Abstract
Background
The pre-ejection period (PEP) and left ventricular ejection time (LVET) are easily measured by impedance cardiography (ICG). We hypothesized that the PEP/LVET measured by ICG would correlate with that measured by echocardiography, and that PEP/LVET measured by ICG would be useful for cardiac resynchronization therapy (CRT) optimization.
Methods
Newly CRT implanted patients were optimized by echocardiography. The PEP/LVET was measured by echocardiography and ICG in two different settings: optimized setting and right ventricle (RV)-only pacing.
Results
The PEP/LVET was significantly decreased in the optimized setting compared with that in RV-only pacing (0.62±0.13 vs 0.75±0.16, p<0.05). The PEP/LVET values calculated by ICG and echocardiography were positively correlated (r=0.553, p=0.003).
Conclusion
ICG was useful for the optimization of CRT.
Keywords: Cardiac resynchronization therapy, Impedance cardiography, Echocardiogram
1. Introduction
Previous studies showed that cardiac resynchronization therapy (CRT) improves quality of life and functional status; reduces hospitalizations for heart failure; and prolongs survival in patients with severe heart failure, low left ventricular (LV) ejection fraction (LVEF), and LV dyssynchrony [1], [2]. Although the beneficial effect of CRT has been demonstrated, 20–30% of patients still do not receive the full clinical benefit of CRT [3]. It is necessary to correct electrical and mechanical dyssynchrony through an optimally timed stimulation of the right ventricle (RV) and LV. Although echocardiographic evaluation is used most frequently when performing device optimization [4], the accuracy of echocardiography is affected by measurement errors [5].
Pre-ejection periods (PEPs) and LV ejection times (LVETs) measured by echocardiography are considered important indicators of LV systolic function. The PEP/LVET is easily measured by impedance cardiography (ICG) together with pulse wave velocity and ankle brachial index.
Thus, we hypothesized that the PEP/LVET measured by ICG would correlate with that measured by echocardiography, and that it would be a more sensitive marker for CRT optimization. The purpose of this study was to elucidate the usefulness of PEP/LVET measured by ICG for CRT optimization.
2. Materials and methods
2.1. Patients
We enrolled 27 consecutive patients (19 men, 8 women; 70.7±10.1 years) who recently received CRT. The patients’ atrio-ventricular (AV) delay and ventricular-ventricular (VV) delay were optimized by using the LV outflow tract velocity-time integral (VTI) on echocardiography 1 week after implantation.
2.2. Echocardiography
Standard transthoracic echocardiography was performed with a Vivid 7 system (GE Vingmed Ultrasound, Buckinghamshire, UK) 1 week after implantation. Pulsed Doppler transmitral flow and aortic ejection flow were assessed in the apical long-axis view. The AV delay and VV delay were adjusted by the VTI of aortic flow (AoVTI). The LVETs were used to determine AoVTI, and PEPs were measured from the onset of the QRS to the initial upstroke of the aortic velocity trace. The LVEFs were calculated by using the modified Simpson׳s method.
2.3. Impedance cardiography
ICG was performed with the VS-1500A System (Fukuda Denshi, Tokyo, Japan), and the PEPs and LVETs were measured. The LVETs were defined as the time from the initial upstroke of the right brachial artery pulse tracing to the incisural notch, the PEPs were defined as the LVET time subtracted from the LV systolic time, and the LV systolic time was defined as the time from the onset of the QRS to the second heart sound [6]. These data were mean value of 5 or 6 beats. We performed ICG and measured the PEP/LVET by using the following two different settings with the same AV delay and pacing rate: the optimized setting and RV-only pacing.
2.4. Statistical analysis
The parameters measured by the two methods were compared with the paired t-test. Relationships between the variables were analyzed by Spearman׳s correlation coefficient analysis [7]. Statistical analysis was performed with IBM SPSS Statistics, version 21 (IBM Corp., Armonk, NY, USA). A p value <0.05 was considered statistically significant.
3. Results
CRT devices were successfully implanted in 27 patients (70.7±10.1 years), and the LV leads were placed in the lateral branch or posterolateral branch. Baseline characteristics of all patients are listed in Table 1. Eighteen of 27 patients received a CRT defibrillator. Fourteen and 5 patients presented dilated cardiomyopathy and ischemic cardiomyopathy, respectively. All patients received β-blockers, and 26 patients received renin-angiotensin system blockers and diuretics. Eight patients presented atrial fibrillation rhythm. Seventeen of patients had CLBBB and 11 of patients were bradycardia less than 50 bpm. The mean QRS duration was 157.4±29.3 ms. The mean plasma brain natriuretic peptide level was 402.4±1746.9 ng/L, before CRT implantation.
Table 1.
Baseline characteristics of the patients.
| N | 27 | |
| Age (years) | 70.7±10.1 | |
| Male | 19 (70.4) | |
| NYHA (II/III/IV) | 12/11/4 | |
| CRT-D/P | 18/9 | |
| Etiology | Dilated cardiomyopathy | 14 (51.9) |
| Ischemic cardiomyopathy | 5 (18.5) | |
| Other | 8 (29.6) | |
| Medication | β Blockers | 27 (100) |
| ACEIs or ARBs | 26 (96.3) | |
| Diuretics | 26 (96.3) | |
| Mineralocorticoid receptor antagonists | 17 (63.0) | |
| Electrocardiography | Atrial fibrillation | 8 (29.6) |
| CLBBB | 17 (63.0) | |
| Bradycardia | 11 (40.7) | |
| QRS duration (ms) | 157.4±29.3 | |
| Brain natriuretic peptide (ng/L) | 402.4±1746.9 | |
Data are expressed as n (%) or mean±SD.
In the etiology, “Other” includes dilated phase hypertrophic cardiomyopathy (3 subjects), after cardiac operation (Post-cardiac operation) (3 subjects), amyloidosis (1 subject), and sarcoidosis (1 subject). Bradycardia is defined as less than 50 bpm and/or paroxysmal symptomatic bradycardia episode.
Abbreviations: ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; CLBBB, complete left bundle branch block; NYHA, New York Heart Association.
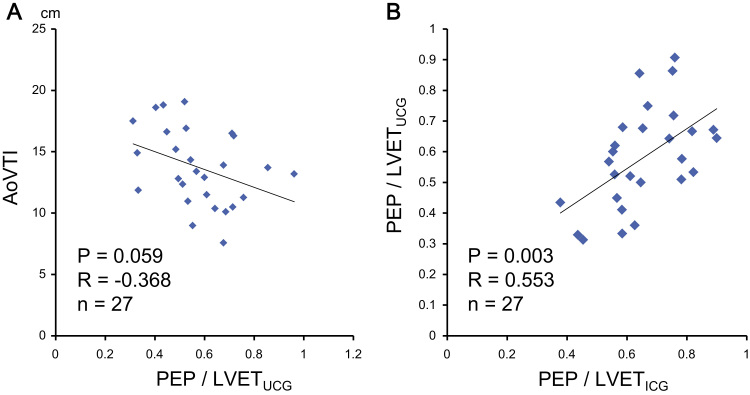
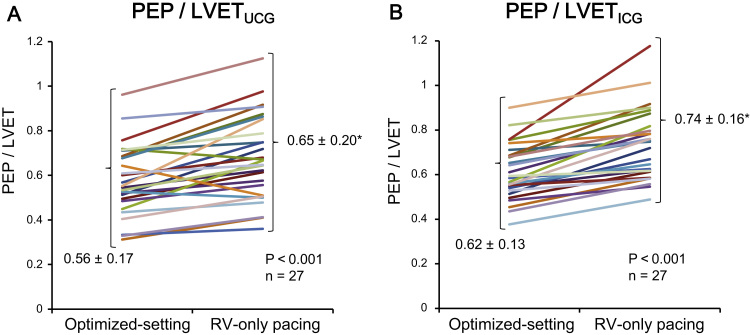
Optimization was performed using AoVTIs measured by Ultrasonic Cardiography (UCG), and the AoVTIs tended to negatively correlate with PEP/LVETs measured by UCG (Fig. 1A). The PEPs and PEP/LVETs measured by both UCG and ICG were increased in RV-only pacing compared with those in the optimized setting, and the AoVTIs and LVETs measured by ICG were decreased in RV-only pacing compared with those in the optimized-setting (Table 2). The PEP/LVETs measured by ICG positively correlated with the PEP/LVETs measured by echocardiography (Fig. 1B). LVEFs measured by echocardiography were comparable between the two settings (Table 2); however, the PEP/LVETs measured by ICG and echocardiography significantly decreased in the optimized-setting compared with those in RV-only pacing (Fig. 2A, B).
Fig. 1.
(A) AoVTIs and PEP/LVETs measured by UCG were tended to correlate negatively (r=−0.368, p=0.059). (B) There was a positive correlation between PEP/LVETs measured by ICG and those measured by Ultrasonic Cardiography (UCG) (r=0.553, p=0.003). Data are expressed as mean±SD. *p<0.05 vs. PEP/LVETs measured by ICG. Abbreviations: AoVTI, velocity-time integral of aortic flow; ICG, impedance cardiography; LVET, left ventricular ejection time; PEP, pre-ejection period.
Table 2.
Hemodynamic and echocardiographic assessments.
| Optimized setting | RV-only pacing | ||
|---|---|---|---|
| Heart rate (bpm) | 74.6±8.9 | ||
| AV delay (ms) | 157.6±22.3 | ||
| LVEF (%) | 34.2±7.8 | 31.6±7.2 | |
| UCG | PEP (ms) | 151.0±43.6 | 172.3±43.9⁎ |
| LVET (ms) | 271.1±40.3 | 270.1±29.4 | |
| PEP/LVET | 0.56±0.17 | 0.65±0.20⁎ | |
| AoVTI (cm) | 13.9±3.5 | 12.4±3.3⁎ | |
| ICG | PEP (ms) | 159.4±25.0 | 186.2±29.8⁎ |
| LVET (ms) | 261.2±21.8 | 255.0±22.8⁎ | |
| PEP/LVET | 0.62±0.13 | 0.74±0.16⁎ | |
Data are expressed as mean±SD.
Pacing rate and A-V delays were not different between the two settings.
Abbreviations: AoVTI, velocity-time integral of aortic flow; AV, atrio-ventricular; ICG, impedance cardiography; LVEF, left ventricular ejection fraction; LVET, left ventricular ejection time; PEP, pre-ejection period; RV, right ventricle; UCG, Ultrasonic Cardiography.
p<0.001 vs. optimized pacing.
Fig. 2.
(A) PEP/LVETs measured by impedance cardiography were decreased in the optimized setting (0.62±0.13) compared with those in RV-only pacing (0.74±0.16). The AV delay and heart rate were comparable between the optimized setting and RV-only pacing (n=27). (B) PEP/LVETs measured by echocardiogram were decreased in the optimized setting (0.60±0.1) compared with those in RV-only pacing (0.74±0.2) (n=27). Data are expressed as mean±SD. *p<0.05 vs. optimize setting. Abbreviations: AV atrio-ventricular; ICG, impedance cardiography; LVET, left ventricular ejection time; PEP, pre-ejection period; RV, right ventricle.
4. Discussion
The major findings of the present study are as follows: (1) The PEP/LVETs measured by ICG correlated with those measured by echocardiography; (2) the PEP/LVETs measured by ICG were decreased in the optimized setting compared with those in RV-only pacing, thus, the PEP/LVETs were useful in the optimization of CRT. These findings suggest that the PEP/LVETs measured by ICG could be a promising method for the optimization of CRT.
CRT is an accepted therapeutic option in patients with severe heart failure, which has been shown to improve LV functional and structural remodeling, symptoms, and functional status [1]. However, 20–30% of patients with CRT receive no benefit [3]. One possible reason for this may be the insufficient optimization of CRT device settings.
Although Doppler echocardiography, performed in a laboratory with special expertize, may be effective to optimize the CRT setting [8], concerns regarding the variability of echocardiography have been pointed out. Alternatively, the PEP/LVETs were reported to be a useful parameter of LV systolic function [9], with less measurement error compared with AoVTI measured by echocardiography [10], and easily measured together with pulse wave velocity and ankle brachial index. We found that the PEP/LVETs measured by ICG were positively correlated with the PEP/LVETs measured by echocardiography. Thus, ICG could be useful for CRT optimization, in combination with echocardiography.
4.1. Study limitations
The present study has several limitations. First, although the LVETs were measured by using the pulse wave of the right brachial artery, there may be some differences between the actual LVET and the measured LVET from pulse wave because of a particular medical condition, such as the stiffness of the arteries or inhomogeneous distribution of arteriosclerosis. Second, the sensitivity of the PEP/LVETs remains to be elucidated. Third, we did not evaluate the AV delay [11]. Forth, although, taken as a whole, the PEP/LVETs measured by ICG and those measured by echocardiography had a positive correlation, individually, there were some differences. One of the reasons might be a measurement error by UCG and/or ICG. Finally, this study was a single-center study with a relatively small number of patients.
5. Conclusion
These results indicate the possibility of the application of ICG for the optimization of CRT device settings.
Conflict of interest
The authors declare that there is no conflict of interest related to this study.
Acknowledgments
We thank the Clinical Engineers (K. Matsuda, M. Akazawa, H. Kajikawa) and Clinical Laboratory Technologists at Iwate Prefectural Central Hospital.
References
- 1.Komajda M. Current challenges in the management of heart failure. Circ J. 2015;79:948–953. doi: 10.1253/circj.CJ-15-0368. [DOI] [PubMed] [Google Scholar]
- 2.Cleland J.G., Daubert J.C., Erdmann E. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 3.Moss A.J., Hall W.J., Cannom D.S. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329–1338. doi: 10.1056/NEJMoa0906431. [DOI] [PubMed] [Google Scholar]
- 4.Kosmala W., Marwick T.H. Meta-analysis of effects of optimization of cardiac resynchronization therapy on left ventricular function, exercise capacity, and quality of life in patients with heart failure. Am J Cardiol. 2014;113:988–994. doi: 10.1016/j.amjcard.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Chung E.S., Leon A.R., Tavazzi L. Results of the predictors of response to CRT (PROSPECT) trial. Circulation. 2008;117:2608–2616. doi: 10.1161/CIRCULATIONAHA.107.743120. [DOI] [PubMed] [Google Scholar]
- 6.Baker C., Love C.J., Moeschberger M.L. Time intervals of cardiac resynchronization therapy in heart failure. Am J Cardiol. 2004;94:1192–1196. doi: 10.1016/j.amjcard.2004.07.094. [DOI] [PubMed] [Google Scholar]
- 7.Noda K., Godo S., Saito H. Opposing roles of nitric oxide and Rho-kinase in lipid metabolism in mice. Tohoku J Exp Med. 2015;235:171–183. doi: 10.1620/tjem.235.171. [DOI] [PubMed] [Google Scholar]
- 8.Sakamaki F., Seo Y., Ishizu T. Tissue doppler imaging dyssynchrony parameter derived from the myocardial active wall motion improves prediction of responders for cardiac resynchronization therapy. Circ J. 2012;76:689–697. doi: 10.1253/circj.cj-11-0774. [DOI] [PubMed] [Google Scholar]
- 9.Umar F., Leyva F. Is the pre-ejection period key to predicting the response to cardiac resynchronization therapy? Circ J. 2012;76:590. doi: 10.1253/circj.cj-12-0095. [DOI] [PubMed] [Google Scholar]
- 10.Chirife R., Ruiz G.A., Gayet E. The systolic index: a noninvasive approach for the assessment of cardiac function: Implications for patients with DDD and CRT devices. PACE. 2013;36:1284–1293. doi: 10.1111/pace.12200. [DOI] [PubMed] [Google Scholar]
- 11.Steinberg B.A., Wehrenberg S., Jackson K.P. Atrioventricular and ventricular-to-ventricular programming in patients with cardiac resynchronization therapy: results from ALTITUDE. J Interv Card Electrophysiol. 2015;44:279–287. doi: 10.1007/s10840-015-0058-5. [DOI] [PMC free article] [PubMed] [Google Scholar]