1. Preamble
The management of acute ST-Elevation Myocardial Infarction (STEMI) has rapidly evolved worldwide during the last two decades with the better understanding of the need for early reperfusion and protocol based pharmacotherapy. Despite global agreement on most issues related to the management of STEMI, wide discrepancies exist in implementation of Western guidelines in most of the developing world. The need has been felt that every country and society should adopt the existing scientific data, in combination with local limitations and strengths, and develop protocols that work best in their community.
India, home to the world’s second largest population, is a country with extreme diversity in terms of geography, race, culture, literacy, infrastructure and economy. All these factors pose serious challenges in the management of acute diseases like STEMI. It is an important responsibility of the medical fraternity, policy makers and all concerned stake-holders to provide the best available therapeutic options in equitable fashion based on the current knowledge and available evidences. It is in this spirit that Cardiological Society of India (CSI) decided to involve leading experts of India, to prepare this ‘Position Statement for the Management of STEMI in India’.
1.1. Historical background
Nearly 3 million STEMI are estimated to occur in India per year. First attempt towards development of STEMI management protocols in India was done in the year 2011.1 Recently a consensus statement has been published jointly by STEMI-INDIA, CSI and Association of Physicians of India (API).2 The concept of “spoke and hub” has been highlighted in this document, which is based on the distance of the place from where the patient is commuting and the location of the primary, secondary or tertiary care centres. But since the whole concept revolved around few locations in India which were having advantages in terms of logistics and infrastructure, there have been challenges in it’s universal application in our diversified country.
1.2. Magnitude of the problem
As per World Health Organisation (WHO) data, the Coronary Artery Disease (CAD) prevalence continues to rise in India with rapid ‘epidemiological transition’. It has already surpassed communicable diseases as the major cause of mortality in India. It has been projected that between 1990 and 2020, there will be 117% and 105% rise in mortality from CAD in men and women respectively in India.3
The rising incidence of CAD in young Indians is of particular concern. The incidence of CAD in young population in Western countries is 2–5%, whereas it is 11–16% in Asian Indians.4 In a study of ethnic differences in patients with Myocardial Infarction(MI) in England, it was observed that young Indians had ten times more risk of developing MI as compared to the white population.5 We have old as well as recent data, especially the registries from different regions of India viz. Himachal Pradesh from North, Assam from North East (NE), Kerala and Chennai from South and multi-city, multi- hospital CREATE Registry.6, 7, 8, 9 The inferences are quite alarming: patients of acute coronary syndrome (ACS) in India have a higher proportion of STEMI as compared to developed countries. Most of these patients are from poor socio-economic status, have delayed presentation, are less likely to get evidence-based treatments and have greater 30-day mortality. Reducing the time to reach hospital and offering affordable optimal therapy could reduce morbidity and mortality.
1.3. Challenges in management of STEMI in India
Economic and geographic diversities along with infrastructural differences make the management of STEMI in India both challenging and discrepant. If this is combined with the high volume of STEMI patients, a very serious picture emerges.
India is labelled as upper low income economy country with a small section of society in the upper socio-economic strata, 18% of the population is in the middle income group category and the rest are in the low or very low income category. It has six metro cities and more than thirty big cities, mostly state capitals, which have world class healthcare facilities. But a vast majority of population lives in villages and smaller towns where only basic primary healthcare is available. Besides, there are many big and small townships, located in the hilly areas, on the sea sides, deltas or in deserts. Infrastructures especially transport systems and hospitals with modern facilities are far from optimal in these remote townships.
The health care in India is the reflection of mixed economy. Only 20% of the population has the affordability to take proper medical care either with government supported schemes or private insurance. The total spending on healthcare is around 4.6% of GDP in India which is much lesser than countries like USA (17.1%), UK (9.1%) and China (5.5%). The government contribution to healthcare is further lower i.e., around 2% of GDP and even this spending is utilized mainly for primary healthcare and communicable diseases. In this scenario, it is not difficult to understand the challenges in delivery of modern evidence based management of STEMI to the majority of the population.3
1.4. Aims and limitations of this statement
The committee constituted by CSI, involved expert cardiologists from across India. The idea was to provide a single document for the entire country that combines the latest scientific information with practical solutions to the common problems specific to our country. However, the main limitations in developing any such recommendations for India remain the lack of large volume of local data and the heterogenous nature of the healthcare delivery system.
The purpose of this position statement is recommendatory in nature and carries no statutory status.
2. Epidemiology of STEMI in India
Cardiovascular disease (CVD) is the number one cause of death in India and accounted for approximately 21% of deaths in the year 2010, with 10% of all deaths occurring due to CAD. The Global Burden of Disease study estimate of age-standardized CVD death rate is 272 per 100 000 in Indian population, which is higher than the global average of 235 per 100 000 population. The WHO estimated that with the current burden of CVD, India would lose $237 billion from the loss of productivity and spending on health care over a 10-year period (2005–2015).10
European scenario assumes that every sixth man and every seventh woman will die from MI. Data from many European nations show different incidences of STEMI in various registries. The most comprehensive data comes from the Swedish registry where the reported incidence is 66 STEMI/100 000 per year. Similar figures are also reported from Belgium and Czech Republic.11, 12 Data from the US also shows similar figures. US estimate nearly 500,000 STEMI events per year according to the NRMI-4 registry.13 Incidence of Non-STEMI is increasing and appears to be more than STEMI.14
In India, the trends are worse compared to Western nations. About 30 million individuals in India have CAD.15 In 2004, WHO reported CVD death rates for all ages as 174.7 per 100,000 in Britain, 178.8 per 100,000 in US, 279.5 per 100,000 in China, and 381.5 per 100,000 in India.10 A study done in Karnataka supported the well established fact that the mean age of occurrence of STEMI in Indians is 5–10 years lower than Western population.16 This is due to numerous risk factors like hypertension, high cholesterol, low HDL cholesterol, diabetes, truncal obesity and many genetic factors.
The INTERHEART-South Asia study identified eight coronary risk factors–abnormal lipids, smoking, hypertension, diabetes, abdominal obesity, psychosocial factors, low fruit and vegetable consumption, and lack of physical activity. These eight factors accounted for 89% of the cases of all acute MI in Indians. Further scrutiny of the INTER-HEART study revealed that Indians who developed STEMI had lower LDL cholesterol levels than others in the study. They also had lower HDL cholesterol. ApoB/ApoA1 showed the strongest association with the risk of acute MI in Indians.17 This shows that Indians deserve a separate consensus in the management of ACS as there are many unknown factors at play.
Though there are no large registries like those maintained in Western countries, we can extrapolate data and latest trends from the few but significant registries like the CREATE registry, KERALA ACS registry, OASIS −2,DEMAT registry,NE registry and Himachal Pradesh Registry.6, 7, 8, 9, 18, 19
2.1. Presentation of ACS patients as STEMI
The CREATE registry showed that 60% of ACS are constituted by STEMI, while in Kerala ACS registry STEMI constituted only 40% of all patients. In many international registries, STEMI constitutes one third of all ACS patients. The Kerala ACS Registry had a similar proportion of men as with the other Indian ACS registries but had a higher proportion of men compared with the National Cardiovascular Data Registry in the US, Euro Heart Survey ACS II registry in Europe and GRACE registry. 20, 21, 22
2.2. Case fatality rates in India
In-hospital mortality rate for STEMI in the Kerala ACS Registry (8.2%) was higher than GRACE (7%) and Euro Heart Survey ACS II (6%), but similar to CREATE (8.6%), which included mortality over 30 days.8, 9, 21, 22 The observed in-hospital mortality rates in the Kerala ACS Registry are similar to those expected after calculating average GRACE Risk Scores, ranging from 0.6 to 7% under plausible estimates of age, heart rate, systolic blood pressure, serum creatinine, Killip classification, ST segment deviation, and cardiac biomarkers.
2.3. Delayed presentation time
It is well established that prompt diagnosis and treatment can reduce mortality, improve prognosis and reduce the duration of hospital stay in patients with STEMI. Reperfusion therapy should be started as soon as possible and preferably within 90 min from first medical contact (FMC). However, the total ischemic time (time between symptom onset and reperfusion therapy) is the most important factor to achieve the best possible outcome for the patient.23 Whatever the choice of reperfusion therapy, patient decision time to seek medical help is crucial. In India various registries have shown trends of late presentation (Table 1).
Table 1.
Average time delay from the onset of symptoms to the first medical contact in the various ACS Registries from India.
2.4. Reperfusion strategies are underutilized
Reperfusion is the key strategy in acute STEMI care and it is time dependent. Shortening the time from symptom to reperfusion and choosing the optimal reperfusion strategy for STEMI patients are great challenges in practice. Thrombolytic therapy and primary Percutaneous Coronary Intervention (PCI) are two commonly used reperfusion strategies and they are conventionally viewed as mutually exclusive alternative therapeutic modalities. Thrombolysis in the setting of STEMI diagnosis was lower in the Kerala ACS Registry than in CREATE as well as GRACE registry.8, 22 Thrombolysis was used in 41% of STEMI patients, however when used,it was generally very prompt with less than one-third patient exceeding the door to needle times of more than 30 min.
Nearly half of the STEMI patients received some form of reperfusion (thrombolysis, PCI or CABG). Primary PCI in STEMI has been proven worldwide as the gold standard of treatment by way of establishing high percentage of complete and lasting reperfusion. However, this treatment modality is available to a very small proportion of STEMI patients in India.
One of the most worrisome factors in the management of STEMI is inappropriate diagnosis and thrombolysis. For instance, in the Kerala ACS registry9 19% of NSTEMI and 10% of unstable angina patients also received thrombolysis. This is much higher than what is reported in the Western population (2.5–5%).26 It is worrisome that such a practice was reported from Kerala, which is one of the most advanced states in managing ACS in India. Such inappropriate thrombolysis increases in-hospital mortality and major adverse cardiovascular events (MACE). Kerala ACS registry reported that inappropriate thrombolysis was more common in rural, non-teaching and low volume centres. Unfortunately, these centres are more likely to handle the bulk of STEMI care in a vast country like India.
2.5. Practice pattern and guideline adherence
Antiplatelet agents, anticoagulants, statins, β-blockers and angiotensin converting enzyme inhibitors(ACEIs)/angiotensin receptor blockers(ARBs)(in ACEI intolerant patients) have been shown to reduce the risk of death and other MACE when given to patients with STEMI. In a secondary analysis of the Kerala ACS Registry, optimal in-hospital and discharge medical care, defined as receiving aspirin, clopidogrel, β-blocker, statin, and heparin (in-hospital only), were delivered in 40% and 46% of admissions, respectively. Wide variability in both in-hospital and discharge medical care was present across the range of participating hospitals, with few participating hospitals reporting consistently high (greater than 90% levels) optimal medical care (Fig. 1).27, 28 In fact, patients who received optimal in-hospital medical care had a 21% lower rate of in-hospital MACE and a trend toward lower in-hospital death rates. Rural, non-academic hospitals were less likely to provide both in-hospital and discharge optimal medical care. There were similarities in receiving optimal in-hospital and discharge medical care from hospitals with or without cardiologists.
Fig. 1.
Proportion of each drug and drug combination for in-hospital and discharge care in Kerala Acute Coronary Syndrome Registry patients. A indicates aspirin; B, β-blocker; C, clopidogrel; H, heparin; and S, statin.9
The CREATE investigators have not reported analyses evaluating pharmaco-therapy for patients with ACS.8 The use of β-blocker and statins were lower, however use of ACE-I/ARB rates were higher in CREATE compared with the Kerala ACS Registry9.
Post discharge rates of optimal medical care in the outpatient setting are unfortunately even lower. Data from the Prospective Urban Rural Epidemiology registry (PURE) suggest that less than half of patients with prevalent CVD take ≥1 medication for secondary prevention.29
2.6. Socioeconomic status and outcomes
CREATE registry described variability in ACS care in India across socioeconomic status (SES).8 Patients with a lower SES were less likely to undergo coronary angiography, PCI, and CABG and were less likely to receive medications for secondary prevention. These differences were associated with a significant 2.7% absolute higher 30-day mortality in the poorest group compared to the richest group, though no differences were seen in the rates of re-infarction or stroke. These differences in mortality were abolished after adjusting for CAD risk factors, location of infarct and treatments, suggesting that better treatment of established CAD offers an opportunity to improve outcome. The differences in mortality across SES in India are not due to differences in risk factors, but almost entirely attributed to differences in treatments and related factors. Because more poor patients might have died before reaching hospital, the actual mortality is likely to be higher than reported here and the difference across SES might be more pronounced. If all patients had access to similar health care facilities, reached hospital rapidly, and received similar treatments, mortality would be reduced, especially for patients in the lower SES.30
2.7. Gender differences in presentation and management
Previous literature from high-income countries has repeatedly shown gender differences in the presentation, diagnosis, and management of ACS, with women having atypical presentations and undergoing less aggressive diagnostic and therapeutic measures. Kerala ACS Registry with 5825 women amongst the 25,748 ACS patients showed that on presentation, women were approximately 5 years older than men and had moderately higher rates of previous MI. In-hospital medical therapy was similar in both groups, though women were slightly more likely to receive reperfusion therapy than men.30 Discharge medication rates also showed similar trends among genders. Even after adjustment for possible confounding factors, there were no significant gender differences in the outcome of death or in the composite outcome of death, re-infarction, stroke, heart failure, and cardiogenic shock. There are limited data available comparing ACS outcomes data between men and women from other, smaller ACS registries in low and middle-income countries. DEMAT registry of 1565 ACS patients from ten tertiary care centres in India demonstrated that after adjustment for age, education, history of CAD, STEMI presentation, and reperfusion of any type, there was no evidence of an effect of increased risk of death at 30 days among women compared to men, nor was there any difference between death, re hospitalization and cardiac arrest at 30 days.19
2.8. The economic impact in India
STEMI imposes large financial burdens on families, with out of pocket expenditure (OOPE) up to 9 times their total household expenditures, depending on their socioeconomic status and type of treatment availed. In fact the magnitude of the OOPE is such that only a small proportion managed to limit their ACS-related expenditure within their overall household expenditure. This included those from the high SES and those who had some form of health security coverage for their illness. The high OOPE and catastrophic health expenditure (CHE) are no doubt critical findings that need to be tackled seriously. However, in order to do that, it is important to identify the triggers that make families vulnerable to CHE and hasten their transition from ‘high’ to ‘catastrophic’. Understanding these pathways is essential if our policies and strategies are to be targeted effectively. The high OOPE and CHE have been reported elsewhere with respect to CVD-related hospitalizations (including ACS) where India (Kerala) had the highest 15-month OOPE and more than 80% of the low- and middle- income groups and more than 60% of the high income group experienced CHE.31
2.9. How can we improve STEMI care in INDIA?
Adherence to guideline-based therapies has been associated with improved in-hospital, 30 day and 1 year clinical outcomes in ACS. The CRUSADE investigators demonstrated a 2.2% absolute difference in hospital mortality between the highest and lowest quartiles of adherence to ACS guidelines. Another study demonstrated that patients who received care greater than 80% concordant with ACS guidelines are 40% more likely to be alive at 1 year, compared to patients who received less than 80% concordant care.26, 32 Such data of ACS setting can be extrapolated to STEMI patients also.
Health care system level programmes for the evaluation and management of STEMI are lacking overall in India, possibly because there are currently no India-specific STEMI clinical practice guidelines. However, success has been demonstrated in a pilot study in Thrissur, Kerala which showed improvements in symptom to-door time, door-to-needle time and appropriate post STEMI discharge prescription rates through community, provider-level education and point of-care interventions. Significant improvements were seen for the discharge prescription of aspirin, beta-blockers, ACE-I, and lipid-lowering drugs.33
Key features of Epidemiology of STEMI in India are summarized in Table 2.
Table 2.
Key features of Epidemiology of STEMI in India.
|
3. Diagnosis of STEMI and early risk stratification—challenges in India
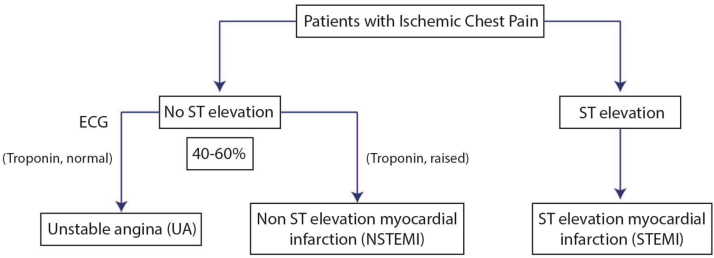
Acute chest pain is the most common presenting symptom of acute myocardial ischemia. ACS encompasses all acute chest pain syndromes resulting from myocardial ischemia.34, 35, 36 Only 20–30% of patients presenting with acute chest pain are ultimately confirmed to have ACS upon detailed evaluation37, 38 (Fig. 2).8, 9, 39 ST segment elevation myocardial infarction (STEMI) is characterized by myocardial ischemia that results in persistent ST segment elevation on electrocardiogram (ECG) and subsequent release of biomarkers of myocardial damage. Increased biomarkers alone in the absence of ST segment elevation constitute Non-ST segment elevation MI (NSTEMI). NSTEMI may manifest with transient/persistent ST segment depression and/or T wave inversion in ECG. Prolonged ischemic chest pain without elevation of markers of myocardial necrosis constitutes unstable angina (USA). STEMI is the most common form of ACS in India, accounting for 40–60% of ACS cases.
Fig. 2.
An approach to diagnosis of acute coronary syndrome.
For the diagnosis of acute myocardial infarction, the presence of any two of the following three features is essential: characteristic chest pain, ECG changes and elevated cardiac enzymes. However, patient’s interpretation of symptoms, availability of ECG and its interpretation, and widespread non-availability of Troponin testing are among the major recognized challenges in the diagnosis of STEMI in India. The diagnosis and early risk stratification is usually done at the point of first medical contact (FMC). In the Western world, the FMC is usually the emergency medical services. However, general practitioners and often non-physicians are the FMC in India, especially in rural and semi-urban areas, which poses unique challenges in the diagnosis of STEMI in India.40 Studies from India6 and abroad have shown that contacting a general practitioner usually delays reperfusion therapy.35
3.1. Chest pain
Typical chest pain of STEMI is a persistent retrosternal discomfort perceived commonly as heaviness or pressure usually lasting for more than 15–20 min, associated with perspiration and vomiting, and not responding to sublingual nitroglycerine. The pain may radiate commonly to left arm, neck and jaw. The pain may be atypical in character (pricking or burning), location (epigastric, back, right arm or only lower jaw) and intermittent in nature. It is advisable to obtain an ECG for anyone beyond 20 years of age presenting to emergency department with acute pain anywhere from jaw to umbilicus.
The physicians must recognize certain atypical presentations of STEMI including recent onset breathlessness, confusion, syncope, nausea and vomiting, fatigue, palpitations and perspiration. These atypical presentations are more common in elderly, women, hospitalized patients with co-morbidities, and in patients with diabetes and chronic renal failure. Prospective registries from the Western population show that up to 30% STEMI patients may present with atypical symptoms.41 However, series of STEMI from India report that more than 90% of patients (91–99%) present with classical chest pain, and commonly associated with sweating (70–80%).7, 42, 43, 44 Thus, atypical presentations seem to be uncommon among Indians. It is not clear whether such atypical presentations are not diagnosed, and hence underrepresented in registries. One plausible explanation for lack of atypical presentation among STEMI in India could be younger age of patients. Most of the series of young MI report a vast majority of male patients presenting with typical rest pain with sweating (in >90% of patients).44 Interestingly, two series from India comparing elderly (>60–65 years) and non elderly patients of STEMI reported that typical angina is observed only in 50% of elderly as compared to 80–85% of non-elderly. Atypical chest pain or no chest pain was seen in nearly 25–50% of elderly as compared to 7.5–10% in younger patients.45, 46 In a nutshell, any unexplained breathlessness, hypotension and hemodynamic collapse should warrant an ECG.
3.2. Electrocardiogram (ECG)
The task force for the universal definition of MI defines “STEMI as new ST elevation at the J point in at least 2 contiguous leads of 2 mm (0.2 mV) in men or 1.5 mm (0.15 mV) in women in leads V2–V3 and/or of 1 mm (0.1 mV) in other contiguous chest leads or the limb leads in the absence of left ventricular (LV) hypertrophy or left bundle-branch block (LBBB)”.47 Apart from this, STEMI may also manifest as new or presumably new LBBB (Table 3).48 However, an old ECG is usually not available for comparison. With increasing age, the population prevalence of asymptomatic LBBB increases. Hence, in the Western countries a vast majority of patients presenting to emergency department with LBBB are not diagnosed to have STEMI upon evaluation. In the presence of pre-existing LBBB, the criteria proposed by Sgarbossa (Table 4) may be a useful guide to confirm STEMI.49 A concordant ST elevation in a lead with the positive QRS complex is the best indicator of STEMI in the presence of LBBB. A score of ≥3 has a specificity of 98% for diagnosing STEMI. However, STEMI may not be ruled out even when none of the features are identified.50 Cases of acute ischemic chest pain with LBBB not accompanied by other ECG evidence of STEMI poses serious management challenge. In such patients Troponin levels and regional wall motion abnormalities may guide reperfusion therapy. If the index of suspicion of STEMI is high, such patients may be taken up for coronary angiogram. Right bundle branch block (RBBB), left anterior fascicular block (LAFB) and left posterior fascicular block do not interfere with the interpretation of ST segment elevation. However, paced rhythm, LV hypertrophy and channelopathies like Brugada syndrome may obscure typical changes in STEMI.
Table 3.
ECG Changes at presentation in STEMI.
| Typical |
|---|
| ST Elevation (at least 2 contiguous leads) |
|
|
| New or presumably new LBBB |
| Atypical |
|---|
| STEMI in Evolution |
|
| Evolving/Evolved STEMI |
|
| Posterior wall MI |
|
| Left main or proximal left anterior descending artery occlusion |
|
Table 4.
Criteria to diagnose STEMI in presence of LBBB.
| Criteria | Score | |
|---|---|---|
| 1 | ST-elevation ≥ 1 mm and concordant with QRS complex | 5 |
| 2 | ST-segment depression ≥ 1 mm in lead V1, V2, or V3 | 3 |
| 3 | ST-elevation ≥ 5 mm and discordant with QRS complex | 2 |
Majority of patients with STEMI will develop Q wave in ECG. In India, it is not uncommon for patients to present late after STEMI with an evolved ECG pattern (Table 3) consisting of Q waves, T wave inversion with variable ST elevation. In such patients, a persistent ST elevation may also be due to a large aneurysm apart from persistent ischemia. Hence, such ST elevation alone beyond 24 h of symptom onset should not be considered an indication for revascularization in the absence of ongoing chest pain. Rarely, a patient may present with hyperacute T wave changes alone in the early phase of STEMI. The ECG is rarely normal even in early stages of STEMI.35 Infrequently, STEMI may present without ST elevation. A true transmural posterior MI may actually present with ST depression in precordial leads along with positive T wave in lead V1 and a left main or proximal left anterior artery (LAD) occlusion may present as ST depression in multiple leads with ST elevation in lead aVR and V1.
ST elevation in the absence of STEMI may occur in a few patients presenting with acute chest pain. Pericarditis, Takatsubo cardiomyopathy, early repolarization syndrome and vasospastic angina are the common conditions confused with STEMI in day to day practice.51 In patients with atypical ECG changes, or concave ST segment elevation, or difficult to ascertain ECG evidence of STEMI may undergo a transthoracic echocardiography to look for regional wall motion abnormalities. However, in doubtful cases immediate referral for angiography may be the best modality to guide therapy.52, 53
Patients may have a first ECG that is not diagnostic of STEMI. In such a situation, a repeat ECG must be obtained at 10–15 min, and at 30 min intervals (Table 5). The ECGs should be carefully looked for even subtle changes. Cardiac biomarkers and echocardiogram may be useful guides to reperfusion therapy in such patients. If the suspicion of ongoing serious myocardial ischemia is high, the patient should be taken for a coronary angiogram to demonstrate coronary artery occlusion or intracoronary thrombus. A CT angiogram is usually reserved for patients with persistent symptoms with low to intermediate likelihood of ischemia or in patients with suspected aortic dissection or pulmonary embolism.35
Table 5.
Recommendations for ECG
| Indications: |
|---|
| 1. Chest pain |
| 2. Acute pain anywhere from jaw to umbilicus (beyond 20 years of age) |
| 3. Atypical symptoms of STEMI |
| 4. Unexplained acute breathlessness, hypotension and hemodynamic collapseo |
| Recommendations |
|---|
| 1. A 12-lead ECG to be performed in all patients with suspected STEMI |
| 2. Presentation to ECG diagnosis of STEMI – < 10 min |
| 3. A low threshold for performance of ECG in patients likely to present with atypical symptoms |
| 4. Continuous ECG monitoring should be started as soon as possible |
| 5. Right precordial leads (V3R, V4R) must be recorded in patients with inferior wall MI |
| 6. True posterior wall MI may be diagnosed as ST elevation (>1 mm) in additional lateral chest leads V7 – V9 |
| Recommendations |
|---|
| 1. Repeat ECG – 10 min, 30 min and as needed |
| 2. Compare with previous ECGs for even subtle changes |
| 3. Troponin I or T to guide therapy |
| 4. Echocardiogram for regional wall motion abnormalities 2. |
| 5. Emergency coronary angiography if high index of suspicion of STEMI |
| 6. CT angiogram only if aortic dissection or pulmonary embolism to be ruled out |
3.3. Cardiac biomarkers
Among the various biomarkers of myocardial necrosis, cardiac Troponin is the preferred biomarker for the diagnosis of STEMI.34, 35 Troponin T or I levels are the most specific and sensitive tests for the diagnosis of myocardial necrosis. Creatine Kinase MB (CK-MB) is less sensitive and specific especially in the early phase of STEMI, but often the only biomarker available in certain parts of India. A blood sample should preferably be collected at presentation and analysed. However, the initiation of reperfusion treatment should not be delayed for biomarker levels in patients with diagnostic ECG changes. High sensitivity Troponin values after 1–2 h of symptom onset may guide reperfusion therapy in patients with presumably new onset LBBB, paced rhythm, post bypass grafting and non-diagnostic ECG. Often the biomarkers need to be repeated when the diagnosis of STEMI is uncertain.35 A negative Troponin assay at 12 h after symptom onset practically excludes STEMI (Table 6).
Table 6.
Role of Biomarker Testing in STEMI.
| Indications for Troponin Testing |
|
| Asymptomatic ECG changes |
| Non diagnostic ECG changes |
| Presumably new onset LBBB without fulfilling STEMI criteria |
| Paced rhythm |
| Post CABG with non diagnostic ST elevation |
|
|
|
|
|
|
|
|
|
|
The lack of availability of standardized cardiac biomarker assays across the country is a major limitation in the early diagnosis and risk stratification of STEMI. Availability of point-of-care Troponin assays across India is even more limited, which results in overreliance on symptoms and repeated ECGs for diagnosing STEMI in doubtful cases. Often only semi-quantitative and qualitative assays of Troponin are available even in referral hospitals. These tests have varying standards and cut offs. A thorough understanding of the Troponin assay available at each centre is essential.
The universal definition of MI demands the demonstration of rise or fall in titre of Troponin with at least one value above the threshold.47 Often an increased level of either Troponin I or T is used for the initial diagnosis of ACS. Troponin I and Troponin T values correlate well, however, Troponin T levels are generally lower than Troponin I. The recommended Troponin cut off for diagnosis of MI is defined as a value exceeding the 99th percentile (with optimal precision defined by total coefficient of variation (CV) <10%) of a normal reference population. This is commonly referred as the ‘99th percentile rule’. This level must be determined for each assay with a good quality control in every centre.35, 54, 55, 56
3.4. Echocardiography
Echocardiography in the acute phase of STEMI is valuable in clarifying the diagnosis in patients presenting with non-diagnostic ECG changes. Regional wall motion abnormalities appear early after coronary artery occlusion. Echocardiogram is useful to identify regional wall motion abnormalities in presence of LBBB. However, LBBB per se may produce a jerky motion of interventricular septum. Echocardiogram can also identify mechanical complications of MI and must be performed when a murmur is identified, or for unexplained hemodynamic collapse and acute heart failure. Echocardiography may also identify certain alternative diagnoses like pulmonary embolism, pericardial disease etc. However, the need for an echocardiogram should not delay the transfer for angiography in high risk patients.35
3.5. Physical examination
A quick medical examination is recommended to risk stratify STEMI patients, recognize complications, identify co-morbidities, and to rule out alternative diagnosis. Left ventricular S4 is a common accompaniment. Hypotension, basal crepitations and LV S3 indicate poor prognosis. A new systolic murmur usually indicates either mitral regurgitation or ventricular septal rupture. Patients with suspected aortic dissection may have inequality in pulses.
3.6. Early risk stratification
The risk factors for early death following a STEMI include advanced age, diabetes mellitus, smoking status, prior infarction, and prior use of aspirin. On presentation, the presence of hypotension, tachycardia, cardiac arrest, heart failure, and mechanical complications of MI augur a poor prognosis. ECG evidence of anterior location of infarct, RBBB, complete heart block in anterior wall MI, and significant arrhythmias also indicate poor early outcomes in STEMI. Biomarker elevation, impaired renal function and echocardiographic LV dysfunction are important investigations pointing to a poor outcome.57, 58
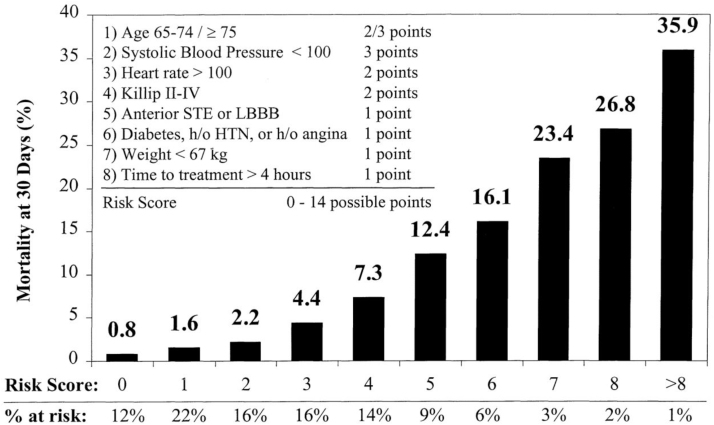
One simple classification, Killip classification (Table 7), which takes into account signs of heart failure and cardiogenic shock must be documented at presentation in all patients with STEMI.59 As risk assessment is a continuous process, they must be reassessed throughout hospital stay till discharge.34, 35 Thrombolysis In Myocardial Infarction (TIMI) risk score takes into account the major prognostic factors in STEMI and is a validated score (Fig. 3).60 The number of risk factors disproportionately increases the adverse outcome. GRACE score is a multifactor risk assessment model which is useful in predicting in-hospital, 30 day and 6-month outcome across ACS including STEMI. It is available as an App. In practice, GRACE score is more often used in the setting of NSTEMI/unstable angina than in STEMI.61
Table 7.
Killip Classification for STEMI risk stratification.
| 1. Class I: No evidence of heart failure |
| 2. Class II: Findings of mild to moderate heart failure (S3 gallop, rales < half-way up lung fields or elevated jugular venous pressure |
| 3. Class III: Pulmonary edema with rales > half-way up lung fields |
| 4. Class IV: Cardiogenic shock defined as systolic blood pressure < 90 mmHg and signs of hypoperfusion such as oliguria, cyanosis, and sweating |
Fig. 3.
TIMI risk score for STEMI.
3.7. Diagnosis of STEMI in India—unique challenges
All the Indian ACS registries report that less than 50% of patients with STEMI have received thrombolysis or PCI.6, 7, 8, 9 Most of the non-reperfusion resulted from late presentation of patients beyond the therapeutic window of reperfusion. Lack of awareness among patients, relatives, paramedics and even doctors is a major factor responsible for suboptimal acute care of STEMI in India.
Two entities that create some confusion and difficulty in diagnosis include stuttering MI and aborted MI. Stuttering MI is extremely rare62 and can be recognized by carefully observing patients with high risk for ACS who do not initially show ST elevation on ECG. When the reperfusion is done early in the course of STEMI, patients do not show rise in Troponin and have only transient ECG changes, which is known as aborted MI. Aborted MI may be seen in nearly 10–25% of patients reperfused very early. Aborted MI is shown to have good short and long term outcomes.63
4. Early triage and pre-hospital management of STEMI in rural and urban India
Timely delivery of reperfusion therapy (whether pharmacological or mechanical) in patients with STEMI is more important than the choice of therapy and the entire emphasis should be to deliver reperfusion therapy to a patient of STEMI as rapidly as possible. Moreover, despite the strength of evidence based medicine pertaining to the benefits of primary PCI in STEMI, treatment options in India are often dictated by resources, logistics, availability and affordability. In our country, not many hospitals offer primary PCI services round the clock in the urban areas and this inadequacy is pronounced more in rural areas where penetration of medical care is modest at best.
Efficient protocols of early triage of patients with STEMI should primarily aim to reduce time delays in patient care since these are associated with adverse outcomes. Delay in patient care in our country may occur at the following levels
-
•Pre-hospital stage
-
○Lack of a definite initial diagnosis: delay in recognizing symptoms by patients and therefore in seeking timely medical help.
-
○
-
•On reaching the hospital
-
○Financial issues
-
○
-
•
Lack of consensus amongst patient relatives and consent regarding procedure
-
•
System delays in hospitals (e.g. registration, transport to emergency department, coronary care units, cardiac catheterization lab etc.)
4.1. The aims of triage protocols
The aims should be to develop a smooth continuum of care including (a) Early recognition of symptoms of STEMI (b) Activation of efficient emergency medical services (EMS) (c) Pre-hospital management (d) Pre-determined hospital destination protocols and (e) Timely establishment of reperfusion.
4.1.1. Timely recognition of symptoms and seeking early medical help
Morbidity and mortality due to acute STEMI can be considerably reduced if symptoms are recognized early allowing timely access to EMS and institution of appropriate therapeutic measures. Since approximately one third of patients with STEMI have symptoms other than chest pain, health care providers need to educate the general public that presence of any of the following symptoms (especially in a patient with past history of cardiac disease) should arise suspicion, prompting call for medical help.
-
•
Chest discomfort with or without radiation to arm[s], back, neck, jaw, or epigastrium
-
•
Shortness of breath
-
•
Diaphoresis, nausea, lightheadedness, weakness
Furthermore, patients and their attendants should be motivated to seek early medical help if the symptoms do not improve or tend to worsen, even if they are not sure about the cause of the symptoms. Public awareness in this regard is of utmost importance.
4.1.2. Organization of a robust and efficient EMS to overcome transfer delays
Well organized EMS with rapid response time are widely prevalent in Western countries and help in early detection, pre-hospital management and timely transfer of patients with STEMI. Transfer to the hospital by ambulance rather than by friends or relatives is ideal since studies have shown that arrival at the emergency department (ED) by ambulance is associated with earlier initiation of reperfusion therapy and superior outcomes.64 Though EMS/ambulance services are available with some degree of penetration in the urban parts of our country, they are almost non-existent in rural India and a substantial proportion of the population does not have access to them leading to inordinate delays in transferring patients to hospitals.
The CREATE registry reported that the median time from the onset of symptoms to hospital arrival was 300 min in patients with STEMI, and only 5% of them utilized ambulances with the majority utilizing private transportation to reach hospitals.8 Only 15.4% patients in the HP-ACS registry used the “108” free ambulance service and a personal vehicle was the commonest mode of transportation, reflecting the poor availability of ambulance services in our country.6 In Indian context, delay in getting STEMI care is multifactorial (Table 8).
Table 8.
Reasons for Delay in STEMI Care.
| 1 | Lack of awareness of symptoms |
| 2 | Attribution of symptoms to gastro-intestinal disorders |
| 3 | Self medication |
| 4 | Family members’ inability to appreciate the gravity of situation |
| 5 | Silent or painless MI |
| 6 | Delays in call for medical attention/ambulance |
| 7 | Transportation difficulty-especially in rural areas |
| 8 | Inaccessibility of remote areas to medical care 24 × 7 |
| 9 | Poor telemedicine technology |
| 10 | Delay in hospital/ambulance response times |
| 11 | Poorly trained paramedics/technicians |
| 12 | Poorly equipped ambulances |
| 13 | Transportation/traffic delays |
| 14 | Lack of primary PCI facilities |
| 15 | Lack of round the clock staff and cath lab access |
| 16 | Economic affordability/lack of health insurance schemes |
| 17 | Delays in patient consent |
The successful delivery of emergency medicine care and services in a geographically diverse country like India is possible and is exemplified by the GVK EMRI (Emergency Management and Research Institute) service, a non-profit organization based on Public Private Partnership model, launched in Hyderabad in collaboration with the Stanford School of Medicine in 2005. It utilizes a centralized 910-type network in which the public can call a single number.108 The service is currently spread across 15 states and two Union Territories and is equipped with around 10,000 ambulances, with an increasing reach into rural, hilly & tribal areas. The ambulances are manned by trained emergency medical technicians who can help make an early diagnosis, deliver quality pre-hospital care and organize transport to an appropriate health care facility.
4.1.3. Pre-hospital ECG and administration of drugs
-
•
ECG: Staffs manning the ambulance services or dispatchers co-ordinating emergency medical calls need to have medical training and be able to perform 12 lead ECG at the time of FMC and transmit it to pre-determined medical care facilities. Prior transmission of ECG while the patient is en-route to the designated receiving hospital not only helps creation of reperfusion checklists but also results in faster time to reperfusion and better clinical outcomes.65, 66 A major facilitator of pre-hospital STEMI care has been the use of mobile technology for quick transmission of information (Use of android/iOS application like WhatsApp for ECG interpretation by expert).
-
•
Aspirin: Patients with ongoing symptoms suggestive of STEMI should be encouraged to take aspirin (165–325 mg, self-administered or by EMS personnel and community health workers). It needs to be remembered that more rapid buccal absorption occurs with non–enteric-coated formulations.
-
•
Nitroglycerin: It is recommended that patients who have been prescribed nitroglycerin previously, should take one nitroglycerin dose in case of any suggestive episode of chest discomfort. The patient or their family members are advised to seek emergency medical help if the chest discomfort does not improve or worsens even after taking 1 sublingual nitroglycerin dose.
-
•
Pre-hospital thrombolysis: The benefit of initiating early Thrombolytic therapy has been consistently demonstrated in several randomized controlled trials, prompting the concept of prehospital Thrombolytic therapy, started at the time of initial evaluation (in the ambulance).67, 68, 69 Since thrombolysis is not without associated risks (which could be life threatening at times), it is important to be certain that it is administered only to patients with a definite STEMI by a qualified medical practitioner.
4.1.4. Pre-determined hospital destination protocols
A set of pre-defined instructions should be made available for the EMS personnel that guide them to appropriate hospitals designated for STEMI care. Pre-decided destination protocols are important to avoid the predicament of EMS personnel to be forced to bear the responsibility of deciding where to take a patient for care. Destination protocols need to be formulated with close involvement of emergency physicians and cardiologists. Local physicians should have a list and contact details of nearby PCI capable centres/Non-PCI hospitals so that fast-track transfers can be organized and further plans of action can be discussed with patients and the cardiologists.
-
•
If the patients arrive directly by self-transport to a PCI-capable hospital, all medical care can potentially be delivered at a single centre.
-
•
For patients arriving via ambulance, the EMS personnel should transport the patient to a PCI-capable or non–PCI-capable hospital following locally established protocols.
4.1.5. Timely establishment of reperfusion
Once the patient reaches a STEMI care centre the choice of reperfusion therapy can be decided by availability of PCI, time to transfer to a PCI capable centre and contraindication of thrombolysis if any.
4.2. The rural and urban disparity
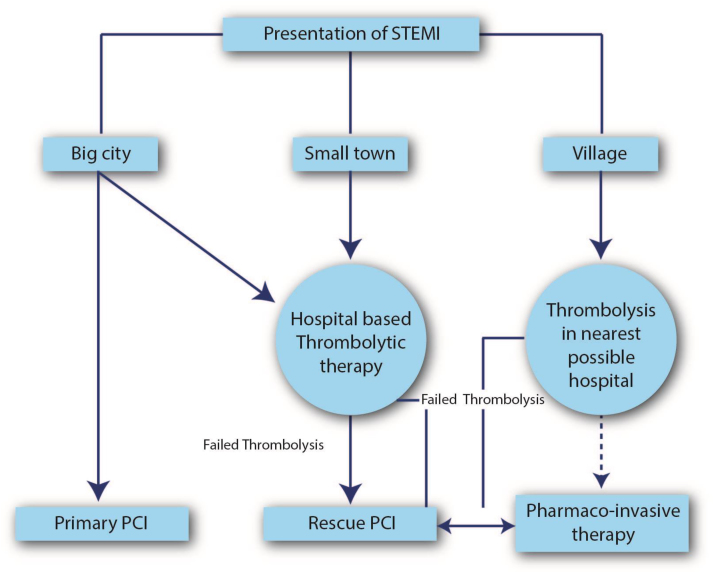
Patients in urban areas are more likely to have access to hospitals with round the clock primary PCI capabilities with short transportation time and should undergo primary PCI whenever feasible. Median hospital delays are reported to be much longer in patients from rural as compared to urban areas. Although the National Health Mission (NHM) caters to the medical needs of rural areas, the majority of health care facilities include primary or community health centres with only basic amenities and penetration to the remote, outlying areas is limited. Hence economically deprived patients from rural areas in India appear to be at greater risk, not only, for acute presentations of CAD but also for worse outcomes following such events. It is advisable that patients in rural areas with limited access to medical care and long transportation times to PCI-capable hospitals should undergo the Pharmaco-invasive (PI) strategy of thrombolysis with a goal of FMC to drug of 30 min or less. Initial thrombolysis should be followed if possible, by referral to PCI capable hospital for cardiac catheterization (if feasible) within 3–24 h of thrombolysis (Fig. 4).
Fig. 4.
Proposed Triage plan for patients of STEMI: Rural and Urban.
Further suggestions for improving triage of patients from rural areas:
-
•
Improving ambulance networks for early transfer.
-
•
Upgrading existent hospitals in rural areas
-
•
Educating and training physicians in the hospitals about the benefits and means of administering PI therapy to patients with STEMI
-
•
State led initiatives of wider reaching insurance schemes and fast track “cashless” approval for emergency procedures can help override financial problems. Two recent successful models include the Arogyasree insurance programme in Andhra Pradesh and the government funded insurance programme in Tamil Nadu. Some states in India provide completely free treatment for certain diseases like STEMI. It is equally important to make such schemes available to the growing Indian middle-class to facilitate optimal STEMI care.
| Recommendations: | |
|---|---|
| 1. | Patients often misperceive their initial symptoms as non-cardiac in origin. So spreading awareness about early recognition of symptoms suggestive of STEMI/ACS by patients or their relatives through intensive public education campaigns via print and non-print media (i.e. television, internet and social networking sites) is important. |
| 2. | It is essential to restructure EMS services in India so that timely transfer to appropriate hospitals can be organized. These can include increasing the number of ambulance networks, “Green Corridors” to allow passage of ambulances and educating the general public for giving priority passage to ambulances. |
| 3. | Immediate ECG to confirm the diagnosis of STEMI and administration of Aspirin at the point of FMC either at the level of EMS personnel or the General Practitioner. |
| 4. | Hospital transfer protocols need to be pre-decided and receiving hospitals should have systems to allow preregistration and direct transport to the catheterization laboratory bypassing the ED especially for patients who do not need urgent resuscitation on arrival. |
5. Selection of reperfusion strategy in STEMI
The prompt restoration of antegrade flow is the core aim of therapy for STEMI. Delay in reperfusion is associated with higher mortality and morbidity rates. Timely reperfusion results in better myocardial salvage and preservation of left ventricular function. Despite recent advances in pharmacological and interventional reperfusion strategies, timely reperfusion still remains suboptimal in patients with STEMI. Initiation of reperfusion therapy varies in India, mandating uniform guidelines across the country. The right reperfusion strategy should be timely, effective, complete, safe and easily accessible. While primary PCI is the preferred mode of reperfusion by most guidelines, only few patients with STEMI can avail this form of reperfusion within recommended timelines. On the other hand, thrombolysis is easily available, economical and evaluated in several clinical studies but fraught with dangers of re-occlusion of infarct related artery (IRA). Initial timely thrombolysis followed by early PCI to improve the patency rates, labeled as PI strategy, is an attractive option of reperfusion in STEMI and may bridge the gaps in systems of care. The contemporary studies from India report that only half the hospitalized patients of STEMI undergo some form of reperfusion (Table 9).
Table 9.
Reperfusion utilization in various Indian STEMI/ACS Registries.
| Study | Total number | Proportion of STEMI (%) among ACS | Thrombolysis (%)* | STK (%)# | TNK & Others (%)# | PCI (%)* |
|---|---|---|---|---|---|---|
| CREATE8 | 20,937 | 60.6 | 58.5 | 96.3 | 3.7 | 8 |
| Kerala ACS9 | 25,748 | 37.0 | 41.4 | 84.8 | 15.2 | 12 |
| HP ACS6 | 5180 | 45.5 | 35.6 | .... | .... | 0.9 |
| North East7 | 704 | 72.4 | 39 | 98.7 | 1.3 | 14.7 |
*Proportion of STEMI patients, #Proportion of thrombolysed patients.
5.1. Reperfusion strategies
There are currently three reperfusion strategies recommended worldwide.
5.1.1. Primary PCI
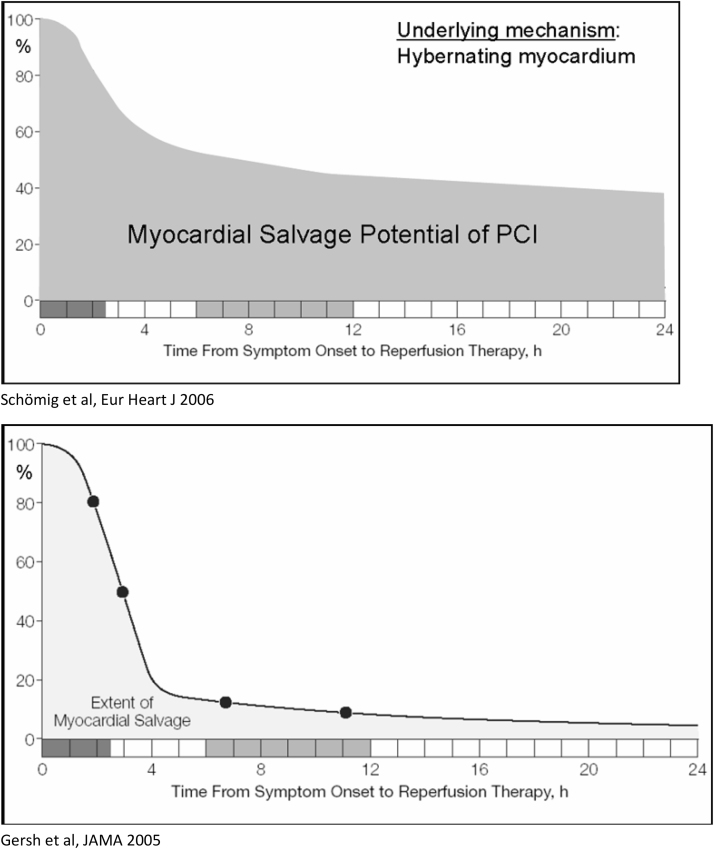
Primary PCI is defined as an ‘emergent percutaneous catheter intervention in the setting of STEMI, without previous thrombolytic treatment’. It is the preferred reperfusion strategy in patients with STEMI, provided it can be performed within guideline-mandated time-frame, by an experienced team.35 Primary PCI produces higher rates of IRA patency, TIMI 3 flow, and lower rates of recurrent ischemia, re-infarction, emergency repeat revascularization procedures, intracranial haemorrhage (ICH), and death.34 Randomized clinical trials have repeatedly shown that primary PCI is superior to thrombolysis, when performed in a timely manner, in high-volume, experienced centres.70, 71 Primary PCI reduces mortality by 25%, re-infarction by 64%, ICH by 95% and stroke by 53% when compared to thrombolytic therapy. Primary PCI results in TIMI 3 flow of IRA in over 90% of patients.72 However, the major disadvantage of primary PCI is the delay in commencing the reperfusion treatment labelled as the ‘PCI-related delay’, defined as ‘the theoretical difference between the time of FMC to balloon inflation, minus the time from FMC to start of Thrombolytic therapy (i.e. ‘door-to-balloon’ minus ‘door-to-needle’) and long delays to primary PCI are associated with worse clinical outcomes.35 PCI related delay of >60 min negates any mortality benefit compared to immediate thrombolysis.73 Current international guidelines recommend primary PCI in patients with STEMI, presenting with symptoms of less than 12 h duration, 12–24 h with ongoing clinical/electrocardiographic evidence of ischemia and those who present with cardiogenic shock or acute heart failure, irrespective of time delay from the onset of symptoms.
USA and Europe have used the nationwide availability of primary PCI as the basis for developing a STEMI system of care. Although these systems are very effective, they are resource intensive and this approach presupposes the accessibility of a fairly evenly distributed catheterization laboratory density coupled with a good EMS system and physical infrastructure for transportation. According to the data from CSI- National Interventional Council, there is a steady increase in the number of primary PCI in India. However, still only a minority of STEMI patients receive this modality of reperfusion due to the limited healthcare infrastructure, financial barriers, lack of awareness and accessibility of EMS services for a majority of the population in India.2, 74
5.1.2. Thrombolysis
Thrombolysis is an important reperfusion strategy, especially when primary PCI cannot be offered to STEMI patients, with a time-dependent reduction in mortality and morbidity rates within 12 h after symptom onset. Thrombolytic therapy has greater benefit in patients treated within 1 h of symptom onset, with a sharp drop off after 3 h. Thrombolysis prevents approximately 30 early deaths per 1000 patients treated within 6 h after symptom onset.67 Thrombolysis is currently the most practiced form of reperfusion method in India. It can be initiated by a physician in an emergency department when there is no plan to transfer the patient to a PCI capable centre for primary PCI. The earlier studies examined thrombolytics, initially with streptokinase and subsequently with tissue plasminogen activator (tPA) and its analogs (Table 10). The term ‘fibrinolytics’ generally refers to fibrin-specific thrombolytics and are currently preferred over non-fibrin-specific thrombolytics. A meta-analysis of thrombolytics showed that this was a good way of reperfusion with improved outcomes across subsets except in those presented beyond 12 h of symptom onset.67 Benefit from thrombolytic therapy in patients with STEMI who present more than 12 h after symptom onset has not been established, although consideration should be given in patients with on-going chest pain, with large myocardium at risk or hemodynamic/electrical instability, if primary PCI is not available.
Table 10.
Thrombolytic Agents Approved For The Treatment Of STEMI.
| Agent | Bolus | Dose | Adverse Effects | Fibrin Specificity | Efficacy (TIMI 2 or 3 flow at 90 min) |
|---|---|---|---|---|---|
| Streptokinase (STK) | No | 1.5 million unit given intravenously over 30–60 minutes | Might cause severe hypotension. Allergic reaction. (+nce of anti-Bodies) Avoid re exposure within 6 months. | No | 60 to 68% |
| Tenecteplase (TNK) | Yes | 30 mg for weight 60 kg; 35 mg for 60–69 kg; 40 mg for 70–79 kg; 45 mg for 80–89 kg; and 50 mg for 90 kg | – | +++ | 85% |
| Reteplase (rPA) | Yes (double bolus) | 10U + 10U intravenous boluses given 30 min apart | – | ++ | 84% |
| Alteplase (rt-PA) |
No | Bolus 15 mg, followed by infusion 0.75 mg/kg (maximum 35) over the next 60 min; total dose should not exceed 100 mg | – | ++ | 75–84% |
Fibrin specific agents have some advantages over streptokinase, but are more expensive and not widely available. Streptokinase is cheap, easily available and is the most frequently used thrombolytic agent in India. However, the final decision of choice of thrombolytic agent is at the discretion of the treating physician and the patient’s choice. There are several contraindications to thrombolysis (Table 11) and the decision to use this therapy is predicated on a risk–benefit analysis that should consider time from onset of symptoms, the clinical and hemodynamic features, patient comorbidities, risk of bleeding, presence of contraindications, and time delay to PCI. Blood pressure should be brought down to below 180/100 mmHg before initiation of thrombolytics, preferably by intravenous nitrate infusion. But undue wastage of time is strongly discouraged. Thrombolytic therapy is associated with an excess of strokes, largely due to ICH, which is more common in patients with advanced age, lower weight, female sex, prior cerebrovascular disease, and hypertension on admission.75 Significant non cerebral bleeding occurs in about 4–13% patients treated with thrombolytics, subject to patients’ comorbidities.76 Re-occlusion of the IRA following successful reperfusion is another important limitation of the thrombolytics and is due to the plasminemia induced by the thrombolytics which in turn activates thrombin formation. GUSTO trials of STK, alteplase and reteplase reported re-occlusion rates of 4.3% during hospitalization at a median time of 3.8 days.76 Benefit of thrombolysis in STEMI is not yet established in patients over 85 years of age.77 Failed thrombolysis can be diagnosed by persisting or worsening chest pain or less than 50% resolution of ST-segment elevation after 90 min of thrombolysis in the lead showing maximum ST-segment elevation at presentation. Rescue PCI is advocated for such patients and patients should be transferred to a PCI-capable centre immediately.
Table 11.
Contraindications to Thrombolysis.
| Absolute | Relative | No Contraindication |
|---|---|---|
| Previous ICH or stroke of unknown origin at any time | Transient ischemic attack in the preceding 6 months | Menstruation |
| Ischemic stroke in the preceding 6 months | Oral anticoagulant therapy | Acute Ischemic stroke within 4.5 h |
| CNS damage or neoplasms or AV malformation | Pregnancy or within 1 week postpartum | |
| Recent major trauma/surgery/head injury (within the preceding 3 weeks) | Refractory hypertension (systolic >180 mmHg and/or diastolic >100 mmHg) | |
| GI bleeding within the past month | Advanced liver disease | |
| Known bleeding disorder (excluding menstration) | Infective endocarditis | |
| Aortic dissection | Active peptic ulcer | |
| Non-compressible punctures in the past 24 h (e.g. liver biopsy, lumbar puncture) | Prolonged or traumatic resuscitation |
5.1.3. Pharmaco-invasive (PI) strategy
PI strategy consists of early thrombolysis followed by either rescue PCI for patients with failed thrombolysis, or non-urgent coronary angiography to determine the need for additional revascularization within 3–24 h.34, 35 Initial timely thrombolysis to open the IRA and early PCI if required, to improve the patency rates, is an attractive option of reperfusion in STEMI and has gained momentum recently. It differs from a ‘facilitated’ approach which consists of an immediate PCI following fibrinolysis and has shown adverse outcomes.78 PCI performed 3 h after thrombolysis precludes the early pro-thrombotic phase and reduces the chances of re-occlusion. Furthermore, this delay may also be the reason for decrease in bleeding complications that were seen with facilitated approach.79 The transfer to the catheterization laboratory is regardless of the response to thrombolytic therapy and advocated as a routine practice. Multiple studies have subsequently shown that this strategy reduces the rate of re-infarction and is superior to the widely prevalent approach of thrombolysis followed by catheterization only for demonstrable ischemia.80 Most PI trials were performed using TNK (Table 12).
Table 12.
Major Trials of PI Strategy.
| Trial | Methods | Conclusion | |
|---|---|---|---|
| GRACIA-281 | Grupo de Analysis de la Cardiopati ‘a Isque’ mica Aguda-2 | RCT in 211 STEMI patients, comparing full-dose TNK followed by stenting within 3–12 h of randomization (early routine post fibrinolysis angioplasty; 104 patients) with primary stenting with abciximab within 3 h of randomization (primary angioplasty; 108 patients | PI approach, resulted in better myocardial reperfusion and TIMI 3 flows compared to primary PCI despite performance of a delayed PCI. |
| TRANSFER-AMI82 | Trial of Routine Angioplasty and Stenting After Fibrinolysis to Enhance Reperfusion in Acute Myocardial Infarction | Total of 1100 patients with high-risk STEMI presenting to non-PCI centres randomized to a PI strategy (transfer for routine PCI within 6 h of fibrinolysis) or to standard treatment after fibrinolysis. | The benefits of PI strategy were seen to exceed those of standard treatment. |
| FAST-MI83 | French Registry on Acute ST-Elevation Myocardial Infarction | Real world and follow-up data up to 1 year of 1714 patients. | PI strategy yields early and 1-year survival rates that are comparable to those of primary PCI. |
| WEST84 | Which Early ST-elevation myocardial infarction Therapy | Open-label, randomized, feasibility study of 304 STEMI patients comparing the effect of TNK and usual care (Group A), TNK and mandatory invasive study in 24 h, including rescue PCI for reperfusion failure (Group B) and primary PCI with 300 mg loading-dose of clopidogrel (Group C). | Pharmacologic regimen rapidly delivered, coupled with routine coronary intervention within 24 h of initial treatment, may not be different from timely expert PCI. |
| NORDISTEMI85 | Norwegian Study of District Treatment of STEMI | 266 patients with STEMI living in rural areas with more than 90-min transfer delays to PCI were treated with TNK, and randomized to immediate transfer for PCI or to standard management in the local hospitals with early transfer, only if indicated for rescue or clinical deterioration | Immediate transfer for PCI reduced the rate of death, reinfarction, or stroke at 12 months in patients with STEMI, treated with thrombolysis. |
| CARESS-in-AMI86 | Immediate angioplasty versus standard therapy with rescue angioplasty after thrombolysis in the Combined AbciximabREteplase Stent Study in Acute Myocardial Infarction | Open, prospective, randomised, multicentre trial of 600 patients treated with half-dose reteplase and abciximab. | Immediate transfer for PCI improves outcome in high-risk patients with STEMI when compared to transfer only if indicated. |
| STREAM87 | Fibrinolysis or Primary PCI in ST-Segment Elevation Myocardial Infarction | 1892 patients with STEMI were randomized to either PI group or primary PCI group | PI strategy resulted in effective reperfusion in patients with STEMI who presented within 3 h after symptom onset. |
‘STREAM’ trial assessed the safety and efficacy of PI strategy versus primary PCI in patients presenting with STEMI. Patients with STEMI were enrolled in the study if they presented within 3 h of symptom onset, and were unable to undergo primary PCI within 60 min of arriving at the hospital. Thrombolysis was done using TNK along with contemporary antiplatelet and anticoagulant therapy. The primary endpoint was a composite of all cause death, shock, congestive heart failure (CHF) or re-infarction at 30 days. Results showed similar outcomes in both groups at 30 days with primary endpoint occurring at 11.4% in the PI group and 14.3% in the primary PCI group (RR = 0.86; 95% CI, 0.68–1.09; p = 0.21). The result was consistent across all pre-specified subgroups in the study. The overall intracranial bleeding rate was not different (0.54% vs 0.26%, respectively; p = 0.45) after the trial protocol was amended to reduce the dose of TNK in patients aged ≥75 years. The PI strategy circumvented an emergent procedure in 64% of the patients. The 1 year results of STREAM showed no difference in mortality rates between both groups.87
‘STEPP-AMI’ is an observational pilot study that compared outcomes of PI versus primary PCI strategies in patients with STEMI.88 200 patients were enrolled into the study. The primary end point was a composite of death, cardiogenic shock, re-infarction, repeat revascularisation of the culprit artery and CHF at 30 days. The primary end point occurred in 10.1% in PI group and in 3.9% in primary PCI group, p = 0.07 (RR = 2.87; 95% CI 0.92 to 8.97). The initial trend towards benefit of primary PCI narrowed over a period of 1 year (13.3% vs. 9%, RR = 1.48, 95% CI 0.60 to 3.62‘p’ = 0.4). There was no difference in bleeding rates between both groups. The average total ischemic time is about 4 h in this study. More importantly, both STREAM and STEPP-AMI showed significantly higher rates of open IRA and better TIMI flow at catheterization in the PI group of patients. These patients also had higher rates of referral for CABG as a part of complete revascularization.
The recent STREAM data and the Indian data from the STEPP AMI study showed that the PI strategy compared well with primary PCI in reducing overall morbidity and mortality.87, 88 The pilot Kovai Erode study and the subsequent pilot Tamil Nadu STEMI program89 have shown the feasibility of combining the two strategies of primary PCI and the PI strategy in systems of care for STEMI patients.
Hence, PI strategy is appropriate for patients with STEMI who are eligible for treatment with thrombolytic drugs and in whom FMC to balloon time is ≥120 min. Current STEMI guidelines from the American College of Cardiology/American Heart Association (ACC/AHA) and the European Society of Cardiology (ESC) also recommended that patients going to a non-PCI-capable hospital should receive fibrinolysis immediately, if the expected FMC to device time is more than 120 min, and then be transferred to a PCI-capable hospital within 24 h for coronary angiogram and if needed PCI.34, 35
In Tamil Nadu STEMI Project, 2420 consecutive STEMI patients were enrolled between 2012 & 2014 in 4 hub and 36 spoke centres in Tamil Nadu. In the pre-implementation 3 month period 898 patients were enrolled and 1522 patients were enrolled in the post-implementation 9 month period. In this group of patients, 407 consecutive patients underwent the PI approach. Streptokinase was used in 94.6% of patients followed by tenectaplase in 4.7% of patients and reteplase in 0.7% of patients. 95% of patients had one month follow up and 90% had one year follow up. Mortality was 1.7% in hospital, 1.8% at one month and 5.8% at one year. In comparison 929 patients had standalone thrombolysis with 96% receiving Streptokinase as thrombolytic. Though this is not a randomized study, standalone thrombolytic treatment had 9.0% mortality while the PI strategy had 1.7% mortality (Personal Communication Dr A Mullasari on behalf of STEMI INDIA).
5.2. Choice of reperfusion therapy
National registry data from 89 cities suggest that Indian patients with STEMI frequently fail to receive an adequate reperfusion therapy.8 Choosing the right mode of reperfusion depends on many factors. The duration of time from symptom onset is a key factor in the choice of reperfusion therapy. When patients are treated within the first 2 to 3 h after symptom onset, patient outcomes are comparable, irrespective of whether thrombolytic or primary PCI is employed.81, 82, 90 A 3-fold increase in hospital death is reported when door-to-balloon times exceeded 2 h, and similarly when door-to-needle times exceeded 1 h.91
Time from FMC to reperfusion therapy is another important metric in optimal reperfusion therapy. Benefit from primary PCI is essentially lost when primary PCI related delay is >60 min, especially since the availability of bolus thrombolytics. Patients presenting to high PCI volume centres with experienced team for primary PCI are found to have significant mortality benefit compared with thrombolytic therapy2 and this too should be considered when choosing the reperfusion strategy, especially in rural areas. There are several factors that are distinctive to Indian subcontinent in the treatment of STEMI. Time from symptom onset to presentation is usually longer than developed countries2 and hence the easily available bolus Thrombolytics cannot be utilized in many patients. On the other hand, ready finances for an expensive procedure such as primary PCI may be a constraint. Most patients with STEMI do not use EMS services for transport,8 and most EMS are not well equipped, hence pre hospital fibrinolysis still remains a challenge (Table 13).
Table 13.
Recommendation for selection of reperfusion strategy.
| Recommendations: |
|---|
| For patients within 12 h of symptom onset and with persistent ST-segment elevation or new or presumed new left bundle branch block, early mechanical or pharmacological reperfusion should be performed as early as possible |
| The reperfusion therapy should be considered if there is clinical and/or electrocardiographic evidence of ongoing ischaemia, between 12 and 24 h. Primary PCI is preferred in these patients |
| Routine PCI of a totally occluded artery >24 h after symptom onset in stable patients without signs and symptoms of ischaemia is not recommended |
| Primary PCI should be performed in patients with STEMI and ischemic symptoms of less than 12 h’ duration who have contraindications to thrombolytic therapy, irrespective of the time delay from FMC |
| Primary PCI should be performed in patients with STEMI presenting with cardiogenic shock, hemodynamic and electrical instability irrespective of time delay from symptom onset |
| Thrombolytic therapy is recommended within 12 h of symptom onset in patients without contraindications if primary PCI cannot be performed by an experienced team within 120 min of FMC(preferably within 90 min) |
| Fibrin specific agents have some advantages over streptokinase but are more expensive and not widely available. Whereas Streptokinase is cheap, easily available and is the most frequently used thrombolytic agent in India. However, the final decision of choice of thrombolytic agent is at the discretion of the treating physician and the patient. |
| All thrombolysed patients should be considered for PI therapy within 3–24 h, if feasible. |
| All patient with failed thrombolysis should undergo prompt Rescue PCI |
6. Management of STEMI at PCI non-capable centres
A significant proportion of population in the developed countries and majority of patients in India present to ‘PCI non-capable centres.
The PCI non-capable centres where patients in India can present with STEMI can range from small clinics with a general practitioner (GP) small nursing homes with physicians to larger hospitals in cities. All of these are in effect ‘PCI non-capable centres ‘and also the ‘first medical contact’ (FMC) for patients.
6.1. First medical contact and first medical facility
The FMC is the first medical person that a patient of STEMI contacts and by definition first medical facility is the first acute care hospital that he goes to. In actual practice, however, many a time the first medical facility and FMC may be the same i.e. the emergency physician in a hospital or a nursing home. Thus an important role of the PCI non-capable centre is to serve as a FMC.
6.2. Role of the PCI non-capable centres in the management of STEMI
Based on available data, the role of the PCI non-capable centres in the management of STEMI includes most or all of the following –
-
(i)
Facilitate and hasten patient care
-
(ii)
Diagnose MI–self/transmit ECG using telemetry or smart phone applications
-
(iii)
Triage of the patient
-
(iv)
Initiate immediate pharmacological therapy. (Aspirin and Pain Relief)
-
(v)
Initiate supportive pharmacotherapy statins/ACEIs/beta blockers
6.2.1. Facilitate and hasten patient care
There are a large number of PCI non-capable centres, which are familiar to the local population and are decidedly the point of first contact. The physicians in these hospitals need to be involved in public education about preventive aspects as well as about the importance of reporting early in case of symptoms suggestive of STEMI. This would build their rapport with the local community and help in patient seeking early help in case of chest pain.
6.2.2. Diagnose MI—self/transmit ECG through telemetry or smartphone applications
The PCI non-capable centre can help by early and accurate diagnosis of STEMI. This is important because the initial ECG may be normal in some patients and some patients may have a non-diagnostic ECG. Since the treatment is extremely time sensitive in case of STEMI, an accurate diagnosis is important. While the physicians on site can be trained in the ECG diagnosis of STEMI, the other effort can be to use our own strengths. India has large number of smart phone users and one can transmit ECGs using smartphone applications. This can be done to confirm the diagnosis and also to alert a PCI capable centre.
6.2.3. Triage of the patient
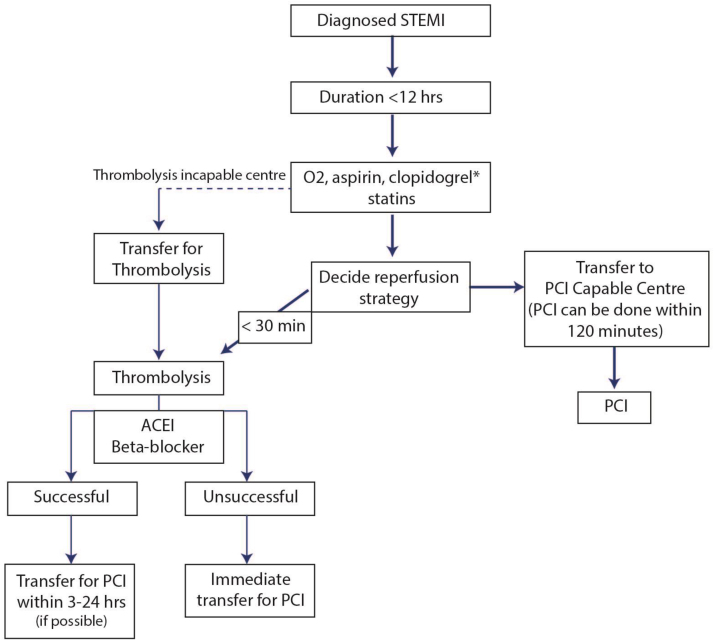
Once a diagnosis has been made, the patient and his family should be taken into confidence and a reperfusion strategy should be planned In case it is possible to shift the patients to a PCI capable centre while maintaining the FMC to Balloon time of <120 min, then the patients should be transferred for PCI. However in practice if the expected transport time is likely to be more, it is better to thrombolyse the patients and shift to a PCI capable centre for a PI approach whenever possible and feasible92 Fig. 5.
Fig. 5.
Mangement plan for patients of STEMI presenting at a PCI non-capable hospital.
Another problem in triaging such patients is due to the late presentation of patients. In CREATE registry8 the median time of patient presentation was over 300 min. Majority of data in the PI studies is of patients who present in the time frame of 0–3 h.87 Therefore expecting the benefit from PI therapy equal to primary PCI even in patients who present late is primarily conjectural. However, in the TN STEMI project low one year mortality could be achieved in actual clinical situation in South India and this fact seems to be encouraging.
6.2.4. Initiate immediate pharmacological and standard medical care
Antiplatelet medications are essential in managing STEMI. Giving 325 mg of soluble Aspirin is of paramount importance and should be given at arrival to all patients of STEMI. The choice of second antiplatelet drug is where the role of PCI non-capable Centre comes in. The choice of second antiplatelet depends on the future course of therapy decided upon and is dealt in other sections of this document (Sections 7 and 9).
In keeping with good practice guidelines, it is mandatory that all patients be rested, given oxygen inhalation if the oxygen saturation is less than 90%, have wide bore i.v. access, administered pain killers like Morphine or other parenteral opioids and given sublingual or i.v. nitrates.
6.2.5. Thrombolytics and anticoagluation
If decision to thrombolyse the patient in the PCI non-capable hospital has been taken then prompt initiation of thrombolysis is of prime importance. The thrombolysis may be started within 30 min with any of the easily available agents. Detailed discussion on thrombolytic therapy has already been done in the earlier section.
Thrombolysis can be initiated by a qualified medical person at a centre where facility of ECG, defibrillator and resuscitation measure are available.
Anticoagluation with heparin is also to be initiated along with thrombolysis (Section 9).
6.2.6. Initiate supportive pharmacotherapy
Another important area where the role of PCI non-capable Centre comes in is the administration of other therapies like high dose statin (Atorvastatin-40–80 mg or Rosuvastatin-20–40 mg), ACE inhibitors/ARB and Beta-blockers as indicated following standard clinical practices (Table 14).
Table 14.
Summary of Management of STEMI in PCI non- capable center.
| Summary – | |
|---|---|
| 1. | PCI non-capable centres in India are an important component in delivery of effective STEMI care. |
| 2. | They can be of a wide spectrum in terms of their nature ranging from small clinics with a GP, and small nursing homes with physicians to larger hospitals. On basis of management strategy they are broadly of two types −PCI non-capable centres that do an ECG and transfer and PCI non-capable centres which do an ECG, thrombolyse the patients and then consider transferring them to a PCI capable centre when feasible. |
| 3. | They help in early diagnosis, triaging, initiating immediate pharmacotherapy and giving supportive treatment, thrombolysing (if so deemed) and transferring patients. |
| 4. | Thrombolysis can be initiated by a Qualified Medical Person at a centre where facility of ECG, defibrillator and resuscitation measure are available. |
| 5. | 5. Occasionally they may be the sole centre offering thrombolysis and/or medical management. |
| 6. | They need to act in tandem with other components of STEMI care for effective patient care. |
7. Management of STEMI at PCI capable center
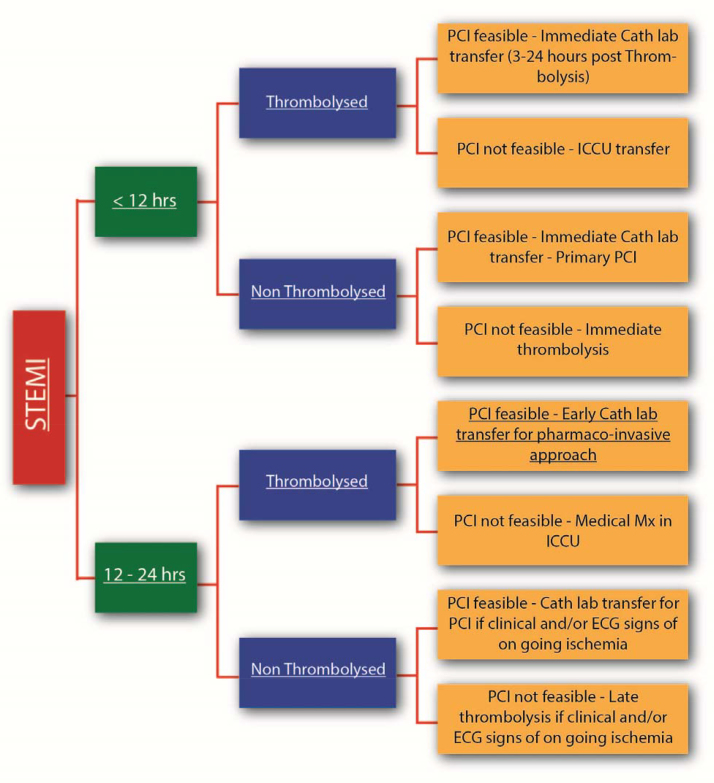
Primary PCI is the gold standard for management of STEMI and all efforts should be directed to maximize its use as the initial reperfusion therapy. The decision making for patient of STEMI presenting to PCI capable hospital depends on eligibility factors and feasibility factors (Table 15; Fig. 6).
Table 15.
Factors to be considered in selection of Reperfusion therapy in STEMI presenting at a PCI capable hospital.
| Eligibility factors | Feasibility factors |
|---|---|
| Time since onset of STEMI Thrombolysis status Presence of chest pain Hemodynamic/electrical Instability Cardiogenic shock Acute heart failure |
Operational catheterization Lab. Economic Feasibility Consent and care takers available or not |
Fig. 6.
Management Strategies for Patients of STEMI Presenting to a PCI Capable Hospital.
7.1. Clinical scenarios
When a patient of STEMI presents to a PCI capable hospital and the PCI is feasible, then depending upon the various eligibility factors, three clinical scenario need to be discussed:-
-
1.
Non Thrombolysed patient presenting <12 h of onset of chest pain.
-
2.
Non Thrombolysed patient presenting 12–24 h of onset of chest pain.
-
3.
Thrombolysed patient presenting <24 hours of onset of chest pain
Management of the patients presenting beyond 24 h is discussed under section of late presenters (Section 8).
7.2. Non-Thrombolysed patient presenting <12 h of onset of chest pain
The early period following STEMI (<12 h) is the time where there is maximum opportunity for saving lives by prompt initiation of appropriate reperfusion therapy. The core philosophy of management in this period is “Time is Muscle”. Every passing minute sans reperfusion indicates lost opportunity for salvaging myocardium.95, 96
7.2.1. Choice of reperfusion
PCI of culprit artery within first 12 h of STEMI onset (Primary PCI) scores over thrombolysis in achieving higher rates of coronary artery patency and TIMI3 flow while preventing potential bleeding complications like ICH. Primary PCI also decreases the rate of recurrent ischemia, re-infarction and death as compared to thrombolysis. These benefits come at the cost of access site bleeding, stent thrombosis, contrast nephropathy and other procedural complications. However, high volume centres with experienced physicians and support staff, as well as shorter treatment delays, help to mitigate these effects.
Multiple randomized studies have validated the superiority of Primary PCI vis-à-vis thrombolysis in decreasing mortality as well as morbidity.70, 71, 97, 98 Even in the subset of patients where thrombolysis is contraindicated due to high bleeding risk, primary PCI has shown benefits.99, 100 Hence, primary PCI should remain the default strategy for reperfusion whenever possible in these groups of patients.
Patients with cardiogenic shock and acute heart failure have high mortality and account for majority of STEMI deaths. Early revascularization with PCI/CABG has been shown to improve 1 year mortality in these patients when compared to thrombolysis. Data from SHOCK trial and NRMI −2 registry support hypothesis that high risk patients gain more mortality advantage with PCI compared to lower risk patients.101, 102, 103, 104
7.2.2. When PCI cannot be performed immediately
As emphasized previously, time delays to reperfusion are associated with worse clinical outcomes, this dogma holds true for both thrombolysis and PCI alike.105, 106 Hence, when due to logistic or economical reasons Primary PCI cannot be performed or can be performed only with a significant delay (>120 min), quick change of strategy to early reperfusion by thrombolysis seems prudent. In countries like India where health insurance coverage is meagre and managing economics for PCI can be time taking, thrombolysis becomes a good initial strategy to ensure coronary artery patency as a part of PI approach.
7.2.3. Acceptable time delays to PCI
From Post hoc analysis of randomized trials and registries, it has been calculated that delay in performance of Primary PCI by >120 min neutralizes the beneficial effects of mechanical reperfusion.107, 108 As a corollary, whenever the expected delay in Passage of wire across culprit artery exceeds 60 min at a PCI capable centre, thrombolytic therapy should be promptly initiated as a part of PI approach. Both American and European guidelines endorse this concept despite majority of population having access to primary PCI.
7.3. Non thrombolysed patient presenting 12–24 h of onset of chest pain
For reasons discussed in previous sections, a sizeable number of patients may present outside the ideal period of 12 h after onset of STEMI. Such patients present a therapeutic dilemma.
7.3.1. If PCI is feasible
Immediate PCI should be performed in patient with clinical or electrocardiographic evidence of ischemia.109, 110 Acute heart failure and cardiogenic shock are two other scenarios reaping unambiguous advantage from PCI irrespective of time of STEMI onset as referred to previously. However, in an asymptomatic individual presenting between 12 and 24 h, who is clinically stable and without any sign of ongoing ischemia, the benefits of PCI may not be as robust. Such patients present a management and social challenge for a hospital which is PCI capable. Small randomized studies have shown benefit even in this subgroup, but large studies are needed. One of them even demonstrated increased survival at 4 years follow up apart from myocardial salvage. Large anterior infarctions and younger patients are the subset of patients who may benefit from more aggressive strategy.111, 112
7.4. Thrombolysed patient presenting <24 h of onset of chest pain
This group of patients are ideally suited for a PI strategy. This approach refers to the administration of thrombolytic therapy followed by immediate transfer to a PCI-capable hospital for early coronary angiography and PCI when appropriate, preferably within 24 h of STEMI onset. By advocating early thrombolysis, the process ensures coronary patency and viability of myocardium before a subsequent PCI.
Numerous large RCT’s and meta-analyses have confirmed feasibility and advantages of this approach over a “wait and watch” policy following thrombolysis.85, 113, 114 The large TRANSER-AMI study demonstrated significant reduction in combined primary endpoint of death, recurrent MI, recurrent ischemia, new or worsening HF, or shock at 30 days when patients were immediately referred to a PCI capable centre.113 A meta-analysis of seven randomized trials referring patients for early transfer for PI therapy found significant reduction in the incidence of death or MI at 30 days and at 1 year, albeit without an increase in major bleeding.115 The STREAM study, went a step ahead and compared the PI approach directly to primary PCl in early presenters.87 The composite primary end point of death, shock, congestive heart failure, or re-infarction up to 30 days was not different between groups. For the patient who demonstrates signs of failed thrombolysis at admission, immediate PCI (Rescue PCI) should be considered. Persistent chest pain, ST segment resolution <50% in lead with maximum ST elevation and absence of reperfusion arrhythmia’s indicate a failed thrombolysis.116 These indicators warrant consideration of immediate Rescue PCI instead of more routine PI approach.
7.5. Management strategies and procedural issues in PCI in patients of STEMI (Primary PCI/Pharmacoinvasive/Rescue PCI)
7.5.1. Anti-Platelets
All patients of STEMI undergoing Primary PCI should receive dual antiplatelet(DAPT) combination of Aspirin plus a P2Y12 inhibitor.
Aspirin is given orally to be chewed preferably in doses of 150–325 mg in emergency department itself. Following PCI, aspirin is to be continued indefinitely. The CURRENT – OASIS 7 and other studies117, 118 failed to find merit in higher doses aspirin (81 mg vs. 325 mg) and hence a maintenance dose of 75 mg seems reasonable.
Clopidogrel, the most widely used P2Y12 inhibitor should be given in a loading dose of 600 mg before primary PCI. Based on beneficial effects seen in CURRENT-OASIS 7, a maintenance dose of 150 mg daily for first week followed by 75 mg daily is recommended.117 Though very economical, antiplatelet effects of clopidogrel are variable being affected by patient phenotype, genotype and drugs. This is because Clopidogrel is a prodrug and needs two step enzymatic action for activation. But given the cost benefit advantage of Clopidogrel it remains the most prescribed second antiplatelet to patients of STEMI. Also many patients who are started initially on newer P2Y12 inhibitor are ultimately switched over to Clopidogrel because of patient or physician preference.
Prasugrel a novel theinopyridine has more rapid onset of action as well as more potent when compared to Clopidogrel. It is given in a loading dose of 60 mg followed by a subsequent maintenance dose of 10 mg daily. In TRITON TIMI 38 study, prasugrel significantly decreased the composite primary end point of 30 day death, MI or stroke in clopidogrel naïve patients undergoing PCI for STEMI and NSTE-ACS.119, 120 There was an additional decline in definite stent thrombosis at 30 days. These benefits were accrued at the cost of increase in non CABG TIMI major bleeding in overall cohort but not in STEMI subgroup. Prasugrel is contraindicated in patients with prior stroke/TIA, age >75 years and body weight <60 kg.
Ticagrelor is non theinopyridene P2Y12 antagonist which is reversible and has quick onset as well as offset of action. It is administered as 180 mg loading dose followed by 90 mg twice daily maintenance dose. Ticagrelor is the antiplatelet agent with most balanced effects i.e. equipoised reduction in thrombotic as well as hemorrhagic effects. In the pivotal PLATO trial, ticagrelor reduced the composite primary end point (cardiovascular death, non-fatal MI, or stroke) as well as cardiovascular mortality across the spectrum of ACS.121, 122 Importantly, benefits were seen irrespective of prior clopidogrel use and invasive strategy for management. The primary side effects of ticagrelor were transient dyspnea and asymptomatic bradycardia both which rarely led to therapy discontinuation.
Intravenous GP IIb/IIIa receptor antagonist were primarily evaluated before the routine use of dual antiplatelet in PCI for STEMI. In the current scenario, their role is debatable. They may be used only in a few specific situations.
-
•
Angiographic visualization of large thrombus burden.
-
•
Slow flow and No reflow phenomenon
-
•
Bail out strategy, in case of use of bivallirudin as sole antithrombotic agent
-
•
Inadequate P2Y12 loading before PCI
The route of administration is also a matter debate. The results of INFUSE-AMI and AIDA-STEMI trials have rekindled interest in their intra-coronary route of administration123, 124
Recommendation of Antiplatelets in STEMI:-
-
1.
For STEMI undergoing Primary PCI, Ticagrelor seem to be more efficacious however it’s limitation are higher cost and BID dose–when compared to Clopidogrel.
-
2.
Prasugrel and Ticagrelor should not be administered to patients who have received thrombolysis.
-
3.
If a thrombolysed patient has received 300 mg of Clopidogrel and is undergoing PI approach then an additional loading with 300 mg clopidogrel should be done.
-
4.
Prasugrel should be considered in a diabetic patient undergoing Primary PCI
7.5.2. Anti-Thrombotics
Unfractionated heparin(UFH) remains the most time tested and experienced choice for parenteral anticoagulation in setting of PCI in STEMI. Weight based bolus regimens titrated to activated clotting time are recommened. (70–100U/ kg and 50–60U/kg when additional GpIIb/IIIa are used)
Enoxaparin has also been used in this setting. The ATOLL trial randomized patients to intravenous UFH or enoxaparin in primary PCI but failed to meet its primary end point. Secondary end points were met and there was no increase in bleeding either.125
Bivalirudin has generated much interest because of its direct thrombin inhibition property. The HORIZONS- AMI study demonstrated significant reduction of bleeding events and survival advantage of Bivalirudin when compared to UFH + GpIIb/IIIa based strategy. There was trend towards increase in acute stent thrombosis which diminished by 30 days.126
Recently, results two large RCT’s HEAT-PPCI and Matrix study have stroked the bivalirudin controversy. Pending the time controversy over bivalirudin is resolved, it cannot be recommended as the first line strategy for anticoagulation. However, in patients with heparin induced thrombocytopenia it remains a viable strategy.
7.5.3. Vascular access—radial versus femoral
The concomitant use of antiplatelet, antithrombotic and thrombolytic therapy in STEMI predisposes a patient to potential risk of bleeding. Arterial access site bleeding remains one vital area of concern during STEMI interventions. The emergence of radial route as an alternate access site has generated tremendous interest in terms of patient satisfaction and bleeding complications. Radial access has now been demonstrated in STEMI scenario to successfully decrease in bleeding complications and mortality advantage in RIVAL and RIFLE STEACS trials.129, 130 Similar trends were observed in MATRIX study as previously enumerated. Thus, radial approach should be preferred for Primary PCI when operator is skilled in radial interventions
7.5.4. Thrombus aspiration
Thrombus aspiration conceptually appears to be very appealing adjunct during primary PCI due to prevailing high thrombotic milieu and burden in STEMI. Distal migration of thrombus during mechanical reperfusion can lead to slow flow or no reflow phenomena which also leads to adverse outcomes. Removal of thrombus should improve myocardial and epicardial reperfusion but its routine use in primary PCI has not translated into clinical benefit. Following significant beneficial results of single centre TAPAS trial and meta-analysis based on it, manual thrombus aspiration was routine advocated in primary PCI.131, 132 However, contemporary multi-center RCT’s have failed to reproduce the similar benefits.135 However, even in the largely negative TASTE/TOTAL trials thrombectomy did improve surrogate markers of myocardial perfusion like ST segment resolution (STR), less distal embolization and Myocardial blush grade which of course did not translate into clinical benefits. One may assume that the technique is correct but the present tools are not.
Pending the answer to this puzzle, thrombus aspiration should be restricted to patient with large thrombus burden, as a bail out for slow flow/no reflow and PCI for acute stent thrombosis.
7.5.5. Stent selection (BMS/DES/BVS)
The use of coronary stents has dramatically altered the outcomes of PCI. In the initial phase, use of bare metal stents (BMS) reduced the threat of acute closure, reinfaction and repeat revascularization when compared to balloon angioplasty.136 Subsequent introduction of drug eluting stent (DES) has led to further reduction of restenosis and target vessel revascularization compared to BMS.137 There were concerns regarding very late stent thrombosis with DES but studies have clearly shown the effects restricted to first generation DES.137 The newer generation, DES as shown in EXAMINATION and COMFORTABLE – AMI have shown excellent outcomes and clear superiority over BMS in all aspects.138, 139, 140 It is recommended to implant a newer generation drug eluting stent in acute MI unless contraindicated. (DAPT contraindicated, hypersensitivity, requirement for urgent surgery)
Bioabsorbable Vascular Scaffolds (BVS) has a theoretical advantage in PCI for coronary artery disease by mitigating long term adverse effects of metal in coronary lumen, restoring vasomotion, and potentially restoring vessel to its natural state. In the setting of STEMI it is studied in the PRAGUE-19,141 BVS-EXAMINATION study142 and ABSORB-STEMI-TROFI II study.143
At present, in absence of large randomized studies routine implantation of BVS in STEMI cannot be advocated. Selected implantation in young patients with discrete lesion can be contemplated.
7.5.6. PCI of non culprit vessel during STEMI
Multi-vessel coronary artery disease is common in STEMI (40–60%) and studies have consistently shown that these patients have worse outcomes.144 Benefits of complete revascularization in STEMI needs to be weighed against potential complication of prolonged PCI in the unstable phase of MI–stent thrombosis, contrast nephropathy, underestimation of stent size and over estimation of lesion due to coronary spasm.
Initial experience with multivessel PCI in STEMI was primarily in the form non randomized and few small randomized studies.145
But now we have encouraging data from 3 contemporary RCT’s – PRAMI, CVLPRIT and DANAMI-PRIMULTI 3.148, 149, 150, 151 Based on these the ACC/AHA update has withdrawn the embargo on performing non IRA PCI in STEMI in hemodynamically stable patients.152
The writing committee recommends:-
-
•
In STEMI patients with cardiogenic shock,complete revascularization should be attempted.
-
•
In patient undergoing Primary PCI and who are not in cardiogenic shock, non culprit lesion may be treated (at index procedure/index hospitalization) provided the lesion is anatomically suitable. (discrete, significant lesion, in proximal epicardial artery). The final decision of the feasibility and timing of such procedure should be taken by the treating physician.
8. Management of STEMI: late presenters (more than 24 h)
Any patient presenting 24 h after onset of chest pain due to STEMI represents a failure of the prevailing STEMI care-system. The “total ischaemic time” (symptom onset to initiation of reperfusion therapy) has two components: (a) Patient delay and (b) System delay (including FMC to diagnosis and diagnosis to initiation of reperfusion therapy). The mean “total ischaemic time” in Kerala ACS Registry was 5 h.9 When a patient presents as late as 24 h after onset of symptoms, patient-related delay accounts for the main reason of delay and system-related delay becomes relatively insignificant. Proportion of latecomers (>12 h) is significant even in data from the west: 11% in GRACE study22 and as high as 40% in the TETAMI study.62 In an Indian study162 done from Uttar Pradesh, 32.3% patients with STEMI had presented after 24 h (Fig. 7).
Fig. 7.
Relationship of outcome and myocardial salvage as a function of total ischemic time.
8.1. Risk stratification in late comers after STEMI
8.1.1. Clinical
In the presence of hemodynamic or electrical instability and/or if patient continues to experience ischemic symptoms, a reperfusion-based strategy using PCI is recommended and endorsed by the current guidelines (ACC/AHA/SCAI and ESC/EACTS).135, 163
8.1.2. ECG
The presence or absence of Q waves by itself is a poor marker of viability or IRA patency.ST elevation may also be due to aneurysm formation in late presenters. Some studies suggest that predictive value of negative T wave as a marker of reperfusion is strong.
8.1.3. Angiographic
There are a few experimental and small clinical studies suggesting that an open IRA is of advantage. However, a single large study,OAT study, failed to show any advantage of the routine opening of IRA beyond 72 h of symptom onset. The importance of an open IRA in patients presenting with a window of 24–72 h is not known. There exists an important yet unrecognized interplay between time to reperfusion and presence of residual coronary flow within the area at risk. Beyond 4 h of symptoms, significant salvage only occurred in the presence of well-developed collateral vessels or preserved antegrade flow in the IRA. The time related transmural progression of infarction is greater in patients with no residual flow in the myocardium in jeopardy and presence of collateral flow or antegrade flow in the IRA may increase the time-window for reperfusion.
8.1.4. LV systolic function
Among patients with STEMI, adverse events are markedly increased in those with LV ejection fraction <40% even when undergoing primary PCI126; however, given the prospect of myocardial salvage, these high risk patients stand to accrue substantial clinical benefits from the procedure as well.
8.1.5. Stress testing
Exercise testing is useful in assessing exercise capacity, identifying persistent ischaemia, and risk stratification for future cardiac events. Submaximal testing may be done in asymptomatic patients after 3–5 days and symptom-limited testing may be done in the same subset of patients after 5 days.
DANAMI-1150 and SWISS164 trials have demonstrated beneficial effects of PCI performed late after MI in patients with persistent ischaemia on stress testing. The INSPIRE trial165 assessed use of myocardial perfusion imaging for defining initial patient risk and in guiding role of intensive medical therapy versus coronary revascularization for reducing the total LV perfusion defect and extent of scintigraphic ischaemia on SPECT imaging. Myocardial perfusion PET provides an option for patient groups who may be more difficult to image with SPECT MPI, including obese patients, women, patients with previous non-diagnostic tests and patients with poor LV function attributable to CAD considered for revascularization.
8.1.6. Myocardial viability
In the VIAMI trial166 patients not treated with primary or rescue PCI but otherwise in the non-high risk category were subjected to either invasive (PCI) or a conservative (ischaemia-guided) strategy if the infarct area was proven to be viable. Benefit in terms of composite end-points of death, myocardial infarction or unstable angina is seen at follow-up of 1 year and at long-term follow-up of median 8 years with initial in-hospital invasive strategy. The same investigators also reported in a sub-study of the trial on the influence of viability and revascuarization on left ventricular remodelling. Viability early after AMI is associated with improvement in LV function after revascularization. When viable myocardium is not revascularized, the LV tends to remodel with increased LV volumes, without improvement of EF. Absence of viability results in ventricular dilatation and deterioration of LVEF, irrespective of revascularization status.
Various modalities which can be utilized to assess myocardial viability are Thallium-201 or technetium–99 m SPECT, FDG-PET, dobtamine stress ECHO, dobutamine stress cardiac MRI and contrast enhanced MRI.
8.2. Reperfusion PCI in late comers with STEMI
8.2.1. Open artery hypothesis
This hypothesis was first proposed by Eugene Braunwald in 1989 when he noted that patients with spontaneous recanalization of the IRA had fewer adverse events during following weeks and months. These effects are not likely to be attributable to small benefits in infarct salvage and probably arise from limitation of infarct expansion, reduction of LV remodeling development, scaffolding effect of reperfused vessel, a conduit for collateral vessel, improvement of blood supply to hibernating myocardium, increased infarct wound healing, development of contraction band necrosis rather that coagulation necrosis in infarcted myocardium, reduction of myocardial apoptosis, increased baroreceptor sensitivityand vagal activity and reduced ventricular arrhythmia. These mechanisms extended the time window of reperfusion to 12 h and possibly 24 h after onset of AMI. However, the period after 24 h of onset of AMI is more challenging. On the basis of OAT study,167 routine PCI 3 days to weeks after AMI is not recommended for persistently occluded IRA in asymptomatic high risk patients, since higher reinfarction rates were seen in the PCI group and yet, no difference were seen between PCI and conservative groups in terms of event-free survival after median follow-up of 3.2 years or LV systolic function at 1 year. Subsequently, Abbate et al. performed a meta-analysis and systematic review of randomized trials (total of 3560 patients from OAT, SWISS III and several other small studies) (Table 1) comparing late PCI (defined as greater than 12 h from symptom onset) with medical therapy in haemodynamically stable patients with primary end-point of survival. PCI was associated with an overall improvement in survival with an odds ratio of 0.49 (95% CI:0.26-0.94). In addition, a greater improvement in cardiac function was observed over time in patients assigned to PCI than medically managed patients supporting the concept that late revascularization may reverse adverse cardiac remodelling over longer term (Table 16).
Table 16.
Randomized clinical Studies of Late Perfusion more than 24 h after onset of AMI.
| Randomized Clinical Studies Of Late Reperfusion More Than 24H After The Onset Of AMI | |||||
|---|---|---|---|---|---|
| Study | No of patients reperfused:+/− | Method of reperfusion | Time to reperfusion | Sustained patency reperfusion:+/− | Overall outcome |
| Topol et al, TAMI-G | 71, 34/37 | tPA+/−PTCA | 12–40 h | 60%/30% at 0 months | Negative (mortality, LV volume, LV systolic function at 0 months) |
| Dzavik V et al TOMIIS |
44, 25/19 | PTCA | 5–42 days, mean 21 days | 43%/19% at 4 months | Negative (clinical outcomes, LV size and CT at 4 months, positive in the subset LVEF at 4 months |
| Horie et al | 83, 44/39 | PTCA | >24 h | 86%/13% at6 months | Positive (LV volume at 6months; death, recurrent MI, congestive heart failure at 56months) |
| Yousef et al | 66,32/34 | Stenting | 3days- 5 weeks: mean-26days | 81%/19% at 12 months | Greater LV dilation but improved exercise tolerance and QOL with reperfusion at 12 months |
| Steg et al DECOPI |
212,109/103 | Stenting | 2–15days | 83%/34% at 6months | Improved LVEF but no difference in clinical outcomes at 2 years |
| Hochman et al. OAT | 2166,1082/1084 | Stenting | 3–28 days, median 8 days | Negative (event free survival at 4 years) | |
| Dzavik V et al TOSCA-2 |
381, 150/136 | Stenting | 3–20 days; median 10 days | 83%/25% at 1 year | Negative (LVEF at 4 years; trend towards less LV dilation in reperfusion) |
Based on these trial results, it is also reasonable to expect that those with a totally occluded IRA on initial diagnostic angiogram are less likely to see a survival benefit from late revascularization than those with subtotal occlusions, unless there are other high-risk features such as inducible ischaemia or significant viability. It is likely that patients with subtotal occlusions are more likely than those with total occlusions to have ongoing ischaemia, which can predispose to further cell death. Late reperfusion probably beneficially interrupts this process and reduces the extent of adverse remodelling occurring in this setting. The BRAVE-2 study enrolled STEMI patients between 12 and 48 h and SPECT study at a median of 7.1 days showed a statistically significant reduction in final infarct size in those assigned to the invasive strategy. Thus, “not so later comes” 12 to 48 h) may stand to gain through reperfusion of hibernating myocardium in peri-infract region.
8.2.2. Challenges of late revascularization
-
•
Success rates, as defined by residual diameter stenosis <20% and epicardial TIMI-3 grade flow, do not exceed 85–90%. On-going micro-vascular damage also interferes with TIMI-3 perfusion grades.
-
•
Total occlusion of an IRA is commonly associated with a significant thrombotic burden. The thrombus is at least partially organized and thus mechanically more resistant to guidewire recanalization.
-
•
However, paradoxically, peri-procedural complications occur only in a minority of patients with adverse coronary lesion features (e.g. unprotected left main lesion, true bifurcation disease and severe calcification) or heavy thrombus burden.
-
•
When thrombus is angiographically evident, aggressive antithrombotic therapy and thrombosuction prior to stent implantation may be helpful in minimizing distal embolization and recurrent myocardial infarction.
-
•
Remaining interventional strategy e.g. choice of bare-metal versus drug-eluting stents, use of debulking or imaging (IVUS or OCT), short versus long term dual antiplatelet therapy should be decided on a case to basis.
Overall, strategy for reperfusion with PCI for latecomers, with STEMI has been summarized in Fig. 8 and Table 17.
Fig. 8.
Proposed algorithm for management of stable patients of acute myocardial infarction presenting more than 24 h from symptom onset.
Table 17.
Principles in Late presenters.
|
|
| In patients presenting beyond 24 h |
|
|
|
|
|
9. Routine in hospital management of patients with STEMI (other than reperfusion therapy)
All patients with STEMI should be admitted to intensive coronary care units (ICCU). Though the initial emphasis is on the reperfusion therapy, the adjunct pharmacotherapy is no less important. Also the routine investigations and discharge medications need to be optimised.
The initial management of patients with STEMI includes admission to the ICCU for rhythm monitoring, hemodynamic monitoring and planning optimal reperfusion strategy. Routine management includes bed rest, analgesia, sedation, use of oxygen, antiplatelet therapy, anticoagulation, nitroglycerine, beta blockers, monitoring and management of arrhythmias, and recognition, evaluation and management of hemodynamic instability.
It is to be noted that more than 50% deaths related to STEMI occur suddenly, outside the hospital environment. The majority of these patients have ventricular fibrillation or pulseless ventricular tachycardia requiring cardiopulmonary resuscitation and direct current defibrillation for survival. With improved out of hospital resuscitation facility, more and more patients are expected to reach the hospital emergency services with ECG evidence of STEMI and varying degrees of neurological deficits due to cerebral hypoxia.34
9.1. ICCU and intermediate care unit
Managing STEMI patients in ICCU historically resulted in significant reduction in mortality even before the advent of reperfusion therapy. Coronary care units are equipped with cardio pulmonary resuscitation facility including defibrillators, temporary pacing, mechanical ventilator support and skilled personnel to recognize and initiate treatment for life threatening arrhythmias, institute hemodynamic monitoring and respiratory support. All STEMI patients should be observed for at least 24 h in the ICCU and then the hemodynamically and electrically stable patients with limited ST elevation myocardial infarction and those having successful coronary reperfusion may be shifted to the intermediate coronary care unit for next 24–48 h. The intermediate coronary care units should be equipped with telemetric ECG monitoring facility and resuscitation facility with skilled personnel for recognizing and instituting appropriate intervention for malignant tachyarrhythmias and bradyarrhythmias available in the immediate vicinity, at short notice. Electrically and hemodynamically unstable patients should be retained in the ICCU longer and decision to transfer should be decided on a case to case basis taking into consideration the electrical and hemodynamic status and occurrence of any mechanical complication.
9.1.1. General measures
As acute STEMI is a major catastrophic illness, care must be exercised in communicating the nature of the illness, the prognosis, the management plan and risk factor modification, life style change, the overall impact on quality of life, physical activity, recreational activity and vocational rehabilitation in a graded manner during the hospital stay. Immediate concern is to provide a quiet hospital environment to allay fear, reduce anxiety, reduce sympathetic drive to prevent hypertension, tachycardia and ventricular tachyarrhythmias using analgesic sedation judiciously as and when necessary. Laxatives should be prescribed routinely unless contraindicated.
Early ambulation is recommended and can be as early as within 24 h of STEMI in stable patients with no hemodynamic or arrhythmic complications, permitting use of bedside commode, sitting upright in a bedside chair etc. It is also advisable to keep the patient fasting for a few hours and then initiate clear liquid diet in the initial 12–24 h to avoid problems of vomiting which is not uncommon in the setting of acute STEMI, often aggravated by drugs like morphine.
9.1.2. Oxygen
Routine administration of supplemental oxygen is not recommended. Patients with arterial oxygen saturation consistently below 90–92% on room air, patients with ongoing chest pain and patients with heart failure should be administered oxygen to maintain oxygen saturation above 92%.High arterial blood oxygen tension can lead to both systemic as well as coronary vasoconstriction. The resultant increase in LV afterload and reduction in coronary perfusion can be detrimental to the patient in the setting of STEMI.
9.1.3. Analgesia
Narcotic analgesics like morphine are preferred for relief of pain and anxiety. Morphine is administered intravenously in aliquots of 1 to 2 mg with close monitoring of arterial oxygen saturation and level of sedation and effort of breathing. It can be administered to a maximum dose of 10–15 mg I/V for an average adult. Assisted ventilation may be necessary if there is significant depression of the respiratory effort. Morphine can lead to significant venodilation and decrease in preload, and can be especially detrimental in setting of right ventricular MI, leading to profound hypotension. It is advisable to avoid morphine in setting of inferior wall MI with suspected RV MI on ECG. Hypotension can lead to reduced hepatic blood flow which can hamper metabolic degradation of morphine accentuating its pharmacological action. Excessive vagomimetic effects of morphine can be countered by administering intravenous atropine.
Nonsteroidal antiinflammatory drugs other than aspirin are to be avoided in patients with STEMI as they are known to increase risk of adverse cardiovascular events including reinfarction, heart failure, and myocardial rupture.
9.1.4. Nitrates
Nitrates cause epicardial artery vasodilation, systemic venodilation, and can relieve ischemia especially in the setting of coronary artery spasm. On admission with ongoing chest pain nitroglycerine can be administered as 0.4 mg sub- lingual and can be repeated for 3 doses over 15 min, closely monitoring the blood pressure. On-going ischemia is an indication for intravenous administration of nitro-glycerine starting at 10 μg/min. and titrating the dose as needed up to 50 to 100 μg/min. Nitro-glycerine may also be useful in reducing systemic hypertension especially if beta blockers are contraindicated.
Nitro-glycerine causes significant venodilation and preload reduction which can be detrimental in patients with inferior wall STEMI with RVMI. It can compound the effects of morphine which also reduces preload due to vanodilation. Patients who are dehydrated or volume depleted tolerate intravenous nitroglycerine poorly and hence volume status should be assessed carefully in the intensive care facility continuously, monitoring the central venous pressure whenever necessary. Nitroglycerine should not be administered if the systolic blood pressure is below 90 mm Hg, or severe bradycardia with hypotension (Bezold – Jarisch reflex), especially in the setting of inferior wall STEMI and RVMI. Hypotension and bradycardia induced by nitrates can be reversed with intravenous atropine. Long acting nitrates are not recommended in the setting of STEMI. History of use of phosphodiesterase 5 inhibitors during the previous 24 h for erectile dysfunction is a contraindication for nitrate use.
9.1.5. Anti coagulation
Anticoagulation with heparin is recommended for all patients with STEMI regardless of the reperfusion strategy (PCI or fibrin specific thrombolytic agents).34 Unfractionated heparin is given as bolus 60units/kg(maximum 4000 units) and infusion 12 units/kg/hour(maximum 1000 units/h) to be titrated to maintain APTT in therapeutic range of 1.5 to 2 times control.
Low molecular weight heparin (LMWH) can be used instead of unfractionated heparin. The advantages are predictable action, ease of administration, superior bio availability, longer half life, non-requirement for laboratory monitoring in most clinical situations, and better outcome in randomized clinical trials for mortality as well as reinfarction (ASSENT3)168 with a small increase in bleeding risk((TIMI 25 EXTRACT).169 Enoxaparin is given as 30 mg I/V bolus followed after 15 min by 1 mg/kg S/C every 12 h. In patients aged >75 years, I/V bolus is omitted and S/C enoxaparin dose is reduced to 0.75 mg/kg twice daily. If GFR Is <30 ml/Min, Enoxaparin is administered as 1 mg/kg once daily.
Fondaparinux 2.5 mg I/V as the initial dose and 2.5 mg S/C on subsequent days during the hospital stay is an equally effective alternative in patients with normal renal function. Fondaparinux is not recommended for patients with serum creatinine more than 3 mg/dl or GFR <30 ml/min. Patients planned for primary PCI or PCI during the course of hospitalization should receive additional unfractionated heparin before the procedure to reduce complications related to catheter thrombosis.
9.1.6. Beta blockers
Various studies have confirmed the beneficial effects of beta blockers when introduced early in the course of STEMI with maximal mortality reduction in the first 24–48 h mainly by reducing re infarction and arrhythmic deaths.34 Oral metoprolol can be initiated 25 mg soon after admission in all acute STEMI patients with no contraindications like heart block or hemodynamic instability such as hypotension and pulmonary congestion or reactive airway disease. Metoprolol tartrate, the short acting molecule can be initiated as 25 to 50 mg and repeated every 6 h. Routine use of intra venous beta-blockers is not recommended in view of higher incidence of pulmonary edema and cardiogenic shock in patients with large infarcts undergoing thrombolysis.
9.1.7. Anti platelet medications: (ref. to Section 7)
9.1.7.1. Aspirin
160 to 325 mg chewable aspirin should be administered at presentation as soon as feasible on diagnosing STEMI and continued at a dose of 75 mg/day indefinitely. The mortality benefit of aspirin in myocardial infarction is as much as mortality benefit obtained by streptokinase (ISIS 2 study).170 In an occasional patient with contraindication to aspirin (past history of significant adverse reaction to aspirin) clopidogrel can be administered instead.
9.1.7.2. P2Y12 inhibitors
It is recommended to administer a P2Y12 inhibitor immediately on admission and continued for one year unless contraindicated.34 Patient requiring mandatory long term anticoagulant therapy as for atrial fibrillation and, mechanical heart valve replacement and chronic venous thromboembolic disease should have a shorter period of dual antiplatelet therapy (Aspirin + P2 Y12 inhibitors) limited to 3–6 months taking into consideration patient risk for recurrent thrombosis, the safety profile of the antithrombotic regime used, and bleeding risk.
The available P2 Y12 receptor inhibitors are clopidogrel loading 300 mg and 75 mg/day maintenance, prasugrel 60 mg loading and 10 mg/day maintenance for patients treated with PCI and ticagrelor 180 mg loading and 90 mg twice daily for patients planned for PCI. When fibrinolysis is the mode of revascularisation, then clopidogrel is the only option as the second antiplatelet with aspirin. A loading dose is not recommended in such situation when the patients age is above 75 years.
9.1.8. Statin
9.1.8.1. Statins
Statins are routinely administered in high doses (high intensity statins: Atorvastatin 40 to 80 mg and Rosuvastatin 20 to 40 mg daily) for their LDL lowering action as well as pleotropic effects(34). Statins are continued indefinitely to achieve and maintain LDL levels less than 70 mg/dL.
9.1.9. ACE Inhibitors/ARB
9.1.9.1. Angiotensin converting enzyme inhibitors
All patients with anterior wall STEMI and depressed LV function and or heart failure with ejection fraction <40% should receive ACEI(34) within 24 h of hospital admission. Use of ACE inhibitor should be considered to all other patients of STEMI. Patients intolerant to ACEI may be given ARB. Renal function and serum potassium should be closely monitored in all patients initiated on ACEI, ARB or aldosterone antagonists.
9.1.9.2. Aldosterone antagonist
Patients of STEMI with LVEF <40% and features of heart failure or diabetes should be started on an aldosterone antagonist after ACEI and beta blockers are given.
9.1.10. Drugs/Interventions NOT found beneficial in acute STEMI
Routine administration of magnesium or glucose insulin infusion is not recommended. Calcium channel blockers, especially the non dihydropyridine calcium channel blockers like verapamil and diltiazem should be avoided as they lead to LV dysfunction. However, in atrial fibrillation with fast ventricular rate and contraindication to beta blockers, verapamil or diltiazem may be administered cautiously in absence of LVF or hypotension. Short acting dihydropyridine calcium channel blockers can increase mortality in setting of acute STEMI and should be avoided.
9.2. Adjunctive therapies to limit myocardial infarction
Rapid advances in the treatment of AMI, mainly through timely reperfusion, have substantially improved outcomes in patients presenting with acute coronary syndrome and particularly ST-segment elevation myocardial infarction. Pharmacological interventions to prevent inflammatory response as well as prevent reperfusion injury have been demonstrated to be effective in animal studies. Cardio-protective agents like adenosine and natriuretic peptides have not been shown to be effective in limiting extent of myocardial necrosis in STEMI. Adjunctive pharmacotherapy to prevent inflammation (Monoclonal antibody against C5 component of complement) has not been found to be of benefit in setting of STEMI.
Presently, it is recommended to institute hypothermia in all resuscitated comatose patients with STEMI admitted to the hospital.
Simple physical methods like ischemic post conditioning by causing short duration repetitive ischemia and remote ischemic preconditioning by causing ischemia in other organs as limb muscles by application of tourniquets, have been shown in small studies to be beneficial, but currently not recommended in absence of large randomized control trials.
9.3. Role of CABG in STEMI
Emergency CABG may be needed in STEMI patients when taken for primary angioplasty but the IRA is found to be unsuitable for PTCA, who have recurrent or ongoing ischemia, cardiogenic shock, severe heart failure or other high risk features. CABG is also indicated if surgical intervention is required for mechanical complications of STEMI.
10. Management of complications of STEMI
10.1. Mechanical complications
10.1.1. Left ventricular failure
Heart failure in STEMI can be due to dysfunction of cardiac muscle due to ischemia, stunning, hibernation and cell death or due to mechanical complications like mitral regurgitation, ventricular septal rupture, and free wall rupture. Bedside echocardiography helps in quantifying LV function and identifying mechanical complications and thus helps plan definitive therapy. Heart failure warrants urgent coronary angiography and revascularization. Viability assessment may be needed before revascularisation if presentation is late and extensive cell death is likely.
Oxygen should be administered and blood gases need to be monitored. Continuous positive airway pressure or endotracheal intubation with ventilator support may be required. Intravenous diuretics and morphine help to reduce pulmonary congestion and allay anxiety. Intravenous nitro-glycerine should be used in all patients to reduce venous congestion and to bring the mean arterial blood pressure to a target of 70 mm of Hg. Intravenous SNP can be added if needed. Inotropic agents or assist device placement may be necessary to aid adding vasodilators. ACE inhibitors have to be given as they improve LV performance and decrease oxygen consumption by reducing afterload.171 Levosemendan has shown benefit in acute heart failure following ACS. Eplerenone can be given to patients with left ventricular ejection fraction < 40% and either diabetes or clinical signs of heart failure. Spironolactone can be used instead of eplerenone. Coexistent renal impairment or hyperkalaemia is a contraindication for eplerenone or spironolactone therapy.172 Beta blockers should be used with caution.
10.1.2. Cardiogenic shock
Of all cardiogenic shock 15% exist at presentation and 85% develop during hospitalization.
Inotropic and vasopressor agents are used to improve the mean arterial pressure. Dopamine and dobutamine improve cardiac hemodynamics but have not shown survival benefit. Dopamine may be associated with excess hazard.173 If the patient remains hypotensive despite dopamine, a direct vasoconstrictor like nor-epinephrine can be added.174
Data on usefulness of IABP in cardiogenic shock are conflicting.175 IABP when used in cardiogenic shock patients who were given Thrombolytic therapy was beneficial. When used in patients treated by primary PCI, there was neither a reduction of infarct size, nor any mortality or morbidity benefit.176
Routine use of IABP in all cases of shock is not be advocated. However, the use of IABP can help to add or up titrate vasodilator therapy.
Temporary mechanical support with left ventricular assist devices may allow time for recovery of stunned or hibernating myocardium.34 However, experience in this setting is limited. Impella device and extracorporeal membrane oxygenation(ECMO) have also been used in this setting.
10.1.3. RV dysfunction and RV failure
Right Ventricular MI and consequent RV dysfunction occurs in about 40% of inferior STEMI patients.RV MI and failure are identified clinically by hypotension, jugular venous distention with clear lungs and electrocardiographically by ST elevation in right sided leads. Echocardiography identifies and quantifies RV dysfunction. Severe right ventricular failure presents with a low cardiac output, oliguria and altered mental state. LV failure can coexist complicating fluid management.
Treatment hinges on maintenance of RV preload by fluid therapy targeting a CVP 15 mm of Hg and reduction of RV afterload. Preload reducing drugs like Nitrates and diuretics should be avoided.34 Restoration of atrioventricular (AV) synchrony with dual chamber temporary pacing or cardioversion from AF should be done when needed. Patients with RV infarction and any bradyarrhythmia with loss of sinus rhythm will benefit from temporary AV sequential pacing. Longer AV delays around 200 ms and a heart rate of about 80–90 give the best hemodynamics.171 RV dysfunction may need positive inotropes like dobutamine which helps to improve RV contractility.
Early revascularisation is crucial. Reperfusion of RV branches improves RV function and lowers 30 day mortality rate.177, 178 RV assist device is indicated for patients who remain in refractory cardiogenic shock in spite of all measures.
10.1.4. Acute MR due to papillary muscle dysfunction
Papillary muscle rupture [PMR] occurs in about 1% of patients with acute MI. PMR contributes to 7% of cardiogenic shock and 5% of mortality following MI. In 50% of the patients, infarct remains relatively small. Posteromedial papillary muscle is more commonly involved due to its single blood supply from RCA.
Prompt diagnosis, supportive measures, resuscitative management and early surgery is the key to salvaging the patient. Vasodilators and inodilators reduce the after load and regurgitant fraction and improve forward stroke volume. Sodium nitroprusside, nitro-glycerine, inotropes, morphine, ACE inhibitors and diuretics play a crucial role in pharmacotherapy. IABP179 by decreasing afterload decreases MR and increases forward flow. Mechanical support with Impella device,180 ECMO181 and Ventricular assist devices182 have been used with benefit in tiding over the acute crisis.
Coronary angiography is necessary as coronary revascularization along with mitral valve surgery is associated with improved short-term and long-term outcomes.183, 184 Intra operatively mitral valve and sub valvar apparatus are inspected to identify evidence of ongoing progression of ischemic injury or evidence of papillary muscle necrosis, which should lead to mitral valve replacement. If not, attempt should be made to repair the mitral valve. With mitral valve replacement, preservation of the sub valvar apparatus is associated with improved survival.185
Prognosis with medical therapy alone for acute MR associated with STEMI is dismal.183 In hospital mortality is about 70–80%.186 In a large series of 116 patients operated for papillary muscle rupture, operative mortality was 26.9% and 15-year survival was 39%. Operative and long-time mortality was better when concomitant coronary revascularisation was done. 5-year mortality of the operative survivors [20.6%] was similar to that of age matched controls with uncomplicated MI.187 Percutaneous mitral valve repair using Mitraclip has been used in salvaging patients with severe MR caused by papillary muscle rupture.188
10.1.5. Free wall rupture
Rupture of the free wall occurs in 0.5–2% of STEMI patients. Incidence has come down in the era of reperfusion. Large acute ruptures of the free wall present as pulseless electrical activity and sudden death. Subacute and small ruptures present with pericarditis with rub, tamponade, low cardiac output state and hypotension. Transthoracic echocardiography shows pericardial effusion usually with echo dense intrapericardial shadows, with or without cardiac tamponade. Absence of pericardial effusion in a patient with AMI excludes the diagnosis of myocardial rupture. CT/MRI may also be useful in determining the extent of free wall rupture.
Mortality of unoperated complete rupture is 100%. Prompt recognition and surgery is the only treatment. Role of medical therapy is to maintain BP, enroute to surgery. This includes fluids, inotropic support, vasopressors, IABP etc. Pericardiocentesis is used for relief of tamponade to save life. It is not routinely recommended as there are concerns that it can increase the size of the rent,189 by displacing thrombus and decompressing the contained rupture190 leading to worsening of tamponade.
Surgery is to relieve tamponade, close the rent to stop bleed and prevention of a second rupture. Direct suture of the myocardium over the infarct zone with reinforcement using Teflon felt strip, or repair by excluding the infarcted tissue is done. Patch and glue technique has shown better results.191 Simple patch and glue technique without coronary revascularisation was associated with better outcome.192 Mortality rate among patients who reach hospital for surgical management remains 60%. Perioperative mortality is high and is around 33–55%.193
10.1.6. Pseudo aneurysm
Pseudo aneurysm is caused by incomplete ruptures of the left ventricle, contained by organising thrombus and the pericardium, which forms the outer wall. It may remain small or undergo progressive enlargement.
2D echocardiography shows an aneurysmal bulge which communicates with the body of the left ventricle through a narrow neck. CT and MRI define the location and extent of the pseudo aneurysm to help plan surgical therapy. Clots inside the pseudo aneurysms can embolise. Spontaneous rupture occurs in 1/3rd of patients. Surgical intervention is required for all patients to prevent sudden death. Surgical repair is done with primary repair or patch closure. If possible the surgery can be delayed for 15 to 30 days, so that myocardial fibrosis that develops can facilitate the surgery.
10.1.7. Ventricular septal rupture
Ventricular septal rupture develops in about 1–2% of STEMI. With early reperfusion therapy with fibrinolysis and primary angioplasty, the incidence has come down to less than 0.5% in most series. Anterior MI causes anterior VSR while Inferior MI causes a basal posterior VSR.
2D echo with color Doppler along with new onset harsh holosystolic precordial murmur clinches the diagnosis. TEE may offer additional information in some cases. The rupture could be large or small and serpiginous, which can become larger over time. Prompt treatment soon after the diagnosis is necessary even if the patient is hemodynamically stable.
Traditionally, surgical correction had been the cornerstone of therapy. Mortality rate in patients with VSD treated medically is 24% at 72 h and 75% at 3 weeks. With cardiogenic shock and multiorgan failure surgical mortality is increased. RV dysfunction and RV failure are poor prognostic indicators. Hence early surgery soon after the diagnosis, is the cornerstone of therapy. The lower mortality observed in patients operated later,194 that led to a suggestion that corrective surgery could be delayed to improve the outcome, is now understood to be due to natural history selection bias, where stable patients are operated late leading to better outcome.195 However watchful waiting to delay surgery in stable patients can improve surgical outcome
There are two common techniques used for repair of VSR, the Daggett and David procedures.194, 196 Surgical mortality for basal VSR is higher [70% vs 30%] as it is technically more challenging.
Concomitant CABG is done after the surgery for VSR is completed.197 Earlier studies showed CABG along with VSR repair did not confer mortality benefit.198 Perotta et al. concluded that concomitant CABG is associated with improved 30 day and long term survival.199 Hence, concomitant CABG is recommended for patients undergoing surgical repair of VSR.200
Percutaneous device closure of periinfarct VSRs are being increasingly reported, with good long term survival.201 Good results are reported in acute settings too particularly in smaller defects202 less than 10 mm is size. Device closure in large defects can be a bridge to definitive surgery by reducing the left to right shunt and improving the hemodynamics.203
10.1.8. Ventricular aneurysm
Ventricular aneurysm formation after STEMI occurs in about 5–38% of patients. It occurs 5 times more frequent in those with anterior infarction. Incidence rates have declined with timely reperfusion and myocardial salvage.204 Anticoagulation is given for patients with mural thrombus. Refractory heart failure or refractory ventricular arrhythmias or recurrent thromboembolism in patients with aneurysms is an indication for surgical repair like Dor procedure.205 These procedures are also performed in some centres along with CABG, aiming to redcue LV diastolic volume and improve ejection fraction.
10.2. Arrhythmic complications of STEMI
Various kind of tachy and brady arrhythmia complicate the course of STEMI.
10.2.1. Tachyarrhythmia associated with MI could be supraventricular or ventricular
10.2.1.1. Supraventricular tachyarrhythmia
Supraventricular arrhythmia more likely to be due to due to excessive sympathetic stimulation, atrial stretch due to LV or RV pressure or volume overload, pericarditis, atrial infarction, hypoxia, electrolyte abnormalities, underlying pulmonary disease than ischemia.
Atrial fibrillation is the most common supraventricular arrhythmia and it occurs in about 8–22%. Contemporary studies have shown lower incidence of about 6.3%.206 New onset AF in STEMI is associated with higher incidence of cardiogenic shock, stroke and 90 day mortality. Rate control can be achieved by intravenous beta blockers or calcium blockers. Intravenous digoxin is used in patients with LV dysfunction/failure. Unstable patients need direct electrical cardioversion. With late presentation, which is common in India, the duration of AF may be unclear or more than 48 h. In this setting, TEE should be done to rule out LA clot, before cardioverting. Atrial flutter in this setting can be cardioverted with 50 J synchronised DC shock. Intravenous amiodarone may be used if there is recurrence of atrial fibrillation/flutter.
Sinus tachycardia does not need a specific therapy. Management of the inciting cause is the therapy of choice.
Atrial premature complexes should not be treated. Predisposing factors like electrolyte imbalance should be looked for and corrected.
10.2.1.2. Ventricular tachyarrhythmia
Ventricular premature complexes, hemodynamically stable non-sustained VT and accelerated idioventricular rhythms that come after reperfusion are not indicative of increased SCD risk and do not require specific therapy in the acute phase of STEMI.34 Betablocker should be started early as it reduces ventricular arrhythmia.
In GUSTO I trial of thrombolysed patients, 10% had sustained VT, while in APEX AMI study in patients undergoing primary PCI, only 5.7% had. 90% of these were within 48 h. Patients with very early VT within completion of Primary PCI had a 2 fold increase in mortality compared to those without VT; Patients with VT after Primary PCI but within 48 h of STEMI had a 5 fold increase in mortality.49 Polymorphic VT occurs in 0.3% of STEMI.207 It is associated with ischemia or reperfusion. Sustained monomorphic VT[SMVT] is associated with a permanent arrhythmic substrate and is associated with higher in hospital mortality and long term recurrence.
Primary VF is VF that occurs within 48 h. It is not often associated with recurrent ischemia or heart failure. In GUSTO-1 Study49 in hospital SMVT and VF were associated with higher in hospital mortality. But only SMVT was associated with higher post discharge one year mortality. Electrical cardioversion must be given for VF and pulseless VT. Intravenous amiodarone can be given for those who have VT with normal BP. Ongoing ischemia, residual ischemia, electrolyte and acid base abnormalities should be corrected
VT/VF that occurs beyond 48 h in the absence of reversible ischemia, reinfarction or metabolic abnormalities is an indication for ICD implantation, before discharge.34 Radiofrequency catheter ablation may be offered to patients with recurrent symptomatic SMVT despite medical therapy, incessant VT/VT storm not due to a reversible cause, Bundle branch reentrant VT, interfascicular VT, recurrent refractory sustained polymorphic VT and VF with a suspected trigger like partially injured purkinje fibres.208
10.2.2. Bradyarrhyrthmias
10.2.2.1. Sinus bradycardia
Sinus bradycardia is common in inferior and posterior MI due to reflex cholinergic stimulation. No specific treatment is needed if there is no hypotension or bradycardia triggered ventricular ectopy. Atropine can be used to raise the target HR to around 60 beats/min.
10.2.2.2. First-degree AV block
No specific treatment is required, however continued monitoring is important, as it can progress to higher degrees of block. Betablockers must be used with caution and avoided if PR interval is >240 ms.
10.2.2.3. Second-degree AV Block
Mobitz type I (Wenckebach) Second-degree AV block is due to AV nodal ischemia. No special treatment is needed. Atropine can be used if needed.
Mobitz type II Second-degree AV block is due to infra Hisian ischemia. It often progresses to complete heart block, and hence needs temporary pacing.
10.2.2.4. Third-degree AV block
Complete AV block (CAVB) in inferior infarctions is due to supra-Hisian or intranodal ischemia. It has a stable narrow QRS escape rhythm with rates exceeding 40/min in 70% of cases. It often responds to atropine, particularly if it developes in the first 6 h. Pacing is needed with lower escape rates, hypotension, pump failure and ventricular ectopy and if CAVB develops later in the course of STEMI.
CAVB in anterior infarction is due to extensive septal necrosis involving the bundle branches and associated with attendant high mortality. It is often preceded by an intraventricular block and often a type II second degree AV block. Escape rhythm is unstable with wide QRS complexes and rates less than 40 beats/min. Ventricular asystole may occur suddenly. Pacing in anterior wall MI has not shown survival benefits. However, it protects against asystole and against transient hypotension, which can extend the infarction and precipitate malignant ventricular arrhythmia.
10.2.3. Indications for permanent pacing after STEMI209
-
1.
Symptomatic persistent 2nd or 3rd degree AV block
-
2.
Persistent infra Hisian 3rd degree AV block
-
3.
Persistent second degree AV block in the His-Purkinje system with alternating bundle-branch block
-
4.
Transient advanced second- or third-degree infra nodal AV block and associated bundle-branch block
10.3. Ischemic complications of STEMI
Recurrent chest pain may be due to re-occlusion of recanalized vessel or thrombotic/mechanical occlusion or new ischemia at non infarct related artery or extension of occlusion to neighbouring vessels/side branches. Post infarction discomfort due to ischemia has come down to about 5% due to aggressive approach of recanalization like primary PCI or PI PCI and usage of anti thrombin, anti platelet drugs.210
10.4. Inflammatory complications
10.4.1. Pericarditis and pericardial effusion
Pericardial effusion associated with pericarditis are usually small and rarely progress to tamponade except the one associated with rupture of walls. They generally resolve slowly over a period of time. Anticoagulation is often stopped on detection of pericardial effusion. However, anticoagulation can be continued if strongly indicated.
Aspirin in doses of 650 mg 4 hourly is used for treatment of chest discomfort associated with pericarditis. NSAIDs and steroids are to be avoided, as they interfere with scar formation.
10.4.2. Dressler syndrome (Post myocardial infarction syndrome)
This delayed pericarditis usually occuring 1 to 8 weeks after infarction is of unknown etiology, probably autoimmune. Associated malaise, fever, leukocytosis and elevated ESR point to the diagnosis. Dressler syndrome responds well to aspirin 625 mg 4 hourly. NSAIDs are used for treatment. Recurrent cases may need colchicine. Steroids can be used if symptoms are severe.
10.5. Thromboembolic and bleeding complications
10.5.1. Venous thrombosis & pulmonary embolism
Deep vein thrombosis and pulmonary embolism are uncommon in infarcts due to early mobilization and discharge. However, they can happen in sicker patients who need prolonged bed rest and hospitalization. Deep vein thrombus from the lower extremities is a more likely source of pulmonary embolism, than a thrombus formation over the area of RV dysfunction.173
Early mobilization and optimization of anticoagulation does help in prevention. When deep vein thrombosis or pulmonary embolism develops, management is similar to the patients without myocardial infarctions.
10.6. Left ventricular thrombus & arterial embolization
Mural thrombus occurs in about 5 to 20% of patients, more common in those with large infarcts. About 10% of them can embolize resulting in stroke, renal infarcts, mesenteric ischemia, peripheral limb ischemia etc. Those in whom mural thrombus has been detected in the first 2 to 3 days often have a worse prognosis.211
Anticoagulation with intravenous heparin should be started in the absence of bleeding to maintain an aPTT around 60–70 s. Since these patients need long term oral anticoagulation for about 3 to 6 months, warfarin should be started soon to reach target INR of 2 to 3, overlapping with heparin. Heparin should be continued till INR is in range.
10.7. Bleeding complications
Bleeding is associated with increased risk of mortality, with hazard ratio ranging from 1.6 for minor bleed to 10.6 for major bleed in a large meta-analysis.212
All attempts should be made to prevent bleeding. Due consideration for age, sex, body weight, renal function, liver function, should be given while planning anticoagulant, antiplatelet and Thrombolytic drugs Due attention should be given to sheath size, catheter size, sheath removal, ACT etc while planning PCI. Radial access is associated with reduction in bleeding complications.
Intracranial haemorrhage warrants stopping all anticoagulant and antiplatelet drugs. Neuroimaging and neurosurgical consultation should be organised.
Suspected retroperitoneal bleed warrants prompt CT imaging of abdomen and pelvis. Often conservative management should suffice. Vascular interventional or surgical management may be needed. Gastrointestinal bleed may warrant urgent diagnostic cum therapeutic endoscopic procedures.
Protamine, fresh frozen plasma, prothrombin complex concentrates, activated factor VII and platelets can be used as necessary.213, 214 However, it is important to note that no particular therapy is associated with improved survival in intracranial haemorrhage.215, 216
Blood transfusion in acute MI setting had been shown to be associated with increased morbidity and mortality. So in the absence of ongoing ischemia, transfusion should be avoided unless the haemoglobin level is <8 mg/dl.34
10.8. Psychosocial complications
65% of patients with STEMI report symptoms suggestive of depression, and 15–22% of them have major depression. 36–60% of the patients continue to have depressive symptoms beyond 1 month of MI.217
STEMI patient should be evaluated for depressive symptoms and guided appropriately. Counselling and behaviour therapy will help in milder cases. Antidepressant medications should be considered if the depression is severe, persistent or recurrent depression, if there is family history of depression and if the patient is not able to cooperate with psychotherapy.217
AHA advisory states that selective serotonin reuptake inhibitor (SSRI) antidepressants-sertraline and citalopram, are safe antidepressants for patients with MI on the basis of randomised controlled studies.218 Use of SSRIs post MI has shown to have 42% decrease in mortality or recurrent myocardial infarction in a post hoc analysis of the an non randomised study Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) study.219
In our country where large segment of STEMI occurs in the rural population, the economic impact of STEMI on the family can be substantial. Every attempt should be made to help them through the available social security systems and NGOs involved in helping such families.
11. Secondary prevention and rehabilitation after STEMI
Lifestyle changes after MI greatly improves prognosis in randomized trials and in observational studies. A meta-analysis by Wilson et al. on smoking cessation after a myocardial infarction showed a relative risk reduction for coronary mortality of 46% and a Cochrane meta-analysis published in 2003 found a 36% reduction in all-cause mortality.220
However, the EUROASPIRE IV, conducted in 24 European countries, demonstrated a high prevalence of unhealthy lifestyles like smoking and little or no physical activity; uncontrolled modifiable risk factors like hypertension, obesity, diabetes and dyslipidemia; and underutilization of drugs with proven benefits for secondary prevention. Also, only 50.7% of post MI patients had been advised to participate in a cardiac rehabilitation or secondary prevention program.221
Secondary prevention and rehabilitation after STEMI should start as soon as the patient comes with the first medical contact after a STEMI. Though the priority during the acute event is revascularization and tackling the complications, the secondary prevention in terms of healthy life style and initiation of long term drugs should start simultaneously. The advantages of this period are that the patient is most motivated to accept the changes in life style like cessation of smoking and dietary modifications; he is a captive learner in the hospital bed and also the initial tolerability of the drugs are easily assessed while the patient is in hospital.
As the second MI always carries a worse prognosis than the initial event and the rate of a second MI is quite significant, the importance of the secondary prevention cannot be over emphasized. The key medicines need to be started, the doses need to be optimized and the duration of therapy must be clearly explained to the patient.
Rehabilitation after an MI is part and parcel of comprehensive management of STEMI. The ultimate goal of STEMI management is to send back the patient to his pre STEMI physical and psychological health. To that end, structured rehabilitation program plays a very important role.
11.1. Secondary prevention after STEMI
Secondary prevention after STEMI involves life style management and drug therapy. Both are complementary to each other.
11.1.1. Smoking
All people should be strongly advised to stop smoking after an MI. Professional counselling should be offered. If the patient is unwilling to accept counselling, pharmacotherapy like nicotine patch is an alternative. Passive smoking is also harmful and work environment needs to be modified to avoid passive smoking.
11.1.1.1. Diet
A healthy diet to prevent cardiovascular disease is equally advisable for primary as well as secondary prevention settings. A Mediterranean-style diet based on brown bread and rice, fresh fruits and vegetables, fish and lean meat, and oil rich in monounsaturated and polyunsaturated oil like mustard oil/barn oil is recommended as far as practicable. It is recommended to avoid red meat, cheese, butter, refined carbohydrates and deep fried food. Recently the Food and Diet Advisory committee in the USA has declared that whole egg, prawn and full fat dairy products do not adversely affect cardiovascular health. Avoidance of trans-fat and consumption of diet rich in fresh fruits and vegetables is highly recommended. Beta carotene and other antioxidants as well as folic acid supplement have no proven role in primary or secondary prevention of MI. Diet needs to be based on local habits, cultures, availability and affordability to be of any meaningful impact on the health of the individual or community. Total calorie must be adjusted to attain the ideal body weight which is a BMI of 20–25 kg/m2 and a waist circumference of 94 cm for men and 80 cm for women. Of course, physical activity is as important as diet in this respect.222
Key dietary recommendations are:-
-
•
Plenty of vegetable/fruit intake
-
•
Avoid simple sugar – should be replaced by natural complex sugar
-
•
Avoid trans fat and excess of saturated fat
-
•
Increase fibre content of food – non refined cereals
-
•
Avoid all processed food including processed meat
-
•
Balanced oils like mustard oil is recommended
-
•
Avoid excess salt
-
•
Calorie intake limitation to optimize body weight
11.1.2. Alcohol
Though there is a notion that alcohol is good for heart, it is never recommended to start alcohol to keep the heart healthy. However, people who drink alcohol may continue to do so. Although there is no strong data to suggest moderate alcohol consumption has harmful effect in patients with STEMI but the writing committee feels that alcohol consumptions should be discouraged especially in patients with LV dysfunction and binge drinking should be prohibited.
11.1.3. Physical activity
It is recommended that a regular aerobic physical activity of 20 to 30 min a day for at least 5 days a week is good after STEMI, if there is no significant heart failure. The exercise should be of that intensity which makes the person mildly breathless. Those who are unaccustomed to exercise before the MI should be preferably taken up in a supervised exercise program in a gradually increasing fashion. If exercise capacity is more than 5 METs without symptoms, the patient can return to routine physical activity. If not, patient should start at 50% of his maximum exercise capacity and gradually build up.
Exercise training should start at 2 h per week of aerobic exercise at 55–70% of the METs or to the onset of symptoms.
Practice of Yoga although advocated has limited scientific evidence.
11.1.4. Pharmacological therapy
Certain drugs have proven benefits for secondary prevention after a STEMI and those should be given and aimed to be given to the highest recommended doses unless they are contraindicated or not tolerated. These include aspirin, a second antiplatelet drug (clopidogrel, prasugral or ticagrelor), statin, beta blocker and angiotensin converting enzyme inhibitor (ACEI) or an angiotensin II receptor blocker (ARB) if ACEI is contraindicated or not tolerated.
11.1.5. Antiplatelet
Aspirin at a daily dose of 75 mg is recommended after all MI and that need to be continued indefinitely. In case of aspirin intolerance (eg, allergy), single therapy with clopidogrel is recommended. In people with high risk for peptic ulcer disease a proton pump inhibitor(PPI) is recommended prophylactically. With clopidogrel use, omeprazole may be replaced by other PPIs.
Apart from the loading dose of the second antiplatelet agent in the acute phase of STEMI, the second antiplatelet agent should be continued for at least 1 year after STEMI. The doses are 75 mg daily clopidogrel, or 10 mg daily prasugrel or 90 mg twice daily ticagrelor.
Both after implantation of a bare metal stent and drug eluting stent, the minimum duration of DAPT recommended is 12 months. In low bleeding risk people, it may be extended beyond 12months. On the other hand, in patients with oral anticoagulants or with high bleeding risk the duration may be shortened to 6 months. Prasugrel should not be used in people with high bleeding risk and with a history of stroke or TIA. Patients with STEMI who received thrombolytic therapy but no stent, clopidogrel is the recommended P2Y12 inhibitor and it should be used for ideally 12 months or more.
11.1.6. ACEI/ARB
ACEI should be started after a STEMI as soon as the patient is hemodynamically stable. (vide Section 10.2) Special indications for ACEI where the benefits are maximum are anterior MI, MI with clinical left ventricular failure (LVF) and MI with left ventricular ejection fraction (LVEF) below 45%. Diabetic has special indication for ACEI. ACEI should be continued indefinitely.
ACEI should be started at a low dose and rapidly escalated to the target dose, at most within 4 to 6 weeks. Renal function and electrolytes must be checked at baseline and then every 7 to 14 days during the drug titration period. Once stable, an annual check-up is usually sufficient. Blood pressure should be cautiously monitored giving special attention to postural hypotension. In case of deteriorating renal function (serum creatinine increasing by more than 30% of the baseline), dose need to be decreased or sometimes, the drug may need to be withdrawn.
Hyperkalemia may be prevented by co-prescription of a thiazide or loop diuretic.
Those who cannot tolerate ACEI should be treated with an ARB, exercising the cautions against renal function deterioration, hyperkalemia and hypotension as for ACEI. A combination of ACEI and ARB is not recommended.
11.1.7. Beta blockers
Every patient with STEMI must be started on a beta blocker, unless contraindicated, as soon as the patient is hemodynamically stable after an MI. and to be continued for at least three year. In presence of left ventricular systolic dysfunction or left ventricular failure, beta blocker should be continued indefinitely. The maximum tolerated approved dose should be targeted to be achieved. Special co-morbidities may dictate choice of beta blockers.
11.1.8. Statins
It is now universally accepted that all patients with cardiovascular disease including STEMI require high intensity statins, in the form of atorvastatin 40 to 80 mg daily or Rosuvastatin 20 to 40 mg daily, with an aim to reduce LDL cholesterol to below 70 mg/dl or by more than 50% from the baseline.
The statin should be continued indefinitely in the same dose, if tolerated. Periodic check up for liver function (ALT, AST) and myopathy (serum CPK) are no longer recommended in absence of symptoms and high risk status. A base line check of AST or ALT should be done. After 6 to 12 weeks a second estimation may be done. If a rise of more than 3 times the upper limit of normal is documented then a primary cause for liver disease should be sought. The statin dose is reduced to check if the enzymes improve. In certain cases, statin may have to withdrawn and restarted very cautiously at a lower dose and may be with a different agent.
In presence of muscle symptoms, estimation of serum CPK is indicated and if the value exceeds 10 times of normal, the statin is withdrawn and is usually contraindicated to be restarted. Certain high risk groups of patients like old age, female sex, low body weight, chronic kidney dysfunction, hypothyroidism, vitamin D deficiency need to be more closely watched for statin induced myopathy.
If the target dose of statin or the target LDL cholesterol value is not achieved due to side effects, then ezetimibe may be added to a lower dose of statin. PCSK-9 inhibitors are the newer agents but are yet to be approved in STEMI setting.
Low HDL cholesterol is a perpetual risk factor for ischemic heart disease but no pharmacological agent has been proved to be of clinical benefit till date. Life style modifications with physical exercise, avoidance of obesity and certain food like nuts help in raising the HDL cholesterol in a beneficial manner.
High triglyceride (TG) levels are proven risk associates for ischemic heart disease. But the clinical benefit of reducing TG with drugs is controversial. Once hyperglycemia, dysthyroidism, nephrotic syndrome and other causes of high TG are excluded or treated, a value above 200 mg/dl may be addressed with a fibrate.But none of them has any evidence of benefit in post STEMI situation. Fibrate if used with statin needs to be carefully monitored for renal function deterioration. However, if serum TG level is above 500 mg/dl, then the treatment is necessary to prevent pancreatitis and a fibrate is the drug of choice here.
11.1.9. Calcium channel blockers
Calcium Channel Blockers have no role in routine treatment after an MI. However, in presence of hypertension, tachycardia or angina, verapamil or diltiazem may be used if beta blockers are contraindicated. But they should not be used in presence of left ventricular dysfunction or left ventricular failure.
11.2. Other anti-anginal drugs
Potassium Channel Activators like Nicorandil, fatty acid oxidation inhibitor like Trimetazidine, late INa inhibitor like Ranolazine and If inhibitor like Ivabradine which are used in chronic stable angina, have no proven benefit in the post STEMI scenario.
11.2.1. Aldosterone antagonists: (vide Section 10.2)
Spironolactone and Eplerenone should be considered in patients with acute MI with left ventricular systolic dysfunction or left ventricular failure to be initiated within 3 to 14 days of the MI, preferably after ACE inhibitor therapy.
Renal function and serum potassium need to be monitored before and during treatment. If hyperkalemia is a problem, the dose of the aldosterone antagonist need to be halved or the drug may have to be withdrawn permanently.
11.3. Comprehensive cardiac rehabilitation
All patients should be offered a cardiac rehabilitation program after STEMI. It involves structured exercise training, healthy life style advice, psychosocial counselling and specific problems met by individual patients in going back to their occupation, sports and recreation and sexual life.
Cardiac rehabilitation programs should be flexible with a range of options, and patients may attend all or some of those as per their clinical needs. If a patient has cardiac or other conditions that may worsen during exercise, these should be addressed beforehand. In some situations, the exercise may be supervised by an appropriately qualified healthcare professional. Patients with left ventricular dysfunction who are stable can safely be offered the exercise component of cardiac rehabilitation.
Cardiac rehabilitation should be imparted in a non-judgemental, respectful, and culturally sensitive manner.
11.4. Sexual activity
After complete recovery from an uncomplicated MI sexual activity does not impose any extra risk for a second MI or MI related complications. However, an average period of 4 weeks should be given before resuming sex life. For treating male erectile dysfunction a phosphodiesterase type 5 (PDE5) inhibitor may be considered only after 6 months, and then also it should not be used if the person is taking a nitrate or nicorandil, for chance of precipitous drop of blood pressure.
11.5. Driving
Every country has its own regulations regarding licensing for driving light vehicle, heavy vehicle and public service vehicles. Those norms must be strictly followed. There are separate regulations for special occupational groups like air craft pilots.
11.6. Conclusion
Structured cardiac rehabilitation and secondary prevention programs reduce further cardiovascular event rates, promote healthy behaviour pattern, and improve active lifestyles. Improved survival has been found for all types of CAD patients, independent of the intervention modality.223, 224 It compares favourably in terms of cost effectiveness analysis as well.
To be useful a secondary prevention and cardiac rehabilitation program after STEMI should address the following issues in a sensitive and committed manner.
CARDIAC REHABILITATION
-
•
Health education and information requirements
-
•
Encouraging patients to attend
-
•
Exercise component
-
•
Psychological and social support
-
•
Sexual activity
-
•
Sports and recreation
LIFESTYLE CHANGES
-
•
Changing diet (e.g., Mediterranean-style diet)
-
•
Physical activity for 20–30 min a day
-
•
Alcohol consumption
-
•
Smoking cessation
-
•
Weight management
PHARMACOLOGICAL THERAPY
-
•
Angiotensin-converting enzyme (ACE) inhibitors
-
•
Dual antiplatelet therapy
-
•
Beta-blockers.
-
•
Statins
Acknowledgements
We acknowledge the contribution of the following doctors in the compilation of this document. Dr Ambukeshwar Singh, Dr K. L. Umamaheshwar, Dr Anant Agarwal, Dr Parminder Singh Manghera, Dr Bappaditya Kumar, Dr Sumanta Chatterjee, Dr Dharam Prakash and Dr. Vinod Singla.
Appendix A. Guidelines Committee
Guidelines Committee
| Chairman: | Dr. Santanu Guha |
| Chief Co-ordinator for STEMI Position Statement: | Dr. Rishi Sethi |
| Convener: | Dr. Saumitra Ray |
| Members: | Dr. V.K. Bahl |
| Dr. S Shanmugasundaram | |
| Dr. Praful Kerkar | |
| Dr. S. Ramakrishnan | |
| Dr. Rakesh Yadav | |
| Deputy Co-Ordinator STEMI Position Statement: | Dr. Gaurav Chaudhary |
Authors and Reviewers
| Dr. A Mullasari | Dr. JM Tharakan | Dr. S.N. Routray |
| Dr. Aditya Kapoor | Dr. Justin Paul | Dr. Sameer Dani |
| Dr. Ajay Mahajan | Dr. K Venogopal | Dr. Sandeep Bansal |
| Dr. Akshyaya Pradhan | Dr. K.C. Goswami | Dr. Sanjay Tyagi |
| Dr. Amal Kumar Banerjee | Dr. M.K. Das | Dr. Santanu Guha |
| Dr. B.P. Singh | Dr. M.S. Hiremath | Dr. Satyendra Tewari |
| Dr. Balachander J | Dr. N.N Khanna | Dr. Saumitra Ray |
| Dr. Brian Pinto | Dr. P.K. Deb | Dr. Sharat Chandra |
| Dr. C.N. Manjunath | Dr. P.P. Mohanan | Dr. Soumitra Kumar |
| Dr Debabrata Roy | Dr. Prafull G Kerkar | Dr Suma M Victor |
| Dr. Gaurav Chaudhary | Dr. Rakesh Yadav | Dr. Sundeep Mishra |
| Dr Geevar Zachariah | Dr. Rishi Sethi | Dr. Thomas Alexander |
| Dr. H.C. Kalita | Dr. S Ramakrishnan | Dr. V.K.Bahl |
| Dr. H.K. Chopra | Dr. S Shanmugasundaram | Dr. Vijay Trehan |
Member of CSI Executive Committee (2015–16)—Reviewed, Commented and Approved the Draft
| Dr. Ajay Kumar Sinha | Dr. P.B. Jayagopal |
| Dr. Chandrashekhar Makhale | Dr. P.K. Asokan |
| Dr. Dhiman Kahali | Dr. (Col.) R. Girish |
| Dr. K.B. Baksi | Dr. Rabindra Nath Chakraborty |
| Dr. Kajal Ganguly | Dr. Rakesh Gupta |
| Dr. M. Somasundaram | Dr. Shishu Shankar Mishra |
| Dr. M.K. Chhetri | Dr. Soura Mookerjee |
| Dr. M.S. Ravi | Dr. Umesh Chandra Samal |
References
- 1.Banerjee A.K., Kumar S. Guidelines for the management of acute myocardial infarction. JAPI. 2011;59:37–42. [PubMed] [Google Scholar]
- 2.Alexander T., Mullasari A.S., Kaifoszova Z. Framework for a national STEMI program: consensus document developed by STEMI INDIA, Cardiological Society of India and Association Physicians of India. Indian Heart J. 2015;67:497–502. doi: 10.1016/j.ihj.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . World Health Organization; Geneva, Switzerland: 1998. World Health Statistics Annual. [Google Scholar]
- 4.Murray L.J., Lopez A.D. The Harvard School of Public Health; Boston: 1996. The Global Burden of Disease. A Comprehensive Assessment of Mortality and Disability from Diseases, Injuries and Risk Factors in 1990 and Projected to 2020. [Google Scholar]
- 5.Whincup P.H., Gilg J.A., Papacosta O. Early evidence of ethnic differences in cardiovascular risk: cross sectional comparison of British South Asian and white children. BMJ. 2002;324:1–6. doi: 10.1136/bmj.324.7338.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Negi P.C., Merwaha R., Paday D. Multicentre HP ACS registry. Indian Heart J. 2015:07–027. doi: 10.1016/j.ihj.2015.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iqbal F., Barkataki J.C. Spectrum of acute coronary syndrome in North Eastern India −a study from a major center. Indian Heart J. 2013;68:128–131. doi: 10.1016/j.ihj.2015.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xavier D., Pais P., Devereaux P.J., Xie C., Prabhakaran D., Reddy K.S. CREATE registry investigators: treatment and outcomes of acute coronary syndromes in India (CREATE): a prospective analysis of registry data. Lancet. 2008;371(9622):1435–1442. doi: 10.1016/S0140-6736(08)60623-6. [DOI] [PubMed] [Google Scholar]
- 9.Mohanan P.P., Mathew R., Harikrishnan S. Presentation, management, and outcomes of 25 748 acute coronary syndrome admissions in Kerala, India: results from the Kerala ACS Registry. Eur Heart J. 2013;34(2):121–129. doi: 10.1093/eurheartj/ehs219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO, WHO Fact sheet No 310, updated June 2011. 2011.
- 11.Widimsky P., Wijns W., Fajadet J. Reperfusion therapy for ST elevation acute myocardial infarction in Europe: description of the current situation in 30 countries. Eur Heart J. 2010;31:943–957. doi: 10.1093/eurheartj/ehp492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Widimsky P., Zelizko M., Jansky P., Tousek F., Holm F., Aschermann M. The incidence, treatment strategies,outcomes of acute coronary syndromes in the reperfusion network of different hospital types in the Czech Republic: results of the Czech evaluation of acute coronary syndromes in hospitalized patients (CZECH) registry. Int J Cardiol. 2007;119:212–219. doi: 10.1016/j.ijcard.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 13.Wiviott S.D., Morrow D.A., Giugliano R.P. Performance of the thrombolysis in myocardial infarction risk index for early acute coronary syndrome in the National Registry of Myocardial Infarction: a simple risk index predicts mortality in both ST and non-ST elevation myocardial infarction. J Am Coll Cardiol. 2003;41:365A–366A. doi: 10.1016/j.jacc.2004.05.045. [DOI] [PubMed] [Google Scholar]
- 14.Manus D.D., Gore J., Yarzebski J., Spencer F., Lessard D., Goldberg R.J. Recent trends in the incidence, treatment, and outcomes of patients with STEMI and NSTEMI. Am J Med. 2011;124:40–47. doi: 10.1016/j.amjmed.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta R. Recent trends in coronary heart disease epidemiology in India. Indian Heart J. 2008;60(2 Suppl. B):B4–18. [PubMed] [Google Scholar]
- 16.Sharma R., Bhairappa S., Prasad S.R., Manjunath C.N. Clinical characteristics, angiographic profile and in hospital mortality in acute Coronary syndrome patients in south indian population. Heart India. 2014;2(3) [Google Scholar]
- 17.Karthikeyan G., Teo K.K., Islam S. Lipid profile, plasma apolipoproteins, and risk of a first myocardial infarction among Asians: an analysis from the INTERHEART Study. J Am Coll Cardiol. 2009;53(3):244–253. doi: 10.1016/j.jacc.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 18.Prabhakaran D., Yusuf S., Mehta S. Two-year outcomes in patients admitted with non-ST elevation acute coronary syndrome: results of the OASIS registry 1 and 2. Indian Heart J. 2005;57(3):217–225. [PubMed] [Google Scholar]
- 19.Pagidipati N.J., Huffman D., Jeemon P., Gupta R., Negi P., Jaison T.M. Association between gender, process of care measures, and outcomes in ACS in India: results from the detection and management of coronary heart disease (DEMAT) registry. PLoS One. 2013;8(4):e6206. doi: 10.1371/journal.pone.0062061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brindis R.G., Fitzerald S., Anderson H.V., Shaw R.E., Weintraub W.S., Willia J.F. The american college of cardiology-National cardiovascular data registry: building a national clinical data repository. J Am Coll Cardiol. 2001:2240–2245. doi: 10.1016/s0735-1097(01)01372-9. [DOI] [PubMed] [Google Scholar]
- 21.Mandelzweig L., Battler A., Boyko V. The second Euro Heart Survey on acute coronary syndromes: characteristics, treatment, and outcome of patients with ACS in Europe and the Mediterranean Basin in 2004. Eur Heart J. 2006;27(19):2285–2293. doi: 10.1093/eurheartj/ehl196. [DOI] [PubMed] [Google Scholar]
- 22.Fox K.A., Goodman S.G., Klein W., Brieger D., Steg P.G. Dabbous: management of acute coronary syndromes. Variations in practice and outcome: findings from the global registry of acute coronary events (GRACE) Eur Heart J. 2002;23:1177–1189. doi: 10.1053/euhj.2001.3081. [DOI] [PubMed] [Google Scholar]
- 23.Ericsson I.T., Hellström K., Isaksson R.M., Sederholm Lawesson S. First medical contact in patients with STEMI and its impact on time to diagnosis; an explorative cross-sectional study. BMJ Open. 2015;5 doi: 10.1136/bmjopen-2014-007059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.George E., Savitha D., Pais P. Pre-hospital issues in acute myocardial infarction. J Assoc Phys India. 2001;49:320–323. [PubMed] [Google Scholar]
- 25.Rajagopalan R.E., Chandrasekaran S., Pai M. Pre-hospital delay in acute myocardial infarction in an urban Indian hospital: a prospective study. Natl Med J India. 2001;14:8. [PubMed] [Google Scholar]
- 26.Allen L.A., O'Donnell C.J., Giugliano R.P., Camargo C.A., Jr., Lloyd-Jones D.M. Care concordant with guidelines predicts decreased long-term mortality in patients with unstable angina pectoris and non-ST elevation myocardial infarction. Am J Cardiol. 2004;93(10):1218–1222. doi: 10.1016/j.amjcard.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 27.Huffman M.D., Prabhakaran D., Abraham A.K., Krishnan M.N., Nambiar A.C., Mohanan P.P. Optimal in-hospital and discharge medical therapy in acute Coronary syndromes in Kerala: results from the kerala ACS registry. Circ Cardiovasc Qual Outcomes. 2013;6(4) doi: 10.1161/CIRCOUTCOMES.113.000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.M Huffman, D Prabhakaran, PP Mohanan. Study of a Quality Improvement Toolkit in Kerala, India, Among Hospitals Treating Acute Coronary Syndrome Patients (ACS QUIK) Clinical Trials. gov Identifier:NCT02256657.
- 29.Gupta R., Islam S., Mony P. Socioeconomic factors and use of secondary prevention therapy for cardiovascular diseases in South Asiaa. The PURE study. Eur J Prev Cardiol. 2015;10:1261–1271. doi: 10.1177/2047487314540386. [DOI] [PubMed] [Google Scholar]
- 30.Patel A., Vishwanathan S., Mark D. 2015. Differences in the Presentation, Diagnosis, and Management of Acute Coronary Syndromes findings From the Kerala (India) ACS Registry GLOBAL HEART. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daivadanam M., Thankappan K.R., Sarma P.S., Harikrishnan S. Catastrophic health expenditure & coping strategies associated with acute coronary syndrome in Kerala, India. Indian J Med Res. 2012;136:585–594. [PMC free article] [PubMed] [Google Scholar]
- 32.Ruiz Bailén M., Rucabado Aguilar L., Aguayo de Hoyos E., Brea-Salvago J.F. The CRUSADE study, evaluation model of quality in percutaneous coronary intervention. Med Intensiva. 2006;30(6):276–279. doi: 10.1016/s0210-5691(06)74524-7. [DOI] [PubMed] [Google Scholar]
- 33.Prabhakaran D., Jeemon P., Mohanan P.P. Management of acute coronary syndromes in secondary care settings in Kerala: impact of a quality improvement programme. Natl Med J India. 2008;21:107–111. [PubMed] [Google Scholar]
- 34.O’Gara P.T., Kushner F.G., Ascheim D.D. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the american college of cardiology Foundation/American heart association task force on practice guidelines. J Am Coll Cardiol. 2013;61:e78–140. doi: 10.1016/j.jacc.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 35.Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC), Steg P.G., James S.K. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33(20):2569–2619. doi: 10.1093/eurheartj/ehs215. [DOI] [PubMed] [Google Scholar]
- 36.O'Gara P.T., Kushner F.G., Ascheim D.D. ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST-elevation myocardial Infarction: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention and the 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv. 2015;87:1001–1019. doi: 10.1002/ccd.26325. [DOI] [PubMed] [Google Scholar]
- 37.Lee T.H., Goldman L. Evaluation of the patients with acute chest pain. N Eng J Med. 2000;342:1187–1195. doi: 10.1056/NEJM200004203421607. [DOI] [PubMed] [Google Scholar]
- 38.Pope J.H., Aufderheide T.P., Ruthazer R. Missed diagnosis of acute cardiac ischemia in the emergency department. N Eng J Med. 2000;342:1163–1170. doi: 10.1056/NEJM200004203421603. [DOI] [PubMed] [Google Scholar]
- 39.Daga L.C., Kaul U., Mansoor A. Approach to STEMI and NSTEMI. J Assoc Phys India. 2011;59(Suppl):19–25. [PubMed] [Google Scholar]
- 40.Chopra H.K. Challenges of STEMI care in India & the real world. Indian Heart J. 2015;67:15–17. doi: 10.1016/j.ihj.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brieger D., Eagle K.A., Goodman S.G. Acute coronary syndromes without chest pain, an underdiagnosed and undertreated high-risk group: insights from the Global Registry of Acute Coronary Events. Chest. 2004;126:461–469. doi: 10.1378/chest.126.2.461. [DOI] [PubMed] [Google Scholar]
- 42.Kunwar B.K., Hooda A., Joseph G. Recent trends in reperfusion in ST elevation myocardial infarction in a South Indian tier-3 city. Indian Heart J. 2012;64:368–373. doi: 10.1016/j.ihj.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seetharama N., Mahalingappa R., Ranjith Kumar G.K., Veerappa V., Aravindh C.L. Clinical profile of acute myocardial infarction patients: a study in tertiary care centre. Int J Res Med Sci. 2015;3:412–419. [Google Scholar]
- 44.Shah V., Jain U. Clinical profile of acute myocardial infarction in young adults. Int J Med Sci Public Health. 2016;5:1709–1713. [Google Scholar]
- 45.Sharma A., Kumar R., Ashotra S., Thakur S. Comparative evaluation of clinical profile, risk factors, and outcome of acute myocardial infarction in elderly and nonelderly patients. Heart India. 2016;4:96–99. [Google Scholar]
- 46.Bhatia L.C., Naik R.H. Clinical profile of acute myocardial infarction in elderly patients. J Cardiovasc Dis Res. 2013;4:107–111. doi: 10.1016/j.jcdr.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thygesen K., Alpert J.S., Jaffe A.S. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035. doi: 10.1161/CIR.0b013e31826e1058. [DOI] [PubMed] [Google Scholar]
- 48.Jain S., Ting H.T., Bell M. Utility of left bundle branch block as a diagnostic criterion for acute myocardial infarction. Am J Cardiol. 2011;107:1111–1116. doi: 10.1016/j.amjcard.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 49.Sgarbossa E.B., Pinski S.L., Barbagelata A. Electrocardiographic diagnosis of evolving acute myocardial infarction in the presence of left bundle-branch block GUSTO-1 (Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries) Investigators. N Engl J Med. 1996;334:481–487. doi: 10.1056/NEJM199602223340801. [DOI] [PubMed] [Google Scholar]
- 50.Tabas J.A., Rodriguez R.M., Seligman H.K., Goldschlager N.F. Electrocardiographic criteria for detecting acute myocardial infarction in patients with left bundle branch block: a meta-analysis. Ann Emerg Med. 2008;52:329–336. doi: 10.1016/j.annemergmed.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 51.Wang K., Asinger R.W., Marriott H.J. ST-segment elevation in conditions other than acute myocardial infarction. N Engl J Med. 2003;349:2128–2135. doi: 10.1056/NEJMra022580. [DOI] [PubMed] [Google Scholar]
- 52.De Winter R.J., Verouden N.J.W., WellensH.J.J. Wilde A.A. A new ECG sign of proximal LAD occlusion. N Engl J Med. 2008;359:2071–2073. doi: 10.1056/NEJMc0804737. [DOI] [PubMed] [Google Scholar]
- 53.Jong G.P., Ma T., Chou P., Shyu M.Y., Tseng W.K., Chang T.C. Reciprocal changes in 12-lead electrocardiography can predict left main coronary artery lesion in patients with acute myocardial infarction. Int Heart J. 2006;47:13–20. doi: 10.1536/ihj.47.13. [DOI] [PubMed] [Google Scholar]
- 54.Mahajan V.S., Jarolim P. How to interpret elevated cardiac troponin levels. Circulation. 2011;124:2350–2354. doi: 10.1161/CIRCULATIONAHA.111.023697. [DOI] [PubMed] [Google Scholar]
- 55.Apple F.S., Parvin C.A., Buechler K.F., Christenson R.H., Wu A.H.B., Jaffe A.S. Validation of the 99th percentile cutoff independent of assay imprecision (CV) for cardiac troponin monitoring for ruling out myocardial infarction. ClinChem. 2005;51:2198–2200. doi: 10.1373/clinchem.2005.052886. [DOI] [PubMed] [Google Scholar]
- 56.Bhoi S., Verma P., Vankar S., Galwankar S. High sensitivity troponins and conventional troponins at the bedside. Int J Crit Illn Inj Sci. 2014;4(3):253–256. doi: 10.4103/2229-5151.141471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Van de Werf F., Bax J., Betriu A. Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: the Task Force on the Management of ST-Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J. 2008;29:2909 29–45. doi: 10.1093/eurheartj/ehn416. [DOI] [PubMed] [Google Scholar]
- 58.Mehta R.H., Califf R.M., GargJ. White H.D. The impact of anthropomorphic indices on clinical outcomes in patients with acute ST-elevation myocardial infarction. Eur Heart J. 2007;28:415–424. doi: 10.1093/eurheartj/ehl329. [DOI] [PubMed] [Google Scholar]
- 59.Killip T., 3rd, Kimball J.T. Treatment of myocardial infarction in a coronary care unit: a two year experience with 250 patients. Am J Cardiol. 1967;20(4):457–464. doi: 10.1016/0002-9149(67)90023-9. [DOI] [PubMed] [Google Scholar]
- 60.Morrow D.A., Antman E.M., Charlesworth A. TIMI risk score for ST-elevation myocardial infarction: a convenient, bedside, clinical score for risk assessment at presentation: an intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation. 2000;102(17):2031–2037. doi: 10.1161/01.cir.102.17.2031. [DOI] [PubMed] [Google Scholar]
- 61.Granger C.B., Goldberg R.J., Dabbous O. Global Registry of Acute Coronary Events Investigators Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med. 2003;163(19):2345–2353. doi: 10.1001/archinte.163.19.2345. [DOI] [PubMed] [Google Scholar]
- 62.Cohen M., Gensini G.F., Maritz F., Gurfinkel E.P., Huber K., Timerman A. TETAMI Investigators. Prospective evaluation of clinical outcomes after acute ST-elevation myocardial infarction in patients who are ineligible for reperfusion therapy: preliminary results from the TETAMI registry and randomized trial. Circulation. 2003;108(16 Suppl. 1):III14–III21. doi: 10.1161/01.CIR.0000091832.74006.1C. [DOI] [PubMed] [Google Scholar]
- 63.Verheugt F.W., Gersh B.J., Armstrong P.W. Aborted myocardial infarction: a new target for reperfusion therapy. Eur Heart J. 2006;27:901–904. doi: 10.1093/eurheartj/ehi829. [DOI] [PubMed] [Google Scholar]
- 64.Hedges J.R., Feldman H.A., Bittner V. for the REACT Study Group: impact of community intervention to reduce patient delay time on use of reperfusion therapy for acute myocardial infarction: rapid Early Action for Coronary Treatment (REACT) trial. Acad Emerg Med. 2000;7:862–872. doi: 10.1111/j.1553-2712.2000.tb02063.x. [DOI] [PubMed] [Google Scholar]
- 65.Sørensen J.T., Terkelsen C.J., Nørgaard B.L. Urban and rural implementation of pre-hospital diagnosis and direct referral for primary percutaneous coronary intervention in patients with acute ST-elevation myocardial infarction. Eur Heart J. 2011;32:430–436. doi: 10.1093/eurheartj/ehq437. [DOI] [PubMed] [Google Scholar]
- 66.Rokos I.C., French W.J., Koenig W.J. Integration of pre-hospital electrocardiograms and ST-elevation myocardial infarction receiving center (SRC) networks: impact on door-to-balloon times across 10 independent regions. J Am Coll Cardiol Intv. 2009;2:339–346. doi: 10.1016/j.jcin.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 67.Fibrinolytic Therapy Trialists’ (FTT) Collaborative Group Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomized trials of morethan 1000 patients. Lancet. 1994;343:311–322. [PubMed] [Google Scholar]
- 68.GruppoItaliano per lo Studio dellaStreptochinasinell’InfartoMiocardico (GISSI) Effectiveness of intravenous thrombolytic treatment in acute myocardial infarction. Lancet. 1986;1:397–402. [PubMed] [Google Scholar]
- 69.Armstrong P.W., Collen D., Antman E. Fibrinolysis for acute myocardial infarction: the future is here and now. Circulation. 2003;107:2533–2537. doi: 10.1161/01.CIR.0000072930.64775.DC. [DOI] [PubMed] [Google Scholar]
- 70.Widimský P., Budesínský T., Vorác D. Long distance transport for primary angioplasty vs: immediate thrombolysis in acute myocardial infarction. Final results of the randomized national multicentre trial—PRAGUE-2. Eur Heart J. 2003;24:94–104. doi: 10.1016/s0195-668x(02)00468-2. [DOI] [PubMed] [Google Scholar]
- 71.Andersen H.R., Nielsen T.T., Rasmussen K. A comparison of coronary angioplasty with fibrinolytic therapy in acute myocardial infarction. N Engl J Med. 2003;349:733–742. doi: 10.1056/NEJMoa025142. [DOI] [PubMed] [Google Scholar]
- 72.Gregg Stone W. Advances in interventional cardiology-angioplasty strategies in ST-segment–elevation myocardial infarction. Circulation. 2008;118:552–566. doi: 10.1161/CIRCULATIONAHA.107.739243. [DOI] [PubMed] [Google Scholar]
- 73.Antman E.M., Anbe D.T., Armstrong P.W. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction-executive summary: a report of the american college of Cardiology/American heart association task force on practice guidelines (Writing committee to revisethe 1999 guidelines for the management of patients with acute myocardial infarction) Circulation. 2004;110(5):588–636. doi: 10.1161/01.CIR.0000134791.68010.FA. [DOI] [PubMed] [Google Scholar]
- 74.Victor S.M., Gnanaraj A., Vijayakumar S., Pattabiram S. Door-to-balloon: where do we lose time? Single centre experience in India. Indian Heart J. 2012;64(6):582–587. doi: 10.1016/j.ihj.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jolly S.S., Pogue J., Haladyn K. Effects of aspirin dose on ischaemic events and bleeding after percutaneous coronary intervention: insights from the PCI-CURE study. Eur Heart J. 2009;30:900–907. doi: 10.1093/eurheartj/ehn417. [DOI] [PubMed] [Google Scholar]
- 76.Lundergan C.F., Reiner J.S., McCarthy W.F., Coyne K.S., Califf R.M., Ross A.M. Clinical predictors of early infarct-related artery patency following thrombolytic therapy: importance of body weight, smoking history, infarct-related artery and choice of thrombolytic regimen: the GUSTO-I experience. Global Utilization of Streptokinase and t-PA for Occluded Coronary Arteries. J Am Coll Cardiol. 1998;32(3):641–647. doi: 10.1016/s0735-1097(98)00278-2. [DOI] [PubMed] [Google Scholar]
- 77.Iyengar S.S., Godbole S.G. Thrombolysis in the era of Intervention. Suppl JAPI. 2011:2011. [PubMed] [Google Scholar]
- 78.Werf F.V.D. Primary versus tenecteplase-facilitated percutaneous coronary intervention in patients with ST-segment elevation acute myocardial infarction (ASSENT-4 PCI): randomised trial. Lancet. 2006;367(9510):569–578. doi: 10.1016/S0140-6736(06)68147-6. [DOI] [PubMed] [Google Scholar]
- 79.Chopra HK, Ramakrishnan S, Pancholia AK, Bansal M. Cardiological Society of India: Cardiology Update 2014. 1st ed. India: Jaypee Brothers Medical Publisher (P) Ltd, 2015. Chapter 20: Pharmacoinvasive strategy in the treatment of STEMI;p. 109–113.
- 80.Antman E.M., Van de Werf F. Pharmacoinvasive therapy. Circulation. 2004;109:2480–2486. doi: 10.1161/01.CIR.0000128736.57259.48. [DOI] [PubMed] [Google Scholar]
- 81.Fernandez-Avilés F., Alonso J.J., Castro-Beiras A. GRACIA-2 (Groupo de Análisis de CardiopatíaIsquémicaAguda) Investigators Primary angioplasty vs. early routine post-fibrinolysis angioplasty for acute myocardial infarction with ST-segment elevation: the GRACIA-2 non-inferiority, randomized, controlled trial. Eur Heart J. 2007;28:949–960. doi: 10.1093/eurheartj/ehl461. [DOI] [PubMed] [Google Scholar]
- 82.Cantor W.J., Fitchett D., Borgundvaag B. TRANSFER-AMI trial investigators: routine early angioplasty after fibrinolysis for acute myocardial infarction. N Engl J Med. 2009;360:2705–2718. doi: 10.1056/NEJMoa0808276. [DOI] [PubMed] [Google Scholar]
- 83.Danchin N., Coste P., Ferrières J. Comparison of thrombolysis followed by broad use of percutaneous coronary intervention with primary percutaneous coronary intervention for ST-segment?elevation acute myocardial infarction. Data From the French Registry on Acute ST-Elevation Myocardial Infarction (FAST-MI) Circulation. 2008;118:268–276. doi: 10.1161/CIRCULATIONAHA.107.762765. [DOI] [PubMed] [Google Scholar]
- 84.Armstrong P.W. WEST Steering Committee: a comparison of pharmacologic therapy with/without timely coronary intervention vs. primary percutaneous intervention early after STelevation myocardial infarction: the WEST (Which Early STelevation myocardial infarction Therapy) study. Eur Heart J. 2006;27:1530–1538. doi: 10.1093/eurheartj/ehl088. [DOI] [PubMed] [Google Scholar]
- 85.Bøhmer E., Hoffmann P., Abdelnoor M., Arnesen H., Halvorsen S. Efficacy and safety of immediate angioplasty versus ischemia-guided management after thrombolysis in acute myocardial infarction in areas with very long transfer distances results of the NORDISTEMI (NORwegian study on DIstrict treatment of ST-elevation myocardial infarction) J Am Coll Cardiol. 2010;55(2):102–110. doi: 10.1016/j.jacc.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 86.Di Mario C., Dudek D., Piscione F. Immediate angioplasty versus standard therapy with rescue angioplasty after thrombolysis in the Combined AbciximabREteplase Stent Study in Acute Myocardial Infarction (CARESS-in-AMI): an open, prospective, randomised, multicentre trial. Lancet. 2008;371(9612):559–568. doi: 10.1016/S0140-6736(08)60268-8. [DOI] [PubMed] [Google Scholar]
- 87.Sinnaeve PR, Armstrong PW, Gershlick AH, Gold Stein P, Wilcox R, Lambert H, et al. ST- Segment- elevation myocardial infarction patients randomized to a pharmacoinvasive strategy or primary percutaneous coronary intervention: Strategic reperfusion early After Myocardial Infarction (STREAM) 1-year mortality follow-up. Circulation. 2014:130:1139–45. [DOI] [PubMed]
- 88.Victor S.M., Subban V., Alexander T G., Srinivas A B.C., S S., Mullasari A.S. A prospective, observational, multicentre study comparing tenecteplase facilitated PCI versus primary PCI in Indian patients with STEMI (STEPP-AMI) Open Heart. 2014;1(1) doi: 10.1136/openhrt-2014-000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Alexander T., Victor S.M., Mullasari A.S., Veerasekar G., Subramaniam K., Nallamothu B.K. Protocol for a prospective, controlled study of assertive and timely reperfusion for patients with ST-segment elevation myocardial infarction in Tamil Nadu: the TN-STEMI programme. BMJ Open. 2013;3 doi: 10.1136/bmjopen-2013-003850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Steg P.G., Bonnefoy E., Chabaud S. Impact of time to treatment on mortality after prehospital fibrinolysis or primary angioplasty: data from the CAPTIM randomized clinical trial. Circulation. 2003;108(23):2851–2856. doi: 10.1161/01.CIR.0000103122.10021.F2. [DOI] [PubMed] [Google Scholar]
- 91.Ting H.H., Rihal C.S., Gersh B.J. Regional systems of care to optimize timeliness of reperfusion therapy for ST-elevation myocardial infarction: the Mayo Clinic STEMI Protocol. Circulation. 2007;116(7):729–736. doi: 10.1161/CIRCULATIONAHA.107.699934. [DOI] [PubMed] [Google Scholar]
- 92.Vora A.N., Holmes D.N., Rokos I., Roe M.T., Granger C.B., French WJet al. Fibrinolysis use among patients requiring interhospital transfer for ST-Segment elevation myocardial infarction care a report from the US national cardiovascular data registry. JAMA Intern Med. 2015;175(2):207–215. doi: 10.1001/jamainternmed.2014.6573. [DOI] [PubMed] [Google Scholar]
- 95.Milavetz J.J., Giebel D.W., Christian T.F., Schwartz R.S., Holmes D.R., Jr., Gibbons R.J. Time to therapy and salvage in myocardial infarction. J Am Coll Cardiol. 1998;31:1246–1251. doi: 10.1016/s0735-1097(98)00088-6. [DOI] [PubMed] [Google Scholar]
- 96.De Luca G., Suryapranata H., Ottervanger J.P., Antman E.M. Time delay to treatment and mortality in primary angioplasty for acute myocardial infarction: every minute of delay counts. Circulation. 2004;109:1223–1225. doi: 10.1161/01.CIR.0000121424.76486.20. [DOI] [PubMed] [Google Scholar]
- 97.Zijlstra F., Hoorntje J.C., de Boer M.J., Reiffers S., Miedema K., Ottervanger J.P. Long-term benefit of primary angioplasty as compared with thrombolytic therapy for acute myocardial infarction. N Engl J Med. 1999;341:1413–1419. doi: 10.1056/NEJM199911043411901. [DOI] [PubMed] [Google Scholar]
- 98.Keeley E.C., Boura J.A., Grines C.L. Primary angioplasty vs. intravenous thrombo- lytic therapy for acute myocardial infarction: a quantitative review of 23 rando-mised trials. Lancet. 2003;361:13–20. doi: 10.1016/S0140-6736(03)12113-7. [DOI] [PubMed] [Google Scholar]
- 99.Grzybowski M., Clements E.A., Parsons L. Mortality benefit of immediate revascularization of acute ST-segment elevation myocardial infarction in patients with contraindications to thrombolytic therapy: a propensity analysis. JAMA. 2003;290:1891–1898. doi: 10.1001/jama.290.14.1891. [DOI] [PubMed] [Google Scholar]
- 100.Zahn R., Schuster S., Schiele R. Maximal Individual Therapy in Acute Myocardial Infarction (MITRA) Study Group: comparison of primary angioplasty with conservative therapy in patients with acute myocardial infarction and contraindications for thrombolytic therapy. Catheter Cardiovasc Interv. 1999;46:127–133. doi: 10.1002/(SICI)1522-726X(199902)46:2<127::AID-CCD2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 101.Hochman J.S., Sleeper L.A., Webb J.G., Sanborn T.A., White H.D., et al Talley J.D. for the SHOCK Investigators: early revascularization in acute myocardial infarction complicated by cardiogenic shock. N Engl J Med. 1999;341:625–634. doi: 10.1056/NEJM199908263410901. [DOI] [PubMed] [Google Scholar]
- 102.Hochman J.S., Lamas G.A., Buller C.E. Coronary intervention for persistent occlusion after myocardial infarction. N Engl J Med. 2006;355:2395–2407. doi: 10.1056/NEJMoa066139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Thune J.J., Hoefsten D.E., Lindholm M. Simple risk stratification at admission to identify patients with reduced mortality from primary angioplasty. Circulation. 2005;112:2017–2021. doi: 10.1161/CIRCULATIONAHA.105.558676. [DOI] [PubMed] [Google Scholar]
- 104.Wu A.H., Parsons L., Every N.R., Bates E.R. Hospital outcomes in patients presenting with congestive heart failure complicating acute myocardial infarction: a report from the Second National Registry of Myocardial Infarction (NRMI-2) J Am Coll Cardiol. 2002;40:1389–1394. doi: 10.1016/s0735-1097(02)02173-3. [DOI] [PubMed] [Google Scholar]
- 105.Boersma E., Maas A.C., Deckers J.W., Simoons M.L. Early thrombolytic treatment in acute myocardial infarction: reappraisal of the golden hour. Lancet. 1996;348:771–775. doi: 10.1016/S0140-6736(96)02514-7. [DOI] [PubMed] [Google Scholar]
- 106.Chareonthaitawee P., Gibbons R.J., Roberts R.S., Christian T., Burns R., for the CORE investigators (Collaborative Organisation for RheothRx Evaluation) The impact of time to thrombolytic treatment on outcome in patientswith acute myocardial infarction. Heart. 2000;84:142–148. doi: 10.1136/heart.84.2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Boersma E. Does time matter?: A pooled analysis of randomized clinical trials comparing primary percutaneous coronary intervention and in-hospital fibrin- olysis in acute myocardial infarction patients. Eur Heart J. 2006;27:779–788. doi: 10.1093/eurheartj/ehi810. [DOI] [PubMed] [Google Scholar]
- 108.Pinto D.S., Kirtane A.J., Nallamothu B.K., Murphy S.A., Cohen D.J., Laham R.J. et al ., Hospital delays in reperfusion for ST-elevation myocardial infarction: implications when selecting a reperfusion strategy. Circulation. 2006;114:2019–2025. doi: 10.1161/CIRCULATIONAHA.106.638353. [DOI] [PubMed] [Google Scholar]
- 109.Hackett D., Davies G., Chierchia S., Maseri A. Intermittent coronary occlusion in acute myocardial infarction: value of combined thrombolytic and vasodilator therapy. N Engl J Med. 1987;317:1055–1059. doi: 10.1056/NEJM198710223171704. [DOI] [PubMed] [Google Scholar]
- 110.Schömig A., Mehilli J., Antoniucci D. Mechanical reperfusion in patients with acute myocardial infarction presenting more than 12 hours from symptom onset: a randomized controlled trial. JAMA. 2005;293:2865–2872. doi: 10.1001/jama.293.23.2865. [DOI] [PubMed] [Google Scholar]
- 111.Gierlotka M., Gasior M., Wilczek K. Reperfusion by primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction within 12 to 24 h of the onset of symptoms (from a prospective national observational study PL-ACS) Am J Cardiol. 2011;107:501–508. doi: 10.1016/j.amjcard.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 112.Ndrepepa G., Kastrati A., Mehilli J., Antoniucci D., Schomig A. Mechanical reperfu- sion and long-term mortality in patients with acute myocardial infarction pre- senting 12 to 48 hours from onset of symptoms. JAMA. 2009;301:487–488. doi: 10.1001/jama.2009.32. [DOI] [PubMed] [Google Scholar]
- 113.Cantor W.J., Fitchett D., Borgundvaag B. Routine early angioplasty after fibrinolysis for acute myocardial infarction. N Engl J Med. 2009;360:2705–2718. doi: 10.1056/NEJMoa0808276. [DOI] [PubMed] [Google Scholar]
- 114.Sanchez P.L., Gimeno F., Ancillo P., Sanz J.J., Alonso-Briales J.H., Bosa F. Role of the paclitaxel-eluting stent and tirofiban in patients with ST-elevation myocardial infarction undergoing postfibrinolysis angioplasty: the GRACIA-3 randomized clinical trial. Circ Cardiovasc Interv. 2010;3:297–307. doi: 10.1161/CIRCINTERVENTIONS.109.920868. [DOI] [PubMed] [Google Scholar]
- 115.Borgia F., Goodman S.G., Halvorsen S. Early routine percutaneous coronary intervention after fibrinolysis vs: standard therapy in ST- segment elevation myocardial infarction: a meta-analysis. Eur Heart J. 2010;31:2156 21–69. doi: 10.1093/eurheartj/ehq204. [DOI] [PubMed] [Google Scholar]
- 116.Sutton A.G., Campbell P.G., Price D.J. Failure of thrombolysis by streptokinase: detection with a simple electrocardiographic method. Heart. 2000;84:149 1–56. doi: 10.1136/heart.84.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mehta S.R., Bassand J.-P., Chrolavicius S. Dose comparisons of clopidogrel and aspirin in acute coronary syndromes. N Engl J Med. 2010;363:930–942. doi: 10.1056/NEJMoa0909475. Erratum in: N Engl J Med. 2010;363:1585. [DOI] [PubMed] [Google Scholar]
- 118.Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. Erratum in: BMJ. 2002;324:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wiviott S.D., Braunwald E., McCabe C.H. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 120.Montalescot G., Wiviott S.D., Braunwald E. Prasugrel compared with clopidogrel in patients undergoing percutaneous coronary intervention for ST-elevation myocardial infarction (TRITON-TIMI 38): double-blind, randomised controlled trial. Lancet. 2009;373:723–731. doi: 10.1016/S0140-6736(09)60441-4. [DOI] [PubMed] [Google Scholar]
- 121.Steg P.G., James S., Harrington R.A., Ardissino D., Becker R.C., Cannon C.P. Ticagrelor versus clopidogrel in patients with ST-elevation acute coronary syndromes intended for reperfusion with primary percutaneous coronary intervention: a Platelet Inhibition and Patient Outcomes (PLATO) trial subgroup analysis. Circulation. 2010;122:2131–2141. doi: 10.1161/CIRCULATIONAHA.109.927582. [DOI] [PubMed] [Google Scholar]
- 122.Storey R.F., Becker R.C., Harrington R.A. Characterization of dyspnoea in PLATO study patients treated with ticagrelor or clopidogrel and its association with clinical outcomes. Eur Heart J. 2011;32:2945–2953. doi: 10.1093/eurheartj/ehr231. [DOI] [PubMed] [Google Scholar]
- 123.Stone G.W., Maehara A., Witzenbichler B. Intracoronary abciximab and aspiration thrombectomy in patients with large anterior myocardial infarction: the INFUSE-AMI randomized trial. JAMA. 2012;307:1817–1826. doi: 10.1001/jama.2012.421. [DOI] [PubMed] [Google Scholar]
- 124.Thiele H., Wohrle J., Hambrecht R. Intracoronary versus intravenous bolus abciximab during primary percutaneous coronary intervention in patients with acute ST-elevation myocar- dial infarction: a randomised trial. Lancet. 2012;379:923–931. doi: 10.1016/S0140-6736(11)61872-2. [DOI] [PubMed] [Google Scholar]
- 125.Montalescot G., Zeymer U., Silvain J. Coronary intervention for ST-elevation myocardial in- farction: the international randomized open-label ATOLL trial. Lancet. 2011;378:693–703. doi: 10.1016/S0140-6736(11)60876-3. [DOI] [PubMed] [Google Scholar]
- 126.Mehran R., Lansky A.J., Witzenbichler B. Bivalirudin in patients undergoing primary angioplasty for acute myocardial infarction (HORIZONS-AMI): 1-year results of a randomized controlled trial. Lancet. 2009;374:1149–1159. doi: 10.1016/S0140-6736(09)61484-7. [DOI] [PubMed] [Google Scholar]
- 129.Jolly S.S., Yusuf S., Cairns J. Radial vs. femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomized, parallel group, multicentre trial. Lancet. 2011;377:1409–1420. doi: 10.1016/S0140-6736(11)60404-2. [DOI] [PubMed] [Google Scholar]
- 130.Romagnoli E., Biondi-Zoccai G., Sciahbasi A. Radial vs. femoral randomized investigation in ST eleva- tion acute coronary syndromes: the RIFLE STEACS study. J Am Coll Cardiol. 2012 doi: 10.1016/j.jacc.2012.06.017. [in press] [DOI] [PubMed] [Google Scholar]
- 131.Vlaar P.J., Svilaas T., van der Horst I.C. Cardiac death and reinfarction after 1 year in the Thrombus Aspiration during Percutaneous coronary intervention in Acute myocardial infarction Study (TAPAS): a 1-year follow-up study. Lancet. 2008;371:1915–1920. doi: 10.1016/S0140-6736(08)60833-8. [DOI] [PubMed] [Google Scholar]
- 132.Burzotta F., De Vita M., Gu Y.L. Clinical impact of thrombectomy in acute ST-elevation myocardial in- farction: an individual patient-data pooled analysis of 11 trials. Eur Heart J. 2009;30:2193–2203. doi: 10.1093/eurheartj/ehp348. [DOI] [PubMed] [Google Scholar]
- 135.Levine G.N., O’Gara P.T., Bates E.R. ACC/AHA/SCAI focused update on primary percutaneous Coronary intervention for patients with ST-Elevation myocardial infarction: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous Coronary intervention and the 2013 ACCF/AHA guideline for the management of ST-Elevation myocardial infarction: a report of the american college of Cardiology/American heart association task force on clinical practice guidelines and the society for cardiovascular angiography and interventions. J Am Coll Cardiol. 2015;(October (21)) Epub ahead of print. [Google Scholar]
- 136.Nordmann A.J., Hengstler P., Harr T., Young J., Bucher H.C. Clinical outcomes of primary stenting vs: balloon angioplasty in patients with myocardial infarction: a meta-analysis of randomized controlled trials. Am J Med. 2004;116(4):253–262. doi: 10.1016/j.amjmed.2003.08.035. [DOI] [PubMed] [Google Scholar]
- 137.Kastrati A., Dibra A., Spaulding C. Meta-analysis of randomized trials on drug-eluting stents vs. bare- metal stents in patients with acute myocardial infarction. Eur Heart J. 2007;28:2706–2713. doi: 10.1093/eurheartj/ehm402. [DOI] [PubMed] [Google Scholar]
- 138.De Luca G., Dirksen M.T., Spaulding C. Drug-eluting vs bare-metal stents in primary angioplasty: a pooled patient-level meta-analysis of randomized trials. Arch Intern Med. 2012;172:611–621. doi: 10.1001/archinternmed.2012.758. [DOI] [PubMed] [Google Scholar]
- 139.Sabate M., Cequier A., Iñiguez A. Everolimus-eluting stent versus bare-metal stent in ST-segment elevation myocardial infarction (EXAMINATION): 1 year results of a randomized controlled trial. Lancet. 2012;380:1482–1490. doi: 10.1016/S0140-6736(12)61223-9. [DOI] [PubMed] [Google Scholar]
- 140.Raber L., Kelbaek H., Ostojic M. Effect of biolimus-eluting stents with biodegradable polymer vs.bare-metal stents on cardiovascular events among patients with acute myocar- dial infarction: the COMFORTABLE AMI randomized trial. JAMA. 2012;308(8):777–787. doi: 10.1001/jama.2012.10065. [DOI] [PubMed] [Google Scholar]
- 141.Kočka V., Malý M., Toušek P. Bioresorbable vascular scaffolds in acute ST-segment elevation myocardial infarction: a prospective multicentre study ‘Prague 19'. Eur Heart J. 2014;35:787–794. doi: 10.1093/eurheartj/eht545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Brugaletta S., Gori T., Low A.F. Absorb bioresorbable vascular scaffold versus everolimus-Eluting metallic stent in ST-Segment elevation myocardial infarction: 1-Year results of a propensity score matching comparison: the BVS-EXAMINATION study (Bioresorbable vascular scaffold-A clinical evaluation of everolimus eluting Coronary stents in the treatment of patients with ST-segment elevation myocardial infarction) J Am Coll Cardiol Intv. 2015;8(1_PB):189–197. doi: 10.1016/j.jcin.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 143.Sabaté M., Windecker S., Iñiguez A. Everolimus- eluting bioresorbable stent vs: durable polymer everolimus-eluting metallic stent in patients with ST- segment elevation myocardial infarction: results of the randomized ABSORB ST-segment elevation myocardial infarction-TROFI II trial. Eur Heart J. 2016;37:229–240. doi: 10.1093/eurheartj/ehv500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sorraja P., Gersh B.J., Cox D.A. Impact of multivessel disease on reperfusion success and clinical outcomes in patients undergoing primary coronary intervention for acute myocardial infarction. Eur Heart J. 2007;28:1709–1716. doi: 10.1093/eurheartj/ehm184. [DOI] [PubMed] [Google Scholar]
- 145.Politi L., Sgura F., Rossi R. A randomised trial of target-vessel versus multi-vessel revascularisation in ST-elevation myocardial infarction: major adverse cardiac events during long-term follow-up. Heart. 2010;96:662–667. doi: 10.1136/hrt.2009.177162. [DOI] [PubMed] [Google Scholar]
- 148.Wald D.S., Morris J.K., Wald N.J. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med. 2013;369:1115–1123. doi: 10.1056/NEJMoa1305520. [DOI] [PubMed] [Google Scholar]
- 149.Gershlick A.H., Khan J.N., Kelly D.J. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am CollCardiol. 2015;65(10):963–972. doi: 10.1016/j.jacc.2014.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Engstorm T., Kalbaek H., Helqvist S. Completerevascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3—PRIMULTI): an open-label, randomised controlled trial. Lancet. 2015;386:665–671. doi: 10.1016/s0140-6736(15)60648-1. [DOI] [PubMed] [Google Scholar]
- 151.El-Hayek G.E., Gershlick A.H., Hong M.K. Meta-analysis of randomized controlled trials comparing multivessel versus culprit- only revascularization for patients with ST-segment elevation myo- cardial infarction and multivessel disease undergoing primary per- cutaneous coronary intervention. Am J Cardiol. 2015;115(11):1481–1486. doi: 10.1016/j.amjcard.2015.02.046. [DOI] [PubMed] [Google Scholar]
- 152.Levine G.N., Bates E.R., Blankenship J.C. ACC/AHA/SCAI focused update on primary percutaneous Coronary intervention for patients with ST-Elevation myocardial infarction: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous Coronary intervention and the 2013 ACCF/AHA guideline for the management of ST-Elevation myocardial infarction. J Am Coll Cardiol. 2015;67(10):1235–1250. doi: 10.1016/j.jacc.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 162.Agarwal V., Lohiya B.V., Sihag B.K., Prajapati R. Clinical profile with angiographic correlation in naïve acute coronary syndrome. J Clin Diagn Res. 2016;10(9):10–14. doi: 10.7860/JCDR/2016/21166.8519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Windecker S., Kolh P., Alfonso F. ESC/EACTS guidelines on myocardial revascularzation. Eur Heart J. 2014;35(37):2541–2619. doi: 10.1093/eurheartj/ehu278. [DOI] [PubMed] [Google Scholar]
- 164.Erne P., Schoenenberger A.W., Burckhardt D. Effects of percutaneous coronary interventions in silent ischemia after myocardial infarction the SWISS II randomized controlled trial. JAMA. 2007;297(18):1985–1991. doi: 10.1001/jama.297.18.1985. [DOI] [PubMed] [Google Scholar]
- 165.Mahmarian J.J., Shaw L.J., Olszewski G.H. Adenosine sestamibi SPECT post-infarction evaluation (INSPIRE) trial: a randomized prospective multicenter trial evaluating the role of adenosine Tc-99 m sestamibi SPECT for assessing risk and therapeutic outcomes In survivors of acute myocardial infarction. J Nucl Cardiol. 2004;11(4):458–469. doi: 10.1016/j.nuclcard.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 166.van Loon R.B., Veen G., Baur L.H., Twisk J.W., van Rossum A.C. Long-term follow-up of the viability guided angioplasty after acute myocardial infarction (VIAMI) trial. Int J Cardiol. 2015;186:111–116. doi: 10.1016/j.ijcard.2015.03.152. [DOI] [PubMed] [Google Scholar]
- 167.Hochman J.S., Lamas G.A., Buller C.E. Coronary intervention for persistent occlusion after myocardial infarction. N Engl J Med. 2006;355:2395–2407. doi: 10.1056/NEJMoa066139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Armstrong P.W., Westerhaut C.M., Van de Werf F. Refining clinical trial composite outcome: an application to the assessment of the safety and efficacy of a new thrombolytic–3(AASENT-3) trial. Am Heart J. 2011;161:848. doi: 10.1016/j.ahj.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 169.Weish R.C., Armstrong P.W. Contemporary pharamalogical reperfusion in S T elevation myocardial infarction. Curr Opoin Cardiol. 2012;27:340. doi: 10.1097/HCO.0b013e3283546670. [DOI] [PubMed] [Google Scholar]
- 170.Coller B.S. Historical perspective and future directions in platelet research. J Thromb Haemost. 2011;9(Suppl. 1):374. doi: 10.1111/j.1538-7836.2011.04356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.AW Grasso, FJ Brener. Complications of myocardial infarction, Center for continuing education, Cleveland clinic July 2014.
- 172.Scottish Intercollegiate Guidelines Network – SIGN; 2007. Cardiac Arrhythmias in Coronary Heart Disease. [Google Scholar]
- 173.Jessica L., Morrow M., Morrow D.A. ST-elevation myocardial infarction: management. In: Mann D.L., Douglas P.Z., Libby P., Bonow R.O., editors. Braunwald’s Heart Disease. 10th ed. Saunders; Philadelphia: 2015. [Google Scholar]
- 174.Nieminen M.S., Buerke M., Cohen-Solál A. The role of levosimendan in acute heart failure complicating acute coronary syndrome: a review and expert consensus opinion. Int J Cardiol. 2016;2016:150–157. doi: 10.1016/j.ijcard.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 175.Patel M.R., Smalling R.W., Thiele H. Intra-aortic balloon counterpulsation and infarct size in patients with acute anterior myocardial infarction without shock: the CRISP AMI randomized trial. JAMA. 2011;306(September (12)):1329–1337. doi: 10.1001/jama.2011.1280. [DOI] [PubMed] [Google Scholar]
- 176.Thiele H., Zeymer U., Neumann F.J., for the IABP-SHOCK II trial investigators Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367:11287–11296. doi: 10.1056/NEJMoa1208410. [DOI] [PubMed] [Google Scholar]
- 177.Dell'Italia L.J. Reperfusion for right ventricular infarction. N Engl J Med. 1998;338:978–980. doi: 10.1056/NEJM199804023381408. [DOI] [PubMed] [Google Scholar]
- 178.Bowers T.R., O’Neill W.W., Grines C., Pica M.C., Safian R.D., Goldstein J.A. Effect of reperfusion onbiventricular function and survival after right ventricular infarction. N Engl J Med. 1998;338:933–940. doi: 10.1056/NEJM199804023381401. [DOI] [PubMed] [Google Scholar]
- 179.Ryan T.J., Antman E.M., Brooks N.H. 1999 update: ACC/AHA guidelines for the management of patients with acute myocardial infarction. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management ofAcute Myocardial Infarction) J Am Coll Cardiol. 1999;34:890–911. doi: 10.1016/s0735-1097(99)00351-4. [DOI] [PubMed] [Google Scholar]
- 180.Dixon S.R., Henriques J.P., Mauri L. A prospective feasibility trial investigating the use of the Impella 2.5 system in patients undergoing high-risk percutaneous coronary intervention (the PROTECT 1 trial): initial US experience. JACC: Cardiovasc Interv. 2009;2:91–96. doi: 10.1016/j.jcin.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 181.Sheu J.J., Tsai T.H., Lee F.Y. Early extracorporeal membrane oxygenator-assisted primary percutaneous coronary intervention improved 30-day clinical outcomes in patients with ST-segment elevation myocardial infarction complicated with profound cardiogenic shock. Crit Care Med. 2010;38:1810–1817. doi: 10.1097/CCM.0b013e3181e8acf7. [DOI] [PubMed] [Google Scholar]
- 182.Leshnower B.G., Gleason T.G., O’Hara M.L. Safety and efficacy of left ventricular assist device support in postmyocardial infarction cardiogenic shock. Ann Thorac Surg. 2006;81:1365–1370. doi: 10.1016/j.athoracsur.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 183.Chevalier P., Burri H., Fahrat F. Perioperative outcome and long-term survival of surgery for acute post-infarction mitral regurgitation. Eur J Cardiothorac Surg. 2004;26(2):330–335. doi: 10.1016/j.ejcts.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 184.Lorusso R., Gelsomino S., De Cicco G., Beghi C., Russo C., De Bonis Met al. Mitral valve surgery in emergency for severe acute regurgitation: analysis of postoperative results from a multicentre study. Eur J Cardiothorac Surg. 2008;33(4):573–582. doi: 10.1016/j.ejcts.2007.12.050. [DOI] [PubMed] [Google Scholar]
- 185.Bouma W., Wijdh den hammer I.J., Koene B.M. Long term survival after mitral valve surgery post MI papillary muscle rupture. J Cardiothorac Surg. 2016:2015. doi: 10.1186/s13019-015-0213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kishon Y., Oh J.K., Schaff H.V. Mitral valve operation in postinfarction rupture of a papillary muscle: immediate results and long-term followup of 22 patients. Mayo Clin Proc. 1992;67:1023–1030. doi: 10.1016/s0025-6196(12)61116-1. [DOI] [PubMed] [Google Scholar]
- 187.Russo A., Suri R.M., Grigioni F. Clinical outcome after surgical correction of mitral regurgitation due to papillary muscle rupture. Circulation. 2008;118:1528–1534. doi: 10.1161/CIRCULATIONAHA.107.747949. [DOI] [PubMed] [Google Scholar]
- 188.Bilge M, Alemdar R, Yasar AS. Successful percutaneous mitral valve repair with the mitraclip system of acute mitral regurgitation due to papillary muscle rupture as complication of acute myocardial infarction. Cathet Cardiovasc Intervent. 2014 Jan 1;83(1):E137–40. [DOI] [PubMed]
- 189.Fitch M.T., Nicks B.A., Pariyadath M., McGinnis H.D., Manthey D.E. Videos in clinical medicine: emergency pericardiocentesis. N Engl J Med. 2012;366(12):e17. doi: 10.1056/NEJMvcm0907841. [DOI] [PubMed] [Google Scholar]
- 190.Purcaro A., Costantini C., Ciampani N. Diagnostic criteria and management of subacute ventricular free wall rupture complicating acute myocardial infarction. Am J Cardiol. 1997;80:397–405. doi: 10.1016/s0002-9149(97)00385-8. [DOI] [PubMed] [Google Scholar]
- 191.Zoffoli G., Battaglia F., Venturini A. A novel approach to ventricular rupture: clinical needs and surgical technique. Ann Thorac Surg. 2012;93(3):1002–1003. doi: 10.1016/j.athoracsur.2011.11.048. [DOI] [PubMed] [Google Scholar]
- 192.Aris A. Surgical repair of left ventricular free wall rupture. Multimedia Manual of Cardio-Thoracic Surgery, 2005, 167. doi:10.1510/MMCTS.2004.000653 [DOI] [PubMed]
- 193.Sutherland F.W., Guell F.J., Pathi V.L., Naik S.K. Postinfarction ventricular free wall rupture: strategies for diagnosis and treatment. Ann Thorac Surg. 1996;61:1281–1285. doi: 10.1016/0003-4975(95)01160-9. [DOI] [PubMed] [Google Scholar]
- 194.The GUSTO Angiographic Investigators The effects of tissue plasminogen activator, streptokinase, or both on coronaryartery patency, ventricular function, and survival after acute myocardial infarction. N Engl J Med. 1993;329:1615–1622. doi: 10.1056/NEJM199311253292204. [DOI] [PubMed] [Google Scholar]
- 195.Stazka J., Olszewski K., Elzbieta K., Rybak J. Myocardial revascularization for acute myocardial infarction. Ann Univ Mariae Curie Sklodowska Med. 2004;59:368–372. [PubMed] [Google Scholar]
- 196.David T.E., Dale L., Sun Z. Postinfarctionventricularseptal rupture: repair by endocardial patch with infarct exclusion. Thorac Cardiovasc Surg. 1995;110(5):1315–1322. doi: 10.1016/S0022-5223(95)70054-4. [DOI] [PubMed] [Google Scholar]
- 197.Cox F., Morshius W., ThijsPlokker H. Importance of coronary revascularization for late survival after postinfarction ventricular septal rupture. A reason to perform coronary angiography prior tosurgery. Eur Heart J. 1996;17:1841–1845. doi: 10.1093/oxfordjournals.eurheartj.a014801. [DOI] [PubMed] [Google Scholar]
- 198.Dalrymple-Hay M.J., Langley S.M., Sami S.A. Should coronary artery bypass grafting be performed at the same time as repair of a post-infarct ventricular septal defect? Eur J Cardiothorac Surg. 1998;13(3):286–292. doi: 10.1016/s1010-7940(98)00010-4. [DOI] [PubMed] [Google Scholar]
- 199.Perrotta S., Lentini S. In patients undergoing surgical repair of post-infarction ventricular septal defect, does concomitant revascularization improve prognosis? Interact Cardiovasc Thorac Surg. 2009;9(5):879–887. doi: 10.1510/icvts.2009.210658. [DOI] [PubMed] [Google Scholar]
- 200.Deleted in press.
- 201.Attia R., Blauth C. Which patients might be suitable for a septaloccluder device closure of postinfarction ventricular septal rupture rather than immediatesurgery? Interact Cardiovasc Thorac Surg. 2010;11(5):626–629. doi: 10.1510/icvts.2010.233981. [DOI] [PubMed] [Google Scholar]
- 202.Maltais S., Ibrahim R., Basmadjian A.J., Carrier M., Bouchard D., Cartier R. Postinfarction ventricular septal defects: towards a new treatment algorithm? Ann Thorac Surg. 2009;87:687–692. doi: 10.1016/j.athoracsur.2008.11.052. [DOI] [PubMed] [Google Scholar]
- 203.Costache V.S., Chavanon O., Bouvaist H. Early Amplatzeroccluder closure of a postinfarct ventricular septal defect as a bridge to surgical procedure. Interact Cardiovasc Thorac Surg. 2007;6(4):503–504. doi: 10.1510/icvts.2006.150649. [DOI] [PubMed] [Google Scholar]
- 204.Dallan LAP, Dallan LRP, Lisboa LAF, Dallan LAO. Mechanical Complications of Acute Myocardial Infarction, Cardiac Surgery - A Commitment to Science, Technology and Creativity, Prof. Miguel Maluf (Ed.), InTech, DOI: 10.5772/57110. Available from: https://www.intechopen.com/books/cardiac-surgery-a-commitment-to-science-technology-and-creativity/mechanical-complications-of-acute-myocardial-infarction
- 205.Chen X., Qiu Z.B., Xu M., Liu L.L., Jiang Y.S., Wang L.M. Surgery for left ventricular aneurysm after myocardial infarction: techniques selection and results assessment. Chin Med J (Engl) 2012;125(24):4373–4379. [PubMed] [Google Scholar]
- 206.Lopes R.D., Elliott L.E., White H.D. Antithrombotic therapy and outcomes of patients with atrial fibrillation following primary percutaneous coronary intervention: results from the APEX-AMI trial. Eur Heart J. 2009;30:2019 20–28. doi: 10.1093/eurheartj/ehp213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wolfe C.L., Nibley C., Bhandari A., Chatterjee K., Scheinman M. Polymorphous ventricular tachycardia associated with acute myocardial infarction. Circulation. 1991;84:1543. doi: 10.1161/01.cir.84.4.1543. [DOI] [PubMed] [Google Scholar]
- 208.Aliot EM, Stevenson WG, Almendral-Garrote JM, Bogun F, Calkins CH, Delacretaz E, et al. EHRA/HRS Expert Consensus on Catheter Ablation of Ventricular Arrhythmias. Heart Rhythm. 6(6):886–933. [DOI] [PubMed]
- 209.Epstein A.E., DiMarco J.P., Ellenbogen K.A. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2008;117:e350–e408. Erratum in: Circulation. 2009;120:e34–5. [Google Scholar]
- 210.Ajit S.M., Balaji P., Khando T. Managing complications in acute Myocardial infarction. JAPI. 2011;59(Suppl):43–59. [PubMed] [Google Scholar]
- 211.Weinsaft J.W., Kim H.W., Shah D.J. Detection of left ventricular thrombus by delayed enhancement cardiovascular magnetic resonance prevalence and markers in patients with systolic dysfunction. J Am Coll Cardiol. 2008;52(2):148–157. doi: 10.1016/j.jacc.2008.03.041. [DOI] [PubMed] [Google Scholar]
- 212.Rao S.V., O’Grady K., Pieper K.S. Impact of bleeding severity on clinical outcomes among patients with acute coronary syndromes. Am J Cardiol. 2005;96:1200–1206. doi: 10.1016/j.amjcard.2005.06.056. [DOI] [PubMed] [Google Scholar]
- 213.Levine G.N., Bates E.R., Blankenship J.C. ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the american college of cardiology Foundation/American heart association task force on practice guidelines and the society for cardiovascular angiography and interventions. Circulation. 2011;124:e574–e651. doi: 10.1161/CIR.0b013e31823ba622. [DOI] [PubMed] [Google Scholar]
- 214.Mayer S.A., Brun N.C., Begtrup K. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008;358:2127–2137. doi: 10.1056/NEJMoa0707534. [DOI] [PubMed] [Google Scholar]
- 215.Goldstein J.N., Marrero M., Masrur S. Management Of thrombolysis associated symptomatic intracerebral hemorrhage. Arch Neurol. 2010;67(8):965–969. doi: 10.1001/archneurol.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Keeling. D, Baglin. T, Tait. C, Watson. H, Jerry. D, Baglin. C, Kitchen. S, Makris. M, and British Committee for standards in Haematology (2011), Guidelines on oral anticoagulation with warfarin-fourth edition. British Journal of Haematology, 154:311–324. doi:10.1111/j.1365-2141.2011.08753.x [DOI] [PubMed]
- 217.Guck T., Kavan M.G., Elasser G.N., Barone E.J. Assessment and treatment of depression following myocardial infarction. Am Fam Phys. 2001;64:641–648. 651-2. [PubMed] [Google Scholar]
- 218.Lichtman Judith H., Bigger J. Thomas, Blumenthal James A. Depression and coronary heart disease; recommendations for screening, referral, and treatment a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research-endorsed by the American Psychiatric Association. Circulation. 2008;118:1768–1775. doi: 10.1161/CIRCULATIONAHA.108.190769. [DOI] [PubMed] [Google Scholar]
- 219.Taylor C.B., Youngblood M.E., Catellier D. ENRICHD Investigators: effects of antidepressant medication on morbidity and mortality in depressed patients after myocardial infarction. Arch Gen Psychiatry. 2005;62:792–798. doi: 10.1001/archpsyc.62.7.792. [DOI] [PubMed] [Google Scholar]
- 220.Critchley J., Capewell S. Smoking cessation for the secondary prevention of coronary heart disease. Cochrane Database Syst Rev. 2004;1 doi: 10.1002/14651858.CD003041.pub2. CD003041. [DOI] [PubMed] [Google Scholar]
- 221.Piepoli M.F., Corrà U., Benzer W. Cardiac Rehabilitation Section of the European Association of Cardiovascular Prevention and Rehabilitation Secondary prevention through cardiac rehabilitation: from knowledge to implementation. A position paper from the Cardiac Rehabilitation Section of the European Association of Cardiovascular Prevention and Rehabilitation. Eur J Cardiovasc Prev Rehabil. 2010;17(1):1–17. doi: 10.1097/HJR.0b013e3283313592. [DOI] [PubMed] [Google Scholar]
- 222.U.S Department of Health and Human Services and U.S Department of Agriculture. 2015-2020 Dietary Guidelines for Americans. 8th edition, December 2015. Available at http://health.gov/dietaryguidelines/2015/guidelines/.
- 223.Clarck A.M., Hartling L., Vandermeeer B., McAlister F.A. Meta-analysis: secondary prevention programms for petients with coronary artery disease. Ann Intern Med. 2005;143(9):659–672. doi: 10.7326/0003-4819-143-9-200511010-00010. [DOI] [PubMed] [Google Scholar]
- 224.Heran B.S., Chen L.M., Ebrahim S. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2011;7 doi: 10.1002/14651858.CD001800.pub2. CD001800. [DOI] [PMC free article] [PubMed] [Google Scholar]