Abstract
Background
Little is known about physiological anticoagulation effects via antithrombin III (AT III) and protein C/S (PC/PS) in patients using new oral anticoagulants (NOACs).
Methods
We evaluated 120 consecutive patients with non-valvular atrial fibrillation (AF) receiving NOACs. Patients were randomly divided into three groups: a dabigatran group (DG, N=40), a rivaroxaban group (RG, N=40) or an apixaban group (AG, N=40). A warfarin group (WG, N=40) was matched with NOAC groups for age, sex and type of AF during the same time period. Blood samples were obtained in pretreatment, trough and peak phases to measure the activity of physiological coagulation inhibitors, including AT III and PC/PS or thrombus formation markers such as D-dimer and thrombin–antithrombin complex (TAT).
Results
D-dimer, TAT and AT III values for the NOAC groups were equivalent in the peak and trough phases. PC/PS activity in both phases was equally maintained in the pretreatment phase in the NOAC groups, while the activity in the WG was significantly suppressed in steady state. Moreover, no differences in trends for PC/PS activity were observed among NOAC groups.
Conclusions
PC/PS activity was constant in both peak and trough phases in the patients on NOACs compared with activity of those on warfarin. In addition, there was no difference in the findings among NOACs.
Keywords: Novel oral anticoagulant, Atrial fibrillation, Antithrombin III, Protein C/S
1. Introduction
Anticoagulation therapy for patients with atrial fibrillation (AF) is essential for prophylaxis against ischemic stroke and systemic embolism [1], [2]. There has been a rapid shift in anticoagulants used for this purpose, from conventional anticoagulants, vitamin K antagonists (VKA) to novel oral anticoagulants (NOACs) [3]. NOACs include the direct thrombin inhibitor, dabigatran, and factor Xa (FXa) inhibitors, rivaroxaban and apixaban. Recently published randomized clinical trials have supported the efficacy and safety of NOACs compared with the VKA, warfarin [4], [5], [6].
Warfarin acts as an anticoagulant by inhibiting the production of the vitamin K-dependent coagulation factors II, VII, IX, and X. In contrast, NOACs selectively and reversibly target thrombin or FXa. Additionally, NOACs have a rapid onset and short half-lives. This causes fluctuations in their effects between peak and trough phases compared with warfarin, which develops a constant anticoagulation effect throughout the entire day [7]. The aforementioned clinical trials have demonstrated a similar incidence of stroke and systemic embolism despite the unique pharmacological features of NOACs [4], [5], [6]. Meanwhile, Protein C/Protein S (PC/PS) and AT III have additional antithrombotic effects as physiological anticoagulation factors. However, little is known about whether NOAC use has influence on trends in these physiological anticoagulant factors. Therefore, the purpose of the study is to reveal the trends in physiological inhibitors such as AT III, PC or PS, and markers of thrombus formation in patients receiving NOACs compared with those using warfarin.
2. Methods
2.1. Study population
We prospectively investigated 120 consecutive patients with non-valvular AF who were prescribed NOACs at the Chubu Rosai Hospital between April 2015 and May 2016. The 120 patients were randomly divided into three groups: dabigatran group (DG, N=40), rivaroxaban group (RG, N=40), or apixaban group (AG, N=40). This study was approved by our Institutional Committee on Human Research. In addition, all patients provided written informed consent for study participation. Exclusion criteria were as follows: patients with congenital coagulation defects or creatinine clearance (Ccr)<30 mL/min. Ccr was determined using the Cockcroft Gault formula. The dabigatran dose was decided according to the renal function or age of patients. A low dose of dabigatran (110 mg twice daily) was administered to patients who had the following conditions: moderate renal dysfunction (Ccr 30–50 mL/min), advanced age (≥70 years), a history of upper gastrointestinal ulcer, or co-administration of glycoprotein inhibitors (amiodarone or verapamil). A low dose of rivaroxaban (10 mg once daily) was administrated to patients with mild renal dysfunction (Ccr 30–50 mL/min). The apixaban dose was decided according to age, body weight, or renal function. A low apixaban dose (2.5 mg twice daily) was administered to patients with any two of the following characteristics: advanced age (≥80 years), renal dysfunction (serum creatinine concentration ≥0.5 mg/dL) and lower body weight (≤60 kg). The patients in the RG were administrated rivaroxaban as a morning dose. The warfarin group (WG) consisted of the same number of patients as each NOAC group, matched for age, sex, and type of AF during the same time period. The warfarin dose was adjusted to maintain a target international normalized ratio (INR) of 1.6–2.6 for older (≥70 years) and 2.0–3.0 for younger individuals (<70 years).
3. Blood sampling
Blood samples for NOAC groups were obtained from each patient before beginning the administration, immediately before morning dose (trough phase) and approximately 3 hours after they had received the anticoagulants (peak phase) when the peak plasma concentration of NOACs had been reached.
In addition, the samples in the peak and trough phases were collected >7 days after the start of anticoagulant therapy as a steady state measurement in the DG, RG, or AG, while the samples in the WG were obtained at random times. Measurement parameters in each phase included prothrombin time (PT), activated partial thromboplastin time (APTT), D-dimer, thrombin–antithrombin complex (TAT), AT III, and PC/PS, which were compared across phases and anticoagulant groups. In tests of blood coagulation, values for PT and APTT were obtained using Thromborel S® and Thrombocheck aPTT-SLA® as the reagent, respectively. D-dimer or TAT was determined using a quantitative latex agglutination assay or enzyme immunoassay, respectively. AT III, PC or PS activity was measured using the Factor Xa-based method, chromogenic method, or free protein S antigen latex immunoassay method, respectively.
3.1. Statistical analysis
All continuous variables were expressed as mean±SD or as median and interquartile ranges. All categorical variables were reported as number (percentage) of patients. A paired Student׳s t test, Mann–Whitney U test, one-way analysis of variance (ANOVA), or Kruskal–Wallis test was used to compare the continuous variables, and categorical variables were compared using a chi-square or Fisher exact test. Differences were considered statistically significant at P<0.05. All the results were analyzed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA).
4. Results
4.1. Patients’ characteristics
Blood samples were collected from a total of 120 patients using NOACs. Groups of 40 patients were allocated to the DG, RG, or AG. The 40 patients receiving warfarin were extracted matched for age, sex and type of AF with the patients in each NOAC group during the same time period. The baseline characteristics of each group are summarized in Table 1. Overall, no significant difference in any factor was observed among the groups except for the data on the rate of low-dose treatments.
Table 1.
Patient characteristics.
| DG | RG | AG | WG | P value | |
|---|---|---|---|---|---|
| N=40 | N=40 | N=40 | N=40 | ||
| Age (years) | 69±8 | 70±7 | 70±6 | 69±10 | 0.85 |
| Sex (female) | 10 (25) | 10 (25) | 13 (33) | 12 (30) | 0.84 |
| Body weight (kg) | 60±18 | 58±11 | 58±11 | 56±7 | 0.79 |
| Paroxysmal AF | 20 (50) | 18 (45) | 19 (48) | 18 (36) | 0.98 |
| Coronary artery disease | 9 (23) | 9 (24) | 8 (20) | 7 (18) | 0.94 |
| Hypertension | 21 (53) | 16 (40) | 23 (58) | 18 (45) | 0.41 |
| Diabetes mellitus | 15 (38) | 8 (20) | 10 (25) | 10 (25) | 0.21 |
| History of heart failure | 6 (15) | 7 (18) | 3 (8) | 4 (10) | 0.52 |
| Prior stroke/TIA | 5 (13) | 3 (8) | 3 (8) | 4 (10) | 0.85 |
| CHADS2 score | 1.7±1.5 | 1.6±1.1 | 1.8±1.2 | 1.6±1.3 | 0.94 |
| 0 | 5 (13) | 6 (15) | 4 (10) | 8 (20) | 0.62 |
| 1 | 12 (30) | 13 (33) | 11 (28) | 12 (30) | 0.97 |
| ≥2 | 23 (58) | 21 (53) | 25 (63) | 20 (50) | 0.32 |
| CHA2DS2-VASc score | 3.2±2.0 | 2.8±1.3 | 3.2±1.5 | 2.2±1.6 | 0.26 |
| LA size (mm) | 41±4 | 43±6 | 44±5 | 44±7 | 0.53 |
| LVEF (%) | 68±7 | 59±10 | 67±10 | 65±6 | 0.17 |
| BNP (pg/mL) | 95 (15, 286) | 194 (87, 513) | 140 (90, 232) | 149 (46, 300) | 0.41 |
| Ccr (mL/min) | 59±13 | 59±11 | 54±14 | 54±14 | 0.61 |
| Low dose | 28 (70) | 20 (50) | 10 (25) | – | <0.001 |
Values are the mean±standard deviations (SD) or n (%). Abbreviations: WG, warfarin group; DG, dabigatran group; RG, rivaroxaban group; AG, apixaban group; AF, atrial fibrillation; TIA, transient ischemic attack; LA, left atrium; LVEF, left ventricular ejection fraction; BNP, brain natriuretic peptide; Ccr, creatinine clearance; PT, prothrombin time; INR, international normalized ratio; APTT, activated partial thromboplastin time.
4.2. Trends in the coagulation markers in each anticoagulant group
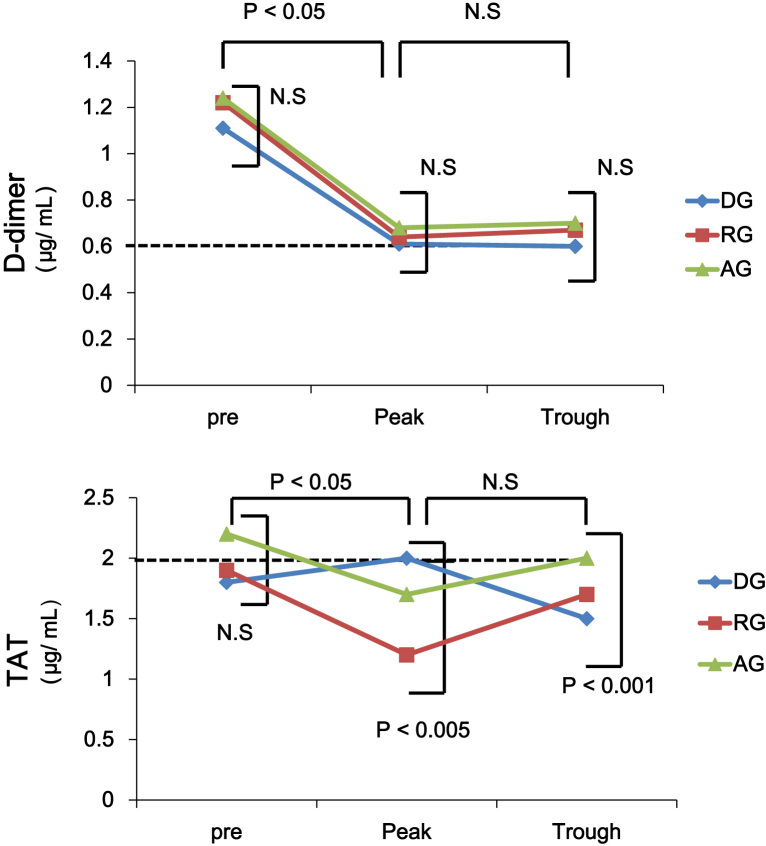
Trends for coagulation markers in each anticoagulant group are shown in Table 2. In the peak phase, the PT value for the RG and WG was longer than that of the DG and AG (13±2 s, 17±2 s, 13±1 s and 27±4 s in the DG, RG, AG and WG, respectively; Table 2) while the APTT for the DG and RG was longer than that of the other groups (46±3 s, 47±5 s, 35±2 s, and 41±3 s in the DG, RG, AG, and WG, respectively; Table 2). Moreover, APTT values in the DG and RG in the peak phase were significantly longer than those in the pretreatment phase or trough phase (46±3 s, 28±2 s, 38±5 s and 47±5 s, 27±3 s, 33±4 s in the DG and RG, respectively; Table 2). The mean INR was 2.2±0.1 in the WG (Table 2). D-dimer levels were equivalent in all phases among all the groups (Table 2, Fig. 1). In the RG, the TAT value in the peak phase was lower than that of the other groups (1.2±0.4 μg/L, 2.0±0.5 μg/L, 1.7±0.5 μg/L, and 2.0±0.7 μg/L in the RG, DG, AG, and WG, respectively; Table 2) while TAT in the trough phase was lower in the DG than in the other groups, shown in Table 2 (1.5±0.2 μg/L, 1.7±0.6 μg/L, 2.0±0.7 μg/L, and 2.0±0.7 μg/L in the DG, RG, AG, and WG, respectively). No significant differences in D-dimer and TAT were observed between the pretreatment phase and peak/trough phases in any of the NOAC groups (Fig. 1).
Table 2.
Trends in coagulation markers among anticoagulants.
| DG | RG | AG | WG | P value | ||
|---|---|---|---|---|---|---|
| N=40 | N=40 | N=40 | N=40 | |||
| PT (s) | Pre | 12±3 | 12±4 | 11±3 | – | 0.10 |
| Peak | 13±2 | 17±2 | 13±1 | 27±4 | <0.001 | |
| Trough | 12±1 | 13±1 | 14±2 | 27±4 | <0.001 | |
| PT-INR | – | – | – | – | 2.2±0.1 | – |
| APTT (s) | Pre | 28±2 | 27±3 | 28±3 | – | 0.87 |
| Peak | 46±3 | 47±5 | 35±2 | 41±3 | <0.001 | |
| Trough | 38±5 | 33±4 | 33±4 | 41±3 | <0.001 | |
| D-dimer (μg/mL) | Pre | 1.1±0.2 | 1.2±0.5 | 1.2±0.7 | – | 0.28 |
| Peak | 0.6±0.7 | 0.6±0.3 | 0.7±0.3 | 0.6±0.2 | 0.31 | |
| Trough | 0.6±0.4 | 0.7±0.2 | 0.7±0.2 | 0.6±0.2 | 0.37 | |
| TAT (μg/L) | Pre | 1.8±0.2 | 1.9±0.7 | 2.2±0.7 | – | 0.47 |
| Peak | 2.0±0.5 | 1.2±0.4 | 1.7±0.5 | 2.0±0.7 | <0.005 | |
| Trough | 1.5±0.2 | 1.7±0.6 | 2.0±0.7 | 2.0±0.7 | <0.001 | |
| AT III (%) | Pre | 94±10 | 98±12 | 94±9 | – | 0.39 |
| Peak | 110±8 | 121±19 | 127±16 | 101±10 | <0.005 | |
| Trough | 96±4 | 96±10 | 105±7 | 101±10 | 0.08 | |
| PC (%) | Pre | 94±15 | 98±16 | 105±12 | – | 0.46 |
| Peak | 117±10 | 103±10 | 100±15 | 51±7 | <0.001 | |
| Trough | 104±8 | 107±20 | 101±12 | 51±7 | 0.90 | |
| PS (%) | Pre | 90±14 | 91±8 | 89±9 | – | 0.82 |
| Peak | 88±4 | 84±10 | 88±8 | 43±4 | <0.001 | |
| Trough | 80±6 | 89±11 | 85±10 | 43±4 | <0.001 |
Values are the mean±standard deviations (SD). Data in the WG are values in steady state, Abbreviations: DG, dabigatran group; RG, rivaroxaban group; AG, apixaban group; PT, prothrombin time; APTT, activated partial thromboplastin time; TAT, thrombin–antithrombin complex; AT III, antithrombin III; PC, protein C; PS, protein S.
Fig. 1.
Trends in D dimer, TAT in patients for each anticoagulant group in the pretreatment, peak, and trough phase. A dotted line shows the value in the WG. DG, dabigatran group; RG, rivaroxaban group; AG, apixaban group; WG, warfarin group; TAT, thrombin–antithrombin complex.
Meanwhile, AT III activity in the WG in the peak and trough phases was lower than that in the NOAC groups (Table 2). PC/PS activity was demonstrated for each group (Table 2). In the NOAC groups, the activity in the peak/trough phases was equal to that in the pretreatment phase while the value for the WG was significantly suppressed as compared with that of the NOAC groups as shown in Table 2. Additionally, there was no difference in physiological markers including AT III, PC, and PS activity among NOAC groups (Table 2). In comparison between low and high dose groups within each NOAC group, the only differences found was for D-dimer values in the peak/trough phases in the DG and AT III activity in the peak/trough phases in the RG and AG (Table 3).
Table 3.
Comparison of trends in coagulation markers between high and low dose group in each NOAC group.
| DG N=40 |
||||
|---|---|---|---|---|
| HG, N=12 | LG, N=28 | P value | ||
| PT (s) | Pre | 12±3 | 12±1 | 0.62 |
| Peak | 14±2 | 13±2 | 0.16 | |
| Trough | 12±2 | 12±1 | 0.77 | |
| APTT (s) | Pre | 28±4 | 29±6 | 0.56 |
| Peak | 48±4 | 45±8 | 0.34 | |
| Trough | 37±11 | 38±4 | 0.41 | |
| D-dimer (μg/mL) | Pre | 1.2±0.1 | 1.1±0.2 | 0.29 |
| Peak | 0.8±1.1 | 0.5±0.3 | <0.005 | |
| Trough | 0.6±0.4 | 0.7±0.2 | <0.005 | |
| TAT (μg/L) | Pre | 2.0±0.5 | 1.9±0.5 | 0.44 |
| Peak | 1.8±0.6 | 2.1±0.4 | 0.32 | |
| Trough | 1.3±0.2 | 1.4±0.3 | 0.06 | |
| AT III (%) | Pre | 98±13 | 91±14 | 0.30 |
| Peak | 107±15 | 103±23 | 0.50 | |
| Trough | 97±4 | 95±8 | 0.38 | |
| PC (%) | Pre | 102±16 | 105±12 | 0.19 |
| Peak | 118±11 | 116±12 | 0.80 | |
| Trough | 103±15 | 109±14 | 0.59 | |
| PS (%) | Pre | 89±12 | 95±11 | 0.18 |
| Peak | 95±4 | 76±16 | 0.15 | |
| Trough | 82±6 | 81±10 | 0.37 | |
| RG, N=40 |
||||
|---|---|---|---|---|
| HG, N=20 | LG, N=20 | P value | ||
| PT (s) | Pre | 13±3 | 12±1 | 0.35 |
| Peak | 18±2 | 15±2 | 0.23 | |
| Trough | 13±1 | 13±2 | 0.88 | |
| APTT (s) | Pre | 32±4 | 31±3 | 0.94 |
| Peak | 46±4 | 48±8 | 0.63 | |
| Trough | 33±5 | 34±4 | 0.89 | |
| D-dimer (μg/mL) | Pre | 1.3±0.2 | 1.2±0.5 | 0.48 |
| Peak | 0.7±1.1 | 0.6±0.3 | 0.41 | |
| Trough | 0.5±0.4 | 0.7±0.2 | 0.09 | |
| TAT (μg/L) | Pre | 2.0±0.5 | 1.8±0.7 | 0.58 |
| Peak | 1.2±0.9 | 1.3±0.4 | 0.71 | |
| Trough | 1.7±0.2 | 1.6±0.4 | 0.87 | |
| AT III (%) | Pre | 104±13 | 109±10 | 0.69 |
| Peak | 136±15 | 106±23 | <0.05 | |
| Trough | 104±4 | 90±10 | 0.17 | |
| PC (%) | Pre | 102±16 | 97±20 | 0.66 |
| Peak | 105±8 | 107±10 | 0.77 | |
| Trough | 119±15 | 107±25 | 0.32 | |
| PS (%) | Pre | 74±11 | 81±10 | 0.23 |
| Peak | 78±6 | 91±10 | 0.18 | |
| Trough | 85±7 | 94±18 | 0.26 | |
| AG, N=40 |
||||
|---|---|---|---|---|
| HG, N=30 | LG, N=10 | P value | ||
| PT (s) | Pre | 11±3 | 11±1 | 0.66 |
| Peak | 14±2 | 12±2 | 0.16 | |
| Trough | 14±1 | 14±1 | 0.70 | |
| APTT (s) | Pre | 28±4 | 29±6 | 0.94 |
| Peak | 37±4 | 34±8 | 0.18 | |
| Trough | 35±5 | 31±4 | 0.15 | |
| D-dimer (μg/mL) | Pre | 1.2±0.1 | 1.2±0.2 | 0.71 |
| Peak | 0.8±1.1 | 0.9±0.3 | 0.41 | |
| Trough | 0.9±0.4 | 1.0±0.2 | 0.45 | |
| TAT (μg/L) | Pre | 2.2±0.5 | 2.1±0.8 | 0.59 |
| Peak | 1.6±0.5 | 1.9±0.4 | 0.49 | |
| Trough | 2.0±0.2 | 2.0±0.6 | 0.91 | |
| AT III (%) | Pre | 94±13 | 94±17 | 0.98 |
| Peak | 141±10 | 115±15 | <0.05 | |
| Trough | 141±4 | 112±14 | <0.005 | |
| PC (%) | Pre | 104±12 | 107±15 | 0.60 |
| Peak | 104±12 | 98±10 | 0.38 | |
| Trough | 100±16 | 103±20 | 0.51 | |
| PS (%) | Pre | 91±10 | 89±11 | 0.21 |
| Peak | 88±10 | 95±16 | 0.10 | |
| Trough | 80±6 | 92±10 | 0.21 | |
Values are the mean±standard deviations (SD). Abbreviations: DG, dabigatran group; RG, rivaroxaban group; AG, apixaban group; HG, high dose group; LG, low dose group; PT, prothrombin time; APTT, activated partial thromboplastin time; TAT, thrombin–antithrombin complex; AT III, antithrombin III; PC, protein C; PS, protein S.
5. Discussion
5.1. Main findings
The present study has demonstrated that the effects of physiological factors including PC/PS, in patients using NOACs were constantly maintained in both the peak and trough phases of the steady state condition compared with those of patients of receiving warfarin. In addition, no difference in trends for these factors was observed among NOAC groups.
5.2. Monitoring of anticoagulant effects in patients treated with NOACs
Conventional anticoagulation tests, PT and APTT are known to be suboptimal for evaluating the anticoagulation effects of NOACs. These methods are still inadequate for precise measurements and the sensitivity varies among the reagents used in the tests [8], [9], [10], [11]. Meanwhile, reports that anti-Xa activity or the level of prothrombin fragment 1+2 reflects the anticoagulation effects of apixaban or rivaroxaban have been presented recently, which might lead to the daily clinical application of these tests [12], [13]. At present, diluted thrombin time or ecarin clotting time is reported to be useful in patients receiving dabigatran, but these might not be practical methods for use as high-specificity laboratory tests [14]. Simple methods for estimating the anticoagulation effects of NOACs at low cost are desirable in patients treated with NOACs.
5.3. Role of physiological factors in patients with NOACs
Data on the role of physiological anticoagulant factors including AT III or PC/PS are currently limited in patients receiving NOACs. AT III is an inhibitory physiological anticoagulation factor. Its primary action is to inhibit both thrombin and FXa by lysing them, which prevents blood coagulation. The present results showed that AT III activity in the NOAC groups was equivalently maintained in all phases indicating that the use of NOACs has no significant effect on AT III activity. PC/PS is an important physiological anticoagulation factor. PC is rapidly converted to activated PC by the thrombomodulin–thrombin complex using PS as a coenzyme. Finally, PC hinders both factors V and VIII [15]. Moreover, PC/PS is inactivated early by the VKA, warfarin, which leads to the incidence of thromboembolic events, especially in induction. The present study showed that PC/PS activity in patients treated with NOACs was equal and maintained in all phases although the activity was inactivated by warfarin (Table 2). These results suggest that NOAC use might decrease the occurrence of embolism in the induction period of anticoagulants compared with warfarin use.
5.4. Trends in thrombus formation makers in each phase
D-dimer and TAT measurements used in the present study are available as markers of thrombus formation in clinical practice. D-dimer is an established marker that reflects the generation of fibrin or thrombin. An increase in the value has been reported to predict thromboembolic events in patients with non-valvular AF [16]. The TAT measurement is clinically essential for the diagnosis of thromboembolic events, as is D-dimer. Elevated concentrations of TAT are found in patients predisposed to thrombosis. The present study has shown that both D-dimer and TAT values decreased in the pretreatment phase with the induction of each anticoagulant in a steady state (Fig. 1). In addition, there were no differences in D-dimer value among the NOACs or between NOAC groups and the WG for both the peak and trough phases (Fig. 1, Table 2). The results demonstrate that optimal anticoagulation conditions might be maintained with proper NOAC use, as with warfarin use (Table 2). In comparison among NOAC groups, a slight increase in the TAT value in the peak phase was observed only in the DG, although the rise was not statistically significant. Furugohri et al. demonstrated that use of a direct thrombin inhibitor could cause inhibition of the negative-feedback system involving thrombin, thrombomodulin, and PC, which leads to the enhancement of thrombin generation [17]. Moreover, the phenomenon was not observed with FXa inhibitor use [17]. On the other hand, the RE-LY trial showed that dabigatran slightly increased the incidence of myocardial infarction (MI) compared with warfarin (0.74% per year vs. 0.53% per year) [4]. However, the results showing that dabigatran leads to MI are still questionable considering the data for Asian patients [18]. In the present study, the increase in the TAT value for the DG might reflect the result of the RE-LY trial4. However, the rise did not lead to an increase in D-dimer value (Fig. 1). Therefore, the increase in TAT in the peak phase might have little impact in clinical practice with regard to the occurrence of thromboembolic events.
5.5. Limitations
This study was performed in a single center. In addition, the sample size was small in each anticoagulant group. Thus, the results should be interpreted along with previously published outcomes. Secondly, several of the values for coagulation factors measured in the present study are affected by the coagulation assays used in institutions. PT and APTT values are especially variable depending on the reagents. AT III values might undergo interference because of the FXa-based assay used in the RG and AG. Therefore, the result needs to be interpreted cautiously. Thirdly, blood sampling in the peak phase was performed three hours after the administration of NOACs. The timing might be different from the actual peak phase because trends in the blood concentration of NOACs after intake vary slightly among individuals.
6. Conclusions
This prospective study showed that PC/PS activity was maintained in both the peak and trough phases as in the pretreatment phase for patients using NOACs compared with those using warfarin. Additionally, there was no difference in the effects of these physiological anticoagulation factors among NOACs.
Funding sources
None declared.
Disclosures
None declared.
Conflicts of interest
All authors declare no conflict of interest related to this study.
Acknowledgments
None declared.
References
- 1.Wolf P.A., Abbott R.D., Kannel W.B. Atrial fibrillation as an independent risk factor for stroke: the framingham study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 2.Gage B.F., Waterman A.D., Shannon W. Validation of clinical classification schemes for predicting stroke: results from the national registry of atrial fibrillation. J Am Med Assoc. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 3.Barnes G.D., Lucas E., Alexander G.C. National trends in ambulatory oral anticoagulant use. Am J Med. 2015;128(1300-5):e1302. doi: 10.1016/j.amjmed.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Connolly S.J., Ezekowitz M.D., Yusuf S. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 5.Patel M.R., Mahaffey K.W., Garg J. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 6.Granger C.B., Alexander J.H., McMurray J.J. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 7.De Caterina R., Husted S., Wallentin L. New oral anticoagulants in atrial fibrillation and acute coronary syndromes: ESC working group on thrombosis-task force on anticoagulants in heart disease position paper. J Am Coll Cardiol. 2012;59:1413–1425. doi: 10.1016/j.jacc.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Weitz J.I., Quinlan D.J., Eikelboom J.W. Periprocedural management and approach to bleeding in patients taking dabigatran. Circulation. 2012;126:2428–2432. doi: 10.1161/CIRCULATIONAHA.112.123224. [DOI] [PubMed] [Google Scholar]
- 9.Douxfils J., Mullier F., Loosen C. Assessment of the impact of rivaroxaban on coagulation assays: laboratory recommendations for the monitoring of rivaroxaban and review of the literature. Thromb Res. 2012;130:956–966. doi: 10.1016/j.thromres.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Samama M.M., Martinoli J.L., LeFlem L. Assessment of laboratory assays to measure rivaroxaban – an oral, direct factor xa inhibitor. Thromb Haemost. 2010;103:815–825. doi: 10.1160/TH09-03-0176. [DOI] [PubMed] [Google Scholar]
- 11.Kanemoto M., Kuhara H., Ueda T. Association of apixaban therapy and prothrombin time in patients with atrial fibrillation. Circ J. 2014;78:2651–2656. doi: 10.1253/circj.cj-14-0512. [DOI] [PubMed] [Google Scholar]
- 12.Tajiri K., Sato A., Harunari T. Impact of rivaroxaban compared with warfarin on the coagulation status in Japanese patients with non-valvular atrial fibrillation: a preliminary analysis of the prothrombin fragment 1+2 levels. J Cardiol. 2015;65:191–196. doi: 10.1016/j.jjcc.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Osanai H., Ajioka M., Masutomi T. Measurement of anti-factor xa activity in patients on apixaban for non-valvular atrial fibrillation. Circ J. 2015;79:2584–2590. doi: 10.1253/circj.CJ-15-0470. [DOI] [PubMed] [Google Scholar]
- 14.Ellis C.R., Kaiser D.W. The clinical efficacy of dabigatran etexilate for preventing stroke in atrial fibrillation patients. Vasc Health Risk Manag. 2013;9:341–352. doi: 10.2147/VHRM.S28271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kisiel W. Human plasma protein c: isolation, characterization, and mechanism of activation by alpha-thrombin. J Clin Investig. 1979;64:761–769. doi: 10.1172/JCI109521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nozawa T., Inoue H., Hirai T. D-dimer level influences thromboembolic events in patients with atrial fibrillation. Int J Cardiol. 2006;109:59–65. doi: 10.1016/j.ijcard.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 17.Furugohri T., Sugiyama N., Morishima Y. Antithrombin-independent thrombin inhibitors, but not direct factor xa inhibitors, enhance thrombin generation in plasma through inhibition of thrombin–thrombomodulin–protein c system. Thromb Haemost. 2011;106:1076–1083. doi: 10.1160/TH11-06-0382. [DOI] [PubMed] [Google Scholar]
- 18.Chan Y.H., Yen K.C., See L.C. Cardiovascular, bleeding, and mortality risks of dabigatran in Asians with nonvalvular atrial fibrillation. Stroke. 2016;47:441–449. doi: 10.1161/STROKEAHA.115.011476. [DOI] [PubMed] [Google Scholar]