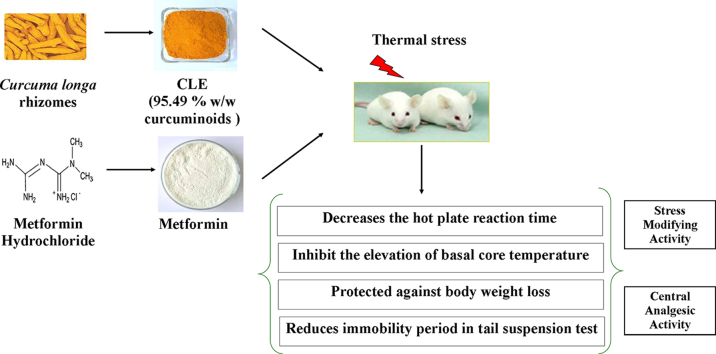
Abstract
The reported experimental study was conducted to compare the effects of repeated daily oral doses of curcuminoids (CLE) with metformin as potential antidepressants and analgesics. Effects of a single and ten daily oral doses of CLE (5, 20, 80 mg/kg/day) and of 50 mg/kg/day metformin (MET) were compared in mice hot plate test (HPT) for analgesics. On the 11th treatment day, all animals were subjected to foot shock stress triggered hyperthermia test, and on the 12th treatment day to tail suspension test (TST) for antidepressants. Immediately thereafter, their blood levels of glucose, insulin and cortisol were quantified. Dose dependent analgesic activity of CLE was observed in HPT, whereas the metformin dose tested suppressed only pain hypersensitivity in the test. But statistically significant effects of both of them were observed in TST, and both of them also afforded protections against body weight loss and slight elevation in core temperatures induced by daily handling and repeated testing. CLE or metformin had no significant effects in foot shock stress triggered transient hyperthermic responses or on blood glucose, insulin and cortisol levels. Reported results reveal that curcuminoids as well as metformin are stress response modifiers with antidepressants like activities, but only low dose curcuminoids possess centrally acting analgesics like activities. They suggest that the bio-assay system used in this study is well suited for identifying curcuminoids like plant metabolites with analgesic and anti-stress activities, and that low dose curcuminoids are more effective as analgesics than low dose metformin.
Keywords: Curcuma longa, Curcuminoids, Hot plate test, Foot shock stress, Hyperthermia, Tail suspension test
Graphical abstract
1. Introduction
Central hypersensitivity to pain is often encountered in patients suffering from chronic inflammatory diseases as well as diverse spectrums of somatic symptom disorders.1, 2 In traditionally known Indian and Chinese systems of medicine and health care, turmeric (Curcuma longa rhizomes) is often used for prevention and cure of such medical conditions. Curcumin and structurally analogous diarylheptanoids encountered in turmeric, often collectively referred to as turmeric curcuminoids, are now attracting considerable attention of modern drug discoverers as therapeutic leads potentially useful for prevention and cure of chronic diseases commonly associated with mental health problems and central sensitivity to pain,3, 4 and number of reports reaffirming anti-nociceptive effects of curcumin and other turmeric derived products in animal models and clinical trials have continued to increase since late decades of the 20th century.5, 6 It has often been suggested also that curcuminoids formulations or their derivative and analogs with improved bioavailability and adverse effect potentials could be universally more acceptable and reliable therapeutic alternatives for prevention and cure of numerous chronic diseases, including cancer and Alzheimer's disease.3, 7, 8 Such suggestions often neglect that like numerous non-systemic9 or covalent drugs,10, 11 blood levels of curcuminoids are not very reliable predictors of their therapeutic potentials or effectiveness.
Oral bioavailability studies conducted with curcuminoids have consistently revealed that even after very high oral doses (up to 1 g/kg) their observed blood levels are often undetectable.12, 13 On the other hand, it has often been reported that low oral doses of curcumin possess antidepressant and stress response suppressing effects in laboratory rodents.14, 15, 16 A report on dose dependant anti-nociceptive effects of curcumin after its single fairly low oral doses has appeared also.17 Other dose finding studies have suggested though, that oral 500 mg/kg curcumin could be its optimal pharmacologically interesting dose in laboratory animals.18 Therefore, in the realm of our ongoing psychopharmacological studies with traditionally known herbal remedies and their bioactive constituents19 we used curcumin and diverse types of curcuminoids enriched turmeric extracts to define their stress response suppressing dose ranges and dosing regimen.20, 21 They have reconfirmed that repeated daily oral 5 mg/kg curcumin or turmeric curcuminoids are high enough for increasing stress resistance in mice and have revealed that several low dose effects of turmeric curcuminoids are qualitatively analogous those of metformin and diverse other food chemicals commonly consumed with every day meals.22, 23, 24, 25, 26 Metformin is currently the antidiabetic drug of first choice for prevention and cure of diverse spectrums of comorbidities associated with diabesity27 and it is also often used for treatments of neuropathic pain.28 Numerous, but not all, therapeutically interesting bioactivities of metformin known to date29 are quite analogous to those of curcuminoids, and it has been reported also that repeated daily oral curcumin administrations also suppresses neuropathic pain in diabetic animals.30, 31
However, like in numerous pharmacological studies with curcumin and turmeric curcuminoids, most preclinical studied revealing their no-conceptive potentials were conducted with their relatively high doses and arbitrarily chosen dosing regimen.5 Therefore, the question whether daily oral intake of lower stress resistance promoting or adaptogenic doses of turmeric curcuminoids could also suppress central hypersensitivity to pain still remain open, or can be speculatively answered only. Results of an experiment conducted to more rationally answering this question has been described and discussed in this communication. Implication of the preclinical observation made to date with low dose curcuminoids for better understanding of Ayurvedic concepts of nutritional therapies or for more rational medicinal uses of turmeric curcuminoids are also pointed out in this article.
2. Materials and methods
2.1. Animals
Swiss albino male mice (20 ± 5 g) obtained from the Central Animal House of the Institute of Medical Sciences, Banaras Hindu University, Varanasi, India (Registration Number: 542/AB/CPCSEA) were housed in groups of six in polypropylene cages at an ambient temperature of 25 ± 1 °C and 45–55% relative humidity, with a 12:12 h light/dark cycle. They were always provided with commercial food pellets and water ad libitum, and were acclimatized for at least 1 week before using them for the experiments. Animals used in this study were pre-selected for their pain sensitivity in the hot plate test described later. For such purposes, reaction time of animals on a hot plate maintained at 55 ± 1 °C were recorded and only those mice reacting within 15 s on the hot plate and which did not show large variation when tested on four separate occasions (each 15 min apart), were randomly allotted to different experimental groups. Principles of laboratory animal care (NIH publication number 85-23, revised in 1985) guidelines were always followed, and prior approval from the Central Animal Ethical Committee of the University (CAECU) was taken for this study protocol (Dean/2014/CAEC/602, dated 30-05-2014).
2.2. Plant extract and other material used
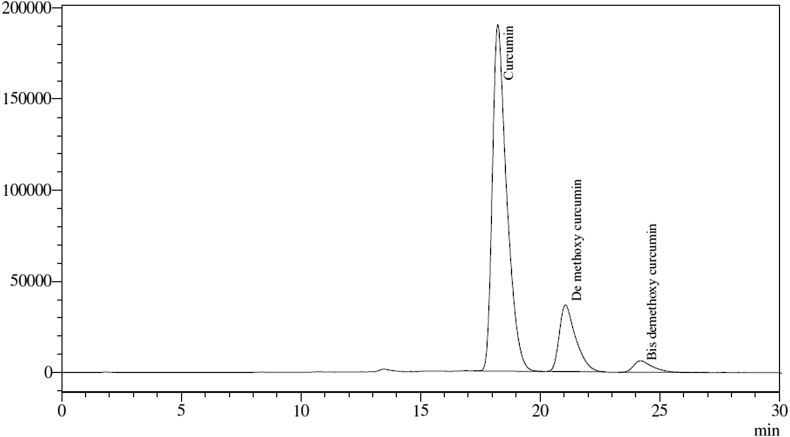
The C. longa rhizome extract (CLE) highly enriched in turmeric curcuminoids (95.49%, w/w) used in this study was generously supplied by R&D Center of Natural Remedies Pvt. Ltd., Bengaluru, India. HPLC chromatogram of CLE is shown in Fig. 1. As quantified by the USP 37-NF32 method, CLE contained curcumin (>77.94%, w/w), demethoxycurcumin (>15.03%, w/w) and bisdemethoxycurcumin (>2.52%, w/w), Metformin (Ranbaxy Laboratories, Gurgaon, India), carboxymethyl cellulose (CMC) (Central Drug House, New Delhi, India) and other chemicals and reagents used in this study were of highest purity commercially available in India.
Fig. 1.
HPLC chromatogram of curcuminoids (CLE).
2.3. Animal grouping and drug treatments
Six groups of six animals each were randomly allotted to different experimental groups. Two of them used as controls viz. CON + HPT (subjected to hot plate test; i.e. HPT) and CON − HPT (not subjected to HPT) were treated orally with the vehicle (0.3% CMC; 10 ml/kg/day) for 11 consecutive days. The four others were similarly treated either with 50 mg/kg/day metformin or with 5 or 20 or 80 mg/kg/day CLE. Except for one of the CMC treated control groups (CON − HPT), all others were subjected to hot plate test on days 1, 5, 7, and 10 of the experiment. For oral administrations, metformin and CLE were suspended in 0.3% CMC. All tests were conducted one hour after the day's oral treatments and all oral treatments on all observational days were given after recording their body weights and rectal temperatures. Further details of the experimental procedure used are graphically summarized in Fig. 2.
Fig. 2.
Graphical representation of experimental procedure used.
2.4. Hot plate test (HTP)
One hour after days treatment, individual mice of a group was gently placed on a hot plate maintained at 55 ± 1 °C and its reaction time (in seconds) for forepaw licking or jumping (whichever occurred first) were recorded.32 Immediately thereafter, the animal was placed back in its home cage, and 10 min thereafter its rectal temperature was recorded again. All rectal temperatures were recorded using a calibrated rectal probe and electronic thermometer. The numerical difference between the basal rectal temperature of an animal and its rectal temperature recorded 10 min after the hot plate test was calculated and used as an index for hot plate test induced hyperthermic response of the animal.
2.5. Foot shock stress induced hyperthermia test
All experimental groups were subjected to this test on the 11th day of the experiment. The experimental procedure used has been described in details elsewhere.21 In short, individual mouse of a group was placed in a black box (24 × 29 × 40 cm) with a grid floor for 1 min. After 10 s of its stay in the box, five consecutive electric foot shocks of 2 mA at 10 s intervals were given through the grid floor (2 mA, 50 Hz of 2 ms duration). Immediately thereafter, the animal was placed back in its home cage and 10 min thereafter its rectal temperature was recorded again. Difference between the rectal temperature of the animal recorded 10 min after the foot shock stress and that of its basal one of that day was considered to be its foot shock stress induced hyperthermic response.
2.6. Tail suspension test and blood glucose, insulin and cortisol estimations
Using the procedure described earlier,21, 22 the tail suspension test for assessing depressive state of animals33 was conducted on the 12th day of the experiment. In short, one hour after recording its body weight and basal rectal temperature, an individual mouse of a group was hung upside down on its tail (using adhesive tapes placed at approximately 1 cm distance from its tail tip) on a horizontal wire placed 50 cm above the floor. After initial vigorous movements, the mouse assumed an immobile posture and the period of immobility during a 5 min observation period was recorded. Immediately after the test, blood samples of the animals were collected by retro-orbital venous plexus sampling method and blood plasma was separated by cold centrifugation. Using appropriate test kits and instructions of the manufacturers, plasma glucose (Autospan Glucose test kit; Beacon Diagnostic Pvt. Ltd., Navasari, India), insulin (ELISA test kit; DRG Instruments GmbH, Germany) and cortisol (ELISA kit; Enzo Life Sciences; PA, USA) were quantified.
2.7. Statistical analysis
Mean ± standard error of mean (SEM) was calculated for the observed values in each experimental group. Statistical analysis was performed by one way analysis of variance (ANOVA) followed by Student–Newman–Keuls multiple comparison test, two way ANOVA followed by Bonferroni post hoc test. GraphPad Prism-5 (GraphPad Software Inc., La Jolla, California, USA) was used for statistical analysis. Origin-Pro 8 (OriginLab Corporation, Massachusetts, USA) software was used for graphs representation. p-value less than 0.05 was considered as statistically significant.
3. Results
3.1. Body weights and core temperatures
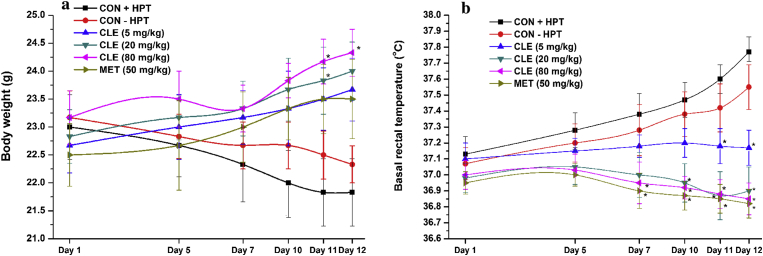
These results are summarized in the Fig. 3a and b. Mean body weights of both the control groups decrease gradually during the course of the experiment, whereas their mean basal rectal temperatures increased gradually. Rates of these changes during the course of the experiments in both the control groups were quite similar. These stress triggered alterations caused by daily handling and occasional testing were not observed in the CLE or metformin treated groups. Even 5 mg daily CLE dose was highly effective in compensating both these alterations. These efficacies of 50 mg/kg/day metformin for antagonizing body weight loss and core temperature elevation were almost identical to those of 5 mg/kg/day and 20 mg/kg/day CLE respectively, and those of the 20 and 80 mg/kg/day CLE against both the mild chronic stress triggered alterations were almost identical.
Fig. 3.
Effects of thermal stimuli induced stress on a) body weight and b) basal rectal temperature of male mice treated with curcuminoids (CLE) and metformin (MET). CON + HPT (Hot plate control), CON − HPT (normal control). Values are mean ± SEM, n = 6. *Denotes statistically significant difference (Two way ANOVA followed by Bonferroni post hoc test) relative to CON + HPT group (*p < 0.05).
3.2. Hot plate test
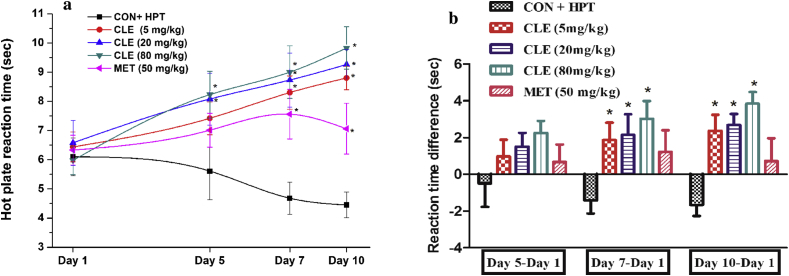
Results summarized in Fig. 4a reveal that one hour after their first oral doses neither metformin nor CLE had any significant effects on the mean reaction time of male mice preselected for their sensitivity to pain responses in the hot plate test. Mean reaction time of the vehicle treated control group (CON + HPT) continued to decrease on the 5th and subsequent observational days, whereas those of the CLE treated ones steadily increased during the course of the experiment. Analogous observed effects of metformin treatments were qualitative similar to those of CLE, but the observed effects of even the lowest tested daily CLE dose (5 mg/kg/day) on the 5th and subsequent observational days were somewhat higher than that of metformin dose (50 mg/kg/day) tested. It is apparent from the calculated values summarized in Fig. 4b that pain response sensitivity of the metformin treated group remained almost constant on all observational days whereas that of CLE continued to increase dose dependently with increasing numbers of treatment days.
Fig. 4.
Effects of curcuminoids (CLE) and metformin (MET) on a) mean hot plate reaction time and b) hot plate reaction time difference of male mice respect to day 1. CON + HPT (Hot plate control). Values are mean ± SEM, n = 6. *Denotes statistically significant difference (Two way ANOVA followed by Bonferroni post hoc test) relative to CON + HPT group (*p < 0.05).
3.3. Hot plate test and foot shock stress triggered hyperthermic responses
Core temperatures of animals recorded 10 min after the hot plate test were always slightly higher than their basal one of the day. These results summarized in Fig. 5 reveal that magnitude of this hot plate testing procedure triggered transient hyperthermic responses in the vehicle treated control group (CON + HPT) increased somewhat during the course of the experiment, whereas those of the CLE or metformin treated ones remained almost constant on all observational days. On the fist observational day magnitude of this response of the CLE or metformin treated groups were statistically not significantly different than the control group. Statistically significant effect of metformin (50 mg/kg/day) treatment on this response was observed only on the 7th treatment day and that of the highest tested CLE dose (80 mg/kg/day) on the 10th observational day. Transient hyperthermic responses triggered by less than 1 min exposures of the animals to foot shock stress on the 11th treatment day were much higher in magnitude than those triggered by hot plate test on earlier observational days. Results of the foot shock stress induced hyperthermia test summarized in Table 1 did not reveal any statistically significant effects of CLE or metformin treatments in this test.
Fig. 5.
Thermal stimuli induced hyperthermia in male mice treated with curcuminoids (CLE) and metformin (MET). CON + HPT (Hot plate control). Values are mean ± SEM, n = 6. *Denotes statistically significant difference (Two way ANOVA followed by Bonferroni post hoc test) relative to CON + HPT group (*p < 0.05).
Table 1.
Effects of 11 daily oral doses of curcuminoids (CLE) and metformin (MET) on foot shock stress induced transient hyperthermic response in male mice.
| Treatment groups | Rectal temperature (°C) |
||
|---|---|---|---|
| Before | After | Difference | |
| CON + HPT | 37.60 ± 0.09 | 38.32 ± 0.07a | 0.72 ± 0.04 |
| CON − HPT | 37.42 ± 0.15 | 38.08 ± 0.16a | 0.67 ± 0.03 |
| CLE (5 mg/kg) | 37.18 ± 0.11 | 37.82 ± 0.11a | 0.63 ± 0.06 |
| CLE (20 mg/kg) | 36.87 ± 0.15 | 37.43 ± 0.13a | 0.57 ± 0.03 |
| CLE (80 mg/kg) | 36.88 ± 0.09 | 37.42 ± 0.08a | 0.53 ± 0.05 |
| MET (50 mg/kg) | 36.85 ± 0.09 | 37.45 ± 0.12a | 0.60 ± 0.06 |
Values are mean ± SEM, n = 6.
Denotes statically significant difference (t-test; p < 0.05) between the mean values of the group recorded before and 10 min after exposure to foot shock stress.
3.4. Tail suspension test and plasma glucose, insulin and cortisol levels
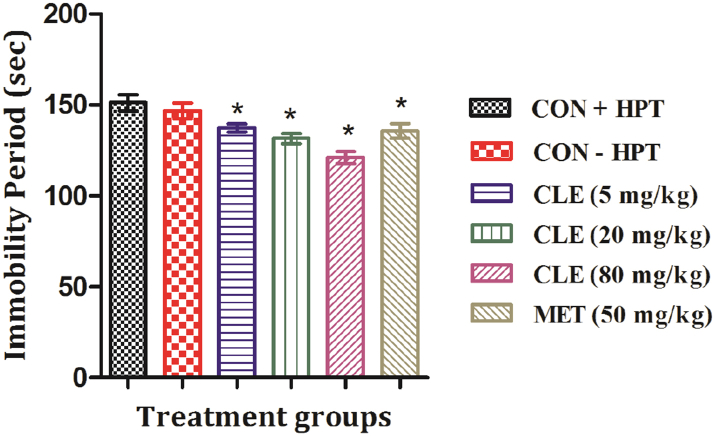
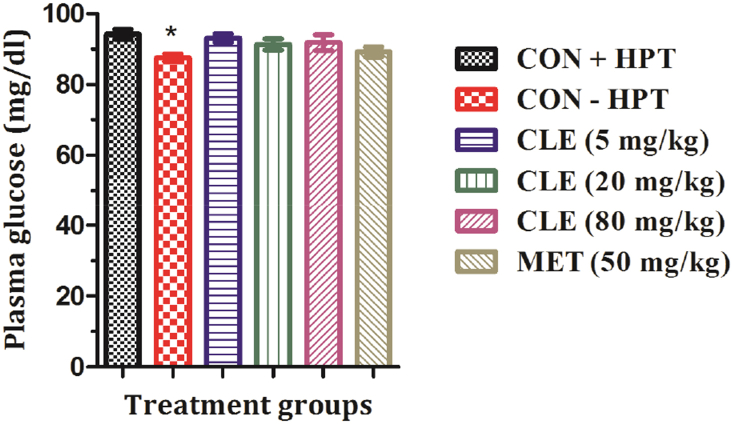
After 11 daily oral treatments, all tested doses of CLE and metformin significantly lowered the immobility period of mice in this test. These results summarized in Fig. 6 revealed that efficacy of CLE increased dose dependently and that the efficacy of the lowest tested daily dose (5 mg/kg/day for eleven consecutive days) of CLE is almost equal to that of 50 mg/kg daily doses of metformin. Mean plasma glucose levels of the control group subjected to hot plate test (CON + HPT) was statistically significantly higher than the other control group not subjected to the test (CON − HPT). These mean values of the two groups were 94.18 ± 1.35 and 87.58 ± 1.02 respectively as shown in Fig. 7. However, mean plasma insulin or cortisol levels of the CLE or metformin treated were not significantly different from those of the CON + HPT group, and no statistically significant differences between the plasma and cortisol levels of the two control groups were observed (results not shown).
Fig. 6.
Effects of curcuminoids (CLE) and metformin (MET) in tail suspension test on day 12. CON + HPT (Hot plate control), CON − HPT (normal control). Values are mean ± SEM, n = 6. *Denotes statistically significant difference (ANOVA) relative to CON + HPT group (*p < 0.05).
Fig. 7.
Effects of curcuminoids (CLE) and metformin (MET) on plasma glucose level in male mice. CON + HPT (Hot plate control), CON − HPT (normal control). Values are mean ± SEM (n = 6). *Denotes statistically significant difference (ANOVA) relative to CON + HPT group (*p < 0.05).
4. Discussion
Reported observations revealing centrally acting analgesics like effects of turmeric curcuminoids strongly suggest that regular intake of their fairly low oral doses could be useful for suppressing central hypersensitivity to pain triggered by repeated exposures to peripheral noxious stimuli. They also add further experimental evidence in support of our working hypothesis that curcuminoids are non-systemic drugs like polyvalent bioactive agents, and that they are regulators of digestive functions involved in controlling body weights and temperature. Like in our earlier dose finding experiments,20, 21 maximal possible protective effects of CLE against body weights changes and core temperature were observed even after its lowest daily dose (5 mg/kg/day) tested. Interestingly though, dose dependant efficacy of CLE in prolonging reaction times in hot plate test increased with increasing number of treatment days, whereas even its highest tested daily dose (80 mg/kg/day) had no protective effects against foot shock stress triggered transient hyperthermic responses after its 11 daily treatments. These findings, taken together with earlier observations21 revealing that 10 daily 5 mg/kg oral doses of CLE are long and high enough for suppressing foot shock stress triggered hyperthermic responses as well as longer lasting alterations in body weight and core temperatures triggered by daily handling and repeated testing20 strongly suggest that bioactivity profiles of CLE depend not only on their daily oral doses and number of treatment days, but also on the earlier stressful experiences of the test subjects. Since except for the analgesic activities all other observed effects of CLE and metformin in this and in our earlier study were analogous, it seems reasonable to assume that such is the case for metformin as well.
Animals used in this study were four times pre-exposed to 55 °C hot plate test for pre-selection purposes and the rate of alterations in the body weights and basal core temperatures of animals of both the control groups observed during the course of the experiment were similar. Therefore, it is apparent that these long lasting alterations are not due to daily handling or testing procedures used during the course of the described experiment only, and that they are consequences, or late effects, of repeated pre-exposures of the animals to hot plate during the pre-selection procedure. These observations encourage us suggest that such preselected animals are well suited not only for increasing the reliability and reproducibility of pharmacological observations made in hot plate test, but also for estimating possible effects of test agents on mild experimental stress triggered alterations in body weights and core temperatures.
The observations that 5 mg/kg daily oral doses CLE is its maximally effective ones in affording protection against repeated exposures to hot plate or foot shock stress triggered alterations in body weight and core temperature strongly suggest that turmeric curcuminoids are more effective modulators of energy metabolism and central pain sensitivity than the metformin dose tested. These observations taken together with available information on oral bioavailability and metabolic stability of curcuminoids12 suggest that either the pharmacological site(s) of action involved in these effects of curcuminoids reside inside the gastrointestinal tract, or some of its bioactive intra-gastric metabolites are involved in its observed effects. In any case, it remains certain that like metformin and numerous other food phytochemical22, 23, 24, 25, 26 low dose curcuminoids are also modulators of brain functions and homeostatic processes regulating body weight and core temperature changes, as well as depressive state of rodents, triggered by external or environmental stimuli. The observations that blood glucose, insulin, and cortisol levels of CLE or metformin treated animals were within the ranges observed in vehicle treated control animals, might suggest that biological processes and mechanisms regulating these blood levels are not involved in their such effects. However, since these observations were made only at one time point and at the end of the experiment only, they are not very definitive evidences for negating the possibility of their involvements in the modes of actions of the test agents. It remains certain though, that dose dependant, and qualitatively metformin like, antidepressants like efficacy of CLE in tail suspension test persists 24 h after its last oral doses, and that repeated daily tested doses of CLE, or of 50 mg/kg metformin, have no long lasting effects on blood glucose, insulin, and cortisol levels of male mice often subjected to hot plate and other tests.
Efforts to pin point the modes of actions of curcuminoids have revealed that curcuminoids increase phosphorylation of AMP-activated protein kinase (AMPK) in hepatoma cells and that one of the turmeric curcuminoids, i.e. curcumin, is several hundred folds more effective than metformin in activating the downstream targets of the kinase.34 AMPK is a nutrient and energy sensor involved in maintenance of energy homeostasis regulating metabolic responses to cellular stress.35, 36, 37, 38, 39 Therefore, it seems reasonable to assume that observed high efficacy of CLE in protecting stress triggered alterations in body weight and core temperature is mainly due to its ability to stimulate AMPK inside the gastrointestinal tract. However, the question whether or not other known bioactivities of curcuminoids observed in cellular and other in vitro models are also involved in often reported analgesics and antidepressants like efficacies of curcuminoids5, 40 still remain open or speculative only.
Our observations clearly reveal, that analgesics and antidepressants like efficacies of CLE observed after its 10 or more daily doses increased with its increasing daily doses, whereas its maximal possible protective effects against stress triggered changes in body weight and core temperature were apparent even after its lowest tested daily doses (5 mg/kg). These observations strongly suggest that either bioavailability of curcuminoids and/or of their metabolites increase with increasing numbers of treatment days, or that their effectiveness to modulate the functions of pharmacological targets other than AMPK are several folds lower. Curcuminoids are extensively metabolized inside the gut,41 and regular oral intake of curcuminoids alters gut microbial ecology and functions.42 Moreover, they also possess antibacterial, antiviral, and antifungal activities.43 Therefore, it could as well be that their observed dose dependant anti-stress, analgesic and antidepressant activities observed after their fairly low oral doses are mainly due to slowly evolving and longer lasting effects of curcuminoids and/or their metabolites on gut microbial ecology and functions.
Although crucial role of gut microbiota in regulating physical and mental health status is now well recognized,44, 45, 46 as yet only very little concentrated efforts have been made to translate available preclinical information on curcuminoids and numerous other food phytochemicals with bactericidal activities in terms of their therapeutic potentials. Observations reported in this and in our earlier reports encourage us suggest that the bioassay procedure used in this study could be a feasible and cost effective one for such purposes. Therefore, efforts to further pharmacological validate the bioassay procedure using other phytochemicals and drugs with microbicidal activities or modulating effects on AMPK can be warranted. Such efforts will not only be helpful for better understanding of pharmacological principles behind traditionally known dietary and herbal therapies, but also could eventually lead to novel therapeutic leads and pharmacological targets urgently need for discovering and developing functionally novel antidepressant drugs with preventive effects against central hypersensitivity to pain and metabolic disturbances.
It has often been suggested that both curcuminoids and metformin can be used for prevention and cure of diverse spectrums of mental health problems and other co-morbidities commonly associated with lifestyle disorders and aging,47, 48, 49 and that turmeric could be an adjuvant diabetic therapy with metformin.50, 51 However, as yet no reports on possible pharmacological interactions between lodes curcuminoids and metformin have appeared. Encouraged by the observation reported in this communication, efforts will now be made in our laboratories to experimentally verify whether combinations of CLE and metformin could have additive or synergistic effects or not. Results of these efforts will not only be useful for more rational development of polypills with metformin and curcuminoids, but also for better understanding of pharmacological principles behind traditionally known medicinal and healthcare uses of turmeric.
5. Conclusion
Both curcuminoids and metformin are orally active stress response modifiers, but unlike metformin even very low doses of curcuminoids can also prevent chronic stress triggered central hypersensitivity to pain. The bio-assay system used in this study is well suited for identifying such activities of herbal extracts and their bioactive constituents, and also for better defining of biological interactions between them. Further efforts to identify the bioactive constituents of other products derived from turmeric and other traditionally known medicinal herbs using the bioassay procedures reported in this study could lead to yet other structurally and functionally novel drug leads for prevention and cure of central sensitivity syndromes.
Conflicts of interest
The authors state that they have no conflicts of interest to declare.
Acknowledgment
Suruchi Verma thankfully acknowledges the Department of Science and Technology, Government of India, New Delhi, for awarding INSPIRE Fellowship (IF131112).
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Contributor Information
Suruchi Verma, Email: suruchi.verma.rs.phe13@iitbhu.ac.in.
Deepak Mundkinajeddu, Email: deepak@naturalremedy.com.
Amit Agarwal, Email: amit@naturalremedy.com.
Shyam Sunder Chatterjee, Email: shyam.chatterjee@web.de.
Vikas Kumar, Email: vikas.phe@iitbhu.ac.in.
References
- 1.Yunus M.B. Editorial review: an update on central sensitivity syndromes and the issues of nosology and psychobiology. Curr Rheumatol Rev. 2015;11:70–85. doi: 10.2174/157339711102150702112236. [DOI] [PubMed] [Google Scholar]
- 2.Tavel M.E. Somatic symptom disorders without known physical causes: one disease with many names? Am J Med. 2015;128:1054–1058. doi: 10.1016/j.amjmed.2015.04.041. [DOI] [PubMed] [Google Scholar]
- 3.Prasad S., Gupta S.C., Tyagi A.K., Aggarwal B.B. Curcumin, a component of golden spice: from bedside to bench and back. Biotechnol Adv. 2014;32:1053–1064. doi: 10.1016/j.biotechadv.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 4.He Y., Yue Y., Zheng X., Zhang K., Chen S., Du Z. Curcumin, inflammation, and chronic diseases: how are they linked? Molecules. 2015;20:9183–9213. doi: 10.3390/molecules20059183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheppudira B., Fowler M., McGhee L. Curcumin: a novel therapeutic for burn pain and wound healing. Expert Opin Investig Drugs. 2013;22:1295–1303. doi: 10.1517/13543784.2013.825249. [DOI] [PubMed] [Google Scholar]
- 6.Morningstar M.W., Strauchman M., Fleischmann G. Controlled-release curcumin for the treatment of pain related to adult degenerative scoliosis: a retrospective, open-label, case-controlled series. J Pain Relief. 2015;4:192. [Google Scholar]
- 7.Prasad S., Tyagi A.K., Aggarwal B.B. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: the golden pigment from golden spice. Cancer Res Treat. 2014;46:2–18. doi: 10.4143/crt.2014.46.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siviero A., Gallo E., Maggini V. Curcumin, a golden spice with a low bioavailability. J Herb Med. 2015;5:57–70. [Google Scholar]
- 9.Filipski K.J., Varma M.V., El-Kattan A.F. Intestinal targeting of drugs: rational design approaches and challenges. Curr Top Med Chem. 2013;13:776–802. doi: 10.2174/1568026611313070002. [DOI] [PubMed] [Google Scholar]
- 10.Singh J., Petter R.C., Baillie T.A., Whitty A. The resurgence of covalent drugs. Nat Rev Drug Discov. 2011;10:307–331. doi: 10.1038/nrd3410. [DOI] [PubMed] [Google Scholar]
- 11.Chatterjee S.S. From covalent bonds to eco-physiological pharmacology of secondary plant metabolites. Biochem Pharmacol. 2015;2952:426–428. doi: 10.1016/j.bcp.2015.07.037. [DOI] [PubMed] [Google Scholar]
- 12.Anand P., Kunnumakkara A.B., Newman R.A., Aggarwal B.B. Bioavailability of curcumin: problems and promises. Mol Phar. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 13.Ji H.F., Shen L. Can improving bioavailability improve the bioactivity of curcumin? Trends Pharmacol Sci. 2014;35:265–266. doi: 10.1016/j.tips.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Xu Y., Ku B.S., Yao H.Y. Antidepressant effects of curcumin in the forced swim test and olfactory bulbectomy models of depression in rats. Pharmacol Biochem Behav. 2005;82:200–206. doi: 10.1016/j.pbb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Xu Y., Ku B.S., Yao H.Y., LinYH Ma X., Zhang Y.H., Li X.J. The effects of curcumin on depressive-like behaviors in mice. Eur J Pharmacol. 2005;518:40–46. doi: 10.1016/j.ejphar.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Zhao X., Wang C., Zhang J.F. Chronic curcumin treatment normalizes depression-like behaviors in mice with mononeuropathy: involvement of supraspinal serotonergic system and GABA receptor. Psychopharmacology. 2014;231:2171–2187. doi: 10.1007/s00213-013-3368-2. [DOI] [PubMed] [Google Scholar]
- 17.Zhu Q., Sun Y., Yun X., Ou Y., Zhang W., Li J.X. Antinociceptive effects of curcumin in a rat model of postoperative pain. Sci Rep. 2014;4:4932. doi: 10.1038/srep04932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin Y.G., Kunnumakkara A.B., Nair A. Curcumin inhibits tumor growth and angiogenesis in ovarian carcinoma by targeting the nuclear factor-κB pathway. Clin Cancer Res. 2007;13:3423–3430. doi: 10.1158/1078-0432.CCR-06-3072. [DOI] [PubMed] [Google Scholar]
- 19.Chatterjee S.S., Kumar V. Holistic psychopharmacology and promiscuous plants and principles of ayurveda. Am J Plant Sci. 2012;3:1015–1021. [Google Scholar]
- 20.Verma S., Chatterjee S.S., Kumar V. Comparative adaptogenic activity of bioavailable extracts of Curcuma longa and pure curcumin in rodents. Indian J Pharmacol. 2014;46:93–94. [Google Scholar]
- 21.Verma S., Chatterjee S.S., Kumar V. Metformin like stress response modulating effects of turmeric curcuminoids in mice. SAJ Neurol. 2015;1:102–110. [Google Scholar]
- 22.Langstieh A.J., Verma P., Thakur A.K., Chatterjee S.S., Kumar V. Desensitization of mild stress triggered responses in mice by a Brassica juncea leaf extract and some ubiquitous secondary plant metabolites. Pharmacologia. 2014;5:326–338. [Google Scholar]
- 23.Shrivastava N., Chatterjee S.S., Kumar V. Stress response desensitizing efficacies of triethylene glycol and quercetin in mice. Indian J Pharmacol. 2014;46:S91–S92. [Google Scholar]
- 24.Shivavedi N., Chatterjee S.S., Kumar V. Evaluation of pharmacologically interesting dose range of ascorbic acid in mice. SAJ Neurol. 2014;1:101–108. [Google Scholar]
- 25.Shivavedi N., Chatterjee S.S., Kumar V. Stress response modulating effects of lactic acid in mice. Ther Targets Neurol Dis. 2014;1:418–423. [Google Scholar]
- 26.Khan S.A., Chatterjee S.S., Kumar V. Potential anti-stress, anxiolytic and antidepressant like activities of mono-hydroxybenzoic acids and aspirin in rodents: a comparative study. Austin J Pharmacol Ther. 2015;3:1073–1082. [Google Scholar]
- 27.Straughan J.L. Focus on metformin-a major cardiovascular medication. ‘Diabesity-the biggest epidemic in human history’. Cardiovasc J Afr. 2007;18:331–333. [PubMed] [Google Scholar]
- 28.Szkudlinska M., Taylor A., Guruguri P., Annabi E., Price T., Yassine H. A retrospective study on metformin efficacy in neuropathic pain. Diabetes Pharmacother. 2012:191. [Google Scholar]
- 29.Pulito C., Sanli T., Rana P., Muti P., Blandino G., Strano S. Metformin: on ongoing journey across diabetes, cancer therapy and prevention. Metabolites. 2013;3:1051–1075. doi: 10.3390/metabo3041051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y., Zhang Y., Liu D.B., Liu H.Y., Hou W.G., Dong Y.S. Curcumin attenuates diabetic neuropathic pain by downregulating TNF-α in a rat model. Int J Med Sci. 2013;10:377–381. doi: 10.7150/ijms.5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma S., Kulkarni S.K., Agrewala J.N., Chopra K. Curcumin attenuates thermal hyperalgesia in a diabetic mouse model of neuropathic pain. Eur J Pharmacol. 2006;536:256–261. doi: 10.1016/j.ejphar.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Turner R.A. Analgesics. In: Turner R., Ebborn P., editors. Screening Methods in Pharmacology. Academic Press; New York: 1965. pp. 100–102. [Google Scholar]
- 33.Can A., Dao D.T., Terrillion C.E., Piantadosi S.C., Bhat S., Gould T.D. The tail suspension test. J Vis Exp. 2012;28:e3769. doi: 10.3791/3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim T., Davis J., Zhang A.J., He X., Mathews S.T. Curcumin activates AMPK and suppresses gluconeogenic gene expression in hepatoma cells. Biochem Biophys Res Commun. 2009;388:377–382. doi: 10.1016/j.bbrc.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 35.Hardie D.G., Ross F.A., Hawley S.A. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hardie D.G. Regulation of AMP-activated protein kinase by natural and synthetic activators. Acta Pharm Sin B. 2015:187–225. doi: 10.1016/j.apsb.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calabrese M.F., Rajamohan F., Harris M.S. Structural basis for AMPK activation: natural and synthetic ligands regulate kinase activity from opposite poles by different molecular mechanisms. Structure. 2014;22:1161–1172. doi: 10.1016/j.str.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 38.Pelosse M., Tokarska-Schlattner M., Schlattner U. AMP-activated protein kinase: a metabolic stress sensor in the heart. Card Cytoarchitecture. 2015:187–211. [Google Scholar]
- 39.Zhang B.B., Zhou G., Li C. AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell Metab. 2009;9:407–416. doi: 10.1016/j.cmet.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 40.Seo H.J., Wang S.M., Han C. Curcumin as a putative antidepressant. Expert Rev Neurother. 2015;15:269–280. doi: 10.1586/14737175.2015.1008457. [DOI] [PubMed] [Google Scholar]
- 41.Wang K., Qiu F. Curcuminoid metabolism and its contribution to the pharmacological effects. Curr Drug Metab. 2013;14:791–806. doi: 10.2174/13892002113149990102. [DOI] [PubMed] [Google Scholar]
- 42.Dey N., Wagner V.E., Blanton L.V. Regulators of gut motility revealed by a gnotobiotic model of diet-microbiome interactions related to travel. Cell. 2015;163:95–107. doi: 10.1016/j.cell.2015.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moghadamtousi S.Z., Kadir H.A., Hassandarvish P., Tajik H., Abubakar S., Zandi K. A review on antibacterial, antiviral, and antifungal activity of curcumin. BioMed Res Int. 2014:186864. doi: 10.1155/2014/186864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moloney R.D., Dinan T.G., Cryan J.F. Stress & the microbiota-gut-brain axis in visceral pain. Psychoneuroendocrinology. 2015;61:8. [Google Scholar]
- 45.Jia W., Li H., Zhao L., Nicholson J.K. Gut microbiota: a potential new territory for drug targeting. Nat Rev Drug Discov. 2008;7:123–129. doi: 10.1038/nrd2505. [DOI] [PubMed] [Google Scholar]
- 46.Petra A.I., Panagiotidou S., Hatziagelaki E., Stewart J.M., Conti P., Theoharides T.C. Gut-microbiota-brain axis and its effect on neuropsychiatric disorders with suspected immune dysregulation. Clin Ther. 2015;37:984–995. doi: 10.1016/j.clinthera.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ying A.M., Maruschak N., Mansur R., Carvalho A., Cha D., McIntyre R. Metformin: repurposing opportunities for cognitive and mood dysfunction. CNS Neurol Disord Drug Targets. 2014;13:1836–1845. doi: 10.2174/1871527313666141130205514. [DOI] [PubMed] [Google Scholar]
- 48.Hu S., Maiti P., Ma Q. Clinical development of curcumin in neurodegenerative disease. Expert Rev Neurother. 2015;15:629–637. doi: 10.1586/14737175.2015.1044981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dulbecco P., Savarino V. Therapeutic potential of curcumin in digestive diseases. World J Gastroenterol. 2013;19:9256–9270. doi: 10.3748/wjg.v19.i48.9256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Selvi N.M.K., Sridhar M.G., Swaminathan R.P., Sripradha R. Efficacy of turmeric as adjuvant therapy in type 2 diabetic patients. Indian J Clin Biochem. 2014;30:180–186. doi: 10.1007/s12291-014-0436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kumar V., Thakur A.K., Verma S., Yadav V., Chatterjee S.S. Potential of some traditionally used edible plants for prevention and cure of diabesity associated comorbidities. TANG. 2015;5:1–22. [Google Scholar]