Abstract
In spite of incredible advances in modern science, technology and allopathic medicine a large we are unable to provide quality healthcare to all. Traditional medicine particularly herbal medicine considered as a major healthcare provider around the globe particularly in rural and remote areas. A large section of people depends on such medicine for their primary healthcare mainly in underdeveloped or developing countries. Indian traditional medicinal system like Ayurveda, Siddha and Unani has a very rich history of their effectiveness; modern research also acknowledged the importance of such medicine. Indian traditional medicine or medicinal plants are also considered as a vital source of new drug. Mainstreaming of such medicine is important for the people. Several steps have been taken in India to promote such medicine and to integrate them into clinical practice. Evidence based incorporation of Indian traditional medicine in clinical practice will help to provide quality healthcare to all.
Keywords: Indian traditional medicine, Ayurveda, Mainstreaming, Health, Clinical practice
Graphical abstract
1. Introduction
In last century, medical science has made incredible advances all over the globe. Overall mortality rate decreased, expectancy of life increased, a lot of new life saving drugs discovered which helps us to fight against several infectious and other diseases, and new advancement in the field of technology has boosted the capacity of modern science. In spite of such incredible advancement whether such benefit of modern science/medicine has reach to the every door of the world? World Health Organization (WHO) in an International Conference on Primary Health Care in 1978 commonly known as ‘Declaration of Alma-Ata’ express the need to achieve the goal ‘Health for All’ step by step manner through tackling the poverty, illiteracy and poor sanitation. In 1998, WHO incorporates a new global health policy “Health for All in the 21st Century” and set the goal to achieve health security, health equity, increased healthy life expectancy and to ensure access to essential quality healthcare for all.1, 2 Modern medical science, despite so many achievements and progress, is finding itself difficult to reach to every people and deal with the ever-increasing diseases and disorders. Still, majority of world population mainly in developing and underdeveloped countries does not have access to modern medicine and depends on the time-tested traditional/alternative or complementary systems of medicine, many of these systems is much older compared to the allopathic medical wisdom.3, 4 Therefore, the major questions still exist – (1) whether the goal has achieved? (2) whether ‘Health for All’ can be possible without scientific integration of traditional herbal medicine in clinical practice?
In the 21st century, pollution, unhealthy lifestyle, environmental toxins increases the risk of diseases. The side effects, overuse/misuses of allopathic drugs are also a major concern. In 2013, WHO developed and lunched ‘WHO Traditional Medicine Strategy 2014–2023’ and emphasised to integrate traditional and complementary medicine to promote universal healthcare and to ensure the quality, safety and effectiveness of such medicine.5 Therefore, the world is looking for cost effective, easily available, better physiological compatible traditional systems of medicine and holistic approach to avert such problem and provide the basic healthcare to all.
2. Indian society and traditional medicine
Knowledge regarding the therapeutic, toxicological effect of plants, minerals and other substances go back to the prehistoric times when people have migrated to into the Indian subcontinent. Several evidences indicated that in Indian subcontinent medical intervention like dentistry and trepanation were exercised as early as 7000 BCE. Current archaeo-botanical excavations pointed towards the evidence regarding the use of medicinal plants in the Middle Gangetic region since the 2nd millennium BCE which are still found in Ayurvedic folk medicine.6 India is a land of different group of people who have their own religion, beliefs, culture, language and dialects. Thus, diverse medicinal systems have developed in this region. A number of medicinal systems also introduced here from outside and enriched in India. Since ancient time, Indian society depends on traditional medicinal systems practiced here. Introduction of allopathic drug during British era and neglecting Indian traditional medicine by British ruler are responsible for significant erosion of Indian traditional medicine. High scientific progress in allopathic medicine and modern facilities also resists the growth of traditional medicine. Still, about 70% rural populations of India are believed in traditional medicine for primary healthcare.7, 8
2.1. Ayurveda
Ayurveda is a comprehensive scientific medicinal system indigenous to India. The term Ayurveda means ‘knowledge of life’, which comprises two Sanskrit words, Ayu (life) and Veda (knowledge or science). Four Vedas, considered as the oldest Indian literature (5000–1000 BC) contain information about natural remedies. Ayurveda was established as a fully grown medicinal system.9, 10 Charaka Samhita (focussing on internal medicine) and Susruta Samhita (focussing on surgery) were written systematically and considered as classical text of Ayurveda. Vital details of Charaka Samhita and Susruta Samhita were complied together and updated additionally in Astanga Sangraha and Astanga Hrdaya. Some other ancient classics which include minor work of Ayurveda includes Madhava Nidana (focussing on diagnosis of disease), Bhava Prakasa (focussing on additional information related to plant and diet), Sarngadhara Samhita (focussing on formulation and dosage form).9, 11 Ayurveda was divided into eight major clinical subdivisions – Kayacikitsa (internal medicine), Salya Tantra (surgery), Salakya (diseases of supra-clavicular origin), Kaumarabhrtya (paediatrics, obstetrics and gynaecology), Bhutavidya (psychiatry), Agada Tantra (toxicology), Rasayana Tantra (rejuvenation and geriatrics), Vajikarana (aphrodisiology and eugenics).9, 10, 11
2.2. Siddha
Siddha system of medicine is believed as a brilliant achievement and symbol of Tamil culture which originated in Southern parts of India. Siddha medicine invented from Dravidian culture and is grown in the time of Indus valley civilization. Chinese alchemy, Taoism, and Taoist Patrology are considered as a main source of inspiration for Siddha alchemy. It is believed that in ancient time, the system was developed by eighteen siddhar (a class of Tamil sages). Though Siddha system of medicine resembles with Ayurveda in many aspects it has own philosophy and concept, holistic approach, and lifestyle oriented measures.12, 13, 14, 15
2.3. Unani
Unani system of medicine is the fusion of contemporary traditional medicinal system in Egypt, Syria, Iran, Iraq, China, India and several other east countries. It was originated in Greece and letter developed in Arab. Arab and Persian settlers in 11th century introduced Unani medicine in India, the system gets recognition and enriched during Mughul rule.16, 17, 18
2.4. Amchi
Amchi or Sowa-Rigpa is another ancient well documented traditional medicinal system, which was popular in Tibet, Mongolia, Nepal, Bhutan, Himalayan region of India, some parts of China and former Soviet Union. Though conflicts exist on the origin place of Amchi medicine as some believed it originated in India, some say Tibetan region and other considered it as Chinese origin. Amchi has close similarity with Ayurveda, though influence of Chinese traditional medicine and Tibetan folklore also observed in this system.19
2.5. Folk medicine
Other than codified traditional medicinal system the uncodified folk medicine also plays a vital role in maintenance of health and cure of diseases for large number of people belongs to rural/indigenous/ethnic communities. This type of knowledge is not documented properly and propagates verbally from ancestors. Nearly 8000 plants species are utilized in folk medicine and approximately 25,000 effective plant-based formulations used by the rural and ethnic communities in India.17
3. Herbal medicine and its importance
Plants are always the key source of drug or treatment strategy in different traditional medicinal systems. In recent years, many people are choosing to plant based medicines or products to improve their health conditions or as curative substance either alone or in combination with others. According to the WHO, herbs or herbal products are used by the large number of populations for basic healthcare needs. Herbal medicine includes herbs, herbal materials (like plant parts) or preparations, processed and finished herbal products, active ingredients.20, 21 In recent years, a huge resurgence of the use of herbal product due to the side effects of modern drugs, failure of modern therapies for against chronic diseases, and microbial resistance. It is estimated that nearly 75% of the plant based therapeutic entities used worldwide were included from traditional/folk medicine. In India, approximately 70% of modern drug are discovered from natural resources and number of other synthetic analogues have been prepared from prototype compounds isolated from plants.20, 22, 23 It was reported that more than 60% of cancer drug available in market or in testing are based on natural products. Currently, about 80% of antimicrobial, immunosuppressive, cardiovascular, and anticancer drugs are derived from plant sources. More than 70% entities among 177 anticancer drugs approved are based on natural products or mimetic. About 25% prescription drug found globally are derived from plant sources, and nearly 121 such drugs entity are in use. Thirteen drugs of natural origin are approved in United States between 2005 and 2007, and clinical trials are going on more than 100 natural product-based drugs. It was also estimated that 11% of the total 252 drugs found in essential medicine list of WHO are exclusively of plant origin.24, 25 In Indian traditional medicine a large number of plants are used. It was estimated that Ayurveda uses 1200–1800 plants, Siddha medicine includes 500–900 plants, Unani utilize 400–700 medicinal plants and Amchi medicine uses nearly 300 plants while folk healers of India use more than 7500 medicinal plants in different medicine. Three classical Ayurvedic literature Charaka Samhita, Sushruta Samhita and Astanga Hridaya mentioned about 526,573 and 902 number of plants.17, 26, 27
4. Promotion of herbal medicine – problems need to be addressed
In spite of global reorganization and very sound history of traditional uses, promotion of herbal medicine faces number of challenges around the glove mainly in developed nations. Following problems need to be overcome before the promotion of traditional herbal knowledge around the world.28, 29, 30, 31, 32, 33, 34
-
•
Quality issues: Adulteration, misidentification of plant, faulty collection and preparation, incorrect formulation process are the main problems that reduces the effectiveness of herbal preparation and can be considered as key factors affecting quality and purity of herbal medicines.
-
•
Processing and harvesting issues: Indiscriminate harvesting, poor agriculture and propagation method, poor pre and post harvest practices, lack of processing techniques leads to the substandard quality of herbal drugs.
-
•
Quality control related issues: Standardization, poor quality control procedure and lack of Good Manufacturing Practices (GMP) are the main hurdle to maintain the quality of herbal drugs. Lack of awareness regarding the guideline among growers and manufacturers, lack of implementation and regulation of the guideline are also frequent in small and medium scale industries.
-
•
Administrative issues: Lack of regulation and controlling authority in herbal sector, lack of proper monitoring and controlling are absolute need for the quality of drugs.
-
•
Infrastructure related issue: Lack of processing technique, trained personal, sophisticated instrument, utilization of modern techniques, facility to fabricate instrument locally are the major problems.
-
•
Pharmacogivilane: Proper pharmacogivilane in herbal sector is the need of time to find the toxicological data and adverse drug reaction of herbal drugs. Adverse reactions, contraindications, interactions with other drug, food and existing orthodox pharmaceuticals need to be monitor properly.
-
•
Clinical trial: Since the safety continues to be a foremost issue with the use of herbal remedies therefore, clinical trials are necessary to understand the safety and efficacy of these drugs before introduced them in global market.
-
•
IPR and biopiracy: Biopiracy is the major difficulty in promotion of herbal traditional medicine. Documentation of folk knowledge thus important for our future.
-
•
Irrational use: It is generally believed that herbal products don't have any side effects, interaction, but unfortunately is not true. Thus, irrational practice of these drugs can lead to various problems which can hinder the promotion of such drugs.
-
•
R&D: Research and development on dosage, processing, techniques are the key need for any drug, but in herbal sector it is quite less compare to allopathic medicine. Although in recent years, the trend is changing. Research to understand the mode of action and pharmacokinetics phenomenon, improvement/creation of monographs and reference standards for marker-based analysis are necessary of time. Decisive gap in current ethnopharmacological and modern medicinal plant research is another problem for sustainable, socio-culturally equitable and safe supply of herbal medicines.
-
•
Other issues: Unethical practice of herbal medicine, lack of qualified physician, exposure of unreliable and misleading information, lack of sufficient fund, absence of focused marketing and branding, lack of knowledge sharing also hold back the global promotion of herbal medicine. Lack of protection of biodiversity and protecting the traditional medicinal plants are also a big challenge.
5. Modernization & integration of herbal medicine in clinical practice – experience from India
In spite of number of hurdles, the traditional medicine of India in acknowledged widely around the world and the demand is increasing continuously. Combined effort of public and government sector is essential for the promotion of herbal medicine. Here we are discussing about the situation and possibilities of promotion of Indian traditional herbal medicine in India.
5.1. Rules, regulation & governing body
In India, the national policy on traditional and alternative medicine was introduced in 1940 in the form of Drug and Cosmetic Act 1940 and Drug and Cosmetic Rule, which was updated in several instate. In 1959, Govt of India recognized traditional Indian System of Medicine (ISM) and updated Drug and Cosmetic Act. Several expert committees for different ISM were established time to time and the earliest was established in 1962. In the year 1969, separate chapter related to Ayurveda, Siddha and Unani drugs was inserted by act 13 of 1964 in the Act, which partly similar as those for conventional pharmaceuticals. Later the act was modified again with some substitutions in the year 1983, 1987, 1994 and 2002. In 2006 and 2008 guideline for evaluation and analysis of drugs under ISM was given under Drug and Cosmetic Rule 1945. The Central Council of Indian Medicine (CCIM) is constituted in the year 1970, which involved in the framing and implementing different regulations including the curricula and syllabii in ISM (i.e. Ayurveda, Siddha and Unani). In 2012, Sowa Rigpa system of medicine is incorporated in the CCIM. Department of Indian Medicine and Homeopathy (ISM & H) was formed with the objective to develop the ISM. In 2003, this Department was renamed as Department of Ayurveda, Yoga and Naturopathy, Unani, Siddha and Homoeopathy (AYUSH), and in 2014 separate ministry on AYUSH was formed.35, 36, 37, 38, 39
5.2. AYUSH and health policy
Department of AYUSH concentrates on the overall governance, education, regulation, development and growth of ISM in the India and abroad. The department has few subordinate offices, several autonomous bodies in the form of research councils, professional council, pharmacopoeia laboratories, national institutes, academy and hospitals. In the year 2002, National Policy on Indian Systems of Medicine & Homoeopathy was introduced. Major objectives of this policy are,37, 40
-
•
Utilize the AYUSH to endorse good health and spread out the outreach of healthcare to our people (mainly who cannot afford or reach to the modern healthcare facilities) through preventive, promotive, mitigative and curative approaches.
-
•
To provide affordable AYUSH services & drugs which are safe and efficacies;
-
•
To ensure the availability and genuine of raw drugs as required by pharmacopoeial standards to help improve quality of AYUSH drugs, for domestic and/or export purpose.
-
•
Incorporate AYUSH in healthcare delivery system and national programmes and to ensure the best possible utilization of huge infrastructure of hospitals, dispensaries and physicians.
-
•
To offer full opportunity for the expansion and development of ISM and utilization of the potentiality, strength and revival of their glory.
5.3. Quality control, regulation of safety and efficacy issues
In India, several legislative and administrative measures are in place to control the manufacturing and sale of Ayurveda, Siddha and Unani (ASU) medicine. Chapter IVA in the Drugs & Cosmetics Act, 1940 describe the provisions for regulation of manufacturing, packaging, labelling and sale of ASU drugs. Periodic revision of such act is also a part of betterment of ASU drugs, as the latest amendments in this chapter were done on March 2013. A separate Ayurveda, Siddha and Unani Technical Advisory Board (ASUDTAB) was also formed which deals and advice the authorities on the technical matters involved in the regulation of ASU drugs. Another Ayurveda, Siddha and Unani Drugs Consultative Committee (ASUDCC) also constituted which advice to attain the uniformity in the administration of Drugs & Cosmetics Act, 1940 (related to ASU drugs) throughout India. GMP for ASU drugs was under Schedule ‘T’ of the Indian Drugs & Cosmetics Act, 1940 and Rules, 1945 was notified in 2000. In view of world concern related to the presence of heavy metals in ASU drugs guideline was issued to test the ASU drug to find the presence of heavy metal and check the limit of heavy metal if any. Guidelines have also been issued to curb growth of irrational ASU combinations.40, 41
Several rules/guidelines like inclusion of proper botanical name in label, include the caution regarding the consumption of drug under medical supervision if ingredient contain any hazardous/other substance specified in Schedule E(1) of the Drugs and Cosmetics Rules 1945, introduction of rule regarding the maintenance of records of raw materials, guideline of permitted excipients along with their standards, law to test heavy metals, stipulation of expiry date for Ayurvedic medicines.42
In 1970, Pharmacopoeial Laboratory of Indian Medicine was formed to ensure standardization and testing of ASU drugs. Several others government recognized laboratories are also involved to lay down pharmacopoeial standards, preparation of monographs and Standard Operating Procedures (SOPs) for ASU drugs. Pharmacopoeial committees for ASU systems involve in the lay down of standards for quality, purity and strength of drugs and approve drug formularies. Pharmacopoeial Laboratories, Central Council for Research in Ayurveda and Siddha (CCRAS) laboratories, laboratories of Central Council for Research in Unani Medicine (CCRUM), Council for Scientific & Industrial Research (CSIR) laboratories and several other laboratories of private sector are involved in the mammoth job of controlling and maintaining of quality, formulation of standard for ensuring safety and quality of polyherbal/herbomineral preparations.17, 40, 41
5.4. Pharmacopeias and formularies
Both traditional and modern parameters are used to test the quality and to standardize the raw and finished products. Several methods like organoleptic standardization of drugs, chemical investigation, and bioassay are used in this regard. Ayurvedic Pharmacopoeia of India (Part I contain eight volumes and Part II contain three volume of compound formulation), Unani Pharmacopoeia of India (Part I on signal drug contain six volume, Part II on formulation contain two volume), Siddha Pharmacopoeia of India (volume I and II), Ayurvedic Formulary of India (Part I & II), Unani Formulary of India (Part I–VI), Siddha Formulary of India (Part I & II) are playing a significant role in this area. Pharmacognostical, chemical and standards of the plant drugs used in ISM are mentioned in such publications. These pharmacopoeias and formularies contain information about the biological source, synonyms, description, TLC, important formulation, therapeutic indication, and details related to identity, purity, strength. Recently, chromatographic fingerprint profile as a supplementary to Ayurvedic Pharmacopoeia was also published. Identification and estimation of active therapeutic ingredients and marker compounds with reference to which drugs of Ayurveda, Siddha and Unani can be standardized are still developing and all these parameters are being added to pharmacopoeias. Inclusion of monograph on herbal drug in Indian Pharmacopeia and formulation of Herbal Pharmacopeia are also a major step to achieve the goal. Along with pharmacopoeias and formularies other publications like ‘Production of ISM Drugs with Current Good Manufacturing Practices’, ‘Quality Standards of Indian Medicinal Plants’ could also be useful to maintain the standard and quality of ISM.37, 42, 43, 44
5.5. Research, industry, education and practice
Several research works are going on the ASU drugs. Basic research to preclinical or clinical study, investigation on standardization and formulation on ISM are a hot area of research in current time. Central Council for Research in Ayurvedic Sciences, Central Council for Research in Unani Medicine, Central Council for Research in Siddha, Central Council for Research in Yoga & Naturopathy, CSIR, Central Drug Research Institute (CDRI), several private research centre, institution and universities are actively engaged in research, development and promotion of traditional herbal medicine.
Nearly, 9000 manufacturing units of Indian Traditional Medicine are present in the India as on April 2013. Though the majority of them (7744) are involved in manufacturing of Ayurveda drugs, whereas, 485, 344 and 323 manufacturing units were engaging in manufacturing of Unani, Siddha and Homoeopathy drugs respectively. Statistics also suggest that only 0.1% per annum growth was observed realized in total AYUSH drug manufacturing units during last two decade. A recent statistics showed that among the 10,000 Ayurvedic, Siddha and Unani medicine manufacturing unit about 54% is GMP complying units.37, 45
CCIM was established under the Indian Medicine Central Council Act, 1970 and involve in the regulation of education and practice of ISM. A significant increase in AYUSH colleges/teaching institutions has been experienced in last two decade. In 2013, more than 500 AYUSH under graduate colleges with admission capacities more than 25,000 was recorded in India. Ayurveda have 261 under graduate colleges, whereas Homoeopathy, Siddha, Unani and Naturopathy have 188, 9, 41 and 17 under graduate colleges respectively. More than 125 post graduate colleges (Ayurveda 76, Homoeopathy 40, Siddha 3, Unani 8) with more than 2700 admission capacities were in existence in the India in 2013. National Institute of Ayurveda (Jaipur), National Institute of Naturopathy (Pune), Institute of Post Graduate Teaching and Research in Ayurveda (Jamnagar), National Institute of Unani Medicine (Bengaluru), National Institute of Siddha (Chennai) are some of the apex educational institute of traditional medicine in India. Several courses like Bachelor Degree Medicine and Surgery in different Indian System of Medicine, Doctor of Medicine (MD), Doctor of Surgery (MS), PG Diploma Courses, Diploma Citification Courses, PhD courses in several branches of ISM are being offered by the several institutions Certificate course, Currently Pharmacy Degree i.e. D. Pharm, B. Pharm, M. Pharm in Indian traditional medicine also started.40, 45, 46
It was counted more than 3100 AYUSH hospitals with 57,056 beds strength runs in India as on April 2013. Maximum number of hospitals are on Ayurveda (2408), whereas, 255, 267, 29, 201 and 7 hospitals pertain to Unani, Siddha, Naturopathy, Homoeopathy and Yoga systems respectively. More than 26,000 AYUSH dispensaries also deliver the primary healthcare facility around the country. Number of dispensaries in Ayurveda, Unani, Siddha, Yoga, Naturopathy, Homoeopathy and Sowa-Rigpa recorded as 15,927, 1483, 830, 140, 120, 7585 and 22, respectively. In 2013, 686,319 AYUSH registered practitioners (maximum 387,976 practitioners have been registered under Ayurveda System) were available which is increasing continuously. The number of Institutionally Qualified (IQ) registered practitioners has also been increased in last few years, in 2013 the total number of IQ registered practitioners was 461,032.40, 45
5.6. Traditional knowledge digital library (TKDL)
TKDL is an exclusive digital database regarding the medical knowledge mentioned in several ISM that are available in the public domain. The database consists the information about the medicinal plant, formulation of Ayurveda, Siddha, Unani, Yoga etc. TKDL is a collaborative project of CSIR and AYUSH, and the information's are also available in different national and international language. TKDL contain information of 500 Ayurvedic, 500 Unani and 200 Siddha formulations. The information from 150 Ayurvedic/Siddha/Unani books is incorporated in TKDL. The access to 2.5 lakh medicinal formulations different to Patent Offices under TKDL access agreement is considered as unique process to protect traditional knowledge from biopiracy. Based on the third party notes submitted by the TKDL, so far a large number of patent applications in European Patent Offices, Canadian Intellectual Property Office, United States Patent and Trademark Office, United Kingdom Patent and Trademark Office, Controller General of Patents, Designs and Trademarks, India has been either set aside, or withdrawn/cancelled, or declared dead.34
5.7. Promotion, gradual integration
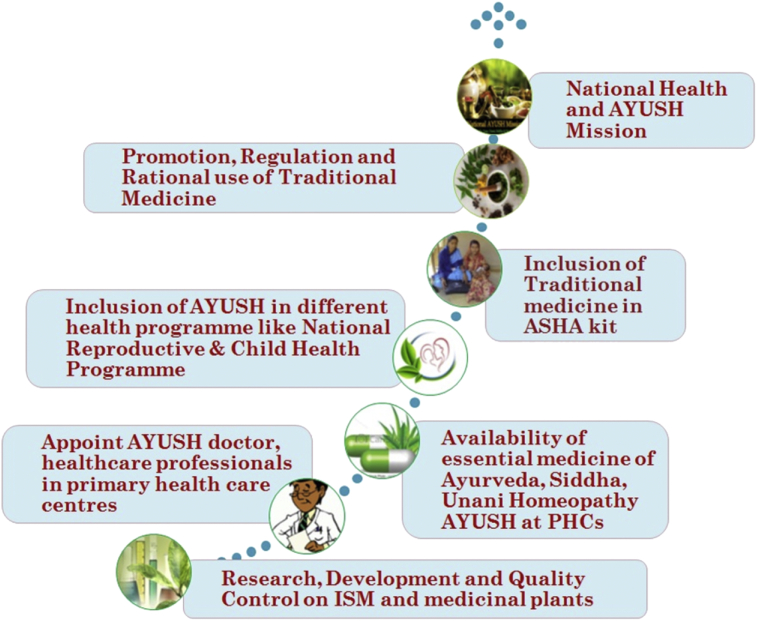
There has been a steady policy support to promote the traditional medicine in India. The government also supporting different plans related to research-development related to medicinal plant research. The budget allocation for the Dept of AYUSH has been increasing gradually over the years. In the 12th Five Year Plan of India (2012–2017), total allocation for AYUSH was INR. 10,044 crore, which was 235% more than the actual expenditure of 11th Plan.47 In the view of integrate the AYUSH system for healthcare system several policies are formulated,40, 48, 49 like:
-
•
Utilization of AYUSH doctors in National Reproductive & Child Health and Population stabilization programmes
-
•
Inclusion of several traditional drugs (i.e. Ayush Ghutti, Bal Rasayana, Soubhagya Shunthi, Ark Ajwain, Ark Pudina, Punarnavadi Mandoor and Ksheerbala Tel.) in the National Reproductive & Child Health (RCH) Programme for use by mothers & children.
-
•
A pilot project to monitor the effect of Ayurveda treatment in the ante-natal and post-natal care
-
•
Utilization of available facilities of ISM in rural health care mission (NRHM). Like, appointing Ayurveda doctors and paramedics in the primary healthcare delivery system and in National Health programs.
-
•
Inclusion of AYUSH drug (i.e. Punarnavadi mandoor for management of anaemia during pregnancy) kit of ASHA (ASHA or Accredited Social Health Activist act as an interface between the community and the public health system in rural India) in addition to generic drugs for common ailments at sub-centre/primary health centre/community health centre.
-
•
Ensure the availability of Ayurvedic, Siddha and Unani essential drug to primary health centre.
-
•
To find the way of inclusion of AYUSH medicine in schemes such as Janani Suraksha Yojana (JSY-AYUSH), ICDS-AYUSH, Reproductive Child Health (RCH), early breastfeeding, growth monitoring of children, ante and post natal care, etc. And find their effectiveness.
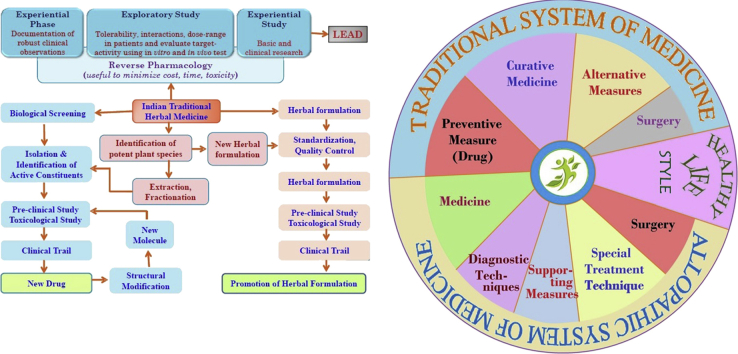
Recently, there are some suggestion to allow the AYUSH doctor to prescribe modern medicine if rural areas in view of shortage of allopathic doctors. But there is strong oppose as it may violate the rule present in India.50 Fig. 1 describes the strategy of integration of traditional herbal medicine in clinical practice.
Fig. 1.
Strategy in include traditional medicine in regular healthcare service in India.
Collaboration, establishment of research institute in foreign, conduct of seminar-conferences are the key way to promote the ISM globally. AYUSH opened an Indo-US joint Center for Research on Indian Systems of Medicine (CRISM) at the University of Mississippi USA in 2008. Ayurveda Education & Research, Gujarat Ayurveda University, Jamnagar singed a MoU with institutions in Japan, Australia, Netherlands, Argentina, Italy, and USA. Several other national institutions also have MoU with foreign institutions in the field of research and education in ISM. Institutions of India offering various courses for the students from Japan, Russia, South Africa, Netherlands, France, Hungary, Canada, USA, Poland, Germany, Brazil, Switzerland, Ukraine, Sri Lanka etc. related to Ayurveda. India and Russia had concluded a MoU on the cooperation in the field of Ayurveda teaching, treatment and research. In 2004, Russian-Indian Centre on Ayurvedic Research was established in Moscow.40, 51
5.8. Protection of biodiversity
Protection of biodiversity and conservation of medicinal plants is essential for the livelihood security and ensure the availability of medicinal plant in future. AYUSH drug manufacturing units use almost 90% of the raw materials of medicinal plants from natural forests, but during such process we overlook the environmental and social issues. Therefore, sustainable use of medicinal plants and good field collection practice is important. In situ (conservation of plants in their natural habitat outside the native habitat) and ex situ conservation (like, seed storage, DNA storage, pollen storage, in vitro conservation, field gene banks and botanical garden etc) are the key approaches to protect and conserve medicinal plant species.52, 53
6. Indian traditional medicine – revival story and globalization
In recent years there is a huge upserge in the use of traditional and complementary medicine around the glove. In Africa nearly 80% of population uses such medicine for their primary healthcare. In China, it was estimated that traditional herbal medicine account for 30–50% of the total medicinal consumption. Majority of the people (around 60%) uses traditional herbal drugs as a first line medicine for treatment high fever resulting from malaria in countries like Ghana, Mali, Nigeria and Zambia. In Australia about 48%, in Canada 70%, in Germany 80%, in USA 42%, in Belgium 39% and in France 76% of population uses traditional/complementary medicine at least once. Around 75% of the HIV positive/AIDS patients living in San Francisco, London and South Africa use traditional and complementary medicine. In Malaysia, people spent more on traditional medicine than allopathic drugs. Importance of herbal medicines in terms of healthcare provider and economy are growing steadily.53, 54, 55 Therefore, India has a great opportunity to promote ISM globally.
6.1. Ayurvedic drugs around the globe
Ayurveda is well recognized in Asian countries like Nepal, Sri Lanka, Bangladesh and other Asian countries. About 75% of 100 million subcontinental people are enjoying the benefits of Ayurveda. In Japan, Osaka Medical School has formed the Society of Ayurveda in 1969, and since last 40 years study, research and spread of Ayurveda is being carried out passionately. Ayurveda is also popular in Thailand, Myanmar. Education and practice of Ayurveda is flourishing in many states of USA. ISM mainly Ayurveda is increasing in Argentina, Brazil, Venezuela, Chile, Nicaragua, Costa Rica, Guatemala, Germany, Austria, Switzerland, France, Czech Republic, Greece, Israel. Countries like South Africa, UAE, Russia, Sweden, Indonesia, Netherlands, Italy, Spain, Australia, New Zealand, Hungary have acknowledged Ayurveda. Several other countries are on the verge of doing the same.56, 57
6.2. ISM, medicinal plants of India and economy
Approximately 25,000 effective plant-based formulations are available in ISM which is commonly used by rural and ethnic people n India and the popularity of such medicine is also increasing among the common people. It was also estimated that >2000 tons of medicinal plant raw material is required annually. More than 1500 herbals are also sold as dietary supplements or ethnic traditional medicines. It was also estimated that nearly 960 species of medicinal plants are in trade, among them 178 species have annual consumption levels more than 100 metric tones. Domestic trade of AYUSH industry is approximately INR. 80–90 billion, and export value of medicinal plants and related products from India is approximately l10 billion. In 2012–2013, the export of AYUSH products was INR. 24,741.2 crores, though in next financial year (2013–2014) it was reduced slightly. The percentage share of AYUSH products in the total trade of India in 2013–2014 was 0.36%. The global market for herbal drugs is increasing in steady manner and the global herbal trade will reach USD 7 trillion by 2050.8, 17, 58
6.3. Preventive and curative approaches of ISM
Ayurveda and other ISMs are involved in the curative and preventive measures to promote the health. Rasayana (rejuvenation therapy), branch of Ayurveda involve in the preservation and promotion of health by promoting longevity and also prevents or delays the ageing process, which another speciality of Ayurveda namely ‘Panchakarma’ (purification therapy) removes the toxins and waste materials from the body and thus purify the biological system to disease completely.10 Ayurvedic formulations are highly effective against various common diseases like common cold, fever, hyperacidity, ulcer, cough, gastro-intestinal problems, diarrhoea, amoebic dysentery, liver diseases, uterine bleeding, urinary tract infection, arthritic condition, gout, bronchial asthma, eye diseases etc. at the primary healthcare level. Formulation of Ayurveda also shown prominent effect in the treatment of several chronic diseases like cardiovascular disease (hypertension, angina, cardiomyopathies, myocardial infarction, congenital heart disease), cancer, dengue, anti-inflammatory disease, kidney diseases etc.59, 60, 61, 62, 63 Some studies have suggested that Ayurvedic medicine is also useful to manage some emergency conditions like severe diarrhoea and vomiting, patient suffering from typhoid suffered from semi consciousness and also muttering delirium, burns and seals, poison, threatened abortion, abortion & miscarriage etc.64 Medicine from Siddha system is used to cure diverse diseases like skin problems (psoriasis), sexual transmitted diseases, urinary tract infections, liver and gastro-intestinal diseases, diabetes, general debility, postpartum anaemia, diarrhoea, rheumatic diseases, prostate enlargement, bleeding piles, peptic ulcer, venereal diseases, fever, allergic disorders and general fevers other than emergency cases.65, 66 Unani drugs are used to treat hepatitis, gastroenteritis & uteritis, fever, cardiovascular problems, palpitation, nausea, vomiting, diarrhoea, gastro intestinal trouble, fever, insomnia, schizophrenia, epilepsy, gonorrhoea, urinary tract infection, kidney stone, headache, dizziness, common cold, migraine, colic pain, arthritis, syphilis, paralysis, diabetes insipidus, bad wetting, anxiety, typhoid fever, measles, small pox, premature ejaculation etc.67 ISMs are also found useful for care of HIV/AIDS patients.68 Medicinal plants found in India and utilized by the different folk and codified medicine are utilized to cure diverse diseases.
6.4. ISM – evidences for effectiveness
Effectiveness of ISM particularly Ayurveda has been evident in sub-continent as people enjoys the benefit of such medicine since the pre-biblical era. Core strength in these systems is the holistic approach to health and disease using natural substances derived from medicinal plants, minerals and animal sources.69 But in current situation proper clinical trial, quality assurance and pharmacogivilance are the important area to promote such drug worldwide. In recent year's national and international level, a lot of clinical studies are conducted to prove effectiveness of ISM. Increase research on medicinal plants and ISMs results raise in clinical studies. A number of collaboration in this sector has been increasingly attracting several entrepreneurs and organizations. AYUSH research portal (http://ayushportal.nic.in/) included information regarding 4175 clinical trial, 8514 pre-clinical research, 6002 drug research and 2926 fundamental research information related to ISM or plants used in these systems as on January 2016.70 Safety evolution and to find the mode of administration or use also a critical criteria of these study. For example, a study showed that polyherbal drug, AB-FN-02 having significant anti-osteoarthritic effect and non-toxic when administered in traditional method with milk, but it may produce toxic effect when administered as a drug otherwise. Indian Council of Medical Research database also contain information about folk medicinal plants. Information about 500 medicinal plants/formulations used by folk medicine practitioners (non-codified system) was included and currently scientific validation (safety, efficacy, mode of action and clinical trials) of few selected leads are going on different parts of India.71 Although it is true that proper high value clinical trial of such medicine is comparatively les due to small size of population, problems with research designs, lack of appropriate control groups etc.
6.5. Indian medicinal plants and drug discovery
Drug discovery from medicinal plants used in different Indian medicinal systems is a hot spot of research. A number of drugs were obtained from the plant sources and several others have discovered by using natural substance as lead. Investigations in India and abroad became played a key role in such research. In 1931, Sen and Bose reported two alkaloids from Rauwolfia serpentina, Siddiqui and Siddiqui in same year isolated five alkaloids which named as Ajmaline, Ajmalinine, Ajmalicine, Scrpentine, and Serpentinine. Chopra and his colleagues in 1933 isolate an alkaloid from the plant and observed the hypotensive and CNS depressant activity. Several others investigations had also been made by the Indian researchers in subsequent years. In 1949, a historical paper by Dr Vakil in British Heart Journal reported the antihypertensive activity of Rauwolfia in patients. During that time nearly 90% of doctors in India used it as a routine hypotensive drug and about 50 million tablets had been sold by a manufacturing agency alone.72, 73 Peruvoside, a cardiac glycoside was isolated from Thevetia peruviana at the Indian laboratory and developed in Germany.72 Taxol, potent anticancer drug discovered from Taxus brevifolia, the plant has been utilized by western Indian cultures as a medicine since long time.74
A national/international research discovered a number of drugs from plant which has been used Indian traditional medicine since ancient time, like, vasicine and vasicinone from Adhatoda vasica, bacosoids from Bacopa monnieri, tylophorine from Tylophora indica, homoharringtonine from Cephalotaxus, camptothecin from Camptotheca acuminate, conessine from Holarrhena antidysentrica, morphine and codeine from Papaver som-niferum, sarsasapogenin, asparanin A and asparanin B from Asparagus adscendens, shatavarin from Asparagus racemosus, atropine from Atropa belladonna, glycyrrhizin from Glycyrrhiza glabra, aloin from Aloe vera, protodioscin from Tribulus terrestris, sophoradin from Sophora subprostrata, quinine from Cinchona spp., trigonelline from Trigonella foenum-graecum, catechin from Acacia catechu, withanolides from Withania somnifera, tinosporic acid from Tinospora cordifolia, cocaine from Erythroxy-lum coca, aegelin and marmelosin from Aegle marmelos, pris-timerin from Celastrus paniculata, asiaticoside from Centella asiatica, emetine from Cephaelis ipecacuanha, psoralen from Psoralea corylifolia, glycyrrhizin from G. glabra, boeravinones from Boerrhavia diffusa, berberine from Berberis aristata, plumbagin from Plumbago indica, curcumin from Curcuma longa, podophyllin from Podophyllum emodi, jatamansone from Nardostachys jatamansi, quassinoids from Ailanthus spp., arjunolic acid from Terminalia arjuna, gingerols from Zingiber officinale, digoxin and digitoxin from Digitalis lantana, paclitaxel from Taxus baccata and Taxus brevifolia, allicin from Allium sativum, nimbidin from Azadirachta indica, forskolin from Coleus forskohlii, pilocarpine from Pilocarpus jaborandi, Dysobinin from Dysoxylum binectariferum, Diosgenin from T. foenum-graecum and plants of Dioscorea spp., vinblastine and vincristine from Catharanthus roseus and many more.17, 75, 76
Novel semisynthetic derivatives of rohitukine (from the plant Amoora rohituka & D. binectariferum) named as flavopiridol and P-276-00 are in the advance clinical trial as anticancer drug.17 Guggulu, an oleo-gum resin obtained from the bark of Commiphora wightii has been used in Ayurveda for the treatment of inflammation, gout, rheumatism, obesity, and disorders of lipids metabolism. Several compounds namely Z-guggulsterone, E-guggulsterone, guggulsterol-I, guggulsterol-II etc. have been isolated from guggulu.77 CSIR and its constituents laboratories are involved in the development of new herbal drug or formulation. Some of the key developments in Central Drug Research Institute (CDRI) in this area are, (i) standardized fraction of gugulipid was developed by CDRI and marketed (Guglip®, Cipla Ltd) as a drug for hyperlipidaemia and atherosclerosis, (ii) arteether (a semisynthetic derivative of artemisinin, the active constituent of Artemisia annua) as antimalarial drug which is marketed by Themis Chemicals Ltd., Mumbai under the trade name E-Mal, (iii) Consap (a local spermicidal cream) contain saponins from Sapindus mukorossi, (iv) picroliv, an iridoid glycoside mixture containing 60% picroside I and kutoside obtained from Picrorhiza kurroa developed as hepatoprotective agent, (v) a standardize herbal preparation derived from the plant B. monnieri as memory enhancer.78 RRL Jammu has commercialized Boswellia serrata gum resin as NSAID (non-steroidal anti-inflammatory drug) (Sallaki® Gufic).79 A number of herbal pain reliever, antifungal cream, anti-dandruff shampoo has been developed by different CSIR labs across the country. Very recently, an antidiabetic drug (BGR-34) has developed jointly developed by scientist of CSIR-NBRI & CSIR-CIMAP. Under the ‘Golden Triangle Partnership’ project between AYUSH, Indian Council of Medical Research (ICMR), CSIR there is an attempt to find few formulations and developing new drugs.
In recent years research on these areas is increasing and lot more drug/formulations investigated by public or private sector in India are in queue or in under clinical trial. India has great pool of diverse medicinal plant sources, a long and well characterized traditional medicinal system which makes India a unique place of new drug discovery.
6.6. Current science & technology in modernization of herbal medicine
Global pharmaceutical companies and researchers equipped with modern scientific knowledge, technology, idea and started to rediscover medicinal plants as a source of new drug candidates based on traditional knowledge.17, 20 Modern technology and techniques have revolutionized the progression of drug discovery from medicinal plants. New approaches/concepts/technologies became a key tool in the development of traditional medicine further. Research utilizing modern equipments or methods helped us to isolate and develop phytoconstituents as new drug present in traditional herbal formulation or medicinal plants. Modern technology and techniques became an essential tool to monitor and maintain the quality of traditional formulation, use phytochemical as lead to discover new drugs, to find pharmacokinetic profile and toxicity, to find out mechanism, to find the new use of existing drug or formulation, acceleration of drug discovery process, synthetic and semisynthetic process to manufacture a natural constituent etc.
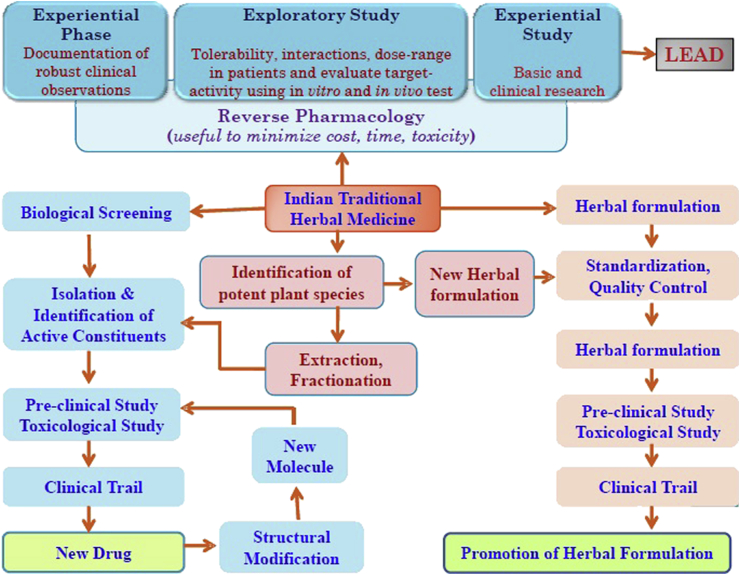
Ayurveda is one of the oldest and well documented health traditions in the world. Drug discovery based on traditional information is a key path towards the discovery of new drug. Reverse pharmacology is an approach where discovery of leads/formulations is based on the documented clinical experiences and scientific observations through series of studies. Reverse pharmacology based on traditional knowledge concentrate on the reversing routine ‘laboratory-to-clinic’ development to ‘clinics-to-laboratories’. Safety is considered as most significant point remains and the effectiveness becomes a matter of validation. This process is highly useful to find better and safer leads.80 Fig. 2 describes the opportunity of drug discovery from Indian System of Medicine.
Fig. 2.
Opportunity of drug discovery from Indian traditional herbal medicine.
7. Towards the achievement of universal healthcare
In 21st century, tremendous advances in healthcare sector coexist with inequities in accessibility, availability and affordability of the healthcare facilities in many parts of the world. Since last few decades, there is a growing interest in traditional medicine in all over the globe. Variety, flexibility, easy availability, religious/social acceptance, relative low side effect and cost became the key factor for the growth of traditional medicine.81 Of course, these also provide us the opportunity to integrate such medicine in primary healthcare to facilitate the people health. However, the path is not so easy. A number of approaches have been formulated toward the mainstreaming of traditional medicine in healthcare system. ‘Romantic approach’ suggested that traditional medicine is good as such and should be remained as it is, ‘trans-cultural and transdisciplinary synergy approach’ support the fact that sciences recognize that they stand for one type of knowledge among others and that information is always culturally entrenched and forming part of historic advance, ‘syncretic approach’ considered to be merging two system to form a new system, in ‘complementarity approach’ one system provides as supportive role to another.81 But evidence based approach to utilize both conventional as well as traditional medicine in conjunction could be the best option to provide healthcare facility to all.
A number of case studies or surveys had shown the importance of traditional medicine in primary healthcare service. A study in rural area of West Bengal shows that folk medicine play a key role to prevent common diseases likes small injuries, skin disease, fever, dehydration, diabetes, high BP, liver disease etc in better way.82 In rural areas of Meghalaya, indigenous medicine play significant role in primary healthcare for prevention/management of common ailments.83 Another study reported the efficacy of Ayurvedic multimodal management in osteoarthritis, the study also proposed that the Ayurvedic drugs can be an alternative of NSAIDs in such treatment.84 Beneficial effect of integration of Ayurveda with modern system of medicine in tertiary care hospital for management of osteoarthritis (knee) also investigated. Ayurvedic treatment proved effective with respect to reducing the symptoms, improving quality of life and reducing the side effect of allopathic pain killers.85 Ayurveda also can be integrated in care for cancer. It was also suggested that Ayurveda offers outstanding drugs and treatments which can be easily included along with the mainstream cancer medicines. Ayurvedic treatment is effective to reduce the side effects of chemotherapy and to improve general well-being of the patient.86 Herbal remedies are found most useful to manage simple healthcare problems like fever, diarrhoea, dysentery, upper respiratory tract infections, worm infestations, certain liver diseases, hepatitis, anaemia, arthritis, few gynaecological problem along with different communicable diseases such as malaria, HIV.86
In India, the ratio of the doctor-patient is 1:1700 if we consider only allopathic doctors, the ratio will come to 1:800 if the AYUSH practitioners are added. This is much better than the WHO recommendation of 1:1000. At present, an acute shortage of allopathic doctors exists in the India and in rural areas this problem is higher and with very less presence in remote areas. AYUSH practitioners however have a much wider presence in rural and remote areas.87
8. Conclusion
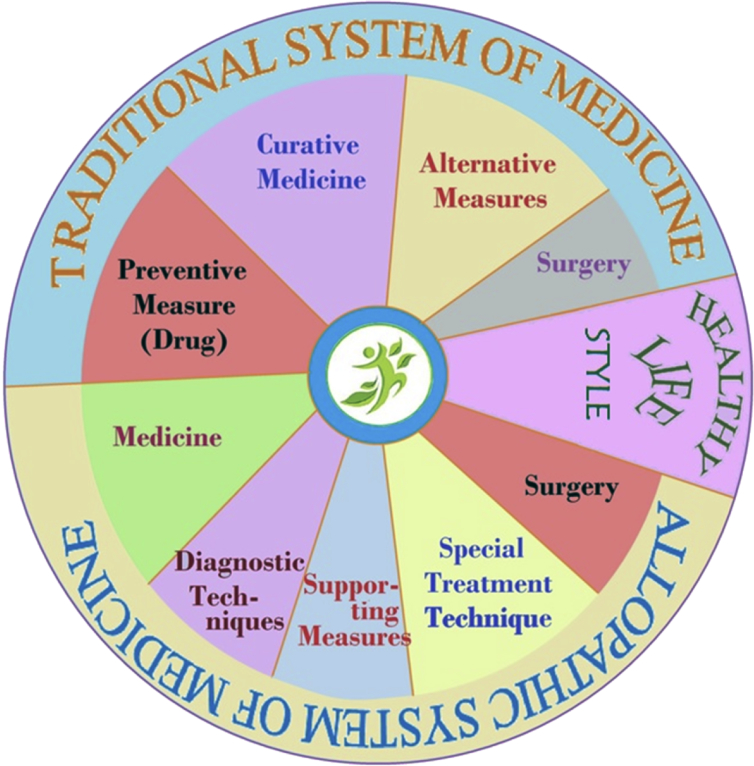
Traditional medicine particularly herbal medicine playing important role in maintain of health in rural and remote areas. Inclusion of traditional herbal medicine in clinical practice will help to achieve the target ‘health for all’ (Fig. 3). Indian traditional medicine like Ayurveda and others have sound scientific background of effectiveness and also acknowledged by the recent researches. Although efforts are needed to overcome barriers like irrational use, quality control and standardization issues, high pharmacovogilance etc. Stick implementation of rules, monitoring and periodic revision of regulations are absolute necessary to promote Indian traditional medicine. Overall, adequate knowledge about the system, high quality clinical trial, proper information about such drugs and their effectiveness among common people required towards the promotion of such medicine. Integration of Ayurvedic and others Indian traditional medicine in clinical practice will helpful to promote the health of the people who are unable to access the modern medicine properly. Utilization of such medicine along with conventional drug surly put more values to promote health or cure diseases in the better way. Therefore, mainstreaming of ISM along with allopathic drugs and healthy lifestyle will be helpful to provide healthcare service in best possible way to all people not only in India but around the globe.
Fig. 3.
Circle of life for achieving universal and quality life.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.van Herten L.M., van de Water H.P.A. New global health for all targets. Br Med J. 1999;319:700–703. doi: 10.1136/bmj.319.7211.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . World Health Organization; Geneva: 1978. Alma-Ata 1978. Primary Health Care. [Google Scholar]
- 3.Hegde B.M. Future medicare system. J Indian Acad Clin Med. 2003;4:92–95. [Google Scholar]
- 4.World Health Organization . World Health Organization; Geneva: 2004. The World Medicines Situation. [Google Scholar]
- 5.World Health Organization . World Health Organization; Geneva: 2013. WHO Traditional Medicine Strategy 2014–2023. [Google Scholar]
- 6.National Centre for Biological Sciences . 2015. Overview of Indian Healing Traditions.https://www.ncbs.res.in/HistoryScienceSociety/content/overview-indian-healing-traditions Accessed from: [Google Scholar]
- 7.Mafuva C., Marima-Matarira H.T. Towards professionalization of traditional medicine in Zimbabwe: a comparative analysis to the South African policy on traditional medicine and the Indian Ayurvedic system. Int J Herb Med. 2014;2:154–161. [Google Scholar]
- 8.Pandey M.M., Rastogi S., Rawat A.K.S. Indian traditional Ayurvedic system of medicine and nutritional supplementation. Evid Based Complement Altern Med. 2013;2013:1–12. doi: 10.1155/2013/376327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anonymous . Department of AYUSH, Ministry of Health & Family Welfare, Govt of India; New Delhi: 2012. Ayurveda the Science of Life. [Google Scholar]
- 10.Chaudhury R.R., Rafei U.M. World Health Organization (Regional Office for South-East Asia); New Delhi: 2001. Traditional Medicine in Asia. [Google Scholar]
- 11.Naswamy V. Origin and development of Ayurveda. Anc Sci Life. 1981;1:1–7. [PMC free article] [PubMed] [Google Scholar]
- 12.Subbarayappa B.V. The roots of ancient medicine: an historical outline. J Biosci. 2001;26:135–144. doi: 10.1007/BF02703637. [DOI] [PubMed] [Google Scholar]
- 13.Karunamoorthi K., Jegajeevanram K., Xavier J., Vijayalakshmi J., Melita L. Tamil traditional medicinal system – siddha: an indigenous health practice in the international perspectives. Tang. 2012;2:1–11. [Google Scholar]
- 14.Tiwari L. Siddha Medicine: Its Basic Concepts. Accessed from: http://www.infinityfoundation.com/mandala/t_es/t_es_tiwar_siddha.htm.
- 15.Subbarayappa B.V. Siddha medicine: an overview. Lancet. 1997;350:1841–1844. doi: 10.1016/s0140-6736(97)04223-2. [DOI] [PubMed] [Google Scholar]
- 16.Ansari AA. Global status of Unani Medicine. In the Proceeding of International Conclave on Traditional Medicine (16–17 November 2006), National Agriculture Science Complex, New Delhi. Available from: http://www.niscair.res.in/conclave/downloadables/Plenary%20Session%203/pdf/Ansari.pdf Accessed on 8 January 2015.
- 17.Sen S., Chakraborty R. Toward the integration and advancement of herbal medicine: a focus on Traditional Indian medicine. Bot Target Ther. 2015;5:33–44. [Google Scholar]
- 18.Siddiqi T. Unani medicine in India. Indian J Hist Sci. 1981;16:22–25. [PubMed] [Google Scholar]
- 19.Gurmet P. “Sowa-Rigpa”: Himalayan art of healing. Indian J Tradit Know. 2004;3:212–218. [Google Scholar]
- 20.Pan S., Litscher G., Gao S. Historical perspective of traditional indigenous medical practices: the current renaissance and conservation of herbal resources. Evid Based Complement Altern Med. 2014;2014:1–20. doi: 10.1155/2014/525340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sen S., Chakraborty R., De B., Ganesh T., Raghavendra H.G., Debnath S. Analgesic and inflammatory herbs: a potential source of modern medicine. Int J Pharma Sci Res. 2010;1:32–44. [Google Scholar]
- 22.Sen S., Chakraborty R., De B., Mazumder J. Plants and phytochemicals for peptic ulcer: an overview. Phcog Rev. 2009;3:270–279. [Google Scholar]
- 23.Sen S., Chakraborty R., De B. Challenges and opportunities in the advancement of herbal medicine: India's position and role in a global context. J Herb Med. 2011;1:7–75. [Google Scholar]
- 24.Wachtel-Galor S., Benzie I.F.F. Herbal medicine: an introduction to its history, usage, regulation, current trends, and research needs. In: Benzie I.F.F., Wachtel-Galor S., editors. Herbal Medicine: Biomolecular and Clinical Aspects. 2nd ed, CRC Press/Taylor & Francis; Boca Raton (FL): 2011. http://www.ncbi.nlm.nih.gov/books/NBK92773/ Accessed from: [PubMed] [Google Scholar]
- 25.Pan S., Zhou D., Gao S. New perspectives on how to discover drugs from herbal medicines: cam's outstanding contribution to modern therapeutics. Evid Based Complement Altern Med. 2013;2013:1–25. doi: 10.1155/2013/627375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Debnath P.K., Banerjee S., Debnath P., Mitra A., Mukherjee P.K. Ayurveda – opportunity for developing safe and effective treatment choice for the future. In: Mukherjee P.K., editor. Evidence-based Validation of Herbal Medicine. Elsevier Science Publishing Co Inc; Amsterdam: 2015. pp. 427–454. [Google Scholar]
- 27.Shankar D., Majumdar B. Beyond the biodiversity convention: the challenges facing the biocultural heritage of India's medicinal plants. In: Bodeker G., Bhat K.K.S., Burley J., Vantomme P., editors. Medicinal Plants for Forest Conservation and Health Care. Food and Agriculture Organization of the United Nations; Rome: 1997. pp. 87–99. [Google Scholar]
- 28.Sahoo N., Manchikanti P. Herbal drug regulation and commercialization: an Indian industry perspective. J Altern Complement Med. 2013;19:957–963. doi: 10.1089/acm.2012.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thillaivanan S., Samraj K. Challenges, constraints and opportunities in herbal medicines – a review. Int J Herb Med. 2014;2:21–24. [Google Scholar]
- 30.Booker A., Johnston D., Heinrich M. Value chains of herbal medicines—Research needs and key challenges in the context of ethnopharmacology. J Ethnopharmacol. 2012;140:624–633. doi: 10.1016/j.jep.2012.01.039. [DOI] [PubMed] [Google Scholar]
- 31.Silva T.D. Industrial utilization of medicinal plants in developing countries. In: Bodeker G., Bhat K.K.S., Burley J., Vantomme P., editors. Medicinal Plants for Forest Conservation and Health Care. Food and Agriculture Organization of the United Nations; Rome: 1997. pp. 34–44. [Google Scholar]
- 32.Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol. 2013;4:177. doi: 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bandaranayake W.M. Quality control, screening, toxicity and regulations of herbal drugs. In: Ahmad I., Aquil F., Owais M., editors. Modern Phytomedicine: Turning Medicinal Plants into Drugs. Wiley-VCH Verlag GmbH & Co; Germany: 2006. pp. 25–27. [Google Scholar]
- 34.Sen S., Chakraborty R. Traditional knowledge digital library: a distinctive approach to protect and promote Indian indigenous medicinal treasure. Curr Sci. 2014;106:1340–1343. [Google Scholar]
- 35.Joshi K. 2008. Indian Herbal Sector.http://www.nistads.res.in/indiasnt2008/t4industry/t4ind19.htm Accessed from: [Google Scholar]
- 36.Mangathayaru K. Pearson; Chennai: 2013. Pharmacognosy: An Indian Perspective. [Google Scholar]
- 37.Anonymous . 2015. AYUSH, About the System.http://www.indianmedicine.nic.in Accessed from: [Google Scholar]
- 38.Anonymous . 1945. Amendments and Notifications Drugs and Cosmetics Rules.www.drugscontrol.org/amendments.asp?act=Drugs%20and%20Cosmetics%20Rules,%201945 Accessed from: [Google Scholar]
- 39.World Health Organization . WHO; Geneva: 2005. National Policy on Traditional Medicine and Regulation of Herbal Medicines – Report of a WHO Global Survey. [Google Scholar]
- 40.Sharma S.K., Katoch D.C. 2006. Current Status & Infrastructure of Ayurveda.http://herbalnet.healthrepository.org/bitstream/123456789/2075/6/3.%20Ayur53-65.pdf Accessed from: [Google Scholar]
- 41.Ministry of Health and Family Welfare, Govt. of India . 2006. Regulation of Manufacture and Sale of ASU Drugs.http://www.pib.nic.in/newsite/erelcontent.aspx?relid=14876 Accessed from: [Google Scholar]
- 42.Mandal S.C., Mandal M. Quality, safety, and efficacy of herbal products through regulatory harmonization. Drug Info J. 2011;45:45–53. [Google Scholar]
- 43.Pharmacopoeial Laboratory for Indian Medicine . 2015. Publications.http://www.plimism.nic.in/index.html Accessed from: [Google Scholar]
- 44.Central Council for Research in Unani Medicine . 2015. Unani Pharmacopeial Committee.http://www.ccrum.net/research/upc/ Accessed from: [Google Scholar]
- 45.Anonymous . 2015. Section 1: Summary of All-India AYUSH Infrastructure Facilities.http://indianmedicine.nic.in/writereaddata/linkimages/0913923524-Summary.pdf Accessed from: [Google Scholar]
- 46.Ministry of AYUSH . 2015. AYUSH for Holistic Healthcare & Healthy Living.http://www.indianmedicine.nic.in Accessed from: [Google Scholar]
- 47.Sharkar M. 2013. Ayush Dept Under-utilises Funds Allocated during Last Three Years.http://www.pharmabiz.com/NewsDetails.aspx?aid=77870&sid=1 Accessed from: [Google Scholar]
- 48.Health Division Planning Commission Government of India . 2015. Report of the Steering Committee on AYUSH for 12th Five Year Plan (2012–17)http://planningcommission.gov.in/aboutus/committee/strgrp12/st_ayush0903.pdf Accessed from: [Google Scholar]
- 49.Jain A.K., Sharma B.K. Developments in the field of Ayurveda – past to present. Ayushdhara. 2014;1:51–64. [Google Scholar]
- 50.Pillai AMP, Aggarwal KK. Ayush Cannot Prescribe Modern Medicine Drugs. Accessed from: www.ima-india.org/ima/left-side-bar.php?scid=245.
- 51.Center for Research on Indian Systems of Medicine . 2015. About Us.http://www.crism.net/about_us.htm Accessed from: [Google Scholar]
- 52.Kasagana V.N., Karumuri S.S. Conservation of medicinal plants (past, present & future trends) J Pharm Sci Res. 2011;3:1378–1386. [Google Scholar]
- 53.National Medicinal Plants Board . 2015. Standard for Good Field Collection Practices of Medicinal Plants [DOC: NMPB-GFCP-01(FD)]http://www.nmpb.nic.in/WriteReadData/links/9695357829Standard%20for%20Good%20Field%20Collection%20Practices.pdf Accessed from: [Google Scholar]
- 54.World Health Organization . World Health Organization; Geneva: 2002. WHO Traditional Medicine Strategy 2002–2005. [Google Scholar]
- 55.World Health Organization . 2013. Traditional Medicine.http://www.who.int/mediacentre/factsheets/2003/fs134/en/ Accessed from: [Google Scholar]
- 56.Patel P. 2003. Global Resurgence and International Recognition of Ayurveda.http://iaf-ngo.org/pdf/GLOBAL%20RESURGENCE%20&%20INTL.%20RECOGNITION%20OF%20AYURVEDA%20(No.%2010).pdf Accessed from: [Google Scholar]
- 57.Patel P. Globalisation of Ayurveda – A global vision for next decade. Accessed from: http://iaf-ngo.org/pdf/Globalisation%20of%20Ayurveda%20-%20a%20global%20vision%20for%20next%20decade%20(No.1).pdf.
- 58.Anonymous . 2014. Section: AYUSH Related Foreign Trade.http://www.indianmedicine.nic.in/writereaddata/linkimages/0145351269-AYUSH_related_foreign_trade.pdf Accessed from: [Google Scholar]
- 59.Lokhande P.D., Jagdale S.C., Chabukswar A.R. Natural remedies for heart diseases. Indian J Trad Know. 2006;5:420–427. [Google Scholar]
- 60.Ganesh B.M., Tarkeshwar S.W., Dattatraya S.L., Surajsingh L.T. To study the effects of ayurvedic treatment in cases of mootraghata and chronic renal failure. Unique J Ayurvedi Herb Med. 2014;2:1–5. [Google Scholar]
- 61.Aggarwal B.B., Prasad S., Reuter S. Identification of novel anti-inflammatory agents from Ayurvedic medicine for prevention of chronic diseases: “reverse pharmacology” and “bedside to bench” approach. Curr Drug Targets. 2011;12:1595–1653. doi: 10.2174/138945011798109464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aggarwal B.B., Ichikawa H., Garodia P. From traditional Ayurvedic medicine to modern medicine: identification of therapeutic targets for suppression of inflammation and cancer. Expert Opin Ther Targets. 2006;10:87–118. doi: 10.1517/14728222.10.1.87. [DOI] [PubMed] [Google Scholar]
- 63.Ram Manohar P. Papaya, dengue fever and Ayurveda. Anc Sci Life. 2013;32:131–133. doi: 10.4103/0257-7941.122994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shukla N. Role of Ayurveda in emergency treatment. Emerg Med. 2012;2:8. [Google Scholar]
- 65.Yadav V., Jayalakshmi S., Singla R.K. Traditional systems of medicine- now & forever. WebmedCentral Pharma Sci. 2012;3:WMC003299. [Google Scholar]
- 66.Anonymous . 2015. Siddha.https://www.karnataka.gov.in/ayush/Siddha%20system.pdf Accessed from: [Google Scholar]
- 67.Anonymous . 2015. Classical Unani Products.http://hashmi-herbal.com/classical-unani-products.html Accessed from: [Google Scholar]
- 68.Mandhare P.M., Garje B.H., Khade A.A. Importance of herbal remedies in the treatment of HIV & TB infection. Int J Sci Res Methdol. 2015;1:1–17. [Google Scholar]
- 69.Chopra A., Saluja M., Tillu G. Ayurveda–modern medicine interface: a critical appraisal of studies of Ayurvedic medicines to treat osteoarthritis and rheumatoid arthritis. J Ayurveda Integr Med. 2010;1:190–198. doi: 10.4103/0975-9476.72620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.AYUSH research portal. 2016. Accessed from: http://ayushportal.nic.in/default.aspx.
- 71.Indian Council of Medical Research . 2016. Highlights of ICMR Activities & Achievements.http://www.icmr.nic.in/About_Us/ICMR_Achievements.html#TraditionalMedicine Research__ Accessed from: [Google Scholar]
- 72.Singh H. Medicinal chemistry research in India. Indian J Hist Sci. 2014;49:413–423. [Google Scholar]
- 73.Isharwal S., Gupta S. Rustom Jal Vakil – his contributions to cardiology. Tex Heart Inst J. 2006;33:61–170. [PMC free article] [PubMed] [Google Scholar]
- 74.Atanasov A.G., Waltenberger B., Pferschy-Wenzig E. Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotechnol Adv. 2015;33:1582–1614. doi: 10.1016/j.biotechadv.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sharma C., Rajendar K., Kumari T., Arya K.R. Indian traditional therapies and bio-prospecting: their role in drug development research. Int J Pharma Sci Res. 2014;5:730–741. [Google Scholar]
- 76.Mehrotra N.N., Ojha S.K., Tandon S. Drug development for cardiovascular diseases from ayurvedic plants. Curr R&D Highlights. 2007:1–16. [Google Scholar]
- 77.Anurekha J., Gupta V.B. Chemistry and pharmacological profile of guggul – a review. Indian J Trad Know. 2006;5:478–483. [Google Scholar]
- 78.Central Drug Research Institute . 2016. New Drugs.http://www.cdriindia.org/newdrugs.htm Accessed from: [Google Scholar]
- 79.Patwardhan B., Warude D., Pushpangadan P., Bhatt N. Ayurveda and traditional Chinese medicine: a comparative overview. Evid Based Complement Altern Med. 2005;2:465–473. doi: 10.1093/ecam/neh140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Patwardhan B., Mashelkar R.A. Traditional medicine-inspired approaches to drug discovery: can Ayurveda show the way forward? Drug Discov Today. 2009;14:804–811. doi: 10.1016/j.drudis.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 81.Payyappallimana U. Role of traditional medicine in primary health care: an overview of perspectives and challenges. Yokohama J Soc Sci. 2010;14:57–77. [Google Scholar]
- 82.Tapan R. Role of folk medicine in primary health care: a case study on West Bengal, India. Int J Interdiscip Multidiscip Stud. 2014;1:13–18. [Google Scholar]
- 83.Albert S., Porter J. Is ‘mainstreaming AYUSH’ the right policy for Meghalaya, northeast India? BMC Complement Altern Med. 2015;15:288. doi: 10.1186/s12906-015-0818-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sharma M.R., Mehta C.S., DShukla D.J., Patel K.B., Patel M.V., Gupta S.N. Multimodal ayurvedic management for Sandhigatavata (osteoarthritis of knee joints) Ayu. 2013;34:49–55. doi: 10.4103/0974-8520.115447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bhatt S., Gupta V., Srikanth N. CCRS, WHO India Country Office Collaborative Study: Technical Report CCRAS, Dept. of AYUSH, Ministry of H and FW, Govt. of India; New Delhi: 2007. Feasibility of Integrating Ayurveda with Modern System of Medicine in a Tertiary Care Hospital for Management of Osteoarthritis (Knee): An Operational Study. [Google Scholar]
- 86.Buch Z.M. Role of Ayurveda in integrated cancer rehabilitation: Ayurvaid's integrated cancer rehabilitation program (ICRP) – a role model. Int Ayurvedic Med J. 2014;2:233–238. [Google Scholar]
- 87.Roy V. Time to sensitize medical graduates to the Indian Systems of Medicine and Homeopathy. Indian J Pharmacol. 2015;47:1–3. doi: 10.4103/0253-7613.150301. [DOI] [PMC free article] [PubMed] [Google Scholar]