin) and vascular endothelial growthfactor in infants with retinopathy of prematurity. Am J Ophthalmol 2012;153:327–333. [DOI] [PubMed] [Google Scholar]
- 8.Carneiro AM, Costa R, Falcao MS, et al. Vascular endothelial growth factor plasma levels before and aftertreatment of neovascular age-related macular degeneration withbevacizumab or ranibizumab. Acta Ophthalmol 2012;90:e25–e30. [DOI] [PubMed] [Google Scholar]
- 9.Mireskandari K, Adams GG, Tehrani NN. Recurrence of retinopathy of prematurity following bevacizumab monotherapy: is it only the tip of the iceberg? JAMA Ophthalmol 2013;131:544–545. [DOI] [PubMed] [Google Scholar]
- 10.Tahija SG, Hersetyati R, Lam GC, et al. Fluoresceinangiographic observations of peripheral retinal vessel growth in infants after intravitreal injection of bevacizumab as sole therapy for zone I and posterior zone II retinopathy of prematurity. Br J Ophthalmol 2014;98:507–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baumal CR, Goldberg RA, Fein JG. Primary intravitreal ranibizumab for high-risk retinopathy of prematurity. Ophthalmic Surg Lasers Imaging Retina 2015;46:432–438. [DOI] [PubMed] [Google Scholar]
- 12.Menke MN, Framme C, Nelle M, et al. Intravitreal ranibizumab monotherapy to treat retinopathy of prematurity zone II, stage 3 with plus disease. BMC Ophthalmol 2015;15:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chinese Medical Association. Guidelines for therapeutic use of oxygen and prevention and treatment of retinopathy in premature infants [in Chinese]. Chin J Reprod Health 2004;15:132–133. [Google Scholar]
- 14.International Committee for the Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity revisited. Arch Ophthalmol 2005;123:991–999. [DOI] [PubMed] [Google Scholar]
- 15.Good WV; Early Treatment for Retinopathy of Prematurity Cooperative Group. Final results of the early treatment for retinopathy of prematurity (ETROP) randomized trial. Trans Am Ophthalmol Soc 2004;102:233–248. [PMC free article] [PubMed] [Google Scholar]
- 16.Darlow BA, Ells AL, Gilbert CE, et al. Are we there yet? Bevacizumab therapy for retinopathy of prematurity. Arch Dis Child Fetal Neonatal Ed 2013;98:F170–F174. [DOI] [PubMed] [Google Scholar]
- 17.Jang SY, Choi KS, Lee SJ. Delayed-onset retinal detachmentafter an intravitreal injection of ranibizumab for zone 1plus retinopathy of prematurity. J AAPOS 2010;14:457–459. [DOI] [PubMed] [Google Scholar]
- 18.Lin CJ, Chen SN, Hwang JF. Intravitreal ranibizumab as salvagetherapy in an extremely low-birth-weight infant with rushtype retinopathy of prematurity. Oman J Ophthalmol 2012;5:184–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y, Feng J, Meng XF, et al. Effects of ranibizumab in zone I and zoneII retinopathy of prematurity patients [Chinese]. Chin J Ocul Fundus Dis 2015;31:6–9. [Google Scholar]
- 20.Yizuo HZ, Sun XT, Chen CZ, et al. Effects of intravitrealranibizumab for the treatment of retinopathy of prematurity [Chinese]. Chin J Ocul Fundus Dis 2015;31:10–13. [Google Scholar]
- 21.Sun XT, Sun S, Wang H, et al. The efficacy of laser photocoagulation and intravitrealranibizumab treatment of retinopathy of premature [Chinese]. Chin J Ocul Fundus Dis 2015;31:14–17. [Google Scholar]
- 22.Hwang CK, Hubbard GB, Hutchinson AK, Lambert SR. Outcomes after Intravitreal Bevacizumab versus laser photocoagulation for retinopathy of prematurity: a 5-year retrospective analysis. Ophthalmology 2015;122:1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Isaac M, Mireskandari K, Tehrani N. Treatment of type 1 retinopathy of prematurity with bevacizumab versus laser. J AAPOS 2015;19:140–144. [DOI] [PubMed] [Google Scholar]
- 24.Flynn JT, Chan-Ling T. Retinopathy of prematurity: two distinct mechanisms that underlie zone 1 and zone 2 disease. Am J Ophthalmol 2006;142:46–59. [DOI] [PubMed] [Google Scholar]
- 25.Castellanos MA, Schwartz S, García-Aguirre G, Quiroz-Mercado H. Short-term outcome after intravitreal ranibizumab injections for the treatment of retinopathy of prematurity. Br J Ophthalmol 2013;97:816–819. [DOI] [PubMed] [Google Scholar]
- 26.Hoerster R, Muether P, Dahlke C, et al. Serum concentrationsof vascular endothelial growth factor in an infant treated withranibizumab for retinopathy of prematurity. Acta Ophthalmol 2013;91:e74–e75. [DOI] [PubMed] [Google Scholar]
- 27.Wong RK, Hubschman S, Tsui I. Reactivation of retinopathy of prematurity afterranibizumab treatment. Retina 2015;35:675–680. [DOI] [PubMed] [Google Scholar]


