Abstract
Pseudomonas sp. strain ADP is the model strain for studying bacterial degradation of the s-triazine herbicide atrazine. In this work, we focused on the expression of the atzDEF operon, involved in mineralization of the central intermediate of the pathway, cyanuric acid. Expression analysis of atzD-lacZ fusions in Pseudomonas sp. strain ADP and Pseudomonas putida showed that atzDEF is subjected to dual regulation in response to nitrogen limitation and cyanuric acid. The gene adjacent to atzD, orf99 (renamed here atzR), encoding a LysR-like regulator, was found to be required for both responses. Expression of atzR-lacZ was induced by nitrogen limitation and repressed by AtzR. Nitrogen regulation of atzD-lacZ and atzR-lacZ expression was dependent on the alternative σ factor σN and NtrC, suggesting that the cyanuric acid degradation operon may be subject to general nitrogen control. However, while atzR is transcribed from a σN-dependent promoter, atzDEF transcription appears to be driven from a σ70-type promoter. Expression of atzR from a heterologous promoter revealed that although NtrC regulation of atzD-lacZ requires the AtzR protein, it is not the indirect result of NtrC-activated AtzR synthesis. We propose that expression of the cyanuric acid degradation operon atzDEF is controlled by means of a complex regulatory circuit in which AtzR is the main activator. AtzR activity is in turn modulated by the presence of cyanuric acid and by a nitrogen limitation signal transduced by the Ntr system.
Pseudomonas sp. strain ADP is the best-characterized bacterial strain capable of degrading the S-triazine herbicide atrazine (2-chloro-4-ethylamino-6-isopropylamino-1,3,5-triazine). The atrazine catabolic pathway of Pseudomonas sp. strain ADP proceeds through six enzymatic steps encoded by the atzA, atzB, and atzC genes and the atzDEF operon. All six genes are harbored on the catabolic plasmid pADP-1 (34), and atzA, atzB, and atzC have been shown to be widespread and plasmid-borne in a number of isolates from different parts of the world (10, 11, 40, 47, 49). The products of the atzA, atzB, and atzC genes are responsible for hydrolytic removal of the chlorine, isopropylamine, and ethylamine residues to yield cyanuric acid (2,4,6-trihydroxy-1,3,5-triazine), a central intermediate in the catabolism of atrazine and other s-triazines. The atzDEF operon encodes cyanuric acid amidohydrolase, biuret hydrolase, and allophanate hydrolase activities, involved in cleavage of the s-triazine ring and its conversion to carbon dioxide and ammonium, which is assimilated as a nitrogen source (reviewed by Wackett et al. [49].
We recently described that atrazine catabolism in Pseudomonas sp. strain ADP is induced under nitrogen-limited growth in a manner reminiscent of general nitrogen control as described for the enterobacteria (20). The atzA, atzB, and atzC genes are unlikely to be targets for nitrogen regulation because sequence analysis, Northern blot hybridization, and real-time reverse transcription-PCR suggest that they are constitutively expressed (13, 34). The atzDEF operon is transcribed divergently from orf99, predicted to encode a LysR-type transcriptional regulator (LTTR) (see Schell [42] for a review of LTTRs). Furthermore, a putative LTTR binding site can be found upstream of atzD, suggesting that expression of the atzDEF operon may be regulated and the product of orf99 may have a role in this regulation (34).
General nitrogen control is a global regulatory network that activates expression of a number of genes involved in assimilation of alternative nitrogen sources in response to decreased nitrogen availability (for a review, see Merrick and Edwards [35]). General nitrogen control has been thoroughly characterized in the enterobacteria and involves five different proteins, the products of the glnB, glnD, ntrB, ntrC, and rpoN genes. NtrB and NtrC are the sensor and regulatory elements of a two-component system, the latter being a transcriptional activator and the former being involved in phosphorylation of NtrC, which is essential for its regulatory activity. The product of rpoN, σN, is an alternative σ factor. NtrC specifically activates expression from promoters that are transcribed by RNA polymerase loaded with σN. The glnB and glnD genes encode a small protein named PII and a uridylyltransferase-uridylyl-removing enzyme, both of which are involved in modulation of the activity of NtrB in response to nitrogen status.
Little is known about general nitrogen control in the genus Pseudomonas. However, a number of indications suggest that it may share some features with the system in the enterobacteria. Homologs to all five genes are present in the sequenced genomes of Pseudomonas putida KT2440, Pseudomonas aeruginosa PAO1, and Pseudomonas syringae pv. tomato DC3000 (7, 37, 45). In addition, rpoN mutants of the former two organisms have been characterized, and they are impaired in utilization of a number of nitrogen sources (28, 48). Other P. aeruginosa mutants with pleiotropic defects in nitrogen assimilation carried mutations linked to the glnA gene, suggesting the involvement of ntrB and ntrC, which are located in the vicinity of glnA (25, 26). However, a two-component system different from NtrB-NtrC, named CbrA-CbrB, has been found to mediate regulation in response to altered carbon-nitrogen balance in a σN-dependent fashion in P. aeruginosa (38). This indicates that not all nitrogen control is channeled through NtrB-NtrC in this organism. Finally, a recent paper reports NtrC-dependent regulation of nitrogen fixation in Pseudomonas stutzeri (12).
In this work, we have explored regulation of the expression of the atzDEF operon both in its natural host strain, Pseudomonas sp. strain ADP, and in the model Pseudomonas strain P. putida KT2440. Our results strongly suggest that the product of orf99, renamed here atzR, is the master regulatory element of a complex circuit involving σN- and NtrC-mediated general nitrogen control and cyanuric acid as a specific inducer.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains used in this work and their relevant genotypes are summarized in Table 1. Mineral medium was routinely used for gene expression analysis (33). Sodium succinate (25 mM) was used as a carbon source. The nitrogen sources for Pseudomonas sp. strain ADP derivatives were ammonium chloride, l-serine, cyanuric acid, or atrazine. The final concentration was 3 mM nitrogen, except for atrazine, which was added at a saturating concentration (≈30 mg liter−1) from a reservoir as described (20). The nitrogen sources for P. putida KT2440 derivatives were ammonium chloride and l-serine (1 g liter−1). Cyanuric acid, biuret, or atrazine (0.1 to 1 mM) was added when required as inducers of the atz genes. Luria-Bertani (LB) medium was used as rich medium. Liquid cultures were grown in tubes or flasks with shaking (180 to 200 rpm) at 30 or 37°C (for Pseudomonas and Escherichia coli strains, respectively). For solid media, Bacto-Agar (Difco, Detroit, Mich.) was added to a final concentration of 18 g liter−1.
TABLE 1.
Bacterial strains and plasmids used in this work
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| Strains | ||
| E. coli DH5α | φ80dlacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17(rK− mK+) supE44 thi-1 gyrA relA1 | 22 |
| P. putida KT2440 rpoN | mt-2 hsdR1(r− m+) rpoN::Km | 28 |
| P. putida KT2442 | mt-2 hsdR1(r− m+) Rifr | 19 |
| P. putida MPO201 | mt-2 hsdR1(r− m+) Rifr ΔntrC::Tc | This work |
| Pseudomonas sp. strain ADP | Wild type, Atr+ | 32 |
| Plasmids | ||
| pBBR1MCS-4 | Broad-host-range cloning vector, Apr Tra− Mob+ | 29 |
| pBBR1MCS-4Δplac | pBBR1MCS-4 derivative lacking the plac promoter | This work |
| pBluescript II KS(+) | Multicopy cloning vector, Apr | Stratagene |
| pBluescript II SK(+) | Multicopy cloning vector, Apr | Stratagene |
| pJB861 | Expression vector containing xylS and the Pm promoter, Kmr Tra− Mob+ IncP | 5 |
| pJES379 | lacZ translational fusion vector, Apr | E. Santero, unpublished data |
| pKNG101 | Cloning vector for chromosomal insertion, Strr Sacs Tra− Mob+oriR6K | 27 |
| pKT230 | Broad-host-range cloning vector, Kmr Strr Tra− Mob+ IncQ | 2 |
| pMPO103 | 1.45-kbp fragment containing atzR and the 5′ end of atzD, cloned in pBluescript II SK(+), Apr | This work |
| pMPO104 | atzR-lacZ protein fusion in pMPO200, Apr | This work |
| pMPO108 | 630-bp fragment containing the 5′ ends of atzR and atzD cloned in pBluescript II KS(+), Apr | This work |
| pMPO109 | atzR coding sequence and promoter region cloned in pKT230, Kmr | This work |
| pMPO124 | atzD-lacZ protein fusion in pMPO200 bearing the PatzR-mut promoter derivative | This work |
| pMPO126 | atzR-lacZ protein fusion in pMPO200 bearing the PatzD-mut promoter derivative | This work |
| pMPO200 | Broad-host-range lacZ protein fusion vector based on pBBR1MCS-4, Apr | This work |
| pMPO202 | atzD-lacZ protein fusion in pMPO200, Apr | This work |
| pMPO204 | atzD-lacZ protein fusion in pMPO200 containing atzR, Apr | This work |
| pMPO210 | atzR expressed from the Pm promoter in pJB861, Kmr Tra− Mob+ IncP | This work |
| pMPO213 | Tcr cassette flanked by sequences upstream and downstream from P. putida ntrC in pBluescript II KS(+), Apr | This work |
| pMPO215 | Cointegrate of pMPO213 with pKNG101, Smr Tcr Sucs Tra− Mob+ | This work |
| pRK2013 | Helper plasmid in conjugation, Kmr Tra+ | 15 |
Antibiotics and other additions were used, when required, at the following concentrations: ampicillin (only in E. coli strains), 100 mg liter−1; kanamycin, 20 mg liter−1; streptomycin, 25 mg liter−1; tetracycline, 10 mg liter−1; carbenicillin (used as a substitute for ampicillin in Pseudomonas strains), 500 mg liter−1; rifampin, 10 mg liter−1; and 5-bromo-4-chloro-3-indoyl-β-d-galactopyranoside (X-Gal), 25 mg liter−1. All reagents were purchased from Sigma-Aldrich-Riedel de Haën, except technical grade atrazine (>98% purity), which was a kind gift from Novartis, now Syngenta (Greensboro, N.C.).
Construction of plasmids and strains.
The plasmids used in this work are summarized in Table 1. All DNA manipulations were performed according to standard procedures (41). Restriction and modification enzymes were purchased from Amersham Biosciences (Piscataway, N.J.). The Klenow fragment or T4 DNA polymerase was routinely used to fill in recessed 3′ ends and trim protruding 3′ ends of incompatible restriction sites. Plasmid DNA preparation and purification kits were purchased from Amersham Biosciences (Piscataway, N.J.) or Macherey-Nagel (Düren, Germany) and used according to the manufacturer's specifications. Plasmid DNA was transferred to E. coli and Pseudomonas strains by transformation (24) or by triparental mating (14). E. coli DH5α was used as a host in all cloning procedures.
The broad-host-range lacZ translational fusion vector pMPO200 was constructed to take advantage of the replication and mobilization functions of pBBR1MCS-4 (29) and the 5′-truncated lacZ reporter gene from pJES379. This is a derivative of pMC1403 (8) bearing a BstBI-SalI deletion that eliminates the lacY and lacA genes (E. Santero, unpublished data). First, pBBR1MCS-4 was digested with HindIII and SphI, blunt ended, and self-ligated, to yield pBBR1MCS-4Δplac. The lacZ gene from pJES379 was then cloned as a 3.5-kbp SalI-EcoRI fragment into NaeI- and EcoRI-cleaved pBBR1MCS-4Δplac, yielding pMPO200. Cloning at the unique EcoRI, SmaI, and BamHI sites of pMPO200 can be used to generate in-frame fusions to the eighth codon of lacZ as described for pMC1403 (8).
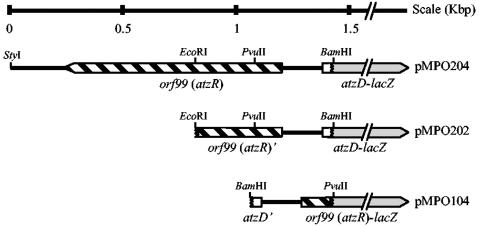
For consistency with Table 1, the new gene designation atzR will be used instead of orf99 throughout. A fragment containing the first 48 bp of atzD, the atzD-atzR intergenic region, and the complete atzR (positions 99501 to 101107 of the published pADP-1 sequence, accession number U66917) was PCR amplified with oligonucleotides AtzD1 (GAGCAGGGTGAGACAGGCGGTGC) and AtzD2 (CGATGGATCCCCAGGGCTGTGGC) (italics indicate mismatches to the original sequence to create a BamHI site). The 1.5-kbp PCR product was cleaved with StyI and BamHI and cloned into EcoRV- and BamHI-digested pBluescript II SK(+), producing pMPO103. The correctness of the DNA sequence of the PCR-amplified fragment was verified by sequencing. A 630-bp EcoRI-BamHI fragment from pMPO103 containing the first 48 bp of atzD, the atzD-atzR intergenic region, and 383 bp of atzR was subcloned in EcoRI- and BamHI-digested pBluescript II KS(+) to yield pMPO108. A 1.45-kbp SalI-BamHI fragment containing the complete insert in pMPO103 was cloned into SmaI- and BamHI-digested pMPO200 to yield the atzD-lacZ in-frame translational fusion plasmid pMPO204 (Fig. 1).
FIG. 1.
Structure of lacZ fusions to atzR (orf99) and atzD. The restriction sites used in cloning procedures are shown. The regulatory gene is labeled with the original designation orf99 as well as with the new gene name atzR. Plasmid names are indicated on the right.
To construct pMPO202 (Fig. 1), a 0.6-kbp EcoRI-BamHI fragment of pMPO103, bearing the 5′ end of atzD, the intergenic region, and about one-third of the atzR coding region was cloned into EcoRI- and BamHI-digested pMPO200. The atzR-lacZ fusion plasmid pMPO104 (Fig. 1) was the result of cloning a 0.4-kbp blunt-ended PvuII-BamHI fragment from pMPO103, containing the 5′ ends of atzR and atzD and the atzR-atzD intergenic region, into the unique SmaI site of pMPO200.
Two more plasmids were constructed that harbored atzR to be provided in trans to the fusion-bearing constructs. Plasmid pMPO109 contains the complete insert in pMPO103, including the complete atzR coding and promoter regions, as an XhoI-SacI fragment cloned between the unique EcoRI and SacI sites in pKT230. To construct plasmid pMPO210, pMPO103 was cleaved with SphI and KpnI. A 1-kbp fragment containing the atzR coding sequence minus the start codon was cloned between the AflIII and KpnI sites in pJB861. The resulting plasmid expresses atzR from the benzoic acid-responsive Pm promoter of the meta pathway for catechol utilization present on the P. putida TOL plasmid (39).
A Pseudomonas putida KT2442 ΔntrC::Tc mutant derivative was constructed by gene replacement with the suicide vector pKNG101 (27). First, a 1.5-kbp PCR fragment containing sequences upstream from ntrC was generated with oligonucleotides NtrC1 (TCGTGGGCCCGCTGGAATACGACC) and NtrC2 (GCCGAATTCCTGACGTGCCAGGCG). This fragment is flanked by ApaI and EcoRI restriction sites (italics). A second 1.5-kbp PCR fragment containing sequences downstream from ntrC was generated with oligonucleotides NtrC3 (CCGAATTCACAGGATCACCTCTGCCCCC) and NtrC4 (GGCTACTAGTCGGCCTGATGCCTCATCA). This fragment is flanked by EcoRI and SpeI restriction sites (italics).
Plasmid pUTminiTn5-Tc was digested with EcoRI, and a 2.2-kbp fragment containing the tetracycline resistance cassette of the mini-Tn5 transposon was isolated. A four-fragment ligation was set up that contained the two PCR products digested at the flanking restriction sites, the 2.2-kbp tetracycline resistance fragment, and ApaI- and SpeI-digested pBluescript II KS(+). One transformant that harbored the tetracycline resistance marker flanked by the two PCR products in the correct orientation in pBluescript II KS(+) was selected. The resulting plasmid was named pMPO213. Finally, pMPO213 was digested with ScaI and cointegrated with SpeI-digested pKNG101 to yield pMPO215. This plasmid was transferred to P. putida KT2442 by triparental mating (14), and plasmid integration was selected on LB medium containing streptomycin, tetracycline and rifampin.
Merodiploids were resolved as previously described (31) by plating on LB medium containing 10% sucrose. Sucrose-resistant (Sucr) colonies were scored for tetracycline and streptomycin resistance. PCR analysis confirmed that Sucr Tcr Strs clones had replaced the wild-type ntrC gene with the disrupted copy. One such mutant, named MPO201, was selected for further study. MPO201 showed slower growth than the wild type on glutamine, urea, arginine, and alanine as sole nitrogen sources (V. García-González, unpublished results).
Site-directed mutagenesis.
Site-directed mutagenesis by overlap extension with PCR was performed essentially as described (23). Mutagenic oligonucleotides PatzR-fwd (GGCACCGATCTTCAATTGACTCGCATG) and PatzR-rev (CATGCGAGTCAATTGAAGATCGGTGCC) (positions altered from the original sequence in italics) were used to modify the putative atzR promoter, while PatzD-fwd (TTCGTTCAAGCTCCACGCCGCCCTGC) and PatzD-rev (GCAGGGCGGCGTGGAGCTTGAACGAA) (positions altered from the original sequence in italics) were used to modify the putative atzDEF promoter. Plasmid pMPO108 was the template for amplification. Independent PCRs were performed with each mutagenic primer and an external oligonucleotide (universal forward or reverse primer) annealing to vector sequences. The products obtained were gel purified, allowed to anneal by the 26- or 27-bp overlap generated by the mutagenic oligonucleotides, and then used as templates for a new PCR with only the external primers. The PCR product containing the modified atzR promoter was digested with SphI and HindIII and cloned into SphI- and HindIII-digested pMPO104 to replace the wild-type promoter fragment, yielding pMPO126. The same strategy was used for the PCR product containing the modified atzDEF promoter, but pMPO202 was used instead, and the resulting plasmid was named pMPO124. The presence of the mutations in the final constructs and absence of additional alterations was verified by commercial sequencing (Sistemas Genómicos, Valencia, Spain).
β-Galactosidase assays.
β-Galactosidase assays were used to examine the expression of atzD-lacZ and atzR-lacZ fusions in Pseudomonas sp. strain ADP and P. putida KT2442 and its derivatives. Preinocula of bacterial strains harboring the relevant plasmids were grown to saturation in mineral medium under nitrogen-sufficient conditions (ammonium chloride, 1 g liter−1). Cells were diluted in mineral medium containing the appropriate nitrogen source (ammonium chloride, cyanuric acid, l-serine, or atrazine for Pseudomonas sp. strain ADP; ammonium chloride or l-serine for P. putida). Cyanuric acid was added when required as an inducer in the non-atrazine-metabolizing P. putida strains. Atrazine and biuret were also tested as inducers in P. putida KT2442. Diluted cultures were shaken for 8 to 16 h to mid-exponential phase (optical density at 600 nm = 0.25 to 0.5). Growth was then stopped, and β-galactosidase activity was determined from sodium dodecyl sulfate- and chloroform-permeabilized cells as previously described (36).
To assay the effect of σN on atzDEF and atzR expression, P. putida KT2442 and P. putida KT2440 rpoN::Km harboring pMPO104 or pMPO204 were grown in mineral medium with glucose (2 g liter−1) and glutamine (1 g liter−1) to an optical density at 600 nm of 0.3. Cells were washed three times with phosphate-buffered saline solution (60 mM sodium-potassium phosphate, 0.5 g of NaCl liter−1, pH 7.0), and resuspended in nitrogen-free mineral medium. Cyanuric acid was added as an inducer when required. Incubation was continued, and expression was monitored as the time course of β-galactosidase activity after transfer to nitrogen starvation conditions.
RNA preparation and primer extension.
Total RNA from Pseudomonas sp. strain ADP or P. putida KT2442 bearing pMPO202 was prepared from cultures grown as described above for β-galactosidase assays. Aliquots (2 ml) were centrifuged for 1 min in a microcentrifuge at top speed at 4°C. Pellets were flash-frozen in liquid nitrogen and stored at −70°C until processed. RNA was prepared by the guanidine thiocyanate method with a commercial reagent (Tripure isolation reagent; Roche Diagnostics, Indianapolis, Ind.), according to the manufacturer's instructions. Primer extension reactions were performed as previously described (21), with 20 or 50 μg of RNA (for detection of atzD and atzR, respectively) as a template, 32P-end-labeled oligonucleotides atzR-PE2 (GAAGAAAGCGTAAATGTTGCATAGGTGGTC) and atzD-PE1 (AGGGCTGTGGCAAGGGATTCGGAAAACGTC), and Superscript II reverse transcriptase (Invitrogen, Carlsbad, Calif.). Sequencing reactions with the same primers and pMPO108 as a template were performed with the Thermo Sequenase cycle sequencing kit (USB, Cleveland, Ohio), according to the manufacturer's instructions. Samples were run on polyacrylamide sequencing cells. Dried gels were exposed to radiosensitive screens, which were subsequently scanned in a Typhoon 9410 scanner (Amersham Biosciences, Piscataway, N.J.).
Sequence analysis.
Sequence comparison was performed with the BLAST package (1), available at the NCBI web page (http://www.ncbi.nlm.nih.gov/BLAST). Similarity to σN-dependent promoters was determined with the promscan.pl Perl script (46), with a scoring matrix derived from a compilation of σN-dependent promoters (3). This tool is available at http://www.promscan.uklinux.net.
RESULTS
The atzDEF operon is regulated by cyanuric acid and nitrogen availability in Pseudomonas sp. strain ADP.
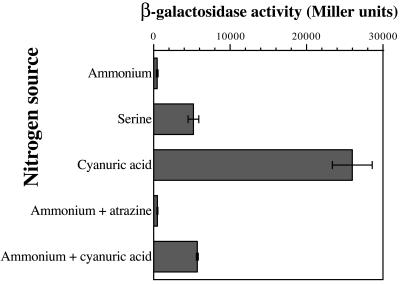
In order to characterize the expression of the atzDEF operon in its natural host, Pseudomonas sp. strain ADP, the broad-host-range atzD-lacZ fusion plasmid pMPO202 was constructed. Plasmid pMPO202 harbors a 630-bp insert, including the first 16 codons of atzD fused in frame to lacZ, the 199-bp intergenic region, and 383 bp of the adjacent gene, orf99 (Fig. 1). Triparental mating was used to transfer pMPO202 to Pseudomonas sp. strain ADP, and expression of the atzD-lacZ fusion was monitored in cells grown on different nitrogen sources by means of β-galactosidase assays. In addition to cyanuric acid and atrazine, which require expression of the atzDEF operon for their catabolism, ammonium was chosen to represent nitrogen-sufficient conditions, while serine was chosen as a growth-limiting nitrogen source (20).
Expression of atzD-lacZ was low when Pseudomonas sp. strain ADP was grown on ammonium as the sole nitrogen source (Fig. 2). The β-galactosidase activity levels were considerably increased (11-fold) in serine-grown cells, suggesting that the atzDEF operon may be subject to regulation dependent on nitrogen availability, as previously observed for atrazine degradation (20). Expression was stimulated further (56-fold) when cyanuric acid was the sole nitrogen source. This result cannot be explained solely by nitrogen limitation due to poor assimilation of cyanuric acid, since Pseudomonas sp. strain ADP grows noticeably more slowly on serine than it does on cyanuric acid (20). In addition, cyanuric acid also stimulated atzD-lacZ expression (12-fold) when added to medium containing ammonium, while atrazine failed to modify the low expression levels obtained under these conditions. Unfortunately, assays could not be performed on cells grown on atrazine as the sole nitrogen source because transconjugants of Pseudomonas sp. strain ADP harboring pMPO202 failed to grow in liquid medium with atrazine as the sole nitrogen source, despite the fact that they formed colonies surrounded by clear halos on agar plates with the same medium composition. Taken together, these results suggest that expression of the atzDEF operon is subject to dual regulation involving two distinct signals, the nitrogen status of the cell and the presence of the substrate of the pathway, cyanuric acid, as an inducer. In consequence, atzDEF expression may be activated by nitrogen limitation in the absence of cyanuric acid or by cyanuric acid under nitrogen-sufficient conditions, but maximum activity may only be achieved when cyanuric acid is present and no better nitrogen source is available.
FIG. 2.
Expression of an atzD-lacZ translational fusion in Pseudomonas sp. strain ADP. β-Galactosidase activity from Pseudomonas sp. strain ADP/pMPO202 grown in mineral medium containing the indicated nitrogen sources are shown. Values are the averages of at least three independent measurements. Error bars denote the standard deviation of the mean.
Dual regulation of atzDEF expression in P. putida KT2440 requires the product of orf99.
Pseudomonas sp. strain ADP is not amenable to genetic studies. Conjugation and electrotransformation occur with low efficiency, making insertion mutagenesis impracticable, and plasmids are often unstable or severely retard growth under selective conditions (V. García-González and F. Govantes, unpublished observations). To avoid these inconveniences, we sought to reproduce the regulation of the atzDEF operon in a heterologous background. P. putida KT2440 was chosen for this purpose because it is a widely used model Pseudomonas strain for which a variety of genetic tools are available (2, 43) and its genome sequence was recently made available (37).
The gene adjacent to atzD, orf99, encodes a putative regulatory protein of the LysR family. Since LTTRs usually activate the expression of genes located next to their own coding sequence and transcribed divergently from them (42), it was hypothesized that the product of orf99 may be involved in regulation of the atzDEF operon (34). In order to test this idea, a second atzD-lacZ fusion plasmid was constructed. Plasmid pMPO204 is similar in structure to pMPO202, but its 1,436-bp insert spans the complete coding region of orf99 (Fig. 1). Plasmids pMPO204 (atzR-atzD-lacZ), pMPO202 (atzD-lacZ), and the control vector pMPO200 were introduced into P. putida KT2442, a Rifr derivative of P. putida KT2440, by mating, and β-galactosidase activity was determined in cultures grown on ammonium (nitrogen-sufficient conditions) or serine (nitrogen-limiting conditions) as the sole nitrogen source. Since P. putida KT2442 cannot metabolize cyanuric acid, the effect of cyanuric acid on atzD-lacZ expression was determined in cells grown on medium containing 0.1 mM cyanuric acid in addition to the corresponding nitrogen source.
The β-galactosidase activity levels measured in P. putida KT2442 cells bearing the control plasmid pMPO200 were negligible under all culture conditions tested (Table 2). The low expression of the atzR-atzD-lacZ fusion in ammonium-grown cells harboring pMPO204 was slightly stimulated (twofold) by the presence of cyanuric acid. Activity levels were substantially higher (21-fold) when serine was the sole nitrogen source, and addition of cyanuric acid to this medium resulted in a final 93-fold increase over the levels obtained with ammonium. These results indicate that the atzDEF operon is also under dual control in P. putida KT2442, requiring both nitrogen limitation and the presence of cyanuric acid for maximum induction. Interestingly, expression of the atzD-lacZ fusion in pMPO202 was low under all conditions tested (Table 2). Nitrogen control and cyanuric acid-dependent regulation were recovered when a second plasmid that bears orf99 transcribed from its own promoter region (pMPO109) was introduced, while no effect was observed with the vector control plasmid (pKT230). Thus, orf99 appears to encode a positive regulatory function absolutely required for regulation of the atzDEF operon in response to both nitrogen limitation and cyanuric acid. To reflect this regulatory role, we renamed it atzR, for atrazine metabolism regulation.
TABLE 2.
Expression of atzD-lacZ and atzR-lacZ fusions in P. putida KT2442a
| Fusion plasmid | Structure | Other plasmid | Avg β-galactosidase activity (Miller units) ± SD (fold induction) with nitrogen source:
|
|||
|---|---|---|---|---|---|---|
| Ammonium
|
Serine
|
|||||
| Without cyanuric acid | With cyanuric acid | Without cyanuric acid | With cyanuric acid | |||
| pMPO200 | Empty vector | <1 (NA) | <1 (NA) | <1 (NA) | <1 (NA) | |
| pMPO204 | atzR-atzD-lacZ | 457 ± 17 (1) | 1,120 ± 31 (2) | 9,480 ± 407 (21) | 42,400 ± 3,320 (93) | |
| pMPO202 | atzD-lacZ | 167 ± 32 (1) | 165 ± 33 (1) | 180 ± 21 (1) | 192 ± 52 (1) | |
| pKT230 (empty vector) | 164 ± 27 (1) | 189 ± 36 (1) | 260 ± 42 (2) | 293 ± 17 (2) | ||
| pMPO109 (PatzR-atzR) | 292 ± 55 (1) | 652 ± 119 (2) | 9,070 ± 1,440 (31) | 39,100 ± 3,320 (134) | ||
| pMPO104 | atzR-lacZ | 8 ± 2 (1) | 7 ± 2 (1) | 637 ± 94 (80) | 721 ± 150 (90) | |
| pKT230 (empty vector) | 12 ± 2 (1) | 13 ± 3 (1) | 543 ± 137 (45) | 564 ± 105 (47) | ||
| pMPO109 (PatzR-atzR) | 13 ± 6 (1) | 15 ± 6 (1) | 81 ± 11 (6) | 46 ± 9 (4) | ||
Values are the average ± standard deviation of at least three independent measurements. Numbers in parentheses indicate induction compared to the same strain grown on ammonium as the sole nitrogen source in the absence of cyanuric acid. NA, not applicable.
The effects of atrazine and biuret as possible inducers of the atzDEF operon were also tested in P. putida KT2442. Neither of them stimulated expression of the atzR-atzD-lacZ fusion in pMPO204-bearing cells grown on ammonium or serine when added at the same concentration used above for cyanuric acid (0.1 mM), suggesting that cyanuric acid is most likely the physiological inducer of the pathway. However, a clear increase in activity (three- to fourfold) was observed when a higher concentration of biuret (1 mM) was used under nitrogen limitation. Thus, biuret may to some extent mimic the inducer effect of cyanuric acid (data not shown). It was also noted that a higher concentration of cyanuric acid (1 mM) stimulated atzD-lacZ expression further in the presence of ammonium (data not shown). This is consistent with the greater induction observed with Pseudomonas sp. strain ADP grown in these conditions (Fig. 2), since the cyanuric acid concentration was also 1 mM in that experiment.
Expression of atzR is under nitrogen control and autoregulated.
Since the atzR gene product appears to be required for both nitrogen limitation- and cyanuric acid-mediated regulation of atzDEF, we considered the possibility that atzR expression is also controlled by the same signals. To test this hypothesis, an atzR-lacZ protein fusion plasmid (pMPO104) was constructed and transferred to P. putida KT2442. Plasmid pMPO104 contains the first 16 codons of atzD, the complete intergenic region, and the first 47 codons of atzR fused in frame to lacZ in pMPO200 (Fig. 1). Expression of this fusion was assayed under the same culture conditions described above for the atzD-lacZ and atzR-atzD-lacZ fusions (Table 2).
Expression of atzR-lacZ was low when cells harboring pMPO104 (atzR-lacZ) were grown on ammonium as the sole nitrogen source. A great increase in β-galactosidase activity (80-fold) was observed when cells were grown on serine, suggesting that expression of atzR is also subject to nitrogen control. The presence of cyanuric acid did not significantly alter the β-galactosidase activity levels regardless of the nitrogen source used. Nitrogen regulation of atzR expression did not require the AtzR protein, since a functional copy of atzR is not present in pMPO104.
To investigate whether AtzR synthesis is also autoregulated, plasmid pMPO109 (see above) was also introduced into the fusion-bearing strain. Production of AtzR in trans from pMPO109 resulted in decreased β-galactosidase levels in serine-grown cells. Expression was lowered regardless of the presence of cyanuric acid, but the effect was somewhat more prominent when cyanuric acid was present in the medium. Low expression levels were again obtained when ammonium was the nitrogen source (Table 2). The vector control (pKT230) failed to alter expression under any conditions tested. These results suggest that AtzR negatively regulates its own synthesis, even in the absence of cyanuric acid. This regulatory mechanism appears to operate to keep AtzR levels low, as described for other LTTRs (42). As a consequence, the effect of nitrogen limitation on the expression of atzR-lacZ is reduced to four- to sixfold.
Nitrogen control of atzDEF expression is not the consequence of nitrogen-regulated synthesis of AtzR.
The results described above are consistent with a regulatory model in which atzDEF expression is controlled by a regulatory cascade. According to this model, nitrogen limitation would promote the synthesis of AtzR, which would in turn activate atzDEF expression in the presence of the specific inducer, cyanuric acid. As a consequence, nitrogen control of atzDEF expression would be indirect, its target being the atzR gene.
To analyze whether this is indeed the case, a plasmid was designed in which the atzR gene is transcribed from a foreign promoter, thus disconnecting AtzR synthesis from nitrogen control. Plasmid pMPO210 is based on the expression vector pJB861 (5) and harbors atzR stripped of its upstream sequences and expressed from the P. putida Pm promoter for the meta pathway of xylene catabolism. Plasmids pMPO210 and pJB861 were transferred to P. putida KT2442 harboring pMPO202 or pMPO104, and their effects on atzD-lacZ and atzR-lacZ expression were determined by β-galactosidase assays under the conditions described above. Addition of an inducer of the Pm promoter (3-methylbenzoic acid) to the growth medium provoked a decrease in the growth rate, while the β-galactosidase activity levels were largely unaffected (data not shown). Therefore, no inducer was used in these assays, and atzR expression was that provided by the basal transcription of the Pm promoter (50).
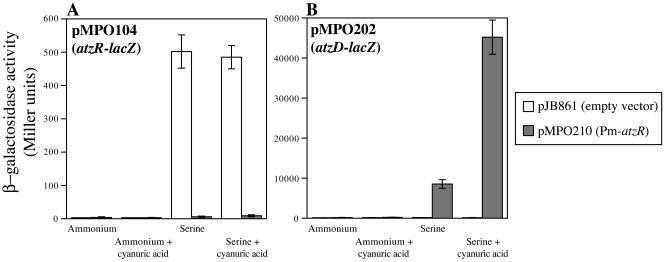
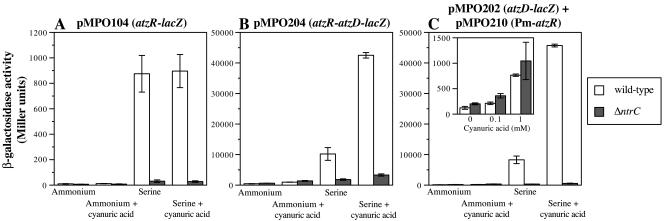
Unregulated synthesis of AtzR from pMPO210 resulted in very low activity of the atzR-lacZ fusion under all conditions (Fig. 3A), indicating that basal expression from the Pm promoter provides sufficient AtzR to shut down atzR expression almost completely. Interestingly, pMPO210 restored both nitrogen and cyanuric acid control of the otherwise unresponsive atzD-lacZ fusion in pMPO202 (Fig. 3B). Expression was induced to levels remarkably similar to those measured for pMPO204 (which harbors a nitrogen-regulated atzR gene), but basal levels in ammonium-grown cells were somewhat (three- to fivefold) decreased. The control plasmid pJB861 did not alter expression of atzD-lacZ or atzR-lacZ significantly. The fact that normal regulation of atzDEF expression is achieved when AtzR synthesis is not nitrogen controlled rules out the simple cascade model described above and indicates that a second mechanism in addition to regulation of AtzR synthesis operates to exert nitrogen control of atzDEF expression.
FIG. 3.
Effect of nitrogen-independent production of AtzR on the expression of atzR-lacZ and atzD-lacZ fusions. β-Galactosidase activity from P. putida KT2442 bearing the atzR-lacZ fusion plasmid pMPO104 (panel A) or the atzD-lacZ fusion plasmid pMPO202 (panel B) is shown. The growth medium was mineral medium containing ammonium or serine as the sole nitrogen source, with or without 0.1 mM cyanuric acid, as indicated. Solid bars indicate the activity levels obtained with atzR transcribed from the Pm promoter in pMPO210. Open bars indicate the activity levels obtained with the control vector pJB861, lacking atzR. Values are the averages of at least three independent measurements. Error bars denote the standard deviation of the mean.
Nitrogen control of atzR and atzDEF expression requires σN.
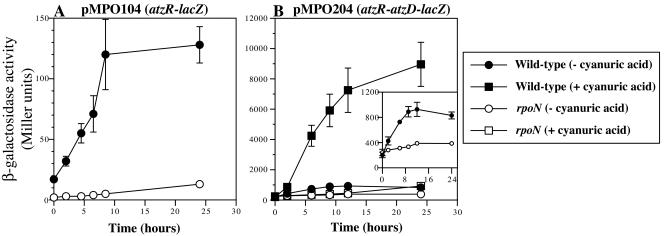
An rpoN derivative of P. putida KT2440 has been isolated and characterized, and it is impaired for growth on a number of nitrogen sources (28), suggesting that regulatory mechanisms involved in general nitrogen control analogous to those in the enteric bacteria may be active in P. putida. To assess whether σN is involved in the expression of the atzDEF and atzR genes, plasmids pMPO104 (atzR-lacZ) and pMPO204 (atzR-atzD-lacZ) were transferred to the rpoN mutant, and expression was compared to that in the wild-type strain under nitrogen limitation. Since the rpoN mutant fails to grow on nitrogen sources that require σN for their assimilation (28), an experimental design was used that provides comparable nitrogen starvation to both strains. To achieve this, cells of the wild-type and rpoN strains harboring the appropriate fusion plasmids were grown under nitrogen-sufficient conditions (glutamine, 1 g liter−1), harvested, washed, and transferred to nitrogen-free minimal medium (see Materials and Methods for details). Cyanuric acid (0.1 mM) was added to some of the pMPO204-bearing cultures as an inducer. Expression of the atzR-lacZ and atzR-atzD-lacZ fusions was subsequently monitored as the time course of β-galactosidase activity accumulation (Fig. 4).
FIG. 4.
Effect of an rpoN mutation on the expression of atzR-lacZ and atzD-lacZ fusions. The time course of β-galactosidase activity from P. putida wild-type and rpoN strains bearing the atzR-lacZ fusion plasmid pMPO104 (panel A) or the atzD-lacZ fusion plasmid pMPO204 (panel B) after a shift to nitrogen-free medium containing (circles) or lacking (squares) 0.1 mM cyanuric acid at time zero is shown. Solid symbols, activity levels obtained with P. putida KT2442. Open symbols, activity levels obtained with P. putida KT2440 rpoN::Km. The inset displays the curves in panel B from cultures not containing cyanuric acid rescaled for better viewing. Values shown are the averages of three independent measurements. Error bars denote the standard error of the mean.
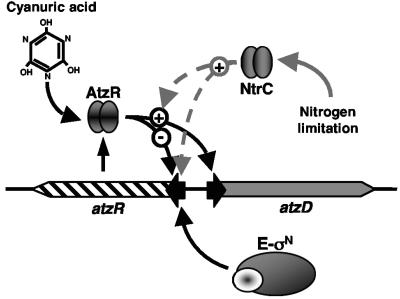
Expression of atzR-lacZ in the wild-type background was elevated after the shift to nitrogen-free medium, to reach an eightfold increase after 8 h (Fig. 4A). The initial β-galactosidase activity levels were very low in the rpoN strain, suggesting that σN is required for atzR expression even under nitrogen sufficiency. Activity accumulated very slowly with time, remaining 10- to 24-fold below that in the wild-type strain throughout the experiment. The fact that the rpoN mutation severely affected atzR expression under both nitrogen excess (initial conditions) and nitrogen limitation suggests that transcription of atzR is likely to be driven from a σN-dependent promoter. A sharp increase in the atzD-lacZ expression levels was observed after transfer of KT2442 harboring pMPO204 (atzR-atzD-lacZ) to nitrogen-free medium containing cyanuric acid (Fig. 4B). β-Galactosidase accumulation during the course of the experiment resulted in 65-fold-higher activity levels after 24 h. When tested in the rpoN strain under the same conditions, expression was not significantly altered during the first 12 h, and only at 12 to 24 h was a modest increase (sevenfold final induction) observed. Moderate induction (fivefold after 24 h) was also observed in the wild-type but not in the rpoN strain in the absence of cyanuric acid (Fig. 4B, inset). Unlike the atzR-lacZ fusion, basal expression of the atzR-atzD-lacZ fusion was not affected by the rpoN mutation. Taken together, these results indicate that σN is required for full activation of the atzDEF operon under nitrogen starvation conditions but not for basal expression during nitrogen-sufficient growth. This suggests that atzDEF may not be transcribed from a σN-dependent promoter, and the effect of the rpoN mutation may be due to the strict requirement of σN for the synthesis of the cognate activator AtzR (see Fig. 7 and Discussion).
FIG. 7.
Working regulatory model for atzR and atzDEF. The diagram displays the regulatory regions of atzR and atzDEF and the regulatory signals and factors unveiled in the present study. Dashed lines for NtrC regulation indicate a possible indirect effect. The dimeric structures of AtzR and NtrC are tentative and not based on experimental evidence. The figure is not drawn to scale.
Identification of the atzR and atzDEF promoters.
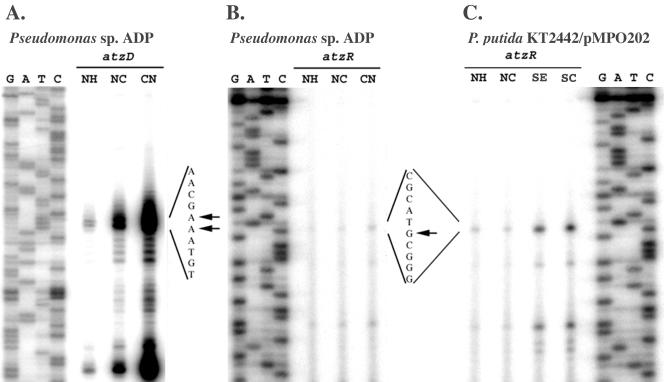
Primer extension analysis was performed in order to map the 5′ end of the atzDEF transcript in the natural host of the atz genes, Pseudomonas sp. strain ADP. An oligonucleotide (atzD-PE1) annealing to positions +13 to +42 was chosen as a primer for reverse transcription. The results are shown in Fig. 5A. Extension from atzD-PE1 with total RNA from Pseudomonas sp. strain ADP as a template produced two bands, corresponding to transcripts initiating at positions −107 and −108 from the atzD start codon, and an additional band initiating at position −77. The intensity of these bands was low when RNA from ammonium-grown cells was used, intermediate with RNA from cultures containing ammonium and cyanuric acid, and maximal when cells were grown in the presence of cyanuric acid as the sole nitrogen source. Additional lower-intensity bands appeared in the samples from the two latter conditions, which may represent processed or degraded forms of the transcript, although the possibility that these are bona fide transcriptional start sites cannot be formally excluded (but see below).
FIG. 5.
Primer extension of atzDEF and atzR transcripts. Total RNA from Pseudomonas sp. strain ADP was prepared from cultures grown on ammonium (NH), ammonium plus cyanuric acid (NC), or cyanuric acid (CN) as nitrogen sources (panels A and B). Total RNA from P. putida KT2442 bearing pMPO202 was prepared from cultures grown on ammonium (NH), ammonium plus 0.1 mM cyanuric acid (NC), serine (SE), or serine plus 0.1 mM cyanuric acid (SC) (panel C). Primer extension was performed with an end-labeled atzD-specific (panel A) or an atzR-specific (panels B and C) oligonucleotide. GATC lanes denote sequencing reactions performed on the atzR-atzD intergenic region with the same oligonucleotides. A 10-nucleotide stretch of the sequence around the transcriptional start (coding strand) is shown for each gene, and arrows indicate the mapped 5′ ends of the transcripts.
In order to map the 5′ end of the atzR transcript, primer extension was also performed with oligonucleotide atzR-PE2, which anneals at positions +59 to +78 from the atzR start codon (Fig. 5B). Two very weak bands were obtained, corresponding to positions +3 and +16 from the annotated atzR translational start. No significant differences in intensity were observed between cells grown on ammonium, ammonium and cyanuric acid, or cyanuric acid, consistent with the fact that AtzR-mediated autorepression results in very low atzR expression.
To confirm the location of the transcriptional start for this transcript, primer extension was also performed with RNA from P. putida KT2442 bearing pMPO202. Since this strain does not produce a functional AtzR protein, expression from the atzR promoter is expected to increase greatly under nitrogen limitation. Primer extension reactions from this strain revealed bands corresponding to the two positions already mapped in Pseudomonas sp. strain ADP as well as additional weaker bands (Fig. 5C). The intensities of these extension products matched the expected regulatory pattern, i.e., expression was increased under nitrogen limitation and unresponsive to cyanuric acid. Again, although the simplest explanation is that the faster-migrating bands correspond to processed or degraded forms of the transcript starting at +3, the presence of multiple 5′ ends cannot be excluded (but see below).
Since the primer extension experiments showed several putative transcriptional start sites for both atzR and atzDEF, we attempted to identify possible promoters upstream from the identified sites. Sequence inspection revealed regions resembling bacterial promoter motifs immediately upstream from the identified 5′ ends. A region with the sequence CAGTCA-N17-TAAGCT, bearing some similarity to the −35 and −10 σ70 promoter consensus sequence TTGACA-N15-18-TATAAT, was found at positions −143 to −115 from the atzD start codon. A nearly perfect match to the −24/−12 σN-dependent promoter consensus (TGGCAC-N5-TTGC) (3), with the sequence CGGCAC-N5-TTGC, is located at positions −25 to −11 from the annotated atzR start codon. A highly significant score of 87 (out of 100) was obtained when this region was tested for similarity to σN-dependent promoters with the promscan.pl Perl script (46).
In order to determine whether these sequences are required for atzDEF and atzR expression, point mutations were introduced at critical positions of the putative promoter sequences and fused to lacZ. The atzD-lacZ fusion plasmid pMPO124 is identical to pMPO202, but the TA dinucleotide at positions 1 and 2 of the −10 hexamer was changed to GG. Plasmid pMPO126 harbors an atzR-lacZ fusion identical to that of pMPO104, but the sequence GCT at positions −12 to −10, including the conserved GC dinucleotide at the −12 box, was replaced with CAA. The fusions to the mutant promoters were designated PatzD-mut and PatzR-mut, respectively. Expression from these constructs in P. putida KT2442 was determined by means of β-galactosidase assays as described above (Table 3).
TABLE 3.
Expression of atzD-lacZ and atzR-lacZ fusions bearing mutations at the putative promoter sequences in P. putida KT2442a
| Fusion plasmid | Structure | Other plasmid | Avg β-galactosidase activity (Miller units) ± SD in nitrogen source:
|
|||
|---|---|---|---|---|---|---|
| Ammonium
|
Serine
|
|||||
| Without cyanuric acid | With cyanuric acid | Without cyanuric acid | With cyanuric acid | |||
| pMPO202 | atzD-lacZ | None | 139 ± 25 | 131 ± 16 | 194 ± 18 | 197 ± 23 |
| pMPO210 (Pm-atzR) | 137 ± 15 | 260 ± 48 | 7,150 ± 1,110 | 36,600 ± 9,630 | ||
| pMPO124 | PatzD-mut | None | 1 ± 0 | 1 ± 0 | 3 ± 0 | 3 ± 0 |
| pMPO210 (Pm-atzR) | 2 ± 0 | 1 ± 0 | 3 ± 0 | 21 ± 4 | ||
| pMPO104 | atzR-lacZ | None | 14 ± 5 | 14 ± 4 | 1,085 ± 390 | 1,060 ± 346 |
| pMPO126 | PatzR-mut | None | 4 ± 1 | 4 ± 1 | 4 ± 0 | 4 ± 0 |
See Table 2, footnote a.
Expression of the PatzD-mut and PatzR-mut fusions was negligible (≤4 Miller units) under all conditions tested. Exceptionally, weak activity (21 Miller units) could still be detected in PatzD-mut under fully inducing conditions (nitrogen limitation, cyanuric acid, presence of AtzR). Although we do not know the origin of such expression, it is nevertheless irrelevant compared to the levels of the wild-type atzDEF promoter region in the same conditions (36,600 Miller units). These results are fully consistent with the location of the atzR transcriptional start site at position +3 from the atzR start codon, and the atzDEF transcriptional start sites at positions −107 and −108 from the atzD start codon, strongly suggesting that additional bands are degraded or processed forms of the full-length transcripts. Taken together, our data indicate that most atzDEF expression is driven from a single promoter resembling the σ70 consensus, while a promoter with high similarity to the σN consensus is responsible for the vast majority of atzR transcription.
NtrC activates atzR and atzDEF expression.
To test whether NtrC has a role in activation of the cyanuric acid degradation pathway, strain MPO201, a ΔntrC derivative of P. putida KT2442, was constructed (see Materials and Methods for details). Deletion of ntrC resulted in slower growth on several poor nitrogen sources (data not shown). Plasmids pMPO104 (atzR-lacZ) and pMPO204 (atzR-atzD-lacZ) were transferred to MPO201 by mating, and expression was monitored by means of steady-state β-galactosidase assays as described above.
The expression levels of the atzR-lacZ and atzR-atzD-lacZ fusions in MPO201 grown on ammonium as the sole nitrogen source were similar to those measured in the wild-type strain regardless of the presence of cyanuric acid in the growth medium (Fig. 6A and B). However, induction of the atzR-lacZ and atzR-atzD-lacZ fusions by nitrogen limitation was greatly decreased in the mutant strain compared to the wild type, indicating that NtrC positively regulates atzR and atzDEF expression in response to low nitrogen availability. Modest induction of both fusions was still observed in the ΔntrC background, which may be due to cross talk activation by other proteins of the NtrC family (44).
FIG. 6.
Effect of an ntrC mutation on expression of atzR-lacZ and atzD-lacZ fusions. β-Galactosidase activity from P. putida wild-type and ΔntrC strains bearing the atzR-lacZ fusion plasmid pMPO104 (panel A), the atzD-lacZ fusion plasmid pMPO204 (panel B), or the atzD-lacZ fusion plasmid pMPO202 in the presence of the AtzR-producing plasmid pMPO210 (panel C) is shown. The growth medium was mineral medium containing ammonium or serine as the sole nitrogen source with or without 0.1 mM cyanuric acid, as indicated. Open bars indicate the activity levels obtained with wild-type P. putida KT2442. Solid bars indicate the activity levels obtained with the ΔntrC mutant MPO201. The inset in panel C displays the activity obtained in medium containing ammonium and 0, 0.1, or 1 mM cyanuric acid. Values are the averages of at least three independent measurements. Error bars denote the standard deviation of the mean.
In order to test whether NtrC is required for activation of atzDEF in addition to controlling atzR expression, the effect of the ΔntrC mutation on expression of the atzD-lacZ fusion in pMPO202 was measured in the presence of nitrogen-independent synthesis of AtzR from plasmid pMPO210, as described above. Low activity comparable to that of the wild-type strain grown on ammonium was observed under all conditions (Fig. 6C), indicating that NtrC positively regulates atzDEF expression under nitrogen limitation regardless of its effect on AtzR synthesis. To test whether NtrC is also involved in cyanuric acid-dependent induction of atzDEF, atzD-lacZ expression was measured in the presence of 0.1 and 1 mM cyanuric acid. Activity was similarly increased in a concentration-dependent fashion in the wild-type and ΔntrC backgrounds when ammonium was the sole nitrogen source (Fig. 6C, inset), and a comparable response to cyanuric acid was also observed with the ΔntrC strain grown on serine (data not shown). These results strongly suggest that cyanuric acid-dependent induction of atzDEF does not require the presence of NtrC.
DISCUSSION
Studies of the regulation of the atrazine utilization pathway in Pseudomonas sp. strain ADP have given discrepant results. On one hand, the structural organization of the catabolic plasmid pADP-1, consistent with recent acquisition of some of the genes, and expression analysis of the atzA and atzB genes suggest that at least the early section of the pathway is constitutively expressed (13, 34). On the other hand, two studies demonstrated differential degradation rates as a function of the nitrogen source (4, 20), suggesting an effect of nitrogen status on the expression of the pathway. The effect of nitrogen on the herbicide degradation pathway is relevant to the use of this strain in bioremediation, since many agricultural fields are rich in nitrogen due to routine fertilization.
In the present study, we have investigated the possibility that the atzDEF operon, encoding the three final steps of atrazine utilization in Pseudomonas sp. strain ADP, is a target for the described regulation. Our work has led to the identification of a general physiological signal, nitrogen limitation, and a specific inducer molecule, cyanuric acid, that coordinately regulate the expression of atzDEF. In addition, we have demonstrated the involvement of two regulatory proteins belonging to different families, AtzR and NtrC, and the alternative σ factor σN in this regulation. Finally, we have identified the promoters that drive the transcription of atzR and atzDEF. In this discussion we integrate the available information in a regulatory model, consistent with the results presented, which may provide some hints on the molecular mechanisms involved. This model is summarized in Fig. 7.
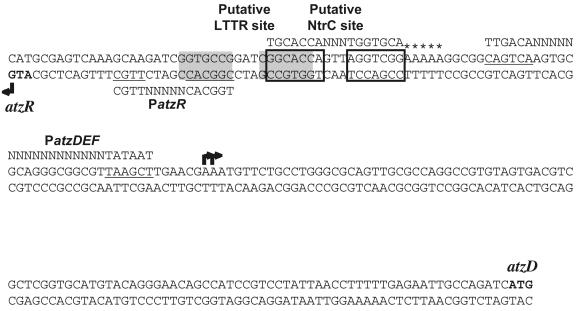
Expression of the cyanuric acid degradation operon atzDEF is specifically induced by the substrate of the pathway, cyanuric acid. The product of the orf99 gene, renamed here atzR, is required for cyanuric acid-mediated activation (Fig. 7). AtzR is homologous to a ubiquitous family of proteins designated LysR-type transcriptional regulators (LTTRs). Like most LTTRs (42), AtzR activates the expression of divergently transcribed genes (the atzDEF operon) in the presence of a small inducing molecule (cyanuric acid) and represses its own synthesis (Fig. 7). Many LTTRs have been found to bind primarily to sites containing the T-N11-A motif enclosed within a region of interrupted dyad symmetry (42). A sequence matching this consensus, which may correspond to the AtzR recognition element, can be found in the atzR-atzD intergenic region (36) (Fig. 8). LTTR recognition sites usually overlap the LTTR's promoter and are centered at approximately position −65 from the transcription start site of their activated promoters (42).
FIG. 8.
atzR-atzD intergenic region, displaying the atzR and atzD promoters and putative regulatory sequences. The sequence displayed spans the region between the annotated start codons of atzR and atzD (34), indicated in bold. Conserved boxes at the atzDEF and atzR promoters (PatzDEF and PatzR) are underlined. The corresponding consensus sequences are shown above fpr PatzDEF and below for PatzR. Two shaded boxes denote the putative AtzR binding site, conforming to the LTTR binding site consensus (T-N11-A within a dyad symmetry region). Two boxed regions mark the best match to the NtrC upstream activation sequence (UAS; consensus shown above the sequence). Finally, five stars indicate an A5 tract, a bendable region that may be relevant to AtzR-dependent activation.
We have mapped the promoters responsible for the vast majority of atzR and atzDEF transcription to locations fully consistent with this model. Accordingly, atzR is transcribed from a region displaying a nearly perfect match to the σN-dependent promoter consensus that overlaps the proposed AtzR binding site, while atzDEF transcription is driven from a σ70-type promoter separated from the putative recognition element by a 21-bp spacer (Fig. 8). An A5 tract is also present between the putative LTTR binding site and the atzDEF promoter. LTTR-induced bending in this region has been reported for other LTTR-activated promoters (42) and may be facilitated by the presence of sequences prone to bending, such as the aforementioned A5 tract. Binding of AtzR to the proposed site would be predicted to prevent binding of RNA polymerase to the atzR promoter, resulting in decreased expression. Since LTTRs bind their recognition sites regardless of the presence of the inducer (42), this prediction is consistent with the fact that AtzR-mediated repression of atzR is also observed in the absence of cyanuric acid.
The notion that atzR is transcribed from a σN-type promoter is fully consistent with the observation that atzR-lacZ expression is almost abolished in an rpoN mutant. In contrast, the lack of nitrogen limitation-dependent activation of atzD-lacZ in this mutant background is likely an indirect effect of the strict σN dependence of AtzR synthesis, since AtzR is required for nitrogen control of atzDEF expression (see below). This idea is supported by the fact that basal levels of atzD-lacZ expression are σN independent. The experimentally mapped 5′ end of the atzR mRNA (position +3 from the proposed start codon) is incompatible with the annotated translational start of the gene. However, sequence comparison with the BlastN program detects similarity between AtzR and other members of the LTTR family only downstream from residue 23, which is a methionine. Based on this evidence, we find it likely that the ATG codon encoding Met-23 is the actual start codon for atzR. Experiments to confirm this hypothesis are currently under way.
General nitrogen control has been reported in many bacteria as a regulatory network that operates to prevent utilization of alternative nitrogen sources when a preferred nitrogen source is present (35). We have proved that expression of both the atzDEF operon and atzR is increased under nitrogen limitation, requiring the intervention of the activator of σN promoters, NtrC (Fig. 7). In addition, our previous work (20) indicated that nitrogen status sensing for regulation of the atrazine degradation pathway requires assimilation of the nitrogen source to intracellular intermediates. General nitrogen control in pseudomonads may therefore operate by mechanisms similar to those thoroughly characterized in the enteric bacteria (35).
Activators of σN-dependent promoters use a unique activation mechanism, characterized by binding to symmetrical sequences located far upstream from the promoter (upstream activation sequences) (30). A search for sequences resembling the NtrC upstream activation sequence did not reveal any clear candidates within the atzR-atzD intergenic region. The best match contains only one conserved half-site, while the other half-site shows poor sequence conservation (Fig. 8). In addition, this region is centered only 13 bp upstream from the σN-dependent atzR promoter. If this site were functional, atzR expression might be activated through a mechanism not previously described and different from that common to enhancer-binding proteins of this family (30) that may involve binding to a site nearby the promoter. Alternatively, activation of the atzR promoter by NtrC may require binding a region with considerable sequence divergence from the consensus or may occur without specific DNA binding, as previously described for the Klebsiella oxytoca nasR gene (51). Finally, the effect of NtrC on regulation of the atzR promoter may be indirect and mediated by a regulatory cascade, as described for the nif genes in Klebsiella pneumoniae (35).
Similar to atzR, atzDEF is also subject to general nitrogen control, resulting in elevated expression during growth on poor nitrogen sources in both Pseudomonas sp. strain ADP and P. putida KT2440. However, several unique regulatory features of atzDEF make it diverge considerably from the model nitrogen-regulated systems. Strikingly, AtzR is absolutely required for activation under nitrogen limitation even in the absence of cyanuric acid (Fig. 7). As discussed above, a sequence resembling LTTR-binding sites is found upstream from atzD, and atzDEF transcription is initiated from a σ70-type promoter. This is in close agreement with the arrangement observed in other LTTR-activated genes. The requirement of AtzR for both nitrogen- and cyanuric acid-mediated regulation of atzDEF suggests that AtzR is the master switch of the regulation and the receiver of both regulatory signals. However, NtrC is still required for increased expression under nitrogen limitation when AtzR is provided from a nitrogen-independent source, suggesting that NtrC has a role in activation of atzDEF in addition to being required for AtzR synthesis (Fig. 7).
We propose four possible mechanisms for NtrC-mediated activation of atzDEF expression. First, regulation may be indirect and mediated by a second regulatory protein whose expression or activity is modulated by NtrC. NtrC-dependent activation of enterobacterial σ70 promoters is mediated by the Nac protein, an LTTR whose synthesis is activated by NtrC under nitrogen limitation (35). Second, NtrC may activate transcription directly at a σN-independent promoter, as previously described for the Rhodobacter capsulatus NtrC protein (6, 16-18). Third, NtrC-mediated regulation may be posttranscriptional, a phenomenon also characterized for NtrC from R. capsulatus (9). Finally, the role of NtrC (or an NtrC-activated factor) in this promoter may be that of a coactivator, acting to modulate AtzR activity rather than directly interacting with RNA polymerase (Fig. 7). We find this hypothesis very attractive, and in this regard it is tempting to speculate that the effect of NtrC may be the result of its interaction with AtzR on the promoter region that may modify the contacts of AtzR with DNA or the RNA polymerase.
Acknowledgments
We thank Inés Canosa, Ana Hervás, and Belén Floriano for critical reading of the manuscript and helpful comments and Maribel Ramírez de Verger for technical help.
The present work was supported by European Union project QLK3-CT-1999-00041 and by a fellowship from the F.P.U. program of the Spanish Ministerio de Educación y Cultura awarded to V.G.-G.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagdasarian, M., B. Lurz, B. Ruckert, F. C. H. Franklin, M. M. Bagdasarian, J. Frey, and K. N. Timmis. 1981. Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors for gene cloning in Pseudomonas. Gene 16:237-247. [DOI] [PubMed] [Google Scholar]
- 3.Barrios, H., B. Valderrama, and E. Morett. 1999. Compilation and analysis of sigma(54)-dependent promoter sequences. Nucleic Acids Res. 27:4305-4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bichat, F., G. K. Sims, and R. L. Mulvaney. 1999. Microbial utilization of heterocyclic nitrogen from atrazine. Soil Sci. Soc. Am. J. 63:100-110. [Google Scholar]
- 5.Blatny, J. M., T. Brautaset, H. C. Winther-Larsen, P. Karunakaran, and S. Valla. 1997. Improved broad-host-range RK2 vectors useful for high and low regulated gene expression levels in gram-negative bacteria. Plasmid 38:35-51. [DOI] [PubMed] [Google Scholar]
- 6.Bowman, W. C., R. G. Kranz, D. Foster-Hartnett, P. J. Cullen, and E. M. Monika. 1998. A bacterial ATP-dependent, enhancer binding protein that activates the housekeeping RNA polymerase. Genes Dev. 12:1884-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buell, C. R., V. Joardar, M. Lindeberg, J. Selengut, I. T. Paulsen, M. L. Gwinn, R. J. Dodson, R. T. Deboy, A. S. Durkin, J. F. Kolonay, R. Madupu, S. Daugherty, L. Brinkac, M. J. Beanan, D. H. Haft, W. C. Nelson, T. Davidsen, N. Zafar, L. Zhou, J. Liu, Q. Yuan, H. Khouri, N. Fedorova, B. Tran, D. Russell, K. Berry, T. Utterback, S. E. Van Aken, T. V. Feldblyum, M. D'Ascenzo, W. L. Deng, A. R. Ramos, J. R. Alfano, S. Cartinhour, A. K. Chatterjee, T. P. Delaney, S. G. Lazarowitz, G. B. Martin, D. J. Schneider, X. Tang, C. L. Bender, O. White, C. M. Fraser, and A. Collmer. 2003. The complete genome sequence of the Arabidopsis and tomato pathogen Pseudomonas syringae pv. tomato DC3000. Proc. Natl. Acad. Sci. USA 100:10181-10186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casadaban, M. J., J. Chou, and S. N. Cohen. 1980. In vitro gene fusions that join an enzymatically active beta-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J. Bacteriol. 143:971-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cullen, P. J., W. C. Bowman, D. F. Hartnett, S. C. Reilly, and R. G. Kranz. 1998. Translational activation by an NtrC enhancer-binding protein. J. Mol. Biol. 278:903-914. [DOI] [PubMed] [Google Scholar]
- 10.de Souza, M. L., J. Seffernick, B. Martinez, M. J. Sadowsky, and L. P. Wackett. 1998. The atrazine catabolism genes atzABC are widespread and highly conserved. J. Bacteriol. 180:1951-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Souza, M. L., L. P. Wackett, and M. J. Sadowsky. 1998. The atzABC genes encoding atrazine catabolism are located on a self-transmissible plasmid in Pseudomonas sp. strain ADP. Appl. Environ. Microbiol. 64:2323-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desnoues, N., M. Lin, X. Guo, L. Ma, R. Carreno-Lopez, and C. Elmerich. 2003. Nitrogen fixation genetics and regulation in a Pseudomonas stutzeri strain associated with rice. Microbiology 149:2251-2262. [DOI] [PubMed] [Google Scholar]
- 13.Devers, M., G. Soulas, and F. Martin-Laurent. 2004. Real-time reverse transcription PCR analysis of expression of atrazine catabolism genes in two bacterial strains isolated from soil. J. Microbiol. Methods 56:3-15. [DOI] [PubMed] [Google Scholar]
- 14.Espinosa-Urgel, M., A. Salido, and J. L. Ramos. 2000. Genetic analysis of functions involved in adhesion of Pseudomonas putida to seeds. J. Bacteriol. 182:2363-2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Figurski, D. H., and D. R. Helinski. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. USA 76:1648-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foster-Hartnett, D., P. J. Cullen, E. M. Monika, and R. G. Kranz. 1994. A new type of NtrC transcriptional activator. J. Bacteriol. 176:6175-6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foster-Hartnett, D., and R. G. Kranz. 1992. Analysis of the promoters and upstream sequences of nifA1 and nifA2 in Rhodobacter capsulatus; activation requires ntrC but not rpoN. Mol. Microbiol. 6:1049-1060. [DOI] [PubMed] [Google Scholar]
- 18.Foster-Hartnett, D., and R. G. Kranz. 1994. The Rhodobacter capsulatus glnB gene is regulated by NtrC at tandem rpoN-independent promoters. J. Bacteriol. 176:5171-5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franklin, F. C., M. Bagdasarian, M. M. Bagdasarian, and K. N. Timmis. 1981. Molecular and functional analysis of the TOL plasmid pWWO from Pseudomonas putida and cloning of genes for the entire regulated aromatic ring meta cleavage pathway. Proc. Natl. Acad. Sci. USA 78:7458-7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.García-González, V., F. Govantes, L. J. Shaw, R. G. Burns, and E. Santero. 2003. Nitrogen control of atrazine utilization in Pseudomonas sp. strain ADP. Appl. Environ. Microbiol. 69:6987-6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Govantes, F. Orjalo, A. V., and R. P. Gunsalus. 2000. Interplay between three global regulatory proteins mediates oxygen regulation of the Escherichia coli cytochrome d oxidase (cydAB) operon. Mol. Microbiol. 38:1061-1073. [DOI] [PubMed] [Google Scholar]
- 22.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 23.Ho, S. N., Hunt, H. D., Horton, R. M., Pullen, J. K., and L. R. Pease. 1989. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 15:51-59. [DOI] [PubMed] [Google Scholar]
- 24.Inoue, H., H. Nojima, and H. Okayama. 1990. High efficiency transformation of Escherichia coli with plasmids. Gene 96:23-28. [DOI] [PubMed] [Google Scholar]
- 25.Janssen, D. B., W. J. Habets, J. T. Marugg, and C. Van Der Drift. 1982. Nitrogen control in Pseudomonas aeruginosa: mutants affected in the synthesis of glutamine synthetase, urease, and NADP-dependent glutamate dehydrogenase. J. Bacteriol. 151:22-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janssen, D. B., H. M. Joosten, P. M. Herst, C. van der Drift, W. J. Habets, and J. T. Marugg. 1982. Characterization of glutamine-requiring mutants of Pseudomonas aeruginosa. J. Bacteriol. 151:1176-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaniga, K., I. Delor, and G. R. Cornelis. 1991. A wide-host-range suicide vector for improving reverse genetics in gram-negative bacteria: inactivation of the blaA gene of Yersinia enterocolitica. Gene 109:137-141. [DOI] [PubMed] [Google Scholar]
- 28.Köhler, T., S. Harayama, J. L. Ramos, and K. N. Timmis. 1989. Involvement of Pseudomonas putida RpoN sigma factor in regulation of various metabolic functions. J. Bacteriol. 171:4326-4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kovach, M. E., P. H. Elzer, D. S. Hill, G. T. Robertson, M. A. Farris, R. M. Roop, 2nd, and K. M. Peterson. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175-176. [DOI] [PubMed] [Google Scholar]
- 30.Kustu, S., E. Santero, J. Keener, D. Popham, and D. Weiss. 1989. Expression of sigma 54 (ntrA)-dependent genes is probably united by a common mechanism. Microbiol. Rev. 53:367-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Llamas, M. A., J. L. Ramos, and J. J. Rodríguez-Herva. 2000. Mutations in each of the tol genes of Pseudomonas putida reveal that they are critical for maintenance of outer membrane stability. J. Bacteriol. 182:4764-4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mandelbaum, R. T., L. P. Wackett, and D. L. Allan. 1995. Isolation and characterization of a Pseudomonas sp. that mineralizes the s-triazine herbicide atrazine. Appl. Environ. Microbiol. 61:1451-1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mandelbaum, R. T., L. P. Wackett, and D. L. Allan. 1993. Mineralization of the s-triazine ring of atrazine by stable bacterial mixed cultures. Appl. Environ. Microbiol. 59:1695-1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martinez, B., J. Tomkins, L. P. Wackett, R. Wing, and M. J. Sadowsky. 2001. Complete nucleotide sequence and organization of the atrazine catabolic plasmid pADP-1 from Pseudomonas sp. strain ADP. J. Bacteriol. 183:5684-5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merrick, M. J., and R. A. Edwards. 1995. Nitrogen control in bacteria. Microbiol. Rev. 59:604-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller, J. H. 1992. A short course in bacterial genetics: a laboratory manual, p. 72-74. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 37.Nelson, K. E., C. Weinel, I. T. Paulsen, R. J. Dodson, H. Hilbert, V. A. Martins dos Santos, D. E. Fouts, S. R. Gill, M. Pop, M. Holmes, L. Brinkac, M. Beanan, R. T. DeBoy, S. Daugherty, J. Kolonay, R. Madupu, W. Nelson, O. White, J. Peterson, H. Khouri, I. Hance, P. Chris Lee, E. Holtzapple, D. Scanlan, K. Tran, A. Moazzez, T. Utterback, M. Rizzo, K. Lee, D. Kosack, D. Moestl, H. Wedler, J. Lauber, D. Stjepandic, J. Hoheisel, M. Straetz, S. Heim, C. Kiewitz, J. Eisen, K. N. Timmis, A. Dusterhoft, B. Tummler, and C. M. Fraser. 2002. Complete genome sequence and comparative analysis of the metabolically versatile Pseudomonas putida KT2440. Environ. Microbiol. 4:799-808. [DOI] [PubMed] [Google Scholar]
- 38.Nishijyo, T., D. Haas, and Y. Itoh. 2001. The CbrA-CbrB two-component regulatory system controls the utilization of multiple carbon and nitrogen sources in Pseudomonas aeruginosa. Mol. Microbiol. 40:917-931. [DOI] [PubMed] [Google Scholar]
- 39.Ramos, J. L., S. Marqués, and K. N. Timmis. 1997. Transcriptional control of the Pseudomonas TOL plasmid catabolic operons is achieved through an interplay of host factors and plasmid-encoded regulators. Annu. Rev. Microbiol. 51:341-373. [DOI] [PubMed] [Google Scholar]
- 40.Rousseaux, S., A. Hartmann, and G. Soulas. 2001. Isolation and characterisation of new Gram-negative and Gram-positive atrazine degrading bacteria from different French soils. FEMS Microbiol. Ecol. 36:211-222. [DOI] [PubMed] [Google Scholar]
- 41.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 42.Schell, M. A. 1993. Molecular biology of the LysR family of transcriptional regulators Annu. Rev. Microbiol. 47:597-626. [DOI] [PubMed] [Google Scholar]
- 43.Schweizer, H. P. 2001. Vectors to express foreign genes and techniques to monitor gene expression in pseudomonads. Curr. Opin. Biotechnol. 12:439-445. [DOI] [PubMed] [Google Scholar]
- 44.Stock, J. B., A. J. Ninfa, and A. M. Stock. 1989. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol. Rev. 53:450-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K. Wong, Z. Wu, I. T. Paulsen, J. Reizer, M. H. Saier, R. E. Hancock, S. Lory, and M. V. Olson. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959-964. [DOI] [PubMed] [Google Scholar]
- 46.Studholme, D. J., and R. Dixon. 2004. In silico analysis of the sigma54-dependent enhancer-binding proteins in Pirellula species strain 1. FEMS Microbiol. Lett. 230:215-225. [DOI] [PubMed] [Google Scholar]
- 47.Topp, E., H. Zhu, S. M. Nour, S. Houot, M. Lewis, and D. Cuppels. 2000. Characterization of an atrazine-degrading Pseudaminobacter sp. isolated from Canadian and French agricultural soils. Appl. Environ. Microbiol. 66:2773-2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Totten, P. A., J. C. Lara, and S. Lory. 1990. The rpoN gene product of Pseudomonas aeruginosa is required for expression of diverse genes, including the flagellin gene. J. Bacteriol. 172:389-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wackett, L. P., M. J. Sadowsky, B. Martinez, and N. Shapir. 2002. Biodegradation of atrazine and related s-triazine compounds: from enzymes to field studies. Appl. Microbiol. Biotechnol. 58:39-45. [DOI] [PubMed] [Google Scholar]
- 50.Winther-Larsen, H. C., Josefsen, K. D., Brautaset, T., and S. Valla. 2000. Parameters affecting gene expression from the Pm promoter in Gram-negative bacteria. Metab. Eng. 2:79-91. [DOI] [PubMed] [Google Scholar]
- 51.Wu, S. Q., W. Chai, J. T. Lin, and V. Stewart. 1999. General nitrogen regulation of nitrate assimilation regulatory gene nasR expression in Klebsiella oxytoca M5a1. J. Bacteriol. 181:7274-7284. [DOI] [PMC free article] [PubMed] [Google Scholar]