Abstract
Dengue is a significant global health problem. Even though a vaccine against dengue is now available, which is a notable achievement, its long-term protective efficacy against each of the 4 dengue virus serotypes remains to be definitively determined. Consequently, drugs directed at the viral targets or critical host mechanisms that can be used safely as prophylaxis or treatment to effectively ameliorate disease or reduce disease severity and fatalities are still needed to reduce the burden of dengue. This review will provide a brief account of the status of therapeutics research and development for dengue.
Keywords: dengue, flavivirus, dengue drug discovery, antivirals, dengue prophylaxis, dengue therapeutics
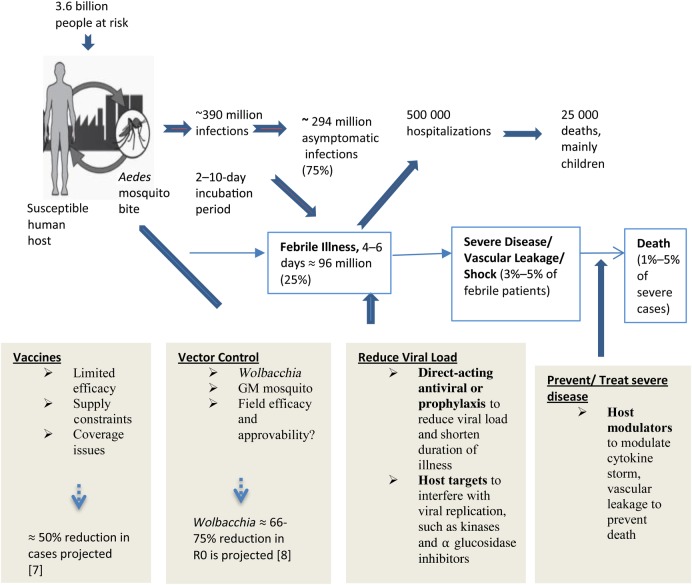
The geographic distribution of dengue has expanded globally in the past 5 decades. This mosquito-borne acute disease is now endemic in >100 countries, with an estimated 400 million infections each year [1]. Recently, Dengvaxia (CYD-TDV), a tetravalent vaccine developed by Sanofi Pasteur that consists of genes encoding the premembrane (prM) and E proteins of dengue virus (DENV) serotypes 1–4 (DENV 1–4) inserted onto the genomic backbone of live attenuated yellow fever vaccine strain, was licensed in several dengue-endemic countries [2]. The vaccine efficacy, however, varied by age and serostatus of the vaccine recipient at baseline and by the DENV serotype causing the infection; lower efficacy was observed for DENV 1 and 2 as compared to DENV 3 and 4 [3–5]. Hence, despite the availability of a dengue vaccine, improvements in case management to reduce the risk of severe dengue are still needed. Current approaches are entirely supportive care in the form of judicious fluid replacement and close clinical monitoring during the critical phase of illness [6]. No antiviral drug has been developed despite the association between higher viremia levels and severe dengue. The current status of dengue burden and impact of various countermeasures is summarized in Figure 1.
Figure 1.
Schematic diagram summarizing the state of the global dengue epidemic, showing countermeasures and their impact on the total dengue burden. Abbreviations: GM, genetically modified; R0, basic reproduction number.
Dengue Drug Targets
The RNA genome of DENV is translated as a single polypeptide that is then cleaved into 3 structural proteins (capsid [C], prM, and E) and 7 nonstructural (NS) proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) by cellular proteases and viral serine protease, composed of NS2B and NS3 [7]. The NS proteins are essential components of replication machinery of the DENV genome. Several recent studies have also shown that their interaction with host factors lead to suppression of natural innate immune responses that may contribute to the epidemiology and pathogenesis that drive the spread of dengue [8].
Antiviral approaches explored thus far have targeted both structural and nonstructural proteins of DENV. Small molecules that target viral entry have been examined, although the most advanced intervention against virus entry is in the form of therapeutic antibodies. These are at various stages of clinical development [9–11]. The search for small-molecule inhibitors has focused on the multifunctional enzymes NS3 and NS5, the supposedly “low-hanging” antiviral targets [12, 13]. In addition, the C protein and NS4B are also being explored as drug targets [14–17]. However, no antiviral that has been developed exclusively for DENV has entered clinical trials. The only drug that is believed to directly target one of the viral proteins (NS5) that has been clinically investigated is balapiravir. This nucleoside analogue, developed by Roche Pharmaceutical originally for hepatitis C, was examined as a short-course indication against dengue because of its useful short-course safety profile [18]. This compound, however, did not meet the efficacy end point, possibly because of altered host cell kinase expression or activity during DENV infection [19].
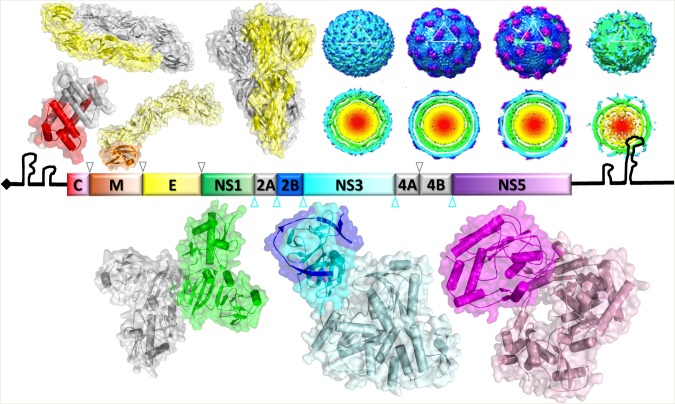
Antiviral drug development can, however, now benefit from advances in molecular and structural virology. Structural information of the virus and several NS proteins that are critical for the virus life cycle have been determined by nuclear magnetic resonance spectroscopy, X-ray crystallography, or cryo–electron microscopy. A portrait of the important elements that can contribute to the drug discovery effort is shown in Figure 2. These high-resolution structures could be combined with molecular tools such as in silico approaches and infectious clone technology to identify new and thus hitherto unexplored drug targets for DENV and possibly other flaviviruses [12, 20].
Figure 2.
The dengue genome and proteome. The 5′ and 3′ untranslated regions and the arrangement of the genes encoding 3 structural and 7 nonstructural (NS) proteins are shown. The structures for capsid protein (C; PDB code: 1R6R), E-dimer (PDB code: 1UZG), premembrane and E (prM/E) proteins (PDB code: 3C6E), E-trimer (PDB code: 1OK8), and various images of the dengue virus virion reconstructed on the basis of cryo–electron microscopy (kindly provided by Dr Shee Mei Lok) are shown above the schematic of the genome, while the NS1 (PDB code: 4O6B), NS3 (PDB code: 2VBC), and NS5 (PDB code: 4V0R) structures are shown below. The figure was provided by Dr Dahai Luo.
An RNA-based approach to inhibit gene expression and serve as antivirals is another strategy that can be potentially exploited if the current limitations such as stability and mode of delivery can be adequately addressed [21].
Target Product Profile That Can Have Maximum Clinical Utility
Dengue is an acute, self-limiting disease in most instances, with a small proportion of patients progressing to severe disease manifested by increased plasma leakage, hemodynamic compromise, shock, and bleeding. If dengue is left untreated, mortality can reach as high as 30%. The acute and self-limiting nature of the disease in the majority of cases thus require that an effective antiviral should have an excellent safety profile and be active against all 4 serotypes of DENV. Ideally, an oral drug that is dissolvable would be available, because there is a large disease burden in the pediatric population. A once-daily dosing schedule would also be useful for good compliance. Pragmatically, however, dosing of up to 3 or 4 times per day may be necessary to maintain drug levels above a minimum effective concentration, as exemplified by antivirals against other acute infections, such as acyclovir for varicella zoster and antibiotics against common acute bacterial infections [22, 23]. The use of biologics such as therapeutic antibodies may overcome the challenges faced in the field with small-molecule drugs, as human immunoglobulin G1 is known to have long half-life. These could be used as a single-dose treatment or as short-term prophylaxis for travelers from countries where dengue in not endemic.
Indeed, the use of antivirals as a tool to prevent infection, either in travelers or in populations living in areas with focal outbreaks, could augment public health measures currently available to prevent dengue. Besides therapeutic antibodies, small molecules administered either once daily or even at longer intervals, such as antimalarial prophylaxis, could be clinically beneficial. In this respect, the pharmacokinetic properties to prevent infection may be less demanding than that needed to rapidly reduce viremia levels in patients with dengue. A strong safety profile in a drug that broadly acts on all DENV serotypes will be necessary for good compliance. However, clinical trials to evaluate such therapy could be challenging to conduct, as they will require treatment of large number of volunteers over long periods, coupled with active surveillance for febrile illness and DENVs.
Therapeutic Development Landscape
Several therapeutic trials performed in Asia and South America that used antivirals or disease modulators have been described since early 2000. Unfortunately, interpretations of results of these early trials are confounded by lack of information on patient demographic characteristics, dengue severity at recruitment, and defined end point measurements [24–30].
Because the pathway to discovery of new small-molecule drugs take a long time to reach the clinic, dengue researchers have taken advantage of the cost-saving and time-saving benefits of drug repurposing [13]. The most recent proof-of-concept clinical trials for dengue have been performed using repurposed or off-patent drugs, namely chloroquine, prednisolone, balapiravir, celgosivir, and lovastatin (Table 1). These trials have all used the conventional double-blinded, randomized, placebo-controlled design with clearly defined primary end points. The drugs were found to be safe in patients with acute dengue, but all of these compounds failed to meet a priori–defined trial end points [18, 31–34].
Table 1.
List of Clinical Studies on Dengue Therapeutics
| Compound | Rationale | Study Site(s) | Study Drug Characteristics | Subject Characteristics | Primary End Point(s) | Results | Reference |
|---|---|---|---|---|---|---|---|
| Chloroquine | Widely used antimalarial drug presumed to interfere with virus entry mechanism by inhibiting fusion between virus and host membrane | OUCRU, Ho Chi Minh City, Vietnam | Placebo vs chloroquine (600 mg on d 1, 600 mg on d 2, 300 mg on d 3) | Age, >18 y; trial size, 307 (154 received placebo, 153 received chloroquine) | Laboratory: time to resolution of viremia, time to resolution of NS1 antigenemia | No change in viremia and NS1 antigenemia | [31] |
| Prednisolone | Antiinflammatory properties, publication of studies supporting modulation of the function of endothelial glycocalyx | OUCRU | Placebo or prednisolone (0.5 mg/kg or 2 mg/kg once daily for 3 d) | Age, 5–20 y; trial size, 225 (75 received placebo, 75 received prednisolone 0.5 mg/kg, 75 received prednisolone 2 mg/kg) | Clinical: safety; laboratory: virological log reduction | Not powered for efficacy; no change in hematological, virological, or clinical end points | [32] |
| Balapiravir | Presumed to be an NS5 nucleoside inhibitor developed for HCV by Roche | OUCRU | Placebo vs balapiravir (1500 mg or 3000 mg twice daily for 5 d) | Age, 18–65 y; trial size, 64 (32 placebo recipients, 10 balapiravir 1500 mg recipients, 22 balapiravir 3000 mg recipients) | Laboratory: viral log AUC from first dose to study d 7, time to first viremia level of <1000 copies/mL, time to resolution of NS1 antigenemia | No change in virological and immunological end points | [18] |
| Celgosivir | Inhibitor of ER-associated α glucosidase | SGH/Duke-NUS, Singapore | Placebo vs celgosivir | Age, 21–65 y; trial size, 50 (26 placebo recipients, 24 celgosivir recipients) | Clinical: fever reduction; laboratory: virological log reduction | No statistically significant reduction of viral load or fever | [33] |
| Lovastatin | Cholesterol synthesis inhibitor thought to limit membrane mobilization required for viral RNA replication complex assembly | OUCRU | Placebo vs lovastatin (80 mg once daily for 5 d) | Age, >18 y; trial size, 300 (149 placebo recipients, 151 lovastatin recipients) | Clinical: safety and tolerability | Not powered to address efficacy; no evidence of beneficial effect on any clinical manifestations or DENV viremia | [34] |
Abbreviations: AUC, area under the curve; DENV, dengue virus; ER, endoplasmic reticulum; HCV, hepatitis C virus; NUS, National University of Singapore; OUCRU, Oxford University Clinical Research Unit in Vietnam; SGH Singapore General Hospital.
Two other trials (involving ivermectin and ketotifen) are currently recruiting in Thailand and Singapore, respectively (clinical trials identifiers NCT02045069 and NCT026773840, respectively). Interestingly, the preliminary findings from the phase 2 ivermectin study suggests a reduction in serum NS1 levels and body temperature with high-dose ivermectin, despite no detectable difference in viremia levels (as measured by real-time quantitative polymerase chain reaction [qPCR]) [35].
Although all of the clinical trials thus far have failed to meet their primary efficacy end points, they have provided unique insights into dengue viremia and NS1 antigenemia. This new information is useful for clarifying efficacy end points for future trials.
Lessons Learned From Using Fever and Viremia as a Primary End Points in Clinical Trials
The rationale of using fever and viral load reduction in these trials stemmed from earlier observational studies that showed a positive correlation between viremia level and disease severity [36, 37]. These observations, together with the known profile of patients with DENV viremia led to the hypothesis that early treatment within 48–72 hours of fever onset with an effective anti-DENV drug could potentially lower the viral load and reduce dengue severity. In reality, however, this approach poses several challenges and limitations in field sites. Patient reporting of fever duration can be highly unreliable in dating the onset of illness. As with management of most acute febrile illnesses, individuals with dengue fever often take a wait-and-see approach with home rest and self-medication, deferring seeing a physician until later stages of illness. In most instances, the stage of peak viremia level would have passed by the time they present to the clinics or get enrolled into a clinical trial. By comparison, the first studies in the clinical development of oseltamivir as an anti-influenza drug started with human challenge trials, where the onset of infection could be clearly defined [38].
DENV detection and quantification using real-time qPCR has become the method of choice in the past 20 years. This method measures RNAemia, rather than quantifies infectious viruses. RNA copy number can exceed infectious viral titers by 2–5 logs. However, direct measurement of infectious viruses is technically difficult because some clinical isolates grow poorly in cell cultures. Moreover, not all unpassaged DENVs form consistent plaques, and hence estimating the number of infectious viral particles in clinical serum samples by using a plaque assay is inherently inaccurate. The most sensitive biological assay available for measuring unpassaged infectious DENV is the mosquito inoculation technique, but the technique is hard to master and requires an insectary, which is not available in most diagnostic virology laboratories [39].
Besides difficulty in the timing of patient enrollment into trials and limitations in viremia measurements, there is also a wide variation in the rate of viral clearance, which is influenced by factors such as DENV serotype and primary versus secondary infection. These factors thus collectively contribute to the large standard deviation often observed in viremia measurements stratified by day from fever onset. Statistical considerations for sample size must thus take into account this expected variability in viremia levels.
DENV NS1 antigen detection is often used to diagnose dengue in patients early, for enrollment into clinical trials [33, 34], and it may have a role in dengue pathogenesis [40, 41]. Its usefulness as a reliable therapeutic efficacy end point through time-to-clearance monitoring, however, is uncertain. A major problem is that the level of NS1 and the duration in which this antigen can be detected in serum differ significantly between DENV serotypes, as well as primary and secondary dengue cases [42]. Nevertheless the recent surge in structural and mechanistic studies of NS1 suggests that more-quantitative NS1 tests whose findings may correlate with disease status, perhaps by using a second host dependent biomarker, may provide reliable end points for application of a therapeutic intervention [40, 41, 43, 44].
Utility of Animal Models for Dengue Drug Efficacy Study
No animal model exists that is capable of approximating human disease [45, 46]. Among the many small-animal models developed, the AG129 mouse, which is deficient in types I and II interferon receptors, has been the most widely used for pathogenesis and immunity studies. It is also the most widely used model to evaluate dengue vaccine and antivirals [47, 48]. The 2 most recent clinical trials of celgosivir and lovastatin were extensively evaluated using this model. Although both compounds showed reduction in viremia levels and increased survival rates in treated mice [49–51], neither compound met efficacy end point in clinical trials. A contributory factor to this disparity between laboratory animal and clinical outcome could be due to the time of dosing. Typically, drug dosing in animals begins soon after viremia onset, whereas in patients with dengue, viremia is mostly in the declining phase by the time they are enrolled into any trial. Dosing regimens in animal studies should thus only be initiated at or after the point of peak viremia level. Consequently, the use of a nonlethal viremia AG129 model could be more useful to inform appropriate dosing for human trials [52].
Nonhuman primates are natural hosts to DENV, with the capability to develop viremia, but they do not manifest the disease and its complications. Although several newer nonhuman primate models have been developed that can capture different aspects of dengue manifestations, their utility is limited by scarce laboratory expertise and cost [22].
For the reasons highlighted above, there is a case for a DENV human infection model that mimics some aspects of natural infection to be developed. Besides being cost saving in the long run, the DENV human infection model has the potential to change the way early phase therapeutic drug trials are conducted and evaluated by allowing for controlled timing of infection and treatment. It can also provide valuable opportunities for optimal pharmacokinetic studies [53]. This work is currently being performed at the State University of New York Upstate Medical University (Syracuse) and John Hopkins University (Baltimore) [54, 55].
Future of Monoclonal Antibodies as Therapeutics Against Dengue
Major advances in our understanding of the structure the DENV virion have been made in the fields of X-ray crystallography and cryo–electron microscopy in the last decade [56–60]. Studies of human monoclonal antibodies isolated from convalescent patients with dengue have led to a greater understanding of the epitopes that need to be targeted for effective virus neutralization. Both serotype-specific and cross-reactive neutralizing monoclonal antibodies are being explored for therapeutic application. The most advanced candidate, Ab513, developed by Visterra (Cambridge, Massachusetts), was engineered to bind domain III of the E protein of all 4 DENV serotypes. This antibody has been shown to bind and neutralize multiple genotypes within each of the 4 serotypes. This antibody also appears to neutralize DENV in target cells that express Fc gamma receptor, such as monocytes, and demonstrates in vivo efficacy despite the presence of cross-reactive antibodies that would otherwise enhance infection [61, 62]. This antibody is poised to enter clinical trials by early 2017 [10, 11, 63].
While Ab513 targets a linear epitope, more-recent discoveries of potent broadly neutralizing antibodies against the quaternary E protein dimer epitope (EDE) by other groups could also have huge therapeutic potential. These antibodies bind across E proteins and act by inhibiting the conformational changes that occur during viral fusion with endosomal membranes. Structural information derived from such studies also has important implications in the future design of new therapeutics and next-generation dengue vaccine development. [64, 65] Management of severe acute viral infections occasionally involved the use of pooled human serum immunoglobulins [66, 67]. The use of intravenous immunoglobulins has, however, not been carefully explored for the treatment of severe dengue, given its antiinflammatory properties. However, the risk of antibody-dependent enhancement could pose some concerns on the use of pooled polyclonal preparation as it may contain subneutralizing levels of antibodies and, paradoxically, enhance infection instead [68–70].
SUMMARY/CONCLUSION
Dengue is the most important epidemic infectious diseases caused by flaviviruses this century, causing immense public health problems with significant morbidity and mortality, particularly in resource-poor countries [71].
A vaccine that is not completely protective and vector-control measures that lack sustainable outcomes even in a highly organized/urbanized area such as Singapore demands that approaches such as antiviral discovery and development remain in the forefront of research. Although no antiviral agent has yet been found to be effective against acute dengue in proof-of-concept trials, the therapeutic development pipeline still contains several compounds and biologics that would soon be evaluated clinically. While significant challenges still exist in the dengue research community in bringing a dengue compound through the entire development process, we are optimistic that there is enough momentum and concerted effort currently in academia, industry, and governmental and charitable organizations to advance and facilitate therapeutic development. The costs and benefits of developing an antiviral drug that can coexist with vaccines are not known at this stage. However, the recurrence of yellow fever outbreaks despite the availability of safe vaccine [72–74] should serve as a reminder and a motivation to capitalize on current momentum in antiviral development against DENV and related flaviviruses, such as Zika virus [75]. Targets such as the DENV protease and polymerase are being captured in the act of carrying out their essential enzymatic activities, and these can contribute enormously to the development of designer compounds that could be potent inhibitors. The goal of finding a cure for dengue in the next decade is highly feasible, judging from the success of potent directly acting antivirals against the Flaviviridae family member hepatitis C virus.
Notes
Acknowledgments. We thank Dr Dahai Luo from the Lee Kong Chian School of Medicine, Nanyang Technological University, for kindly providing the portrait of structures in Figure 2; the Ministry of Health in Singapore (National Medical Research Council [NMRC]), Duke–National University of Singapore Medical School, and SingHealth, for generously supporting dengue drug development; and the NMRC (grant CTGCoD-1001 to J. G. H. L.), for support of ongoing dengue translational studies.
Potential conflicts of interests. E. E. O. was a member of the scientific advisory board on dengue vaccine for Sanofi Pasteur during 2014–2015. S. G. V. holds a consultancy role with BRIM Biotechnology and received research grants from the NMRC. J. G. H. L. had a prior consultancy role with Visterra, Chugai Pharmaceutical, and Janssen Infectious Diseases–Diagnostics and received research grants from the NMRC. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Bhatt S, Gething PW, Brady OJ et al. . The global distribution and burden of dengue. Nature 2013; 496:504–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vannice KS, Durbin A, Hombach J. Status of vaccine research and development of vaccines for dengue. Vaccine 2016; 34:2934–8. [DOI] [PubMed] [Google Scholar]
- 3. Hadinegoro SR, Arredondo-Garcia JL, Capeding MR et al. . Efficacy and long-Term safety of a dengue vaccine in regions of endemic disease. N Engl J Med 2015; 373:1195–206. [DOI] [PubMed] [Google Scholar]
- 4. Villar L, Dayan GH, Arredondo-Garcia JL et al. . Efficacy of a tetravalent dengue vaccine in children in Latin America. N Engl J Med 2015; 372:113–23. [DOI] [PubMed] [Google Scholar]
- 5. Halstead SB, Russell PK. Protective and immunological behavior of chimeric yellow fever dengue vaccine. Vaccine 2016; 34:1643–7. [DOI] [PubMed] [Google Scholar]
- 6. World Health Organization. Global strategy for dengue prevention and control, 2012–2020. Geneva, Switzerland: WHO Press, 2012. [Google Scholar]
- 7. Lescar J, Luo D, Xu T et al. . Towards the design of antiviral inhibitors against flaviviruses: the case for the multifunctional NS3 protein from Dengue virus as a target. Antiviral Res 2008; 80:94–101. [DOI] [PubMed] [Google Scholar]
- 8. Chan YK, Gack MU. Viral evasion of intracellular DNA and RNA sensing. Nat Rev Microbiol 2016; 14:360–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang QY, Patel SJ, Vangrevelinghe E et al. . A small-molecule dengue virus entry inhibitor. Antimicrob Agents Chemother 2009; 53:1823–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Robinson LN, Tharakaraman K, Rowley KJ et al. . Structure-guided design of an anti-dengue antibody directed to a non-immunodominant epitope. Cell 2015; 162:493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Teoh EP, Kukkaro P, Teo EW et al. . The structural basis for serotype-specific neutralization of dengue virus by a human antibody. Sci Transl Med 2012; 4:139ra83. [DOI] [PubMed] [Google Scholar]
- 12. Luo D, Vasudevan SG, Lescar J. The flavivirus NS2B-NS3 protease-helicase as a target for antiviral drug development. Antiviral Res 2015; 118:148–58. [DOI] [PubMed] [Google Scholar]
- 13. Sung C, Kumar GS, Vasudevan SG. Dengue drug development. dengue and dengue hemorrhagic fever. 2nd ed UK: CABI, 2014. [Google Scholar]
- 14. Byrd CM, Dai D, Grosenbach DW et al. . A novel inhibitor of dengue virus replication that targets the capsid protein. Antimicrob Agents Chemother 2013; 57:15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Becker GL, Lu Y, Hardes K et al. . Highly potent inhibitors of proprotein convertase furin as potential drugs for treatment of infectious diseases. J Biol Chem 2012; 287:21992–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scaturro P, Trist IM, Paul D et al. . Characterization of the mode of action of a potent dengue virus capsid inhibitor. J Virol 2014; 88:11540–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van Cleef KW, Overheul GJ, Thomassen MC et al. . Identification of a new dengue virus inhibitor that targets the viral NS4B protein and restricts genomic RNA replication. Antiviral Res 2013; 99:165–71. [DOI] [PubMed] [Google Scholar]
- 18. Nguyen NM, Tran CN, Phung LK et al. . A randomized, double-blind placebo controlled trial of balapiravir, a polymerase inhibitor, in adult dengue patients. J Infect Dis 2013; 207:1442–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen YL, Abdul Ghafar N, Karuna R et al. . Activation of peripheral blood mononuclear cells by dengue virus infection depotentiates balapiravir. J Virol 2014; 88:1740–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Noble CG, Shi PY. Structural biology of dengue virus enzymes: towards rational design of therapeutics. Antiviral Res 2012; 96:115–26. [DOI] [PubMed] [Google Scholar]
- 21. Martinez MA, ed. RNA interference and viruses: current innovations and future trends. UK: Caister Academic Press, 2010. [Google Scholar]
- 22. Whitehorn J, Yacoub S, Anders KL et al. . Dengue therapeutics, chemoprophylaxis, and allied tools: state of the art and future directions. PLoS Negl Trop Dis 2014; 8:e3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gubler DJ, Vasudevan S, Farrar J. Dengue and dengue hemorrhagic fever. 2nd ed UK: CABI, 2014. [Google Scholar]
- 24. Dimaano EM, Saito M, Honda S et al. . Lack of efficacy of high-dose intravenous immunoglobulin treatment of severe thrombocytopenia in patients with secondary dengue virus infection. Am J Trop Med Hyg 2007; 77:1135–8. [PubMed] [Google Scholar]
- 25. Jacobs J, Fernandez EA, Merizalde B, Avila-Montes GA, Crothers D. The use of homeopathic combination remedy for dengue fever symptoms: a pilot RCT in Honduras. Homeopathy 2007; 96:22–6. [DOI] [PubMed] [Google Scholar]
- 26. Kularatne SA, Walathara C, Mahindawansa SI et al. . Efficacy of low dose dexamethasone in severe thrombocytopenia caused by dengue fever: a placebo controlled study. Postgrad Med J 2009; 85:525–9. [DOI] [PubMed] [Google Scholar]
- 27. de Castro RA, de Castro JA, Barez MY, Frias MV, Dixit J, Genereux M. Thrombocytopenia associated with dengue hemorrhagic fever responds to intravenous administration of anti-D (Rh(0)-D) immune globulin. Am J Trop Med Hyg 2007; 76:737–42. [PubMed] [Google Scholar]
- 28. Castro JE, Vado-Solis I, Perez-Osorio C, Fredeking TM. Modulation of cytokine and cytokine receptor/antagonist by treatment with doxycycline and tetracycline in patients with dengue fever. Clin Dev Immunol 2011; 2011:370872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Salgado D, Zabaleta TE, Hatch S, Vega MR, Rodriguez J. Use of pentoxifylline in treatment of children with dengue hemorrhagic fever. Pediatr Infect Dis J 2012; 31:771–3. [DOI] [PubMed] [Google Scholar]
- 30. Cabrera-Cortina JI, Sanchez-Valdez E, Cedas-DeLezama D, Ramirez-Gonzalez MD. Oral calcium administration attenuates thrombocytopenia in patients with dengue fever. Report of a pilot study. Proc West Pharmacol Soc 2008; 51:38–41. [PubMed] [Google Scholar]
- 31. Tricou V, Minh NN, Van TP et al. . A randomized controlled trial of chloroquine for the treatment of dengue in Vietnamese adults. PLoS Negl Trop Dis 2010; 4:e785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tam DT, Ngoc TV, Tien NT et al. . Effects of short-course oral corticosteroid therapy in early dengue infection in Vietnamese patients: a randomized, placebo-controlled trial. Clin Infect Dis 2012; 55:1216–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Low JG, Sung C, Wijaya L et al. . Efficacy and safety of celgosivir in patients with dengue fever (CELADEN): a phase 1b, randomised, double-blind, placebo-controlled, proof-of-concept trial. Lancet Infect Dis 2014; 14:706–15. [DOI] [PubMed] [Google Scholar]
- 34. Whitehorn J, Nguyen CV, Khanh LP et al. . Lovastatin for the treatment of adult patients with dengue: a randomized, double-blind, placebo-controlled trial. Clin Infect Dis 2016; 62:468–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Avirutnan P. Ivermectin: a promising anti-dengue replication treatment [abstract S634] Presented at: 26th European Congress of Clinical Microbiology and Infectious Diseases, Amsterdam, Netherlands,9–12 April 2016. [Google Scholar]
- 36. Gubler DJ, Suharyono W, Tan R, Abidin M, Sie A. Viraemia in patients with naturally acquired dengue infection. Bull World Health Organ 1981; 59:623–30. [PMC free article] [PubMed] [Google Scholar]
- 37. Libraty DH, Young PR, Pickering D et al. . High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J Infect Dis 2002; 186:1165–8. [DOI] [PubMed] [Google Scholar]
- 38. Hayden FG, Osterhaus AD, Treanor JJ et al. . Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenzavirus infections. GG167 Influenza Study Group. N Engl J Med 1997; 337:874–80. [DOI] [PubMed] [Google Scholar]
- 39. Choy MM, Ellis BR, Ellis EM, Gubler DJ. Comparison of the mosquito inoculation technique and quantitative real time polymerase chain reaction to measure dengue virus concentration. Am J Trop Med Hyg 2013; 89:1001–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Modhiran N, Watterson D, Muller DA et al. . Dengue virus NS1 protein activates cells via Toll-like receptor 4 and disrupts endothelial cell monolayer integrity. Sci Transl Med 2015; 7:304ra142. [DOI] [PubMed] [Google Scholar]
- 41. Beatty PR, Puerta-Guardo H, Killingbeck SS, Glasner DR, Hopkins K, Harris E. Dengue virus NS1 triggers endothelial permeability and vascular leak that is prevented by NS1 vaccination. Sci Transl Med 2015; 7:304ra141. [DOI] [PubMed] [Google Scholar]
- 42. Duyen HT, Ngoc TV, Ha do T et al. . Kinetics of plasma viremia and soluble nonstructural protein 1 concentrations in dengue: differential effects according to serotype and immune status. J Infect Dis 2011; 203:1292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Watanabe S, Tan KH, Rathore AP et al. . The magnitude of dengue virus NS1 protein secretion is strain dependent and does not correlate with severe pathologies in the mouse infection model. J Virol 2012; 86:5508–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Muller DA, Young PR. The flavivirus NS1 protein: molecular and structural biology, immunology, role in pathogenesis and application as a diagnostic biomarker. Antiviral Res 2013; 98:192–208. [DOI] [PubMed] [Google Scholar]
- 45. Zompi S, Harris E. Animal models of dengue virus infection. Viruses 2012; 4:62–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cassetti MC, Durbin A, Harris E et al. . Report of an NIAID workshop on dengue animal models. Vaccine 2010; 28:4229–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schul W, Liu W, Xu HY, Flamand M, Vasudevan SG. A dengue fever viremia model in mice shows reduction in viral replication and suppression of the inflammatory response after treatment with antiviral drugs. J Infect Dis 2007; 195:665–74. [DOI] [PubMed] [Google Scholar]
- 48. Chang J, Schul W, Butters TD et al. . Combination of alpha-glucosidase inhibitor and ribavirin for the treatment of dengue virus infection in vitro and in vivo. Antiviral Res 2011; 89:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Martinez-Gutierrez M, Correa-Londono LA, Castellanos JE, Gallego-Gomez JC, Osorio JE. Lovastatin delays infection and increases survival rates in AG129 mice infected with dengue virus serotype 2. PLoS One 2014; 9:e87412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rathore AP, Paradkar PN, Watanabe S et al. . Celgosivir treatment misfolds dengue virus NS1 protein, induces cellular pro-survival genes and protects against lethal challenge mouse model. Antiviral Res 2011; 92:453–60. [DOI] [PubMed] [Google Scholar]
- 51. Watanabe S, Rathore AP, Sung C et al. . Dose- and schedule-dependent protective efficacy of celgosivir in a lethal mouse model for dengue virus infection informs dosing regimen for a proof of concept clinical trial. Antiviral Res 2012; 96:32–5. [DOI] [PubMed] [Google Scholar]
- 52. Watanabe S, Chan KW, Dow G, Ooi EE, Low JG, Vasudevan SG. Optimizing celgosivir therapy in mouse models of dengue virus infection of serotypes 1 and 2: The search for a window for potential therapeutic efficacy. Antiviral Res 2016; 127:10–9. [DOI] [PubMed] [Google Scholar]
- 53. Whitehorn J, Van VC, Simmons CP. Dengue human infection models supporting drug development. J Infect Dis 2014; 209(suppl 2):S66–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Endy TP. Dengue human infection model performance parameters. J Infect Dis 2014; 209(suppl 2):S56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kirkpatrick BD, Whitehead SS, Pierce KK et al. . The live attenuated dengue vaccine TV003 elicits complete protection against dengue in a human challenge model. Sci Transl Med 2016; 8:330ra36. [DOI] [PubMed] [Google Scholar]
- 56. Kuhn RJ, Zhang W, Rossmann MG et al. . Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell 2002; 108:717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Modis Y, Ogata S, Clements D, Harrison SC. Structure of the dengue virus envelope protein after membrane fusion. Nature 2004; 427:313–9. [DOI] [PubMed] [Google Scholar]
- 58. Yu IM, Zhang W, Holdaway HA et al. . Structure of the immature dengue virus at low pH primes proteolytic maturation. Science 2008; 319:1834–7. [DOI] [PubMed] [Google Scholar]
- 59. Li L, Lok SM, Yu IM et al. . The flavivirus precursor membrane-envelope protein complex: structure and maturation. Science 2008; 319:1830–4. [DOI] [PubMed] [Google Scholar]
- 60. Kostyuchenko VA, Zhang Q, Tan JL, Ng TS, Lok SM. Immature and mature dengue serotype 1 virus structures provide insight into the maturation process. J Virol 2013; 87:7700–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sridharan A, Chen Q, Tang KF, Ooi EE, Hibberd ML, Chen J. Inhibition of megakaryocyte development in the bone marrow underlies dengue virus-induced thrombocytopenia in humanized mice. J Virol 2013; 87:11648–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ng JK, Zhang SL, Tan HC et al. . First experimental in vivo model of enhanced dengue disease severity through maternally acquired heterotypic dengue antibodies. PLoS Pathog 2014; 10:e1004031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Beltramello M, Williams KL, Simmons CP et al. . The human immune response to Dengue virus is dominated by highly cross-reactive antibodies endowed with neutralizing and enhancing activity. Cell Host Microbe 2010; 8:271–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dejnirattisai W, Wongwiwat W, Supasa S et al. . A new class of highly potent, broadly neutralizing antibodies isolated from viremic patients infected with dengue virus. Nat Immunol 2015; 16:170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Screaton G, Mongkolsapaya J, Yacoub S, Roberts C. New insights into the immunopathology and control of dengue virus infection. Nat Rev Immunol 2015; 15:745–59. [DOI] [PubMed] [Google Scholar]
- 66. Keller MA, Stiehm ER. Passive immunity in prevention and treatment of infectious diseases. Clin Microbiol Rev 2000; 13:602–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Alexander BT, Hladnik LM, Augustin KM et al. . Use of cytomegalovirus intravenous immune globulin for the adjunctive treatment of cytomegalovirus in hematopoietic stem cell transplant recipients. Pharmacotherapy 2010; 30:554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chan KR, Ong EZ, Ooi EE. Therapeutic antibodies as a treatment option for dengue fever. Expert Rev Anti Infect Ther 2013; 11:1147–57. [DOI] [PubMed] [Google Scholar]
- 69. Halstead SB, O'Rourke EJ. Dengue viruses and mononuclear phagocytes. I.: Infection enhancement by non-neutralizing antibody. J Exp Med 1977; 146:201–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chan KR, Wang X, Saron WAA et al. . Cross-reactive antibodies enhance live attenuated virus infection for increased immunogenicity. Nature Microbiology 2016; 1:16164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gubler DJ. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol 2002; 10:100–3. [DOI] [PubMed] [Google Scholar]
- 72. Julander JG. Experimental therapies for yellow fever. Antiviral Res 2013; 97:169–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ling Y, Chen J, Huang Q et al. . Yellow fever in a worker returning to China from Angola, March 2016. Emerg Infect Dis 2016; 22:1317–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Monath TP. Treatment of yellow fever. Antiviral Res 2008; 78:116–24. [DOI] [PubMed] [Google Scholar]
- 75. Barrows NJ, Campos RK, Powell ST et al. . A screen of FDA-Approved drugs for inhibitors of zika virus infection. Cell Host Microbe 2016; 20:259–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Guy B, Briand O, Lang J, Saville M, Jackson N. Development of the Sanofi Pasteur tetravalent dengue vaccine: One more step forward. Vaccine 2015; 33:7100–11. [DOI] [PubMed] [Google Scholar]
- 77. Ferguson NM, Kien DT, Clapham H et al. . Modeling the impact on virus transmission of Wolbachia-mediated blocking of dengue virus infection of Aedes aegypti. Sci Transl Med 2015; 7:279ra37. [DOI] [PMC free article] [PubMed] [Google Scholar]