Abstract
Background
Atrial fibrillation (AF) is one of the most prevalent cardiac arrhythmias associated with substantially increased risks of ischemic stroke and thromboembolism. Oral anticoagulants (OACs) are the cornerstone of AF management and effectively prevent AF-related stroke. As new non-vitamin K antagonist OACs (NOACs) have become available, the landscape of stroke prevention in AF has changed. However, there are considerable gaps between daily clinical practice and current guideline-based recommendations for anticoagulant therapy in Japan. Consequently, little is known about the real-world setting and the current use of NOACs, especially by practitioners in Japan.
Methods
We conducted a prospective, observational study in 3847 patients with AF who were enrolled in clinics and hospitals located in Kanagawa Prefecture from September 2013 through March 2015. The participating centers included practitioners (small clinics), medium-sized hospitals, and university hospitals. The primary endpoints were epidemiologic characteristics, status of treatment with anticoagulants and antiplatelet agents, outcomes, and adverse events, including cerebrovascular disease, bleeding, and death.
Results
The mean CHADS2 score was 1.81±1.27, the mean CHADS2-Vasc score was 3.02±1.58, and the mean HAS-BLED score was 2.23±1.06, respectively. The usage rate of warfarin was 44.2% overall, and the usage rate of NOACs was 33.5%.
Conclusions
The results of the study are expected to serve as the basis for providing clinical practice guidance to healthcare institutions in Japan, with the ultimate goals of better characterizing the appropriate use of OACs and providing clinical decision support to physicians to facilitate the design of appropriate therapeutic strategies and the selection of anticoagulants for the management of AF.
Keywords: Atrial fibrillation, Anticoagulant, ASSAF-K, Cohort study, Rationale, Design
1. Introduction
Atrial fibrillation (AF) is one of the major arrhythmias, commonly occurring among adults worldwide. In general, the prevalence rates of AF are higher in men than in women and increase with age [1], [2] in both sexes. In Western countries, the prevalence rates of AF range from 1.1% to 1.21% in men and 0.8% to 1.27% in women [2], [3], [4], [5], [6]. In Japan, the prevalence rate is about 0.56%, which is equivalent to about two-thirds of the prevalence rate in the United States [4], [7], [8], [9]. Epidemiologic studies estimated that AF affected 0.716 million patients as early as 2005 in Japan; moreover, the prevalence of AF is expected to gradually and steadily increase to about 1.03 million people by the year 2050 owing to aging of the population and increasing incidences of predisposing conditions [7], [8], [10].
Currently, pharmacological treatment strategies for AF include rate-control strategies, rhythm-control strategies, and anticoagulant therapy designed to prevent systemic thrombotic disease. Among these strategies, the effects of rate-control strategies and rhythm-control strategies on survival remain controversial because consistent results have not been obtained in various pivotal clinical studies, including the AFFIRM trial [11], PIAF trial [12], RACE trial [13], and SPAF trial [14]. The potential effects of these strategies have to be carefully interpreted in actual clinical settings. In fact, patients with AF have a 5-fold higher risk of cardioembolic stroke compared to patients without AF, often leading to poorer outcomes, particularly among elderly individuals [15], [16]. AF-related ischemic stroke has an incidence of about 5% per year in the absence of appropriate prophylaxis [2]. Practically, the successful prevention of thrombotic diseases associated with AF would be a major clinical achievement because disability and morbidity due to thrombotic diseases such as cerebral stroke lead to both socio-physical and financial burdens not only on the individual but also on society as a whole.
Despite the recent advent of direct thrombin inhibitors (e.g., dabigatran) and factor Xa inhibitors (e.g., rivaroxaban, apixaban, and edoxaban), warfarin continues to play a role in preventing cerebral thrombotic diseases, as well as other thromboembolic diseases. However, four new oral anticoagulants (NOACs: dabigatran, apixaban, rivaroxaban, and edoxaban) were shown to be equivalent or superior to warfarin in preventing thrombotic diseases in patients with AF enrolled in phase III clinical trials [17], [18], [19], [20] and have been approved in Japan. The safety and efficacy of these NOACs in patients with AF should now be assessed in various practical clinical settings.
Most of the previous data on the use of anticoagulant therapy for the management of AF were derived from databases of patients enrolled at highly specialized cardiovascular centers or large hospitals in Japan, whereas many patients with AF are treated by primary-care physicians in community-based clinical settings. We therefore planned and executed this prospective observational study (designated as ASSAF-K) in the Kanagawa region of Japan to clarify the clinical features of patients with AF and to examine the safety and efficacy of NOACs for preventing thrombotic diseases. Our major objectives were to clarify current anticoagulant prescribing habits for patients with AF who are admitted to practitioners (small clinics), medium-sized, and university hospitals, and to assess the frequency of the main complications associated with anticoagulant therapy. This paper describes the rationale for ASSAF-K along with the clinical trial protocol and presents the results of cross-sectional analyses at the initial registry.
2. Material and methods
2.1. Design and objectives of the study
ASSAF-K is a multicenter, prospective, observational study designed to clarify the clinical features of patients with AF in the era of NOACs and to assess the safety and efficacy of anticoagulant therapy. Another objective was to examine the current use of anticoagulant therapy in patients with AF in hospitals of various sizes in the Kanagawa region. By conducting follow-up studies in the future, changes in disease status and modifications of diagnostic procedures and treatment regimens will also be investigated. Outcomes, including the development of stroke associated with anticoagulant therapy and the incidence of complications such as cerebral hemorrhage and extracerebral hemorrhage, will also be evaluated. Detailed information about the protocol of the ASSAF-K study is provided as a translated protocol in the Supplementary File of the present manuscript.
2.2. Study participants
Patients were eligible for the study if they meet items (1) and (2) and did not meet (3) the exclusion criteria.
-
(1)
Subjects: Patients with AF
The subjects are patients with all types of AF, including conditions such as AF with valvular heart diseases and AF occurring after valve replacement, and not only patients with non-valvular AF in whom treatment with NOACs is indicated. In other words, not only chronic AF, such as persistent AF and continuous AF amenable to defibrillation, but also paroxysmal atrial fibrillation (PAF) is being studied. This is because patients with PAF have a high risk of stroke, even if they are currently in sinus rhythm. On the basis of the survey results, outcomes, including the occurrence of cerebral infarction associated with anticoagulant therapy and the incidence of complications such as intracerebral hemorrhagic stroke, will also be evaluated.
-
(2)
Eligibility criteria
-
■
Patients with a diagnosis of AF
-
■
Patients who were previously given a diagnosis of paroxysmal AF, even if they are currently in sinus rhythm
-
■
Patients will be registered even if they have received antiarrhythmic therapy, treatment for tachycardia, or ablation.
-
(3)
Exclusion criteria
Patients who were judged by their attending physician to be unsuitable for participation in the study. For example, subjects with limited life expectancy up to one year because of malignant diseases or those with senile cognitive impairments who are unable to continue regular hospital visits and medications at enrollment were excluded.
2.3. Study procedures
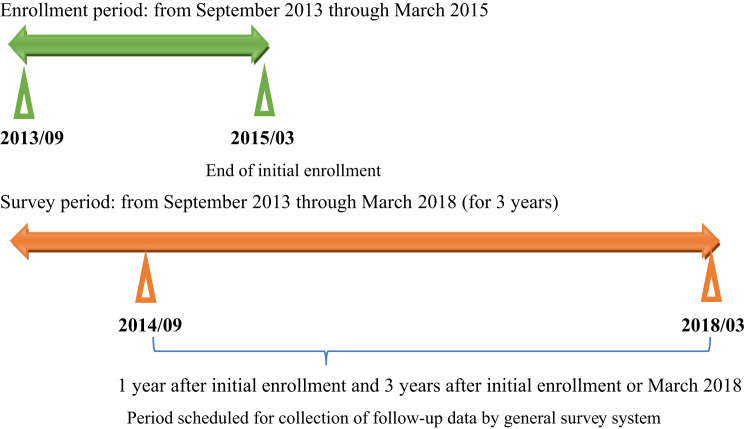
The schematic study procedures are shown in Fig. 1. The enrollment period was from September 2013 through March 2015, and the survey period is from September 2014 through March 2018. Observations, examinations, and implementation methods are as follows: 1) collection of epidemiologic data on patients with AF (age, sex, presence or absence of valvular disease, vital signs, past medical history); 2) the current status of treatment (types of drugs selected); and 3) outcomes (1 and 3 years after enrollment). Patients will be confirmed to meet the enrollment criteria and not to meet the exclusion criteria and will be enrolled by means of case report form (CRF) registration, Web registration, or FileMaker Runtime input. With the exception of Web enrollment, data will be routinely sent to the data center. The items shown in the Supplementary file will be observed and examined, and the data will be used in this study. All of the items will be assessed in routine clinical practice. After completion of the study, the principal investigators will provide the subjects with the most appropriate medical care. The results of this study will be included in treatment-related decisions.
Fig. 1.
Schematic presentation of the study protocol.
2.4. Evaluation of outcomes: primary endpoints and secondary endpoints
The primary and secondary endpoints are shown in detail in the Supplementary file. Follow-up will be continued, even if one of these primary endpoints occurs. In summary, the primary endpoints include status of treatment with anticoagulants and antiplatelet agents, thromboembolic outcomes, and adverse events, including cerebrovascular disease, bleeding, and death. Secondary endpoints include the relationship between treatment and the effectiveness and safety and a comparison of outcomes according to the type of drug therapy. The participating physicians will record all cardiovascular events or death by means of CRF registration, Web registration, or FileMaker Runtime input. We excluded a few subjects from the statistical analyses because of missing pivotal data, such as height, body weight, or age (date of birth). To avoid missing data, the attending physicians were cautioned a total of three times.
2.5. Target sample size and statistical analysis
The target number of patients is 5000. This is an observational study conducted in routine clinical practice. We based the target number of patients on the number of patients that could be enrolled during the study period described above. In the REACH study, a prospective cohort study of patients with coronary artery disease, cerebrovascular disease, peripheral vascular disease, and high-risk patients with these diseases, the annual incidence of cardiovascular death/myocardial infarction/stroke was reported to be 3.22% in 5021 Japanese subjects. These results were also used as a reference. Statistical analysis of the variables shown in the Supplementary file will be performed by a third party unrelated to this study with the use of IBM SPSS, ver. 22.0 software (IBM Japan, Nihonbashi, Tokyo).
2.6. Study organization
This study will be performed by the following organization:
Study Representative:
-
•
Chief Executive Officer: Yutaka Hatori, Director, Hatori Clinic
Members of the Protocol Committee:
-
•
Tomoyuki Kunishima, Director, Kunishima Clinic
-
•
Hiroyuki Sakai, Director, Sakai Clinic (Database construction and data collection)
-
•
Nobuo Hatori, Kobayashi Hospital (Data analysis)
Advisors:
-
•
Professor Naoki Sato, Department of Cardiology, Nippon Medical University Musashi Kosugi Hospital
-
•
Others
Participating hospitals:
-
•
Participating hospitals will be recruited from members of Kanagawa Physicians Association and members of the Japanese Society of Internal Medicine (hospitals in Kanagawa Prefecture). The expected number of participating hospitals was 100, but 105 hospitals were recruited by the end of March 2015). Detailed information of participating hospitals were shown as in Supplemental file.
3. Results
A total of 4014 subjects were enrolled and registered during the initial recruitment period from September 2013 through March 2015. In total, 167 subjects were excluded because of missing pivotal data such as height, body weight, or age (date of birth). Finally, 3847 subjects were analyzed for baseline characteristics as shown in Table 1. The mean CHADS2 score was 1.81±1.27, the mean CHADS2-Vasc score was 3.02±1.58, and the mean HAS-BLED score was 2.23±1.06. The usage rate of warfarin was 44.2% overall, and the usage rate of NOACs was 33.5%.
Table 1.
Background characteristics of the subjects enrolled in ASSAF-K.
| Parameter | Total (N=3847) |
|---|---|
| Age | 72.9±10.1 |
| Male:Female | 2512:1335 |
| Body mass index, kg/m² | 23.4±3.6 |
| SBP/DBP (mmHg) | 126.5±16.3/ |
| 73.1±11.5 | |
| PR (bpm) | 74.5±18.8 |
| Cr (mg/dL) | 1.08±1.17 |
| CCr | 66.6±28.6 |
| eGFR | 59.6±19.5 |
| Clinical Score | |
| CHADS2 Score | 1.81±1.27 |
| CHADS2 Vasc Score | 3.02±1.58 |
| HAS BLED Score | 2.23±1.06 |
| Concomitant diseases | |
| CHF | 22.90% |
| Hypertension | 60.10% |
| Diabetes Mellitus | 20.20% |
| Cerebral Infarction | 13.70% |
| Cerebral Hemorrhage | 0.90% |
| TIA | 2.10% |
| OMI | 5.90% |
| Angina pectoris | 9.00% |
| PAD | 2.30% |
| HCM | 1.70% |
| DCM | 2.10% |
| Liver Dysfunction | 1.40% |
| Hemorrhagic Disease | 1.10% |
| Hemodyalisis | 1.50% |
| Neoplasm | 4.30% |
| Thyroid Dysfunction | 3.00% |
| Interventional procedures | |
| PCI | 10.70% |
| Catheter Ablation | 5.80% |
| Maze Operation | 0.50% |
| DC | 1.00% |
| Pharmacological Defibrillation | 0.20% |
| Concomitant structural heart diseases | |
| Mitral Stenosis | 1.90% |
| Mitral Regurgitation | 14.10% |
| Mechanical Valve Replacement | 1.90% |
| Biological Valve Replacement | 0.50% |
| Other Valvular Disease | 5.50% |
| Medications | |
| Digitalis | 15.90% |
| β-blockade | 32.90% |
| Calcium Channel Blockade | 11.50% |
| Other Anti-arrhythmic Drugs | 16.20% |
| Anti-platelet medications | |
| ASA | 18.50% |
| Ticlopidine | 1.10% |
| Clopidogrel | 3.70% |
| Cilostazol | 1.20% |
| Anti-coagulant medications | |
| Warfarin | 44.20% |
| All NOAC | 33.50% |
| Dabigatran | 15.00% |
| Ribaroxaban | 15.00% |
| Apixaban | 3.50% |
| Edoxaban | 0.02% |
Abbreviations: ASA, acetylsalicylic acid; Cr, creatinine; CCr, creatinine clearance; CHF, chronic heart failure; DC, direct current defibrillation; DCM, dilated cardiomyopathy; eGFR, estimated glomerular filtration rate; HCM, hypertrophic cardiomyopathy; NOAC, new oral anticoagulant; OMI, old myocardial infarction; PAD, peripheral aretery disease; PCI, percutaneous coronary intervention; PR, pulse rate; SBP/DBP, systolic blood pressure/diastolic blood pressure; TIA, transient ischemic attack
4. Discussion
The optimal therapeutic strategies and tools in our current arsenal for patients with AF are as follows: rate-control strategies, rhythm-control strategies, anticoagulant strategies for preventing thrombotic diseases, and catheter-based high-frequency ablation therapies aimed to achieve permanent cessation of the disease. Among these therapeutic strategies, anticoagulant strategies are important because of the huge socioeconomic burden caused by disability and morbidity from cerebral thrombotic diseases associated with AF.
In earlier years, studies comparing antiplatelet agents such as aspirin salicylate and thienopyridine derivatives with vitamin K-dependent oral anticoagulants and coumarin derivatives such as warfarin were performed to evaluate the efficacy and safety of these drugs for the prevention of thrombotic diseases in subjects with AF [21], [22]. Warfarin use with dosage adjustments by prothrombin time-international normalized ratio monitoring strategies was shown to be significantly more effective compared to antiplatelet agents by various clinical trials so far [23], [24], [25]. However, although warfarin effectively prevents the development of thrombotic diseases in patients with AF [26], [27], the inherent laborious and complex dosage adjustments seemed to cause a low utilization rate of warfarin in relatively lower doses in various clinical observational trials performed in Japan, including the Fushimi AF Registry by Akao et al. [28], [29], [30].
In the Fushimi AF Registry, warfarin-based anticoagulant therapies were not widely used in actual clinical settings, with a utilization rate of only 48.5%. In our current analyses, the utilization rate of warfarin was about 44.2%, but the total utilization rate of anticoagulants increased to 77.7% (Table 1). The drugs prescribed to the remaining subjects were antiplatelet agents, such as acetylsalicylic acid, or no drug was prescribed to prevent thrombotic diseases, reflecting the so-called “underuse” of anticoagulants seen in the Fushimi AF Registry.
In addition, potential pharmacological dilemmas have led to risk/benefit conflicts. Inappropriate use of warfarin has sometimes resulted in unexpected hemorrhagic events, such as cerebral hemorrhage and gastrointestinal hemorrhage, which can be lethal. As compared with Caucasians, various clinical trials have shown that Asians have a higher risk of cerebral hemorrhage; patients receiving warfarin or other anticoagulants must therefore be closely monitored [31], [32], [33], [34]. Other factors such as numerous drug/drug and drug/food interactions and an increased risk of excessive bleeding [35], [36] have also discouraged the use of warfarin in Asia. In particular, Asians have a 4-fold higher risk of warfarin-induced intracranial hemorrhage as well as major gastrointestinal bleeding compared to non-Asians [33], [37]. These factors have also had a negative impact on the effective use of warfarin, contributing to substantial underutilization [38]. To resolve such “unmet” needs in actual clinical settings, NOACs, such as dabigatran, apixaban, rivaroxaban, and edoxaban, were recently developed and successively launched in Japan. Finally, we have entered the era of NOACs for the management of AF.
To date, multiple phase III clinical trials and subsequent post-hoc analyses assessing the efficacy and the safety of NOACs have shown either non-inferiority or superiority to warfarin treatment [17], [18], [19], [20]. The efficacy and the safety of dose adjustments in accordance with the individual characteristics of patients, including factors such as post-ischemic stroke [39], [40], advanced age [41], [42], and concurrent renal impairment [43], [44], have been examined under various clinical conditions. Owing to racial differences from patients enrolled in international clinical trials, Japanese clinical trials have consistently shown that NOACs are effective and safe in Japanese patients [33], [45], [46], [47], [48], [49]. In Japan, NOACs as well as warfarin can be used under national coverage by the public health-care insurance system, placing a lower financial burden on patients than that in other countries. Because clinical trials are generally performed in artificially controlled circumstances, the safety and efficacy demonstrated by such studies is generally limited. Unexpected complications and adverse effects or unknown clinical issues that are newly identified should be tested and clarified in actual clinical settings. ASSAF-K is characterized by the participation of medical practitioners in Kanagawa, Japan, which is a representative urban area in a modern developed country. ASSAF-K is expected to reveal the current status of the treatment and outcomes of patients with AF, including rates of compliance with clinical guidelines, and to provide proof of the correctness or inadequacy of current guideline-based treatments for AF in Japan.
The results of our initial cross-sectional analyses assessing the clinical characteristics of the patients participating in ASSAF-K are shown in Table 1. As compared with the “Fushimi AF Registry,” considered a representative Japanese AF registry with an initial registration of 3183 patients [28], the size of ASSAF-K is approximately the same. The Fushimi AF Registry revealed both the underuse and underdosage of warfarin in actual clinical settings. ASSAF-K is expected to show how oral anticoagulants are currently used in the era of NOACs. The mean CHADS2 scores in previous studies in Japan were 2.1 in the RE-LY Japanese population [48], 3.27 in J-ROCKET AF [45], 2.09 in the Fushimi AF Registry, 1.7 in the J-Rhythm Registry [50], and 1.82 in ASSAF-K, indicating a relatively lower thrombotic risk among the participants. Thus, ASSAF-K is expected to reveal changes in risks and benefits associated with the launching of NOACs in Japan.
We found that the subjects enrolled from clinics were characterized by a significantly higher age, prevalence of hypertension, percentage of women, and history of transient ischemic attack and stroke, as compared to the subjects enrolled from hospitals (data not shown). Only the prevalence of congestive heart failure was significantly higher in the subjects enrolled from hospitals than that in the subjects from clinics; therefore, both the CHADS2 and CHADS2-Vasc scores were significantly higher in the subjects enrolled from clinics. Consistent with this trend, the HAS-BLED score was also significantly higher in the subjects enrolled from clinics. These findings suggested that the subjects enrolled from clinics were at high risk for thromboembolic diseases and bleeding complications associated with anticoagulant therapy for atrial fibrillation.
The results of our study are expected to serve as the basis for providing clinical practice guidance to healthcare institutions in Japan, with the ultimate goals of better characterizing the appropriate use of oral anticoagulants and providing clinical decision support to physicians to facilitate the design of appropriate therapeutic strategies and the selection of anticoagulants for the management of AF. Because our current research was designed as an observational cross-sectional and prospective cohort study, the data will provide hypothetical and exploratory conclusions at best. Additionally, one of the limitations of our study may be that we did not perform source data validation procedures for each hospital and clinic. Further clinical trials will be required to confirm the hypotheses generated from the results of the present study.
Sources of funding
The Kawasaki Physicians Association provided financial support to ASSAF-K. No donations, financial support, or other payments potentially associated with a conflict of interest relevant to the ASSAF-K study were received by any individual involved in the study; any member of the Kanagawa Physicians Association or Kawasaki Physicians Association; the manufacturers of the NOACs, anticoagulants, or antithrombotic agents used in the study; or from any other organization or individual.
Conflict of interest
All authors declare no conflict of interest related to this study.
Acknowledgements
We thank Dr. Hisao Ogawa, Deputy Director of the National Cerebral and Cardiovascular Center and former Professor of the Department of Cardiovascular Medicine, Graduate School of Medical Science, Kumamoto University for his scientific advisory comments regarding our study.
Footnotes
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.joa.2016.07.008.
Appendix A. Supplementary material
Supplementary material
Supplementary material
References
- 1.Feinberg W.M., Blackshear J.L., Laupacis A. Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch Intern Med. 1995;155:469–473. [PubMed] [Google Scholar]
- 2.Go A.S., Hylek E.M., Phillips K.A. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and risk factors in atrial fibrillation (ATRIA) study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 3.Wolf P.A., Abbott R.D., Kannel W.B. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke; a J Cereb Circ. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 4.Furberg C.D., Psaty B.M., Manolio T.A. Prevalence of atrial fibrillation in elderly subjects (the Cardiovascular Health Study) Am J Cardiol. 1994;74:236–241. doi: 10.1016/0002-9149(94)90363-8. [DOI] [PubMed] [Google Scholar]
- 5.Majeed A., Moser K., Carroll K. Trends in the prevalence and management of atrial fibrillation in general practice in England and Wales, 1994-1998: analysis of data from the general practice research database. Heart. 2001;86:284–288. doi: 10.1136/heart.86.3.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heeringa J., van der Kuip D.A., Hofman A. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur heart J. 2006;27:949–953. doi: 10.1093/eurheartj/ehi825. [DOI] [PubMed] [Google Scholar]
- 7.Ohsawa M., Okayama A., Sakata K. Rapid increase in estimated number of persons with atrial fibrillation in Japan: an analysis from national surveys on cardiovascular diseases in 1980, 1990 and 2000. J Epidemiol / Jpn Epidemiol Assoc. 2005;15:194–196. doi: 10.2188/jea.15.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inoue H., Fujiki A., Origasa H. Prevalence of atrial fibrillation in the general population of Japan: an analysis based on periodic health examination. Int J Cardiol. 2009;137:102–107. doi: 10.1016/j.ijcard.2008.06.029. [DOI] [PubMed] [Google Scholar]
- 9.Iguchi Y., Kimura K., Aoki J. Prevalence of atrial fibrillation in community-dwelling Japanese aged 40 years or older in Japan: analysis of 41,436 non-employee residents in Kurashiki-city. Circ J. 2008;72:909–913. doi: 10.1253/circj.72.909. [DOI] [PubMed] [Google Scholar]
- 10.Ohlmeier C., Mikolajczyk R., Haverkamp W. Incidence, prevalence, and antithrombotic management of atrial fibrillation in elderly Germans. Europace. 2013;15:1436–1444. doi: 10.1093/europace/eut048. [DOI] [PubMed] [Google Scholar]
- 11.Wyse D.G., Waldo A.L., DiMarco J.P. A comparison of rate control and rhythm control in patients with atrial fibrillation. New Engl J Med. 2002;347:1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 12.Hohnloser S.H., Kuck K.H., Lilienthal J. Rhythm or rate control in atrial fibrillation--Pharmacological Intervention in Atrial Fibrillation (PIAF): a randomised trial. Lancet. 2000;356:1789–1794. doi: 10.1016/s0140-6736(00)03230-x. [DOI] [PubMed] [Google Scholar]
- 13.Van Gelder I.C., Hagens V.E., Bosker H.A. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. New Engl J Med. 2002;347:1834–1840. doi: 10.1056/NEJMoa021375. [DOI] [PubMed] [Google Scholar]
- 14.Flaker G.C., Blackshear J.L., McBride R. Antiarrhythmic drug therapy and cardiac mortality in atrial fibrillation. The Stroke Prevention in Atrial Fibrillation Investigators. J Am Coll Cardiol. 1992;20:527–532. doi: 10.1016/0735-1097(92)90003-6. [DOI] [PubMed] [Google Scholar]
- 15.Kannel W.B., Wolf P.A., Benjamin E.J. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82:2N–9N. doi: 10.1016/s0002-9149(98)00583-9. [DOI] [PubMed] [Google Scholar]
- 16.Miller P.S., Andersson F.L., Kalra L. Are cost benefits of anticoagulation for stroke prevention in atrial fibrillation underestimated? Stroke. 2005;36:360–366. doi: 10.1161/01.STR.0000153002.56324.8c. [DOI] [PubMed] [Google Scholar]
- 17.Connolly S.J., Ezekowitz M.D., Yusuf S. Dabigatran versus warfarin in patients with atrial fibrillation. New Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 18.Granger C.B., Alexander J.H., McMurray J.J. Apixaban versus warfarin in patients with atrial fibrillation. New Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 19.Patel M.R., Mahaffey K.W., Garg J. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. New Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 20.Giugliano R.P., Ruff C.T., Braunwald E. Edoxaban versus warfarin in patients with atrial fibrillation. New Engl J Med. 2013;369:2093–2104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 21.Connolly S., Pogue J., Hart R. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet. 2006;367:1903–1912. doi: 10.1016/S0140-6736(06)68845-4. [DOI] [PubMed] [Google Scholar]
- 22.Adjusted-dose warfarin versus low-intensity, fixed-dose warfarin plus aspirin for high-risk patients with atrial fibrillation: Stroke Prevention in Atrial Fibrillation III randomised clinical trial. Lancet. 1996;348:633–638. [PubMed] [Google Scholar]
- 23.Stroke prevention in atrial fibrillation study. Final results. Circulation. 1991;84:527–539. doi: 10.1161/01.cir.84.2.527. [DOI] [PubMed] [Google Scholar]
- 24.The effect of low-dose warfarin on the risk of stroke in patients with nonrheumatic atrial fibrillation. The Boston Area Anticoagulation Trial for Atrial Fibrillation Investigators. New Engl J Med. 1990;323:1505–1511. doi: 10.1056/NEJM199011293232201. [DOI] [PubMed] [Google Scholar]
- 25.Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. EAFT (European Atrial Fibrillation Trial) study group. Lancet. 1993;342:1255–1262. [PubMed] [Google Scholar]
- 26.Determinants of warfarin use and international normalized ratio levels in atrial fibrillation patients in Japan. Subanalysis of the J-RHYTHM registry. Circ J. 2011;75:2357–2362. doi: 10.1253/circj.cj-11-0427. [DOI] [PubMed] [Google Scholar]
- 27.Inoue H., Okumura K., Atarashi H. Target international normalized ratio values for preventing thromboembolic and hemorrhagic events in Japanese patients with non-valvular atrial fibrillation: results of the J-RHYTHM Registry. Circ J. 2013;77:2264–2270. doi: 10.1253/circj.cj-13-0290. [DOI] [PubMed] [Google Scholar]
- 28.Akao M., Chun Y.H., Wada H. Current status of clinical background of patients with atrial fibrillation in a community-based survey: the Fushimi AF Registry. J Cardiol. 2013;61:260–266. doi: 10.1016/j.jjcc.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Furusho H., Takamura M., Takata S. Current status of anticoagulation therapy for elderly atrial fibrillation patients in Japan: from Hokuriku atrial fibrillation trial. Circ J. 2008;72:2058–2061. doi: 10.1253/circj.cj-08-0290. [DOI] [PubMed] [Google Scholar]
- 30.McBride D., Bruggenjurgen B., Roll S. Anticoagulation treatment for the reduction of stroke in atrial fibrillation: a cohort study to examine the gap between guidelines and routine medical practice. J Thromb thrombolysis. 2007;24:65–72. doi: 10.1007/s11239-006-0002-8. [DOI] [PubMed] [Google Scholar]
- 31.Hankey G.J., Stevens S.R., Piccini J.P. Intracranial hemorrhage among patients with atrial fibrillation anticoagulated with warfarin or rivaroxaban: the rivaroxaban once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation. Stroke. 2014;45:1304–1312. doi: 10.1161/STROKEAHA.113.004506. [DOI] [PubMed] [Google Scholar]
- 32.Wong K.S., Hu D.Y., Oomman A. Rivaroxaban for stroke prevention in East Asian patients from the ROCKET AF trial. Stroke. 2014;45:1739–1747. doi: 10.1161/STROKEAHA.113.002968. [DOI] [PubMed] [Google Scholar]
- 33.Hori M., Connolly S.J., Zhu J. Dabigatran versus warfarin: effects on ischemic and hemorrhagic strokes and bleeding in Asians and non-Asians with atrial fibrillation. Stroke. 2013;44:1891–1896. doi: 10.1161/STROKEAHA.113.000990. [DOI] [PubMed] [Google Scholar]
- 34.Hart R.G., Diener H.C., Yang S. Intracranial hemorrhage in atrial fibrillation patients during anticoagulation with warfarin or dabigatran: the RE-LY trial. Stroke. 2012;43:1511–1517. doi: 10.1161/STROKEAHA.112.650614. [DOI] [PubMed] [Google Scholar]
- 35.Fuster V., Ryden L.E., Cannom D.S. ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2011;2011(123):e269–e367. doi: 10.1161/CIR.0b013e318214876d. [DOI] [PubMed] [Google Scholar]
- 36.Hart R.G., Benavente O., McBride R. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann Intern Med. 1999;131:492–501. doi: 10.7326/0003-4819-131-7-199910050-00003. [DOI] [PubMed] [Google Scholar]
- 37.Shen A.Y., Yao J.F., Brar S.S. Racial/ethnic differences in the risk of intracranial hemorrhage among patients with atrial fibrillation. J Am Coll Cardiol. 2007;50:309–315. doi: 10.1016/j.jacc.2007.01.098. [DOI] [PubMed] [Google Scholar]
- 38.Ogilvie I.M., Newton N., Welner S.A. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med. 2010;123 doi: 10.1016/j.amjmed.2009.11.025. 638-45. e4. [DOI] [PubMed] [Google Scholar]
- 39.Diener H.C., Connolly S.J., Ezekowitz M.D. Dabigatran compared with warfarin in patients with atrial fibrillation and previous transient ischaemic attack or stroke: a subgroup analysis of the RE-LY trial. Lancet Neurol. 2010;9:1157–1163. doi: 10.1016/S1474-4422(10)70274-X. [DOI] [PubMed] [Google Scholar]
- 40.Easton J.D., Lopes R.D., Bahit M.C. Apixaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of the ARISTOTLE trial. Lancet Neurol. 2012;11:503–511. doi: 10.1016/S1474-4422(12)70092-3. [DOI] [PubMed] [Google Scholar]
- 41.Halvorsen S., Atar D., Yang H. Efficacy and safety of apixaban compared with warfarin according to age for stroke prevention in atrial fibrillation: observations from the ARISTOTLE trial. Eur heart J. 2014;35:1864–1872. doi: 10.1093/eurheartj/ehu046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Halperin J.L., Hankey G.J., Wojdyla D.M. Efficacy and safety of rivaroxaban compared with warfarin among elderly patients with nonvalvular atrial fibrillation in the rivaroxaban once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation (ROCKET AF) Circulation. 2014;130:138–146. doi: 10.1161/CIRCULATIONAHA.113.005008. [DOI] [PubMed] [Google Scholar]
- 43.Hohnloser S.H., Hijazi Z., Thomas L. Efficacy of apixaban when compared with warfarin in relation to renal function in patients with atrial fibrillation: insights from the ARISTOTLE trial. Eur heart J. 2012;33:2821–2830. doi: 10.1093/eurheartj/ehs274. [DOI] [PubMed] [Google Scholar]
- 44.Fox K.A., Piccini J.P., Wojdyla D. Prevention of stroke and systemic embolism with rivaroxaban compared with warfarin in patients with non-valvular atrial fibrillation and moderate renal impairment. Eur heart J. 2011;32:2387–2394. doi: 10.1093/eurheartj/ehr342. [DOI] [PubMed] [Google Scholar]
- 45.Hori M., Matsumoto M., Tanahashi N. Rivaroxaban vs. warfarin in Japanese patients with atrial fibrillation - the J-ROCKET AF study. Circ J. 2012;76:2104–2111. doi: 10.1253/circj.cj-12-0454. [DOI] [PubMed] [Google Scholar]
- 46.Hori M., Matsumoto M., Tanahashi N. Rivaroxaban vs. warfarin in Japanese patients with non-valvular atrial fibrillation in relation to age. Circ J. 2014;78:1349–1356. doi: 10.1253/circj.cj-13-1324. [DOI] [PubMed] [Google Scholar]
- 47.Ogawa S., Shinohara Y., Kanmuri K. Safety and efficacy of the oral direct factor xa inhibitor apixaban in Japanese patients with non-valvular atrial fibrillation. -The ARISTOTLE-J study. Circ J. 2011;75:1852–1859. doi: 10.1253/circj.cj-10-1183. [DOI] [PubMed] [Google Scholar]
- 48.Hori M., Connolly S.J., Ezekowitz M.D. Efficacy and safety of dabigatran vs. warfarin in patients with atrial fibrillation--sub-analysis in Japanese population in RE-LY trial. Circ J. 2011;75:800–805. doi: 10.1253/circj.cj-11-0191. [DOI] [PubMed] [Google Scholar]
- 49.Koretsune Y., Yamashita T., Kimura T. Short-term safety and plasma concentrations of edoxaban in japanese patients with non-valvular atrial fibrillation and severe renal impairment. Circ J. 2015;79:1486–1495. doi: 10.1253/circj.CJ-14-0942. [DOI] [PubMed] [Google Scholar]
- 50.Atarashi H., Inoue H., Okumura K. Present status of anticoagulation treatment in Japanese patients with atrial fibrillation: a report from the J-RHYTHM Registry. Circ J. 2011;75:1328–1333. doi: 10.1253/circj.cj-10-1119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material