Abstract
Background
Several studies reported that cavotricuspid isthmus-dependent atrial flutter (typical AFL) frequently coexists with atrial fibrillation (AF); however, the underlying mechanisms have not been fully investigated. This study aimed to reveal the mechanisms of the initiation of typical AFL and the association between typical AFL and AF.
Methods
Among 154 consecutive patients undergoing a first catheter ablation of AF, we investigated the appearance and mechanism of spontaneous initiation of typical AFL during catheter ablation. Then, we retrospectively investigated 67 consecutive patients without a previous AF episode who underwent typical AFL ablation. The occurrence and predictors of AF after catheter ablation were evaluated.
Results
During AF ablation, spontaneous initiation of typical AFL occurred during sinus rhythm in eight (5.2%) patients. The initiations of typical AFL were pulmonary vein (PV) firings except in one patient, in whom paroxysmal AF following superior vena cava firing initiated reverse typical AFL after PV isolation. After typical AFL ablation, AF occurred in 23 (34.3%) patients (mean follow up, 28.2±20.3 months). Kaplan-Meier analysis showed the occurrence of AF after typical AFL ablation to be significantly higher in the patients with a larger left atrial diameter over 40 mm (log-rank test, P=0.046).
Conclusions
PV firing through AF played an important role in initiating typical AFL. The occurrence of AF after typical AFL ablation was high, and a dilated left atrium was associated with increased occurrence of AF. These findings disclosed the close relationship between typical AFL and AF, especially PV firing.
Keywords: Atrial fibrillation, Catheter ablation, Pulmonary vein firing, Typical atrial flutter
1. Introduction
Catheter ablation of cavotricuspid isthmus (CTI)-dependent atrial flutter (typical AFL) is a therapy with a high success rate [1], [2], [3], [4]. However, typical AFL often coexists with atrial fibrillation (AF), and AF frequently appears after catheter ablation of typical AFL. Several studies have reported the prevalence of AF after catheter ablation of typical AFL to range from 21% to 43% [1], [2], [3], [4], [5]. Some reports have described an association between AF and typical AFL; however, previous reports showed that AF was an initiator of typical AFL only in animal models and in postoperative open-heart patients with epicardial electrograms recorded by a wire electrode placed temporarily [6], [7]. In contrast, one report showed that pulmonary vein (PV) firing plays a role in the transition of typical AFL to AF [8]. However, no reports have revealed the contribution of PV firing to the initiation of typical AFL in the clinical setting. This study aimed to reveal the mechanisms of the initiation of typical AFL and the association between typical AFL and AF, and especially PV firing.
2. Materials and methods
2.1. Study population and design
We identified 228 consecutive patients who underwent catheter ablation of AF in our institutions in 2011. Among them, 164 patients who had received a first catheter ablation of AF were investigated because of careful observation of PV firings and typical AFL initiations. We evaluated the association between the initiation of typical AFL and various electrophysiological findings, especially those of AF and PV firings.
Second, we retrospectively investigated 71 consecutive patients who underwent catheter ablation of typical AFL in our institutions from 2000 to 2010 to determine the prevalence of AF after catheter ablation of typical AFL. Patients with a prior episode of AF and AF that converted to typical AFL with class IC agents were excluded. The subjects were divided into two groups according to the occurrence or non-occurrence of AF after the catheter ablation of typical AFL. The clinical parameters were also compared between the two groups to evaluate the predictors of an AF occurrence after catheter ablation.
2.2. First catheter ablation of AF
In the first group of patients, catheter ablation was performed for AF following the cessation of all antiarrhythmic drugs for over five half-lives before the procedure, except for amiodarone. Extensive encircling PV isolation (EEPVI) was performed with a double-lasso technique with the patient sedated with dexmedetomidine hydrochloride. Two 7-Fr decapolar ring catheters (Lasso, Biosense Webster, Inc., Diamond Bar, CA, USA) and a 7.5-Fr irrigation catheter with 3.5-mm distal electrode (ThermoCool, Biosense Webster) were inserted into the left atrium (LA) via a transseptal approach. After selective PV angiography, the two ring catheters were positioned in the ostium of each upper and lower PV. Radiofrequency energy applications were delivered approximately 0.5–1.0 cm away from the PV ostia. The endpoint of the EEPVI was the creation of a bidirectional conduction block between the LA and PVs. After the EEPVI, if AF persisted or was induced with coronary sinus burst pacing at a cycle length down to 180 ms during continuous intravenous administration of isoproterenol (1.0–3.0 μg/min), additional ablation, including an LA roof linear ablation and/or superior vena cava (SVC) isolation, was performed. If AF persisted even with these procedures, ablation of continuous fractionated atrial electrograms was performed. If AF was not terminated after these procedures, sinus rhythm was restored by transthoracic cardioversion.
2.3. Catheter ablation of typical AFL
In the second group of patients, catheter ablation of typical AFL was performed under fluoroscopic and electrophysiological guidance after written informed consent was obtained from each patient. A 5-Fr decapolar catheter with 4-mm interelectrode spacing (Irvine Biomedical Inc., Irvine, CA, USA) was inserted to the proximal coronary sinus from the right subclavian vein, and 5-Fr decapolar catheters with 2-mm interelectrode spacing (Irvine Biomedical Inc.) were positioned in the His bundle region and lateral right atrium along the tricuspid annulus from the right femoral vein. A 7-Fr quadripolar ablation catheter with an 8-mm distal tip electrode and deflectable tip (Ablaze; Japan Lifeline Co., Ltd., Tokyo, Japan) was inserted from the right femoral vein and used for mapping and delivery of radiofrequency energy applications. After the catheter ablation, the creation of a bidirectional block line across the CTI was confirmed by a differential pacing technique [9].
2.4. Follow up
After catheter ablation in the patients with typical AFL, all antiarrhythmic drugs were discontinued. As suggested, anticoagulative therapy was continued for at least 3 months after the catheter ablation of typical AFL. Continuous electrocardiogram (ECG) monitoring was performed until hospital discharge, and periodic monitoring of 12-lead ECGs and Holter ECGs were performed in the hospital outpatient clinic at 3, 6, and 12 months after the catheter ablation of typical AFL. After that, we performed periodic monitoring of Holter and event ECGs to detect the presence of AF according to the patient׳s symptoms. In patients with implantable devices, we investigated the presence of AF by interrogation of the device.
2.5. Definitions
In the analysis of the electrophysiological findings during the catheter ablation of AF, the spontaneous appearance of typical AFL was diagnosed with the same criteria as mentioned above. PV firing was defined as a rapid, spiky potential recorded within the PV preceding the LA potentials. If AF or PV firing preceded a typical AFL, that firing was defined as an initiator of typical AFL.
In patients with persistent AFL, the diagnosis of typical AFL was made when a counterclockwise activation sequence along the tricuspid annulus was present, and the dependency of a tachycardia on the CTI was proven with entrainment mapping. In the patients without persistent AFL, a diagnosis of typical AFL was made based on the characteristics of the AFL documented by the 12-lead ECG. On ECGs, serrated negative flutter waves in leads II, III, aVF, and V6 and positive flutter waves in lead V1 with a regular atrial cycle length were diagnosed as typical AFL. The appearance of AF after the catheter ablation of typical AFL was diagnosed based on periodic 12-lead ECG and Holter ECG monitoring performed during the outpatient follow-up period.
2.6. Statistical analysis
All data are shown as means±standard deviations or numbers and percentages. Differences between the two groups were investigated by an unpaired t-test or a Mann-Whitney U test for continuous data with or without a normal distribution. All categorical data were compared using Fisher׳s exact probability test. A Kaplan-Meier analysis with a log-rank test was performed to determine the freedom from AF occurrence after the catheter ablation of typical AFL. A two-sided P value of <0.05 was considered statistically significant.
3. Results
3.1. Study population undergoing first catheter ablation of AF
The baseline characteristics are summarized in Table 1. Ten of 164 patients were excluded from the study subjects because of a prior history of CTI linear ablation, so 154 subjects were analyzed. There were 58 (38%) patients with persistent AF and 96 (62%) with paroxysmal AF, depending on whether the duration of each AF episode was >7 days. Structural heart disease was present in 13 (8.4%) patients. Complicating diseases included dilated cardiomyopathy (n=2), hypertrophic cardiomyopathy (n=5), ischemic heart disease (n=3), and aortic regurgitation (n=4), with one patient complicated by both hypertrophic cardiomyopathy and aortic regurgitation.
Table 1.
Baseline characteristics of the study population undergoing first catheter ablation of atrial fibrillation.
| Total | AFL (+) | AFL (−) | |
|---|---|---|---|
| (n=154) | (n=8) | (n=146) | |
| Age (y) | 60.4±11.8 | 57.4±13.9 | 60.5±11.7 |
| Male sex | 129 (84%) | 8 (100%) | 121 (83%) |
| Type of AF: persistent | 58 (38%) | 3 (38%) | 55 (38%) |
| History of AFL | 11 (7%) | 0 (0%) | 11 (8%) |
| LVEF (%) | 65.7±0.1 | 63.9±7.7 | 65.8±9.1 |
| LVDd (mm) | 48.4±5.3 | 50.3±3.7 | 48.3±5.4 |
| LAD (mm) | 38.1±6.9 | 38.1±8.1 | 38.1±6.9 |
| Hypertension | 71 (46%) | 4 (50%) | 67 (46%) |
| Dyslipidemia | 51 (33%) | 3 (38%) | 48 (33%) |
| Diabetes | 26 (17%) | 3 (38%) | 23 (16%) |
| Structural heart disease | 13 (8%) | 0 (0%) | 13 (9%) |
| ABL procedure for AF | |||
| EEPVI | 77 (50%) | 3 (38%) | 74 (51%) |
| EEPVI+roof line±SVC-I | 39 (25%) | 2 (25%) | 37 (25%) |
| EEPVI+roof line±SVC-I+CFAE | 38 (25%) | 3 (38%) | 35 (24%) |
| CTI | 131 (85%) | 8 (100%) | 123 (84%) |
Data are shown as the means±standard deviations or number of patients (%).
AAD=anti-arrhythmic drug; ABL=ablation; AF=atrial fibrillation; AFL=atrial flutter; CFAE=continuous fractionated atrial electrogram; CTI=cavotricuspid isthmus; EEPVI=extensive encircling pulmonary vein isolation; LAD=left atrial diameter; LVDd=left ventricular end-diastolic diameter; LVEF=left ventricular ejection fraction; SVC-I=superior vena cava isolation.
3.2. Study population undergoing catheter ablation of typical AFL
Four of 71 (5.6%) patients had recurrences of typical AFL after the catheter ablation with a mean follow-up period of 9.3±1.5 months. Therefore, only 67 subjects with a successful catheter ablation without recurrence of typical AFL were followed up in the outpatient clinic and were included in this study. The baseline characteristics are summarized in Table 2. Structural heart disease was present in eight (11.9%) patients, including ischemic heart disease (n=3), hypertrophic cardiomyopathy (n=2), dilated cardiomyopathy (n=1), and aortic regurgitation (n=2). Seven of 67 patients had implantable devices (five in the AF [+] group and two in the AF [-] group).
Table 2.
Baseline characteristics of the study population undergoing catheter ablation of typical atrial flutter.
| Total (n=67) | AF (+) after AFL ABL (n=23) | AF (−) after AFL ABL (n=44) | Pvalue | |
|---|---|---|---|---|
| Age (y) | 59.6 ± 13.9 | 58.2 ± 13.0 | 60.3 ± 14.4 | 0.456 |
| Male sex | 58 (87%) | 19 (83%) | 39 (89%) | 0.492 |
| Body mass index (kg/m2) | 23.2±4.1 | 23.6±2.4 | 23.0±4.8 | 0.716 |
| LVEF (%) | 58.1±10.7 | 59.7±10.4 | 57.2±10.9 | 0.265 |
| LVDd (mm) | 46.6±6.6 | 46.4±6.4 | 46.7±6.7 | 0.703 |
| LAD (mm) | 38.2±6.6 | 40.6±6.1 | 37.0±6.5 | 0.036 |
| Plasma BNP level (ng/L) | 106.2±184.3 | 125.4±108.0 | 96.3±214.4 | 0.062 |
| Creatinine (µmol/L) | 96.08±122.00 | 81.59±86.16 | 103.70±137.25 | 0.668 |
| Hypertension | 25 (37%) | 9 (39%) | 16 (36%) | 0.824 |
| Dyslipidemia | 21 (31%) | 7 (30%) | 14 (32%) | 0.908 |
| Diabetes | 12 (18%) | 3 (13%) | 9 (20%) | 0.453 |
| Structural heart disease | 8 (12%) | 1 (4%) | 7 (16%) | 0.166 |
| Class I AAD before AFL ABL | 36 (54%) | 13 (57%) | 23 (52%) | 0.741 |
| Class III AAD before AFL ABL | 6 (9%) | 3 (13%) | 3 (7%) | 0.397 |
Data are shown as the means±standard deviation or number of patients.
AAD=anti-arrhythmic drug; ABL=ablation; AF=atrial fibrillation; AFL=atrial flutter; BNP=brain natriuretic peptide; LAD=left atrial diameter; LVDd=left ventricular end-diastolic diameter; LVEF=left ventricular ejection fraction.
3.3. Detailed electrophysiological findings during the first catheter ablation of AF
Of the 154 patients undergoing their first AF ablation, typical AFL appeared during the ablation in eight (5.2%) patients, and it appeared during sinus rhythm in all eight of these patients. The initiation of typical AFL was due to AF, and the triggering AF episodes was initiated by PV firing (four in the left superior PV, two in the right superior PV, and one in the left inferior PV; see the representative case in Fig. 1) except in one patient, in whom reverse typical AFL was initiated following SVC firing after the EEPVI (Fig. 2). A detailed electrophysiological evaluation during the procedure revealed the contribution of AF, especially PV firing, to the initiation of typical AFL (Table 3).
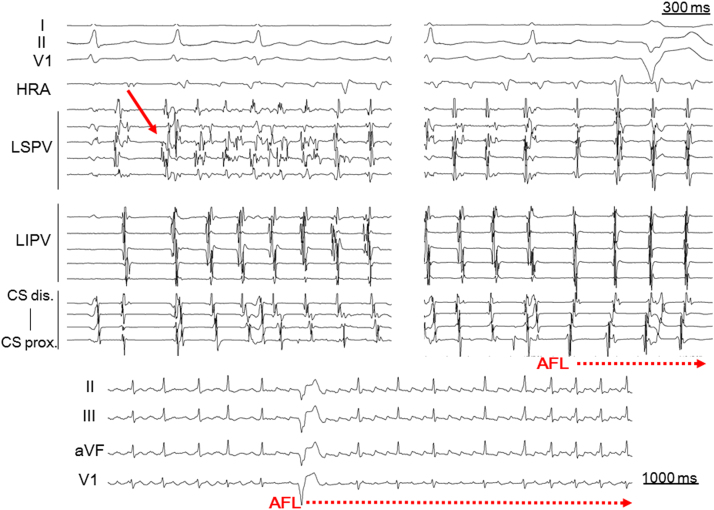
Fig. 1.
Representative case of typical atrial flutter initiated by paroxysmal atrial fibrillation following a pulmonary vein firing. Atrial fibrillation was initiated with a left superior pulmonary vein firing (solid arrow) during baseline sinus rhythm and was subsequently converted to typical atrial flutter. AFL=atrial flutter; CS=coronary sinus; HRA=high right atrium; LIPV=left inferior pulmonary vein; LSPV=left superior pulmonary vein.
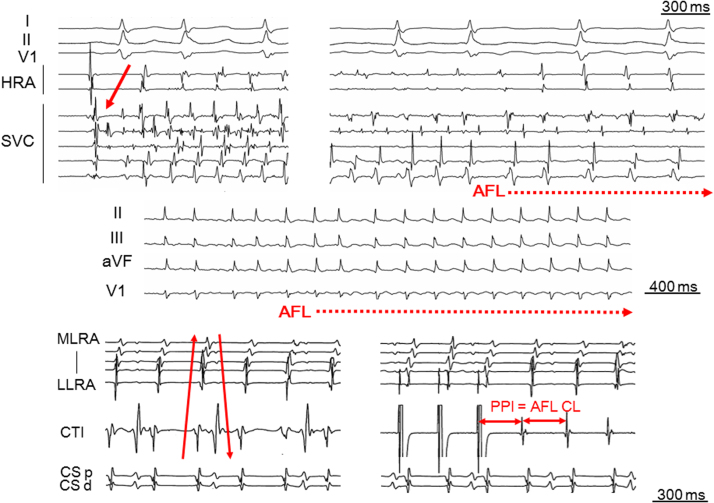
Fig. 2.
Representative case of reverse typical atrial flutter initiated with paroxysmal atrial fibrillation following a superior vena cava firing. The baseline rhythm was sinus rhythm after an extensive encircling pulmonary vein isolation. Atrial fibrillation was initiated with a superior vena cava firing during baseline sinus rhythm and was subsequently converted to reverse typical atrial flutter. The solid arrow in the upper panel shows the superior vena cava firing as the initiator of atrial fibrillation. The solid arrows in the lower panel show the clockwise activation sequence along the tricuspid annulus during reverse typical AFL. AFL=atrial flutter; CL=cycle length; CS d=coronary sinus distal; CS p=coronary sinus proximal; HRA=high right atrium; LIPV=right inferior pulmonary vein; LLRA=low lateral right atrium; MLRA=mid lateral right atrium; PPI=post-pacing interval; SVC=superior vena cava.
Table 3.
Detailed electrophysiological findings of the patients with an appearance of typical AFL during first catheter ablation of atrial fibrillation.
| Case | Age (y)/sex | Type of AF | Baseline rhythm | Trigger of AFL |
|---|---|---|---|---|
| 1 | 65/male | Paroxysmal | SR | AF following RSPV firing |
| 2 | 60/male | Paroxysmal | SR | AF following LIPV firing |
| 3 | 59/male | Persistent | SR | AF following RSPV firing |
| 4 | 68/male | Paroxysmal | SR | AF following SVC firing (after PVI) |
| 5 | 66/male | Persistent | SR | AF following LSPV firing |
| 6 | 25/male | Paroxysmal | SR | AF following LSPV firing |
| 7 | 63/male | Persistent | SR | AF following LSPV firing |
| 8 | 53/male | Paroxysmal | SR | AF following LSPV firing |
AF=atrial fibrillation; AFL=atrial flutter; LIPV=left inferior pulmonary vein; LSPV=left superior pulmonary vein; PVI=pulmonary vein isolation; RSPV=right superior pulmonary vein; SR=sinus rhythm; SVC=superior vena cava.
3.4. Occurrence of AF after catheter ablation of typical AFL
AF occurred in 23 (34%) patients during a mean follow-up period of 28.2±20.3 months after the catheter ablation of typical AFL. The study subjects were divided into two groups according to the presence of an AF occurrence after the catheter ablation of typical AFL. The LA diameter on ultrasound echocardiography was significantly larger (40.6±6.1 mm vs. 37.0±6.5 mm, P=0.036) in the group with an AF occurrence compared to the group without an AF occurrence. There were no significant differences in the biochemical data (Table 2). A Kaplan-Meier analysis showed that the occurrence of AF after the catheter ablation of typical AFL was significantly higher in the patients with a larger LA diameter (>40 mm) than in those with a normal LA diameter (≤40 mm, log-rank test, P=0.046, Fig. 3).
Fig. 3.
Kaplan-Meier analysis of freedom from the occurrence of atrial fibrillation after the ablation of typical atrial flutter between the patients with a left atrial diameter of >40 mm and a normal (≤40 mm) left atrial diameter. ABL=ablation; AF=atrial fibrillation; AFL=atrial flutter; LAD=left atrial diameter.
4. Discussion
4.1. Major findings
The major findings of this study were as follows:
-
1)
Among 154 patients who underwent the first catheter ablation of AF, spontaneous initiation of typical AFL appeared during sinus rhythm in eight (5.2%) patients. 2) PV firing was the initiator of typical AFL in those patients. 3) During the mean follow up of 28 consecutive months after the catheter ablation of typical AFL, ECG-proven AF was observed in over one-third of the patients without previous evidence of AF. 4) A dilated LA was associated with increased occurrence of subsequent AF after the catheter ablation of typical AFL, suggesting the probability that AF might coexist with typical AFL, especially in patients with left atrial enlargement. These findings elucidate the high prevalence of AF in patients with typical AFL and suggest that AF might coexist and trigger AFL even though AF was not detected clinically before the catheter ablation of typical AFL.
4.2. Mechanism of AFL initiation
A functional block between the SVC and inferior vena cava (IVC) is necessary for the development of typical AFL because it acts as a critical lateral boundary that prevents short-circuiting of the AFL reentrant circuit along the tricuspid annulus. AF plays an important role in the formation of such functional block between the SVC and IVC [6], [10], [11]. In the reentrant tachycardia circuit, a portion acting as a “slow conduction zone” must be necessary. The slow conduction zone is located in the low right atrial isthmus during typical AFL, an area surrounded by the IVC, Eustachian valve, coronary sinus ostium, and tricuspid annulus [12]. Moreover, the wavefront of typical AFL travels up the interatrial septum and down the right atrial free wall to the CTI along the tricuspid annulus in a counter-clockwise fashion. Therefore, for the induction of typical AFL with this wavefront direction and a slow conduction zone in the low right atrial isthmus, the triggering rapid rhythm must occur from the left side of the entire atrium, such as the LA including the interatrial septum and coronary sinus. With the high prevalence of AF in the general population and the fact that most initiations of AF originate from the PVs, the triggering rhythm of typical AFL may certainly be AF following PV firing [13]. Indeed, in this study, the initiation of typical AFL was by AF, and all of the AF rhythms were initiated by PV firing.
An exception occurred in one patient in whom reverse typical AFL was initiated by the AF from SVC firing after the EEPVI. It is reported that non-PV ectopy, including SVC firing, can initiate AF [14]. Theoretically, if AF exists, typical AFL could occur. Thus, not only PV firing but also SVC firing can trigger the formation of typical AFL through the initiation of AF. The reason why the initiated AFL in this case was “reverse typical” is unclear. Whether the initiated typical AFL is “typical” or “reverse typical” may depend on the location of triggering SVC firing and the difference of conduction properties of the right atrium after the pulmonary vein isolation (PVI) procedure.
4.3. Coexistence of AF prior to catheter ablation of typical AFL
As suggested in this study, and as Waldo and Feld reported in their experimental study, the existence of AF is a requisite for the initiation of typical AFL [11]. This does not contradict the high rate of AF coexisting with typical AFL. However, not all patients with catheter ablation of typical AFL experienced the occurrence of AF. There are some possibilities to explain this contradiction. First, the periodic ECG and Holter monitoring recordings could not detect the appearance of prior AF if no symptoms were present. Second, a transitional rhythm other than AF might trigger typical AFL. It was reported that a single atrial premature beat could not initiate typical AFL; at least more than several continuous atrial premature beats are necessary to initiate typical AFL [7]. Moreover, atypical AFL has been reported to be able to initiate typical AFL [7]. Atrial tachycardia has been reported to arise from the mitral annulus, PV, coronary sinus ostium, and coronary sinus musculature, all of which are in the LA [15], [16], [17]. Thus, a short run of repetitive atrial premature beats and/or atypical AFL or atrial tachycardia without obvious symptoms might initiate typical AFL without clinical detection of these triggering arrhythmias.
4.4. Clinical implications
After the catheter ablation of typical AFL, the occurrence of AF is high even with no previous episode of AF. If AF appears after the termination of anticoagulative agents, the risk of thromboembolic events becomes high. Therefore, close observation for the appearance of AF after the catheter ablation of typical AFL is needed to prevent unforeseen thromboembolic events. Moreira et al. reported that the incidence of typical AFL was low in the patients with AF who underwent a PVI procedure [18]. Wazni et al. reported that PVI without concomitant CTI linear ablation decreased the occurrence of not only AF but also AFL [19]. Additionally, Mohanty et al. suggested that the concomitant CTI ablation with PVI does not provide any added advantage, and PVI alone may be sufficient for control of both AF and AFL [20]. Schneider et al. also showed that PVI can prevent the recurrence of AFL, even without CTI ablation [21]. These results, combined with the present results, indicate that we should re-evaluate the efficacy of concomitant CTI linear ablation with the PVI procedure in patients with AF without a previous typical AFL episode.
4.5. Study limitations
This is limited by is retrospective nature and the relatively small number of subjects evaluated for the prevalence of AF after the catheter ablation of typical AFL. Despite a large patient population with a detailed electrophysiological analysis during the catheter ablation of AF, the prevalence of typical AFL was quite low (5.2%) in this study. However, it was reported that the prevalence of spontaneous typical AFL during the catheter ablation of AF is less than 2% [22]. Moreover, direct evidence showing that PV firing followed by AF initiates typical AFL in patients without a prior AF episode is needed. However, LA mapping, which requires a transseptal approach, is not routinely attempted during the catheter ablation of typical AFL in the usual clinical setting. Therefore, additional studies of a large number of patients who are scheduled to receive catheter ablation of AF are needed to further reveal and confirm the relationship between typical AFL and AF, and especially that the PVs play a crucial role in these atrial arrhythmias.
5. Conclusions
This study revealed evidence that a dilated LA was associated with increased AF occurrence after the catheter ablation of typical AFL, and AF subsequently following PV firing plays an important role in the initiation of typical AFL. These findings suggested the close relationship of a dilated LA and PV firing with typical AFL and AF. Accordingly, more attention should be paid to concealed AF to avoid thromboembolic events in patients undergoing catheter ablation of typical AFL even without any documentation of an AF episode.
Conflict of interest
None.
Acknowledgments
We thank George B. Powell for providing editorial assistance.
References
- 1.Bertaglia E., Zoppo F., Bonso A. Northeastern Italian Study on Atrial Flutter Ablation Investigators: Long term follow up of radiofrequency catheter ablation of atrial flutter: clinical course and predictors of atrial fibrillation occurrence. Heart. 2004;90:59–63. doi: 10.1136/heart.90.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tai C.T., Chen S.A., Chiang C.E. Long-term outcome of radiofrequency catheter ablation for typical atrial flutter: risk prediction of recurrent arrhythmias. J Cardiovasc Electrophysiol. 1998;9:115–121. doi: 10.1111/j.1540-8167.1998.tb00892.x. [DOI] [PubMed] [Google Scholar]
- 3.Gilligan D.M., Zakaib I.T., Shepard R.K. Long-term outcome of patients after successful radiofrequency ablation for typical atrial flutter. Pacing Clin Electrophysiol. 2003;26:53–58. doi: 10.1046/j.1460-9592.2003.00150.x. [DOI] [PubMed] [Google Scholar]
- 4.Calkins H., Canby R., Weiss R. 100W Atakr II Investigator Group. Results of catheter ablation of typical atrial flutter. Am J Cardiol. 2004;94:437–442. doi: 10.1016/j.amjcard.2004.04.058. [DOI] [PubMed] [Google Scholar]
- 5.Voight J., Akkaya M., Somasundaram P. Risk of new-onset atrial fibrillation and stroke after radiofrequency ablation of isolated, typical atrial flutter. Heart Rhythm. 2014;11:1884–1889. doi: 10.1016/j.hrthm.2014.06.038. [DOI] [PubMed] [Google Scholar]
- 6.Shimizu A., Nozaki A., Rudy Y. Onset of induced atrial flutter in the canine pericarditis model. J Am Coll Cardiol. 1991;17:1223–1234. doi: 10.1016/0735-1097(91)90857-6. [DOI] [PubMed] [Google Scholar]
- 7.Waldo A.L., Cooper T.B. Spontaneous onset of type 1 atrial flutter in patients. J Am Coll Cardiol. 1996;28:707–712. doi: 10.1016/0735-1097(96)00223-9. [DOI] [PubMed] [Google Scholar]
- 8.Hsieh M.H., Tai C.T., Tsai C.F. Mechanism of spontaneous transition from typical atrial flutter to atrial fibrillation: role of ectopic atrial fibrillation foci. Pacing Clin Electrophysiol. 2001;24:46–52. doi: 10.1046/j.1460-9592.2001.00046.x. [DOI] [PubMed] [Google Scholar]
- 9.Shah D., Haïssaguerre M., Takahashi A. Differential pacing for distinguishing block from persistent conduction through an ablation line. Circulation. 2000;102:1517–1522. doi: 10.1161/01.cir.102.13.1517. [DOI] [PubMed] [Google Scholar]
- 10.Da Costa A., Romeyer C., Mourot S. Factors associated with early atrial fibrillation after ablation of common atrial flutter. A single centre prospective study. Eur Heart J. 2002;23:498–506. doi: 10.1053/euhj.2001.2819. [DOI] [PubMed] [Google Scholar]
- 11.Waldo A.L., Feld G.K. Inter-relationships of atrial fibrillation and atrial flutter. J Am Coll Cardiol. 2008;51:779–786. doi: 10.1016/j.jacc.2007.08.066. [DOI] [PubMed] [Google Scholar]
- 12.Tai C.T., Chen S.A., Chiang C.E. Characterization of low right atrial isthmus as the slow conduction zone and pharmacological target in typical atrial flutter. Circulation. 1997;96:2601–2611. doi: 10.1161/01.cir.96.8.2601. [DOI] [PubMed] [Google Scholar]
- 13.Haïssaguerre M., Jaïs P., Shah D.C. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 14.Lin W.S., Tai C.T., Hsieh M.H. Catheter ablation of paroxysmal atrial fibrillation initiated by non-pulmonary vein ectopy. Circulation. 2003;107:3176–3183. doi: 10.1161/01.CIR.0000074206.52056.2D. [DOI] [PubMed] [Google Scholar]
- 15.Kistler P.M., Sanders P., Hussin A. Focal atrial tachycardia arising from the mitral annulus: electrocardiographic and electrophysiologic characterization. J Am Coll Cardiol. 2003;41:2212–2219. doi: 10.1016/s0735-1097(03)00484-4. [DOI] [PubMed] [Google Scholar]
- 16.Kistler P.M., Sanders P., Fynn S.P. Electrophysiological and electrocardiographic characteristics of focal atrial tachycardia originating from the pulmonary veins: acute and long-term outcomes of radiofrequency ablation. Circulation. 2003;108:1968–1975. doi: 10.1161/01.CIR.0000095269.36984.75. [DOI] [PubMed] [Google Scholar]
- 17.Badhwar N., Kalman J.M., Sparks P.B. Atrial tachycardia arising from the coronary sinus musculature: electrophysiological characteristics and long-term outcome of radiofrequency ablation. J Am Coll Cardiol. 2005;46:1921–1930. doi: 10.1016/j.jacc.2005.07.042. [DOI] [PubMed] [Google Scholar]
- 18.Moreira W., Timmermans C., Wellens H.J. Can common-type atrial flutter be a sign of an arrhythmogenic substrate in paroxysmal atrial fibrillation? Clinical and ablative consequences in patients with coexistent paroxysmal atrial fibrillation/atrial flutter. Circulation. 2007;116:2786–2792. doi: 10.1161/CIRCULATIONAHA.107.711622. [DOI] [PubMed] [Google Scholar]
- 19.Wazni O., Marrouche N.F., Martin D.O. Randomized study comparing combined pulmonary vein-left atrial junction disconnection and cavotricuspid isthmus ablation versus pulmonary vein-left atrial junction disconnection alone in patients presenting with typical atrial flutter and atrial fibrillation. Circulation. 2003;108:2479–2483. doi: 10.1161/01.CIR.0000101684.88679.AB. [DOI] [PubMed] [Google Scholar]
- 20.Mohanty S., Mohanty P., Di Biase L. Results from a single-blinded, randomized study comparing the impact of different ablation approaches on long-term procedure outcome in coexistent atrial fibrillation and flutter (APPROVAL) Circulation. 2013;127:1853–1860. doi: 10.1161/CIRCULATIONAHA.113.001855. [DOI] [PubMed] [Google Scholar]
- 21.Schneider R., Lauschke J., Tischer T. Pulmonary vein triggers play an important role in the initiation of atrial flutter: Initial results from the prospective randomized Atrial Fibrillation Ablation in Atrial Flutter (Triple A) trial. Heart Rhythm. 2015;12:865–871. doi: 10.1016/j.hrthm.2015.01.040. [DOI] [PubMed] [Google Scholar]
- 22.Chyou J.Y., Hickey K., Diamond L. Atypical electrocardiographic features of cavotricuspid isthmus-dependent atrial flutter occurring during left atrial fibrillation ablation. Ann Noninvasive Electrocardiol. 2010;15:200–208. doi: 10.1111/j.1542-474X.2010.00364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]