Abstract
Bergenia ciliata Sternb., commonly known as Paashaanbhed, is a well known herb of Sikkim Himalaya with various pharmaceutical properties. However, scientific exploration of B. ciliata, growing in the Sikkim Himalaya, for phytochemicals and pharmacological properties is in infancy. With this view, the present study was undertaken to investigate B. ciliata leaf extracts for antioxidant, antimicrobial activity and bioactive compounds. Three solvents viz., methanol, ethyl acetate and hexane were used for extraction and the respective leaf extracts were analyzed for total phenolic and flavonoid contents along with the antioxidant and antimicrobial activities. Amongst the tested solvents, methanol was found to be the best solvent for extraction with highest total phenolic contents and the lowest IC50 values for the 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assays. Methanol extract also exhibited effective antimicrobial activity, particularly against bacteria and actinomycetes. Further, high performance liquid chromatography (HPLC) analysis revealed that methanolic extract contains the highest amount of all the three analyzed bioactive compounds viz. bergenin, catechin and gallic acid. The current study suggests that the methanol extract of B. ciliata is a potential source of natural antioxidant and antimicrobial compounds that can be used in food and drug industries.
Keywords: Antioxidant, Bergenia ciliata, Phenolic content, Flavonoid content, Sikkim Himalaya
Abbreviation: ABTS, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid); DPPH, 2,2-diphenyl-1-picrylhydrazyl; HPLC, high performance liquid chromatography
Graphical abstract
1. Introduction
Microbial contamination and side effects of synthetic antioxidants are two important major concerns of food and pharmaceutical industries. Increasing propensity for replacing synthetic antioxidant by natural one on one side and development of microbial resistance to existing antibiotics from the other has encouraged researchers toward appraising medicinal plants for dual antioxidant and antimicrobial properties. Though, since immemorial time, medicinal plants have been used to treat and prevent various human ailments and they are considered as reservoir of bioactive compounds.1, 2 Till date, biological properties and bioactive compounds of many medicinal plants are not studied.
Extensive investigation of medicinal plants for biological activities and bioactive compounds is the crucial and the foremost step in development of effective alternative medications. In view of this, in the present study, Bergenia ciliata Sternb. (family Saxifragaceae), a high value plant of the Sikkim Himalaya, has been investigated for antioxidant, antimicrobial activity and bioactive compounds. It is an important perennial medicinal herb that grows widely in the temperate Himalaya between 1500 and 3000 m asl.3 The plant species flourishes well in rocky areas and on the cliffs. The plant has been in use as folklore medicine since ancient times for dissolution of kidney and gall bladder stones.4, 5 In Sikkim Himalaya, Bergenia rhizome is used to treat fractured bones, fresh cuts, wounds, diarrhea, pulmonary infections, vomiting, fever, cough and boils by locales.6, 7, 8 Bergenia is also used for the treatment of heart disease, haemorrhoids, stomach disorders and ophthalmia. In addition, it is accredited with analgesic, antiviral, anti-inflammatory and antimalarial properties.9, 10 The plant's virtues, to a large extent, are attributed to its secondary metabolites such as bergenin, gallic acid and catechin which are therapeutic and account for its use in traditional medicine.11, 12
Despite the widespread use of this plant in traditional medicine, the scientific literature with respect to its biological properties is scanty. To the best of our knowledge, so far, antioxidant, antimicrobial properties and the bioactive compounds associated with the leaf of Bergenia plant, growing in the Sikkim Himalaya, have not been investigated. Therefore, the present study was conducted with the objectives to (1) obtain the most effective solvent for extracting the potent bioactive compounds, especially phenolics and flavonoids, (2) investigate different extracts for antioxidant and antimicrobial activities and, (3) quantify the amount of bergenin, catechin and gallic acid present in the leaf extracts of B. ciliata.
2. Material and methods
2.1. Plant collection
Leaves of B. ciliata were collected during flowering season (March 2014) from the plants growing in the arboretum of G.B. Pant Institute of Himalayan Environment and Development (GBPIHED), Sikkim Unit, Gangtok, India (latitude 27° 21′ 35.7″N; longitude 88° 37′ 24.4″E), situated at the elevation of 2047 m asl. Voucher specimens (GBP3590) were deposited at the Herbarium of GBPIHED.
2.2. Extract preparation
The leaves were washed thoroughly under running tap water and finally with distilled water and dried on blotting paper at room temperature to get consistent weight. The dried leaves were later ground to powder using grinder. To prepare samples, 2 g powder was soaked separately in 10 mL of different solvents (ethyl acetate, methanol and hexane) for 24 h. The supernatant was transferred into a new tube and the residue was re-extracted twice with 10 mL solvent. Extracts were filtered using a Buckner funnel and Whatman No. 1 filter paper. Each filtrate was concentrated to dryness in oven, maintained at 40 °C. Each extract was resuspended in the methanol to yield a 50 mg/mL stock solution. The yield of the extraction was calculated from {(W1/W2) × 100}, where W1 is the weight of extract after evaporation of solvent and W2 is the dry weight of the plant sample.
2.3. Total phenolic content (TPC)
Total phenolic content in Bergenia extracts was determined using Folin–Ciocalteu assay.13 Solutions of each extract (100 μL; 1 mg/mL) were taken individually in test tubes. To this solution, 2.5 mL of 10-fold diluted Folin–Ciocalteu reagent was added, and the test tubes were thoroughly shaken. After 3 min, 2.0 mL of 7.5 % Na2CO3 solution was added and the mixtures were incubated for 30 min. The absorbance of the reaction mixtures was measured at 760 nm by using a spectrophotometer (UV-1800, Shimadzu, Kyoto, Japan). Gallic acid was used as a standard and TPC of Bergenia extracts was expressed in milligram gallic acid equivalents (mg GAE/g extract).
2.4. Total flavonoid content (TFC)
Total flavonoid content was determined by the aluminum chloride calorimetric method,14 with some modifications. Briefly, the test samples were individually dissolved in methanol. Then, the sample solution (2 mL) was mixed with 2 mL of 2% AlCl3. After 10 min of incubation at ambient temperature, the absorbance of the solution was measured at 435 nm by using a spectrophotometer (UV-1800, Shimadzu, Kyoto, Japan). The flavonoid content was expressed as milligram quercetin equivalent (mg QE/g extract).
2.5. Determination of antioxidant activity
2.5.1. DPPH radical scavenging assay
The effect of extracts on 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical was determined using the method of Liyana-Pathiranan and Shahidi.15 A solution of 0.135 mM DPPH in methanol was prepared and 1.5 mL of this solution was mixed with 1.5 mL of extract in methanol. The reaction mixture was mixed thoroughly and left in the dark at room temperature for 30 min. The absorbance of the mixture was measured spectrophotometrically at 517 nm. Butylated hydroxytoluene (BHT) was used as standard. The ability of sample to scavenge DPPH radical was calculated by the following equation:
| DPPH radical scavenging activity (%) = [(Abs control − Abs sample)]/(Abs control) × 100 |
where Abs control is the absorbance of DPPH radical + methanol; Abs sample is the absorbance of DPPH radical + extract/standard. The radical scavenging activity of extracts was determined by IC50 value. IC50 value is the concentration of extracts at which DPPH radicals are scavenged by 50%. The lower IC50 value indicates higher radical scavenging capacity and vice versa.
2.5.2. ABTS radical scavenging assay
The 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical scavenging assay was done according to Re et al.16 with slight modifications. The ABTS radical cation solution was prepared by mixing equal quantities of ABTS (7 mM) and ammonium persulfate (2.45 mM) and allowing them to react for 16–20 h at room temperature, in dark. The working solution was then prepared by diluting the previous solution with methanol until the absorbance at 734 nm was 0.706 ± 0.02. ABTS solution was freshly prepared for each assay. Plant extracts (1.5 mL) were allowed to react with equal volume of the ABTS working solution and the absorbance was taken at 734 nm after 7 min using the spectrophotometer. The ABTS scavenging capacity of the extracts was compared with that of BHT and the percentage inhibition was calculated as:
| ABTS radical scavenging activity (%) = [(Abs control − Abs sample)]/(Abs control) × 100 |
where Abs control is the absorbance of ABTS radical + methanol; Abs sample is the absorbance of ABTS radical + extract/standard. ABTS radical scavenging activity of extracts was determined by IC50 value as mentioned above in DPPH assay.
2.6. Antimicrobial assay
Total eight strains (4 bacteria, 2 actinomycetes and 2 fungi) were taken from Microbial Culture Collection established in the Microbiology laboratory of GBPIHED Institute, Almora, India. Initially test organisms were grown on the respective media i.e. bacteria and actinomycetes in Tryptone Yeast extract (TY) and fungus on Potato Dextrose broth (PD) in conical flask, at 25°C for 24 h. Antimicrobial activity was performed on TY and PD agar plates following disc diffusion assay at 25°C, 2 days for bacteria and 6 days for actinomycetes and fungus. Minimum inhibitory concentration (MIC) of extracts was determined following the protocol of Clinical and Laboratory Standards Institute (CLSI).17, 18 All of the experiments were performed in triplicate, and results were expressed as diameters (mm) of inhibition.
2.7. High performance liquid chromatography (HPLC) analysis
Detection and quantification of catechin, gallic acid and bergenin were carried out using Shimadzu 20 AD, HPLC system (Shimadzu, Japan) consisted of UV detector, a binary pump, a 10 μL injection loop, and reverse phase C-18 column of dimensions 4.6 × 250 mm. The mobile phase used for catechin was 75% A (water + 0.1% trifluoroacetic acid) and 25 % B (methanol) with a flow rate of 0.8 mL/min. The eluted samples were detected by the UV detector at 280 nm. For gallic acid, mobile phase used was 90 % A (water + 1% acetic acid) and 10% B (acetonitrile) with a flow rate of 1.0 mL/min. The eluted samples were detected by UV detector at 271 nm.
For analysis of bergenin, elution was carried out with solvent 75% A (water + 0.1% trifluoroacetic acid) and 25% B (acetonitrile) as mobile phase having flow rate of 1.0 mL/min. Detection was carried out at 248 nm. Calibration curve was constructed by plotting the peak area (y) against concentration in μg/mL of standard solutions (x). The standard equation obtained from the curve was used for quantification of bioactive compounds in the unknown samples.
2.8. Statistical analysis
All the experiments were performed in triplicate and the experimental data were expressed as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) and Duncan's multiple range tests were carried out to determine significant differences (p < 0.05) between the means by SPSS (version 16.0). Correlation coefficients were determined by SPSS (version 16.0) Pearson correlation program.
3. Results and discussion
3.1. Extraction yield
Results showed that leaf extraction yield of B. ciliata varied considerably as a function of solvent nature and ranged from 8.3 to 38.2 % with a descending order of methanol > ethyl acetate > hexane (Table 1). Extraction with methanol resulted in the highest amount of total extractable compounds whereas the extraction yield with hexane was small in comparison to other solvents. Higher extraction yield in methanol might be due to the fact that it easily penetrates the cellular membrane and extracts the intracellular ingredients from the plant material. Moreover, results indicated that the plant contains more of polar substances than the others. Earlier reports have also suggested that methanol give higher extraction yield than the other solvents such as acetone, diethyl ether, ethyl acetate and water.19, 20
Table 1.
Total phenolics, flavonoid and extraction yield of different extracts of Bergenia ciliata.
| Solvent | Extraction yield (% w/w) | Total phenolic content (mg GAE/g extract) | Total flavonoid content (mg QE/g extract) |
|---|---|---|---|
| Methanol | 38.2 ± 3.4 a | 473.4 ± 15.1 a | 89.9 ± 0.1 b |
| Ethyl acetate | 18.5 ± 2.5 b | 249.7 ± 1.3 b | 208.4 ± 0.6 a |
| Hexane | 8.3 ± 1.4 c | nd | nd |
Values are mean ± standard deviation of triplicate experiments. Different letters in columns show significant differences at p < 0.05; nd, not detected.
3.2. Total phenolic content (TPC)
The antioxidant activity of medicinal plants, fruits and vegetables has been reported to be positively correlated to their total phenolic contents due to their ability to scavenge free radicals.21, 22 It becomes, therefore, mandatory to estimate total phenolic compounds present in the Bergenia extracts. In the present study, the content of extractable phenolic compounds was determined through a linear gallic acid standard curve (y = 2.271x + 0.18; R2 = 0.995). The highest content of total phenolic compounds was detected in the Bergenia methanolic extract (473.4 mg GAE/g extract) followed by ethyl acetate extract (249.7 mg GAE/g extract) (p < 0.05). In the hexane extract, phenolic compounds could not be detected (Table 1). These results demonstrate clearly that the content of phenolic compounds is dependent on the polarity of the solvent used; higher the polarity of the solvent, higher the content of phenolic compounds. These results are in agreement with the previous study of Singh et al.20 that reported methanol as an effective solvent for antioxidant extraction, phenolic compounds in particular.
3.3. Total flavonoid content (TFC)
Flavonoids are common secondary metabolites present in plants which are responsible for many plant biological activities.21 The solvent efficiency on TFC, in ascending order was: hexane < methanol < ethyl acetate. The highest flavonoid content of 208.4 mg QE/g extract was observed in the extract of ethyl acetate followed by methanol extract (89.9 QE/g extract). Hexane was found to be ineffective for flavonoid extraction (Table 1). These results are in agreement with the report of Hajji et al.23 which reported that flavonoid content in extract depend on solvent polarity.
3.4. Antioxidant activity
3.4.1. DPPH free radical scavenging activity
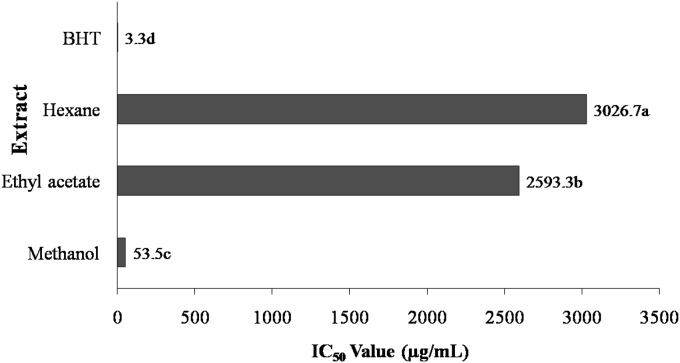
The DPPH free radical scavenging activity in leaf extracts of B. ciliata is presented in Fig. 1. The methanolic leaf extract showed excellent free radical scavenging activity (IC50 = 53.5 μg/mL). In other two leaf solvent extracts, the free radical scavenging activity in ethyl acetate extract (IC50 = 2593.3 μg/mL) was superior to that of the hexane extract (IC50 = 3026.7 μg/mL). Nevertheless, when compared to standard, the BHT, all the tested Bergenia leaf extracts showed significantly (p < 0.05) lower DPPH radical scavenging activity. These results are indicative of the influence of the solvent on the antioxidant activity of the biological extracts. It has been reported that in plant compounds with different polarity and structure are present that dissolve in specific solvents having similar polarity.24, 25 The difference in the DPPH radical scavenging activity in different solvent extracts implies towards the preference of the solvents for extraction of different types and concentrations of bioactive compounds.24, 25 Strong DPPH radical scavenging activity of B. ciliata methanolic extract can be due to higher content of total phenolic compounds.
Fig. 1.
Free radical-scavenging activity of B. ciliata extracts measured by DPPH method in terms of IC50 value (μg/mL of extract). Values are mean of triplicate experiments. Mean values with different letters differ significantly (p < 0.05) according to Duncan's multiple range test.
3.4.2. ABTS radical scavenging assay
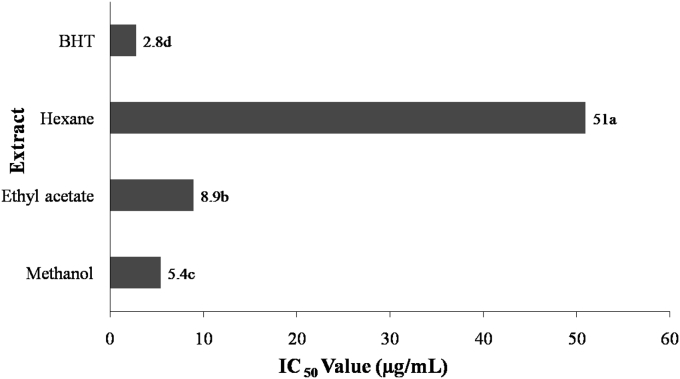
The free radical scavenging activity of Bergenia extracts was also determined using ABTS radical. Significant difference (p < 0.05) was revealed between ABTS scavenging capacities of extracts measured as IC50 value (Fig. 2). The highest ABTS radical scavenging activity was found in methanol extract with an IC50 value of 5.4 μg/mL, whereas hexane extract exerted the lowest ability to scavenge ABTS radical with an IC50 value of 51 μg/mL. Strong ABTS radical scavenging ability of B. ciliata methanol and ethyl acetate extracts can be attributed to the presence of flavonoids. Earlier, it has been reported that ABTS radical scavenging ability of bioactive compounds depends on its molecular weight, structure and presence of number of aromatic rings.26
Fig. 2.
Free radical-scavenging activity of B. ciliata extracts measured by ABTS method in terms of IC50 value (μg/mL of extract). Values are mean of triplicate experiments. Mean values with different letters differ significantly (p < 0.05) according to Duncan's multiple range test.
3.4.3. Correlation of IC50 values of antioxidant activities with TPC and TFC
The correlation coefficients determined between the antioxidant activities and the total phenolic and flavonoid contents in all the B. ciliata leaf extracts revealed the presence of significant high correlation (p < 0.1). The correlation for DPPH IC50 value vs TPC and DPPH IC50 value vs TFC were −0.913 and −0.056, respectively. Results indicate clearly that the DPPH activities of extracts are mainly due to the total phenolic compounds. Negative correlation indicated that with the increase in phenolic and flavonoid content, concentration of extract required to inhibit free radical decreases. In comparison to DPPH assay, strong correlation existed between ABTS assay and TFC. The correlation for ABTS IC50 value vs TPC and ABTS IC50 value vs TFC were −0.911 and −0.782, respectively, imply that both the total phenol and flavonoids of B. ciliata are responsible for ABTS activities. These differences in correlation between phytochemicals and antioxidant assays could be attributed to the different mechanism of the radical antioxidant reaction. In accordance with this study, Li et al.27 have also reported high negative linear correlation between the phenolic contents and IC50 values in Angelicae sinensis confirming that phenolics are likely to contribute to the antioxidant activity of the extracts. However, presence of other metabolites which have significantly contributed in antioxidant activity of B. ciliata extracts cannot be ruled out.
3.5. Antimicrobial activity
The results of the disc diffusion assay (Table 2) revealed that all the extracts tested were effective against the bacteria and actinomycetes studied, but showed no activity against fungi. Among the tested extracts, methanol extract was found to be the most effective which showed the highest effects against Bacillus megaterium strain with inhibition zone of 9.8 mm, followed by Nocardia tenerifensis and Bacillus subtilis with inhibition zones of 9.7 and 8.8 mm, respectively. The ethyl acetate extract showed the similar pattern of microbial inhibition with highest inhibition zone of 7.5 mm for B. megaterium, followed by 6.2 and 5.5 mm inhibition zone for N. tenerifensis and B. subtilis, respectively. In contrary to methanol and ethyl acetate extract, the hexane extract had the greatest inhibitory effects against Serratia marcescense (5.0 mm) and B. subtilis (4.7 mm).
Table 2.
Effect of the extraction solvent on the antimicrobial activity of Bergenia ciliata.
| Microorganism | Zone of inhibition (mm) |
||
|---|---|---|---|
| Methanol extract | Ethyl acetate extract | n-Hexane extract | |
| Bacillus subtilis* | 8.83 ± 0.3 | 5.5 ± 0.5 | 4.66 ± 0.3 |
| Bacillus megaterium* | 9.83 ± 0.2 | 7.5 ± 0.5 | 1.83 ± 0.3 |
| Escherichia coli* | 6.00 ± 0.0 | 1.83 ± 0.3 | na |
| Serratia marcescense* | 7.00 ± 0.2 | 5.00 ± 0.3 | 5.00 ± 0.3 |
| Nocardia tenerifensis** | 9.66 ± 0.9 | 6.16 ± 0.2 | na |
| Streptomyces sp.** | 6.66 ± 0.6 | 1.83 ± 0.3 | na |
| Aspergillus niger# | na | na | na |
| Fusarium oxysporum# | na | na | na |
* = bacteria; ** = actinomycetes; # = fungi; na = no activity.
The minimum inhibitory concentration (MIC) was determined in the methanolic extracts of B. ciliata due to its higher antimicrobial activity in disc diffusion assay against the tested organisms. The methanol extract gave the lowest minimal inhibitory concentration against B. subtilis with MIC of 1250 μg/mL (Table 3). The highest antimicrobial activity of methanolic extract could be due to the high contents of phenolic compounds and flavonoids present in the extract. These results are in accordance with a previous study where similar observations were recorded in case of Bergenia ligulata leaf extracts.28
Table 3.
Minimum Inhibitory Concentration (MIC) of test organism.
| S. no. | Microorganisms | MIC (μg/mL) |
|---|---|---|
| 1 | Bacillus subtillis* | 1250 |
| 2 | Bacillus megaterium* | 2500 |
| 3 | Serratia marcescense* | 2500 |
| 4 | Escherichia coli* | 2500 |
| 5 | Nocardia tenerifensis** | 5000 |
| 6 | Streptomyces sp.** | 2500 |
| 7 | Fusarium oxysporum# | na |
| 8 | Aspergillus niger# | na |
* = bacteria; ** = actinomycetes; # = fungi; na = No activity.
3.6. Quantification of bergenin, catechin and gallic acid by HPLC
B. ciliata is reported to contain a wide array of pharmaceutically important bioactive compounds.9, 29, 30 Among all, three main compounds viz., bergenin catechin and gallic acid are the most sought after compounds of the plant species.30 During the past years, efforts have been made for identification and quantification of the major bioactive compounds from Bergenia spp.5, 28, 30, 31, 32 However, till date, B. ciliata plant of the Sikkim Himalaya has never been analyzed for secondary metabolites. To the best of our knowledge, this is the first report on this aspect.
In the present study, different extracts of B. ciliata were analyzed by HPLC for the identification and quantification of bergenin, catechin and gallic acid. Standards showed high linearity at tested concentrations with correlation coefficients (R2) of 0.99, 0.96 and 0.99 for catechin, gallic acid and bergenin, respectively. The chromatographic peaks of the analytes were confirmed by comparing their retention time with those of the standards. From the standard equation obtained (Table 4), the amount of bergenin, catechin and gallic acid calculated in different extracts is presented in Table 5. Bergenin was detected in all the extracts and amount of bergenin was found in the order: methanol > ethyl acetate > hexane. The methanol extract contained bergenin (2.43 ± 0.08%) in amounts that were orders of magnitude higher than those in the ethyl acetate (1.63 ± 0.17%) and hexane (0.0024 ± 0.00%) extracts. HPLC analysis of catechin and gallic acid revealed that these compounds were present in methanol extracts only (Table 5).
Table 4.
Standard curve analysis for catechin, gallic acid and bergenin.
| Compound | Retention time (min) | Standard equation | R2 |
|---|---|---|---|
| Catechin | 9.43 ± 0.18 | y = 14331x − 78520 | 0.99 |
| Gallic acid | 3.97 ± 0.03 | y = 10949x + 90475 | 0.96 |
| Bergenin | 3.28 ± 0.03 | y = 1626x + 16757 | 0.99 |
Table 5.
Catechin, gallic acid and bergenin content (%) in different extracts of Bergenia.
| Phenolic compounds | Extract |
||
|---|---|---|---|
| Hexane | Ethyl acetate | Methanol | |
| Catechin | nd | nd | 0.08 ± 0.03 |
| Gallic acid | nd | nd | 0.88 ± 0.01 |
| Bergenin | 0.0024 ± 0.00 | 1.63 ± 0.17 | 2.43 ± 0.08 |
Values are mean ± standard deviation of triplicate experiments. nd, not detected.
4. Conclusion
In this study, the antioxidant and antimicrobial activities of B. ciliata plant growing in the Sikkim Himalaya are reported for the first time. The methanolic extracts of B. ciliata leaves, that were shown to contain the highest total phenolic and flavonoid compounds, exhibited high free radical scavenging activity against DPPH and ABTS radicals. Methanolic extract also exhibited strong antimicrobial activity against bacteria and actinomycetes. Further, HPLC analysis of different extracts for catechin, gallic acid, and bergenin showed that methanolic extract contained significantly higher amount of these bioactive compounds.
Conflict of interest
The authors declare “no conflict of interest.”
Acknowledgment
Authors are highly grateful to the Director, GB Pant Institute of Himalayan Environment and Development, Almora, for providing necessary facilities. Ministry of Environment, Forests and Climate Change (MoEF & CC) is thanked for financial support.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Savoia D. Plant-derived antimicrobial compounds: alternatives to medicines. Future Microbiol. 2012;8:979–990. doi: 10.2217/fmb.12.68. [DOI] [PubMed] [Google Scholar]
- 2.Pandey A., Agnihotri V. Antimicrobials from medicinal plants: research initiatives, challenges and the future prospects. In: Gupta V.K., Tuohy M.G., O'Donovan A., Lohani M., editors. Biotechnology of Bioactive Compounds: Sources and Applications in Food and Pharmaceuticals. John Wiley & Sons Ltd; 2015. [Google Scholar]
- 3.Bharati K.A., Sharma B.L. Plants used as ethno veterinary medicines in Sikkim Himalaya. Ethnobot Res Appl. 2001:339–356. [Google Scholar]
- 4.Garimella T.S., Jolly C.I., Narayanan S. In vitro studies on antilithiatic activity of seeds of Dolichos biflorus Linn. and rhizomes of Bergenia ligulata Wall. Phytother Res. 2001;15:351–355. doi: 10.1002/ptr.833. [DOI] [PubMed] [Google Scholar]
- 5.Singh D.P., Srivastava S.K., Govindarajan R., Rawat A.K.S. High-performance liquid chromatographic determination of bergenin in different Bergenia species. Acta Chromatogr. 2007;19:246–252. [Google Scholar]
- 6.Rai L.K., Sharma E. Bishen Singh Mahendra Pal Singh; Dehradun: 1994. Medicinal Plants of Sikkim Himalaya: Status, Uses and Potential. [Google Scholar]
- 7.Rai L.K., Prasad P., Sharma E. Conservation threats to some important medicinal plants of the Sikkim Himalaya. Biol Conserv. 2000;93:27–33. [Google Scholar]
- 8.Pradhan B., Badola H.K. Ethnomedicinal plant use by Lepcha tribe of Dzongu valley, bordering Khangchendzonga Biosphere Reserve, in North Sikkim. India J Ethnobiol Ethnomed. 2008;4:22. doi: 10.1186/1746-4269-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sinha S., Murugesan T., Maiti K., Gayen J.R., Pal M., Saha B.P. Evaluation of anti-inflammatory potential of Bergenia ciliata Sternb. rhizome extract in rats. J Pharma Pharmacol. 2001;53:193–196. doi: 10.1211/0022357011775398. [DOI] [PubMed] [Google Scholar]
- 10.Walter N.S., Bagai U., Kalia S. Antimalarial activity of B. ciliata (Haw) Sternb against Plasmodium berghei. Parasitol Res. 2013;112:3123–3128. doi: 10.1007/s00436-013-3487-z. [DOI] [PubMed] [Google Scholar]
- 11.Dharmender R., Madhavi T., Reena A., Sheetal A. Simultaneous quantification of bergenin, (+)-catechin, gallicin and gallic acid; and quantification of ß-sitosterol using HPTLC from Bergenia ciliata (Haw.) Sternb. Forma ligulata Yeo (Pasanbheda) Pharm Anal Acta. 2010;1:104. [Google Scholar]
- 12.Sajad T., Zargar A., Ahmad T., Bader G.N., Naime M., Ali S. Antibacterial and anti-inflammatory potential of Bergenia ligulata. Am J Biomed Sci. 2010;2:313–321. [Google Scholar]
- 13.Meda A., Lamien C.E., Romito M., Millogo J., Nacoulma O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey as well as their radical scavenging activity. Food Chem. 2005;21:571–577. [Google Scholar]
- 14.Lamaison J.L.C., Carnet A. Teneurs en principaux flavonoides des fleurs de Crataegeus monogyna Jacq et de Crataegeus laevigata (Poiret D.C) en fonction de la vegetation. Pharm Acta Helv. 1990;65:315–320. [Google Scholar]
- 15.Liyana-Pathiranan C.M., Shahidi F. Antioxidant activity of commercial soft and hard wheat (Triticum aestivum L) as affected by gastric pH conditions. J Agric Food Chem. 2005;53:2433–2440. doi: 10.1021/jf049320i. [DOI] [PubMed] [Google Scholar]
- 16.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 17.Clinical and laboratory Standards Institute . 6th ed. M7-A6; Wayne: 2005. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically: Approved Standard. [Google Scholar]
- 18.Clinical and laboratory Standards Institute . 3rd ed. M27-A3; Wayne: 2008. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts: Approved Standard. [Google Scholar]
- 19.Oki T., Masuda M., Kobayashi M., Nishiba Y., Furuta S., Suda I. Polymeric procyanidins as radical-scavenging components in red-hulled rice. J Agric Food Chem. 2002;50:7524–7529. doi: 10.1021/jf025841z. [DOI] [PubMed] [Google Scholar]
- 20.Singh M., Roy B., Tandon V., Chaturvedi R. Extracts of dedifferentiated cultures of Spilanthes acmella Murr. possess antioxidant and anthelmintic properties and hold promise as an alternative source of herbal medicine. Plant Biosyst. 2013;148:259–267. [Google Scholar]
- 21.Cai Y., Luo Q., Sun M., Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004;74:2157–2184. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh R., Kumari N. Comparative determination of phytochemicals and antioxidant activity from leaf and fruit of Sapindus mukorrossi Gaertn. – a valuable medicinal tree. Ind Crops Prod. 2015;73:1–8. [Google Scholar]
- 23.Hajji M., Masmoudi O., Ellouz-Triki Y., Siala R., Gharsallah N., Nasri M. Chemical composition of volatiles and antioxidant and radical-scavenging activities of Periploca laevigata root bark extracts. J Sci Food Agric. 2009;89:897–905. [Google Scholar]
- 24.Upadhyay R., Singh S.P., Jha A., Kumar A., Singh M. Appropriate solvents for extracting total phenolics, flavonoids and ascorbic acid from different kinds of millets. J Food Sci Technol. 2013;52:472–478. [Google Scholar]
- 25.Singh M., Jha A., Kumar A., Hettiarachchy N., Rai A.K., Sharma D. Influence of the solvents on the extraction of major phenolic compounds (punicalagin, ellagic acid and gallic acid) and their antioxidant activities in pomegranate aril. J Food Sci Technol. 2014;51:2070–2077. doi: 10.1007/s13197-014-1267-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hagerman A.E., Riedl K.M., Jones G.A., Sovik K.N., Ritchard N.T., Hartzfeld P.W. High molecular weight plant polyphenolics (tannins) as biological antioxidants. J Agric Food Chem. 1998;46:1887–1892. doi: 10.1021/jf970975b. [DOI] [PubMed] [Google Scholar]
- 27.Li X., Wu X., Huang L. Correlation between antioxidant activities and phenolic contents of radix Angelicae sinensis (Danggui) Molecules. 2009;14:5349–5361. doi: 10.3390/molecules14125349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agnihotri V., Sati P., Jantwal A., Pandey A. Antimicrobial and antioxidant phytochemicals in leaf extract of Bergenia ligulata: a Himalayan herb of medicinal value. Nat Prod Res. 2014;29:1074–1077. doi: 10.1080/14786419.2014.980244. [DOI] [PubMed] [Google Scholar]
- 29.Sinha S., Murugesan T., Pal M., Saha B.P. Evaluation of anti-tussive activity of Bergenia ciliata Sternb. rhizome extract in mice. Phytomedicine. 2001;8:298–301. doi: 10.1078/0944-7113-00039. [DOI] [PubMed] [Google Scholar]
- 30.Dhalwal K., Shinde V.M., Biradar Y.S., Mahadik K.R. Simultaneous quantification of bergenin, catechin, and gallic acid from Bergenia ciliata and Bergenia ligulata by using thin layer chromatography. J Food Comp Anal. 2008:496–500. [Google Scholar]
- 31.Umashankar D.C.R., Chawla A.S., Mundkinajeddu D., Singh D., Handa S.S. High pressure liquid chromatographic determination of bergenin and (+) -afzelechin from different parts of Paashaanbhed (Bergenia ligulata Yeo) Phytochem Anal. 1999;10:44–47. [Google Scholar]
- 32.Srivastava S., Rawat A.K.S. Simultaneous determination of bergenin and gallic acid in different Bergenia species. J Planar Chromatogr—Mod TLC. 2007;20:275–277. [Google Scholar]