Abstract
To evaluate the antibacterial activity of 12 ethanol extracts of Thai traditional herb against oral pathogens. The antibacterial activities were assessed by agar well diffusion, broth microdilution, and time-kill methods. Antibiofilm activity was investigated using a 3-[4,5-dimethyl-2-thiazolyl]-2,5-diphenyl-2H-tetrazolium-bromide (MTT) assay. High performance liquid chromatography (HPLC), thin layer chromatography (TLC) fingerprinting, and TLC-bioautography were used to determine the active antibacterial compounds. Piper betle showed the best antibacterial activities against all tested strains in the minimal inhibitory concentration and minimal bactericidal concentration, ranged from 1.04–5.21 mg/mL and 2.08–8.33 mg/mL, respectively. Killing ability depended on time and concentrations of the extract. P. betle extract acts as a potent antibiofilm agent with dual actions, preventing and eradicating the biofilm. The major constituent of P. betle extract was 4-chromanol, which responded for antibacteria and antibiofilm against oral pathogens. It suggests that the ethanol P. betle leaves extract may be used for preventing oral diseases.
Keywords: Antibacteria, Antibiofilm, Piper betle, 4-Chromanol, Oral pathogens
Graphical abstract
1. Introduction
Herbal medicines are valuable and available resources of primary health care for thousands year in the traditional medicine including the Thai folk medicine. Generally, the bioactive compounds in herbal medicines are secondary metabolites which act as various pharmacological properties.1, 2, 3 They are used as the substances of modern drugs e.g. morphine from Papaver somniferum for pain treatment,4, 5 colchicine from Colchicum autumnale for treatment in pericardial disease.6 Some studies have reported antibacterial activity from various herbs7, 8, 9; however, sufficient evidence to support their active compounds and their effectiveness for antibacteria and antibiofilm, particularly on oral pathogens is limited.
An imbalance of bacteria in the dental biofilm can cause dental caries and periodontitis which are major oral infections.10 The high doses of antimicrobial agents are need for removing the biofilm in clinical approach.11, 12 Moreover, in long term use of chlorhexine (CHX) mouth-rinse can cause side effects; disturbance in taste sensation, brown discoloration at dorsum of tongue and desquamative lesions of oral mucosa.13, 14
Our previous study has presented anticandidal and antibiofilm of some Thai herbs in Thailand.15 All herbs tested were commonly found in tropical areas of Southeast Asia. Herbal medicines may be an alternative way for being antibacterial agents to prevent oral infectious diseases. This extended study was to evaluate the antibacterial and antibiofilm activities of 12 ethanol extracts of Thai traditional herb included Alpinia galanga, Curcuma longa, Curcuma zedoaria, Piper betle, Piper chaba, Piper nigrum, Piper sarmentosum, Mentha cordifolia, Ocimum africanum, Ocimum basilicum, Ocimum sanctum and Zingiber officinale, and to investigate the active compounds of the most effective extract.
2. Materials and methods
2.1. Preparation of medicinal herb extracts
Twelve herbs were purchased from the local market, and the details were shown in Table 1. The herbs were identified by an expert in the Department of Pharmacognosy and Pharmaceutical Botany, Faculty of Pharmaceutical Sciences, Prince of Songkla University, Songkhla, Thailand, where voucher specimens (Table 1) were deposited in the herbarium. Dried plants were macerated with ethanol for 3 days, and then filtrated through Whatman No. 4 filter paper. Each filtrate was dried using a rotary evaporator at 40 °C and kept at −20 °C. For testing, 0.1 mg of each dried extract was initially dissolved with 100 μL of dimethyl sulfoxide (DMSO) and then adjusted to 1 mL by adding sterile distilled water, giving a final concentration of 10% (w/v) extract in 10% (v/v) dimethyl sulfoxide (DMSO).
Table 1.
Plants and susceptibility of oral microorganisms.
| Scientific name | Voucher specimen no. | Used part | Antibacterial activity of microorganisms |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Ef | Lf | Ls | Ss | Sm | Aa | Fn | |||
| Mentha cordifolia Opiz. | SKP 095130301 | Leaf | ++ | + | + | − | − | + | + |
| Ocimum africanum L. | SKP 095150201 | Aerial | + | + | + | − | − | . | + |
| Ocimum basilicum Lour. | SKP 095150101 | Aerial | + | + | + | − | − | − | − |
| Ocimum sanctum L. | SKP 095151901 | Aerial | + | + | + | − | − | − | − |
| Piper betle L. | SKP 146160201 | Leaf | ++ | ++ | ++ | +++ | +++ | +++ | +++ |
| Piper chaba Hunter | SKP 146160301 | Fruit | + | − | − | − | − | − | + |
| Piper nigrum L. | SKP 146161401 | Fruit | + | − | − | − | − | − | + |
| Piper sarmentosum Roxb. | SKP 146161906 | Leaf | + | − | − | − | − | − | ++ |
| Alpinia galanga (L.) Willd. | SKP 206010701 | Rhizome | − | − | − | − | + | ++ | ++ |
| Curcuma longa L. | SKP 206031201 | Rhizome | + | − | − | − | + | − | ++ |
| Curcuma zedoaria Roscoe | SKP 206032601 | Rhizome | − | − | − | − | + | − | ++ |
| Zingiber officinale Roscoe | SKP 206261501 | Rhizome | ++ | − | − | + | − | − | ++ |
+, 0 < zone < 10 mm; ++, 10 ≤ zone < 20 mm; +++, 20 ≤ zone; −, absence of inhibition zone.
2.2. Bacterial strains and growth conditions
A total of 7 oral microorganisms were employed in the study including 5 Gram positive cariogenic bacteria, Enterobacter faecalis ATCC 19433 (Ef), Lactobacillus fermentum ATCC 14931 (Lf), Lactobacillus salivarius ATCC 11741 (Ls), Streptococcus sobrinus ATCC 33478 (Ss) and Streptococcus mutans ATCC 25175 (Sm), and 2 Gram negative periodontopathogenic bacteria, Aggregatibacter actinomycetemcomitans ATCC 33384 (Aa) and Fusobacterium nucleatum ATCC 25586 (Fn). Microorganisms were maintained on either brain heart infusion agar (BHA) with 5% (v/v) blood for facultative bacteria, and supplemented with 0.5% (w/v) yeast extract, hemin and vitamin K for anaerobic bacteria. The strains were grown under aerobic or anaerobic (10% H2, 10% CO2 and 80% N2) conditions as appropriate.
2.3. Antibacterial assay
2.3.1. Agar well diffusion method
One hundred microliters of inoculum, equivalent to 107 CFU/mL, was mixed with 20 mL of warm melted BHA. The mixture was then poured into the plate with a 6 mm diameter metal cup. After solidifying of BHA, the metal cups were removed and the well was added with 100 μL of each plant extract. The plate was incubated at 37 °C for 24 h. A 10% DMSO were taken as a negative control. The antimicrobial activity of each plant extract was determined by measuring the diameter of the zone of the inhibition in millimeters. Duplicates were maintained and the experiment was repeated thrice.
2.3.2. Broth microdilution method
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) were carried out as recommended instruction of the Clinical and Laboratory Standards Institute (CLSI). Briefly, 10% stock solution of each extract was diluted in the brain heart infusion broth (BHI) in two-fold serial dilutions to obtain concentrations from 0.02 to 25 mg/mL at a total volume of 100 μL per well in 96-well microtiter plates. Each tested strain (100 μL) at a final concentration of 1 × 106 CFU/mL was added to each well and incubated at 37 °C in appropriate conditions. The medium, 0.1% (w/v) CHX and 10% DMSO were used as the non-treated, positive and negative controls, respectively.
MIC was defined as the lowest concentration of the extract that completely inhibited growth in comparison with the non-treated control. MBC was defined as the lowest concentration of wells that did not allow visible growth when 10 μL of the well contents was plated on agar and grown at 37 °C in appropriate conditions. All experiments were repeated thrice in duplicate.
2.3.3. Time-kill assay
As the results of the screening of herb extracts revealed that P. betle leaves extract gave the strongest antibacterial activity. Thus, P. betle extract was used for the time-kill assay. The growing cultures (106 CFU/mL) of representative strains, S. mutans ATCC 25175 and A. actinomycetemcomitans ATCC 33384, were incubated in BHI broth supplemented with the extract at concentrations equivalent to 1 ×, 2 ×, and 4 × MIC at 37 °C. Surviving bacteria were observed at 0, 0.5, 1, 2, 4, 6, 8, 10, 12 and 24 h by culturing on agar plates. The procedure was repeated in triplicate, and the log10 CFU/mL was plotted against time. A 0.1% CHX and extract free medium were used as positive and non-treated controls, respectively.
2.4. Antibiofilm assay
2.4.1. Inhibition of biofilm formation
The effect of P. betle extract on biofilm formation of each representative strain, S. mutans ATCC 25175 and A. actinomycetemcomitans ATCC 33384, was examined by using the modified microdilution method.16 Briefly, two-fold serial dilutions of P. betle extract were prepared, with final concentration ranged from 0.02 to 25 mg/mL. A cell suspension of the tested strains was prepared as described in the MIC assay, and 100 μL (1 × 106 CFU/mL) were inoculated in each of a 96-well plate. A 0.1% CHX, phosphate buffered saline (PBS) and extract free medium were used as the positive controls, non-treated and blank controls, respectively. After incubation at 37 °C for 24 h, supernatants were discarded and washed 3 times with PBS. Biofilm formation was quantified by using a 3-[4,5-dimethyl-2-thiazolyl]-2, 5-diphenyl-2H-tetrazolium-bromide (MTT) assay. The numbers of surviving bacteria were determined by measuring their ability to reduce the yellow tetrazolium salt to a purple formazan product at 570 nm. Higher OD values indicate an increased number of surviving microorganisms in the biofilm. Percentage inhibition was calculated by using the equation [1 − (A570 of the test/A570 of non-treated control)] × 100. The minimum biofilm inhibition concentration (MBIC) was defined as the lowest concentration that showed 50% and 90% inhibition of biofilm formation (MBIC50 and MBIC90).
2.4.2. Eradication of biofilm formation
The eradication of biofilm formation of P. betle extract was also examined using the minimum biofilm eradication concentration (MBEC) assay. Briefly, a 200 μL in early log phase (106 CFU/mL) of each representative strain, S. mutans ATCC 25175 and A. actinomycetemcomitans ATCC 33384, was inoculated into each well of a 96-well plate and incubated for 24 h at 37 °C for the development of a multilayer biofilm. The culture was then blotted out, and the well carefully washed 3 times with sterile PBS in order to remove non-adherent cells.16 These biofilms were then exposed to a 200 μL of various concentrations of P. betle extract ranged from 0.02 to 25 mg/mL, incubated for 24 h at 37 °C in appropriate conditions. At the end-point of the treatment of the biofilms with P. betle extract, the adherent cells were washed 3 times with sterile PBS. The numbers of surviving culture were determined using MTT assay. Percentage eradication was calculated by using the equation [1 − (A570 of the test/A570 of non-treated control)] × 100. The MBEC value was defined as the concentrations that showed 50% and 90% eradication of biofilm formation. The 0.1% CHX, PBS and the extract free medium were used as the positive, non-treated and blank controls, respectively.
2.5. Bioactive compound assay
2.5.1. High performance liquid chromatography (HPLC)
The active antibacterial compounds of P. betle extract were analyzed using the high performance liquid chromatography (HPLC) (Agilent® 1100 series, Palo Alto, CA). The reverse column was Apollo C18, 250 mm × 4.6 mm, 5 μm particle size (Alltech Associates Inc., Deerfield, IL). The conditions were set as following; mobile phases (methanol:water 70:30 v/v), flow rate at 0.7 mL/min and UV detector at 280 nm. A injection volume (20 μL) of each sample, 1 mg of P. betle extract, 5 μL of pure eugenol (the agent controller) and 0.5 mg of 4-chromanol (the agent controller), were prepared in 1 mL of 95% (v/v) ethanol. Each prepared sample was taken to analyze by HPLC, and curves were prepared using the Microsoft Excel software package (Excel 2007; Microsoft Corporation, Redmond, WA, USA). The experiments were performed three times.
2.5.2. Thin layer chromatography (TLC) fingerprinting and TLC-bioautography of P. betle extract
For TLC-fingerprinting study, 100 mg of P. betle extract, 5 μL of pure eugenol (the agent controller) and 1 mg of 4-chromanol (the agent controller) were prepared in 1 mL of 95% ethanol. A 10 μL of each sample was applied onto the alumina silica gel GF254 TLC-plate, and was run through a series of solvent systems using a mixture of toluene and ethyl acetate (90:10 v/v) as the mobile phase. The plate was air dried, and was stained with a 20% (w/v) of phosphomolybdic acid in ethanol.17
TLC-bioautography was performed using agar overlay method. TLC-plates were placed on plate contained 6 mL of BHA, and 10 mL of top agar with 100 μL of either S. mutans ATCC 25175 or A. actinomycetemcomitans ATCC 33384 inoculum (107 CFU/mL) was covered on TLC plate. After overnight incubation, the inhibited zone was observed as clear zone. The relative front values (Rf) was calculated as: Rf = distance traveled by solute/distance traveled by solvent.18
2.6. Statistical analysis
Data were expressed as mean and standard deviation (SD) by computational analysis from the three experiments with duplicate or triplicate independent experiments.
3. Results
3.1. Effect of Thai medicinal plants against bacteria
Antibacterial activity of 12 Thai traditional herb extracts against various oral bacteria was revealed in Table 1. The different extracts showed different inhibited zone ranged from 1 to 20 mm. P. betle leaves showed a good result of antibacterial activity against all tested microorganisms using agar well diffusion assay (Table 1). The MIC of P. betle extract ranged from 1.04 to 5.21 mg/mL and MBC ranged from 2.08 to 8.33 mg/mL, respectively (Table 2).
Table 2.
MIC and MBC of P. betle extracts against oral microorganisms.
| Microorganisms | Concentration of extract (mg/mL) |
|
|---|---|---|
| MIC | MBC | |
| Gram positive bacteria: | ||
| E. faecalis ATCC 19433 | 5.21 ± 1.80 | 8.33 ± 3.61 |
| L. fermentum ATCC 14931 | 4.17 ± 1.80 | 8.33 ± 3.61 |
| L. salivarius ATCC 11741 | 4.17 ± 1.80 | 8.33 ± 3.61 |
| S. sobrinus ATCC 33478 | 1.56 ± 0.00 | 3.17 ± 1.80 |
| S. mutans ATCC 25175 | 1.56 ± 0.00 | 3.17 ± 1.80 |
| Gram negative bacteria: | ||
| A. actinomycetemcomitans ATCC 33384 | 1.04 ± 0.45 | 2.08 ± 0.90 |
| F. nucleatum ATCC 25586 | 1.30 ± 0.45 | 2.08 ± 0.90 |
MIC – the minimum inhibitory concentration.
MBC – the minimum bactericidal concentration.
3.2. Time-kill assay of P. betle against bacteria
Time kill assay of P. betle extract against representative strains, S. mutans ATCC 25175 and A. actinomycetemcomitans ATCC 33384, is demonstrated in Fig. 1. It was depended on time and concentrations of the extract. The completed killing of S. mutans ATCC 25175 treated with 1 ×, 2 ×, and 4 × MIC of the extract occurred within 12, 8, and 6 h, respectively. At 1 ×, 2 ×, and 4 × MIC, A. actinomycetemcomitans ATCC 33384 was killed after 6, 2, and 1 h, respectively. The killing of positive control (0.1% CHX) was observed within 30 min.
Fig. 1.
Time-kill curves of S. mutans ATCC 25175 (a) and A. actinomycetemcomitans ATCC 33384 (b) at different concentrations of P. betle extract: 0 × MIC (▵), 1 × MIC (▾), 2 × MIC (○), and 4 × MIC (●); 0.1% (w/v) CHX (■); CFU, Colony Forming Units.
The present study demonstrated that commonly used Thai herbs could exhibit antimicrobial activity against various oral bacteria. Of 12 Thai traditional herb extracts, P. betle extract revealed the best antibacterial activity against both Gram positive bacteria and Gram negative bacteria.
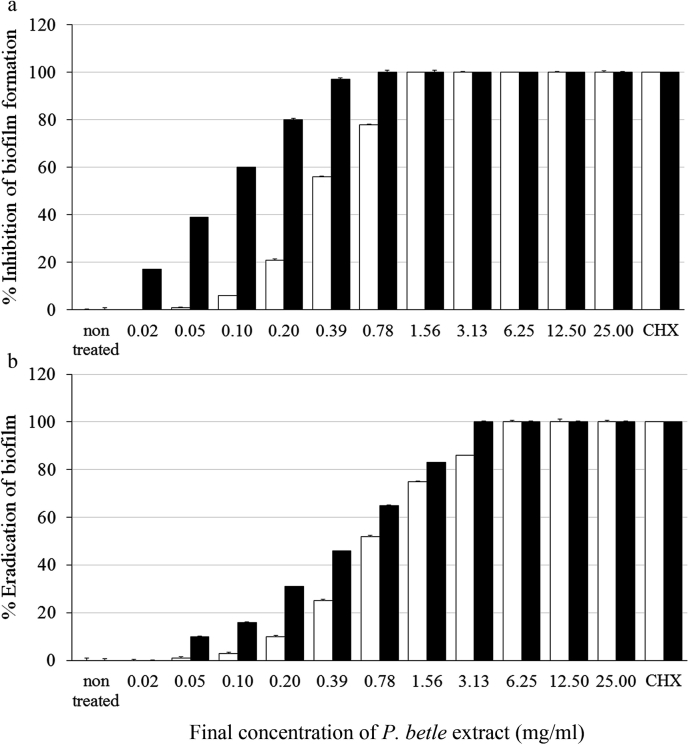
3.3. Effect of P. betle extract on inhibition and eradication biofilm formation
The inhibition and eradication of the biofilm formation of P. betle extract against S. mutans ATCC 25175 and A. actinomycetemcomitans ATCC 33384 is demonstrated in Fig. 2a and b, respectively. The concentrations of P. betle extract required to inhibit for ≥50% biofilm formation (MBIC50) of S. mutans ATCC 25175 and A. actinomycetemcomitans ATCC 33384 were 0.39 ± 0.20 and 0.10 ± 0.03 mg/mL, respectively. And for ≥90% biofilm inhibitions (MBIC90) were 1.56 ± 0.11 and 0.39 ± 0.65 mg/mL, respectively. The concentrations of P. betle extract to eradicate for ≥50% biofilm formation (MBEC50) of S. mutans ATCC 25175 and A. actinomycetemcomitans ATCC 33384 were 0.78 ± 0.74 and 0.78 ± 0.11 mg/mL, respectively. And for ≥90% biofilm eradication (MBEC90) were 6.25 ± 0.58 and 3.13 ± 0.28 mg/mL, respectively. CHX could completely (100%) inhibit or remove biofilm formation of both tested strains.
Fig. 2.
Inhibition (a) and eracdication (b) of biofilm formation of S. mutans ATCC 25175 (□) and A. actinomycetemcomitans ATCC 33384 (■) by P. betle leaves extract at various concentrations, 0.1% (w/v) CHX was used as control. Error bars indicate standard deviations; n = 6.
3.4. HPLC and TLC analysis
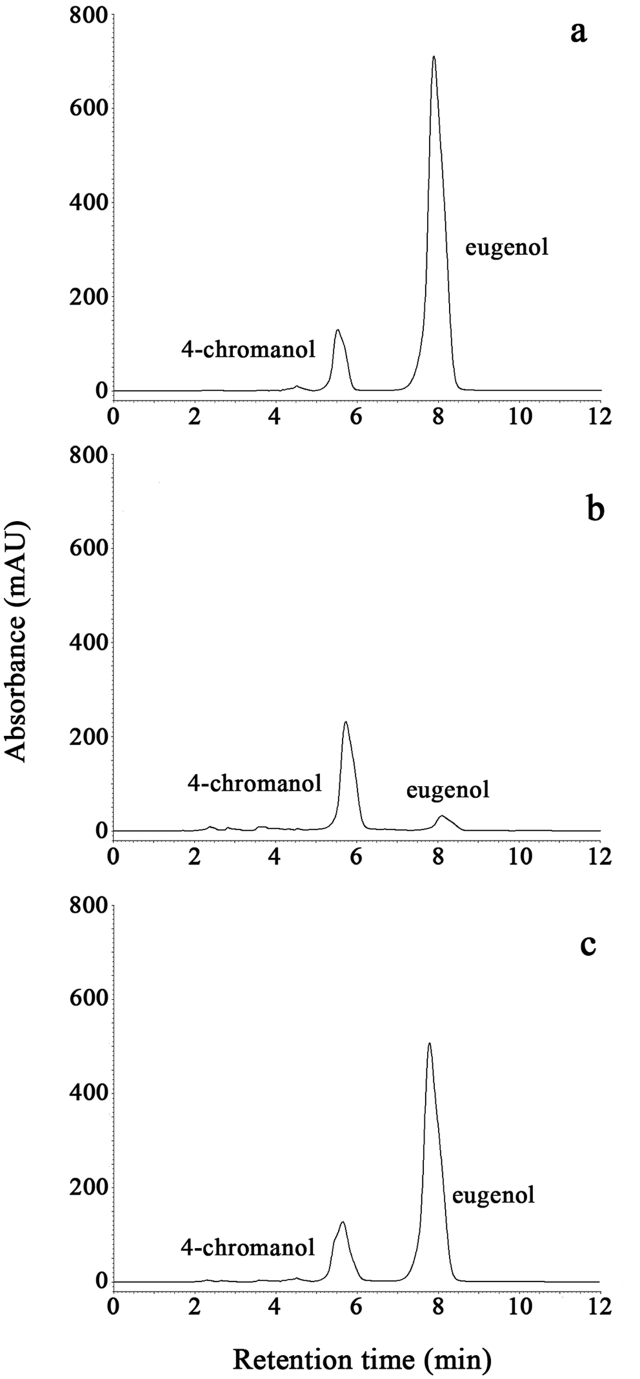
Due to its interesting results, the further study focused on the active compounds of P. betle leaves extract. The HPLC chromatograms of the standards, 4-chromanol and eugenol (Fig. 3a), P. betle extract (Fig. 3b) and the mixture of P. betle extract and the standards (Fig. 3c) are shown. Peak 1 of Fig. 3a–c was identified as 4-chromanol with retention times of 5.486 ± 0.00 min, 5.654 ± 0.04 min and 5.610 ± 0.00 min, respectively. Peak 2 of Fig. 3a–c was eugenol with retention times of 7.853 ± 0.01 min, 7.966 ± 0.09 min and 7.757 ± 0.01 min, respectively. It indicated that at least 4-chromanol and eugenol were the components of P. betle leaves extract.
Fig. 3.
HPLC chromatograms of the standards; 4-chromanol and eugenol (a), P. betle leaf extract (b), and the mixture of the standards and P. betle extract (c).
There were several bands of P. betle extract components appeared on the TLC-plate (Fig. 4a). One major band with Rf at 0.38 matched to the standard 4-chromanol gave the large inhibited clear zone against S. mutans ATCC 25175 in TLC-bioautography assay (Fig. 4b), and this band also gave inhibited clear zone against A. actinomycetemcomitans ATCC 33384 (data not shown). Another minor band with Rf at 0.74 matched to the standard eugenol did not show any inhibited zone.
Fig. 4.
TLC fingerprinting (a) and TLC-bioautography against S. mutans ATCC 25175 (b) of P. betle leaves extract.
4. Discussion
P. betle (family Piperaceae) is known as “Phlu”, which has been extensively used in traditional herbal remedies in Thailand and many other countries in tropical Asia. It is reported having various pharmacological activities such as antimicrobial, immunomodulatory, and anti-inflammatory.19 A significant antimicrobial activity against a wide range of medical bacteria such as Streptococcus pyogenes, Staphylococcus aureus, Proteus vulgaris, Escherichia coli, Pseudomonas aeruginosa etc. was found in the leaves.20 In this study, it has shown that P. betle extract also exhibited antimicrobial activity against various oral microorganisms including cariogenic and periodontogenic pathogens e.g. S. mutans and A. actinomycetemcomitans. Our results agree with the study of Deshpande and Kadam21 who demonstrated the finding of antibacterial activity of ethanol and aqueous crude extract of P. betle leaves against S. mutans. In that report, zone of inhibition of ethanol extract (20.6 ± 1.12 mm) was found to be larger than aqueous extract (18.3 ± 0.53 mm). The MIC value for the aqueous and ethanol extract was 10 mg/mL and 5 mg/mL, respectively. The P. betle leaves ethanol extract in this study was found to be more potent with MIC value of 1.56 mg/mL. This may be due to ecological and geographical conditions, age of herb and time of harvesting. However, there was no information of antibiofilm was mentioned in that study.
The antibiofilm is a desired property that expected from herbs extracts. In this study, P. betle leaves extract has been revealed having dual actions in preventing and eradicating biofilm formation. It may be worth for considering the P. betle leaves extracts to replace CHX mouth-rinse. CHX is a chemical-based antimicrobial agent which is used extensively in the mouth-rinse to maintain dental biofilm at a level compatible with oral health. However, it was reported on some undesired local side effects for long term use.13, 14
Results of both planktonic and biofilm form indicated that P. betle extract was more active against Gram negative bacteria than Gram positive bacteria. The similar results were also reported in Citrus bergamia,22 Woodfordia fruticosa,23 and Artocarpus lakoocha9 showing that Gram negative bacteria were more susceptible to some plant extracts than Gram positive. This contrasted to previous studies showed that plant extracts were more active against Gram positive bacteria than Gram negative bacteria.24, 25 The Gram negative bacteria are considered to be more resistant due to their outer membrane acting as a barrier to many environmental substances including antimicrobial substances. Moreover, they could stimulate the membrane-associated mechanisms of resistance via the expression of efflux pumps to control the antibacterial agents.26, 27
Deshpande and Kadam21 reported that 4-chromanol was a predominant compound in P. betle leaves aqueous extract, which responded for antibacterial activity. Our previously study demonstrated that the major constituents of P. betle leaves extract were 4-chromanol (62.33%) and eugenol (17.10%) in GC-MS analysis, and the 4-chromanol showed good anticandidal activities.15 In this study, the 4-chromanol also exhibited the strong antibacterial activity by observing a clear inhibition zone against oral pathogens in the TLC-bioautography assay. Thus, it indicated that the 4-chromanol could overcome the barrier and membrane-associated resistance of Gram negative bacterial membrane.
5. Conclusion
This study suggested that the ethanol extract of P. betle leaves is a potential source of natural antibacterial and antibiofilm agents. The major constituents of P. betle leaves extract was 4-chromanol exhibiting a good antibacterial and antibiofilm properties that may be used for oral infectious diseases. For further study, the mechanisms of antibacterial activity of 4-chromanol should be investigated in more detail as well as its activity in vivo.
Author contribution
Rawee Teanpaisan: Project initiation and design, Data analysis, Writing and preparing the manuscript, Editing the manuscript. Pajaree Kawsud: Project initiation and design, Laboratory performance, Data analysis, Writing and preparing the manuscript. Nuntiya Pahumunto: Data/samples collection, preparing the manuscript. Jindaporn Puripattanavong: Data/samples collection, preparing the manuscript.
Conflict of interest
None declared.
Acknowledgments
This work was supported by the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission (DEN540547S).
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Cragg G.M., Newman D.J. Medicinals for the millennia: the historical record. Ann N Y Acad Sci. 2001;953:3–25. doi: 10.1111/j.1749-6632.2001.tb11356.x. [DOI] [PubMed] [Google Scholar]
- 2.Neelamkavil S.V., Thoppil J.E. Evaluation of the anticancer potential of the traditional medicinal herb Isodon coetsa. South Indian J Biol Sci. 2016;2:41–45. [Google Scholar]
- 3.Jesonbabu J., Spandana N., Aruna L.K. In vitro antimicrobial potentialities of chloroform extracts of ethanomedicinal plant against clinically isolated human pathogens. Int J Pharm Pharm Sci. 2012;4:624–626. [Google Scholar]
- 4.Pace S., Burke T.F. Intravenous morphine for early pain relief in patients with acute abdominal pain. Acad Emerg Med. 1996;3:1086–1092. doi: 10.1111/j.1553-2712.1996.tb03365.x. [DOI] [PubMed] [Google Scholar]
- 5.Blakemore P.R., White J.D. Morphine, the Proteus of organic molecules. Chem Commun (Camb) 2002;7:1159–1168. doi: 10.1039/b111551k. [DOI] [PubMed] [Google Scholar]
- 6.Deftereos S., Giannopoulos G., Papoutsidakis N. Colchicine and the heart: pushing the envelope. J Am Coll Cardiol. 2013;62:1817–1825. doi: 10.1016/j.jacc.2013.08.726. [DOI] [PubMed] [Google Scholar]
- 7.Chanudom L., Bhoopong P., Khwanchuea R., Tangpong J. Antioxidant and antimicrobial activities of aqueous & ethanol crude extract of 13 Thai traditional plants. Int J Curr Microbiol App Sci. 2014;3:549–558. [Google Scholar]
- 8.Teanpaisan R., Senapong S., Puripattanavong J. In vitro Antimicrobial and antibiofilm activity of Artocarpus lakoocha (Moraceae) extract against some oral pathogens. Trop J Pharm Res. 2014;13:1149–1155. [Google Scholar]
- 9.Mahalakshmi N., Dhanasekaran S., Ravi C., Lingathurai S. In-vitro antimicrobial activities of Pongamia glabra and Phyllanthus niruri. South Indian J Biol Sci. 2016;2:236–244. [Google Scholar]
- 10.Liljemark W.F., Bloomquist C. Human oral microbial ecology and dental caries and periodontal diseases. Crit Rev Oral Biol Med. 1996;7:180–198. doi: 10.1177/10454411960070020601. [DOI] [PubMed] [Google Scholar]
- 11.Nickel J.C., Ruseskal I., Wright J.B., Costerton J.W. Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob Agents Chemother. 1985;27:619–624. doi: 10.1128/aac.27.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donlan R.M., Costerton J.W. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flötra L., Gjermo P., Rölla G., Waerhaug J. Side effect of chlorhexidine mouthwashes. Eur J Oral Sci. 1971;79:119–125. doi: 10.1111/j.1600-0722.1971.tb02001.x. [DOI] [PubMed] [Google Scholar]
- 14.Addy M., Moran J. Mechanisms of stain formation on teeth, in particular associated with metal ions and antiseptics. Adv Dent Res. 1995;9:450–456. [Google Scholar]
- 15.Kawsud P., Puripattanavong J., Teanpaisan R. Screening for anticandidal and antibiofilm activity of herbs in Thailand. Trop J Pharm Res. 2014;13:1495–1501. [Google Scholar]
- 16.Tang H.J., Chen C.C., Ko W.C., Yu W.L., Chiang S.R., Chuang Y.C. In vitro efficacy of antimicrobial agents against high-inoculum or biofilm embedded methicillin-resistant Staphylococcus aureus with vancomycin minimal inhibitory concentrations equal to 2 μg/mL (VA2-MRSA) Int J Antimicrob Agents. 2011;38:46–51. doi: 10.1016/j.ijantimicag.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Department of Medical Sciences, Ministry of Public Health . Office of National Buddhism Press; Bangkok, Thailand: 2007. Thai Herbal Pharma-copoeia Volume II; pp. 63–70. [Google Scholar]
- 18.Rahalison L., Hamburger M.O., Hostettmann K., Hostettmann K., Monod M., Frenk E. A bioautographic agar overlay method for the detection of antifungal compounds from higher plants. Phytochem Anal. 1991;2:199–208. [Google Scholar]
- 19.Nagori K., Singh M.K., Alexander A. Piper betle: a review on its ethnobotany, phytochemistry, pharmacological profile and profiling by new hyphenated technique DART-MS (Direct Analysis in Real Time Mass Spectrometry) J Pharm Res. 2011;4:2991–2997. [Google Scholar]
- 20.Pradhan D., Suri K.A., Pradhan D.K., Biswasroy P. Golden heart of the nature: Piper betlle L. J Pharmacogn Phytochem. 2013;1:147–167. [Google Scholar]
- 21.Deshpande S.N., Kadam D.G. GCMS analysis and antibacterial activity of Piper betle (Linn)leaves against Streptococcus mutans. Asian J Pharm Clin Res. 2013;6:99–101. [Google Scholar]
- 22.Mandalari G., Bennett R.N., Bisignano G. Antimicrobial activity of flavonoids extracted from bergamot (Citrus bergamia Risso) peel, a byproduct of the essential oil industry. J Appl Microbiol. 2007;103:2056–2064. doi: 10.1111/j.1365-2672.2007.03456.x. [DOI] [PubMed] [Google Scholar]
- 23.Parekh J., Chanda S. In vitro antibacterial activity of the crude methanol extract of Woodfordia fruticosa Kurz. flower (Lythraceae) Braz J Microbiol. 2007;38:204–207. [Google Scholar]
- 24.Vlietinck A.J., Van Hoof L., Totte J. Screening of hundred Rwandese medicinal plants for antimicrobial and antiviral properties. J Ethnopharmacol. 1995;46:31–47. doi: 10.1016/0378-8741(95)01226-4. [DOI] [PubMed] [Google Scholar]
- 25.Rabe T., Van Staden J. Antibacterial activity of South African plants used for medicinal purposes. J Ethnopharmacol. 1997;56:81–87. doi: 10.1016/s0378-8741(96)01515-2. [DOI] [PubMed] [Google Scholar]
- 26.Nikaido H., Pagès J.M. Broad-specificity efflux pumps and their role in multidrug resistance of Gram-negative bacteria. FEMS Microbiol Rev. 2012;36:340–363. doi: 10.1111/j.1574-6976.2011.00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fadli M., Chevalier J., Hassani L., Mezrioui N.E., Pagès J.M. Natural extracts stimulate membrane-associated mechanisms of resistance in Gram-negative bacteria. Lett Appl Microbiol. 2014;58:472–477. doi: 10.1111/lam.12216. [DOI] [PubMed] [Google Scholar]