Abstract
We isolated five new temperature-sensitive alleles of the essential cell division gene ftsZ in Escherichia coli, using P1-mediated, localized mutagenesis. The five resulting single amino acid changes (Gly109→Ser109 for ftsZ6460, Ala129→Thr129 for ftsZ972, Val157→Met157 for ftsZ2066, Pro203→Leu203 for ftsZ9124, and Ala239→Val239 for ftsZ2863) are distributed throughout the FtsZ core region, and all confer a lethal cell division block at the nonpermissive temperature of 42°C. In each case the division block is associated with loss of Z-ring formation such that fewer than 2% of cells show Z rings at 42°C. The ftsZ9124 and ftsZ6460 mutations are of particular interest since both result in abnormal Z-ring formation at 30°C and therefore cause significant defects in FtsZ polymerization, even at the permissive temperature. Neither purified FtsZ9124 nor purified FtsZ6460 exhibited polymerization when it was assayed by light scattering or electron microscopy, even in the presence of calcium or DEAE-dextran. Hence, both mutations also cause defects in FtsZ polymerization in vitro. Interestingly, FtsZ9124 has detectable GTPase activity, although the activity is significantly reduced compared to that of the wild-type FtsZ protein. We demonstrate here that unlike expression of ftsZ84, multicopy expression of the ftsZ6460, ftsZ972, and ftsZ9124 alleles does not complement the respective lethalities at the nonpermissive temperature. In addition, all five new mutant FtsZ proteins are stable at 42°C. Therefore, the novel isolates carrying single ftsZ(Ts) point mutations, which are the only such strains obtained since isolation of the classical ftsZ84 mutation, offer significant opportunities for further genetic characterization of FtsZ and its role in cell division.
The FtsZ protein of Escherichia coli is essential throughout the process of cell division (3, 11, 29, 33, 48, 49). Following the segregation of daughter chromosomes, FtsZ polymerizes around the inner circumference at midcell to form the Z ring (9). At least nine proteins (all of which are essential for cell division) are recruited to the division site in an FtsZ-dependent fashion (12, 13), and the Z ring then leads this division apparatus inward, ultimately allowing separation of daughter cells. Division proteins ZipA and FtsA are recruited to the Z ring by direct interaction with FtsZ, and the recruitment of at least one of these proteins is essential for productive Z-ring formation (21, 23, 25, 31, 32, 45, 56, 59). All other division proteins are thought to localize to the Z ring in a hierarchical fashion (that is, the localization is dependent on the prior presence of other cell division proteins). Therefore, all proteins which make up the divisome ultimately depend on the presence of the Z ring for their localization to the septum.
The timing of the arrival at the division site of these proteins is thought to reflect the order in which they are involved during cell division (4, 13, 22, 30). An exception is FtsW, which has been ascribed either an early (10, 24) or late (35) role in the division process. Recent data demonstrating a direct in vitro interaction between Mycobacterium tuberculosis FtsW and FtsZ (16) points to an early role for FtsW, at least in actinobacterial species, which interestingly have neither ftsA nor zipA genes (33, 55).
FtsZ is both a structural and functional homologue of eukaryotic tubulins (26, 39, 43, 50, 54), in that it has a high GTPase activity and is able to polymerize in vitro in a GTP-dependent fashion into higher-order structures (3, 48). Although many biochemical and biophysical properties of FtsZ and FtsZ polymers have been characterized, crucial questions concerning the mechanism of Z-ring nucleation and constriction remain unanswered (48). Conditional mutations in ftsZ have proven to be difficult to isolate in vivo, and of the two such mutants which exist, the ftsZ84 and ftsZ26 mutants (8), only the former is due to a single amino acid change. Furthermore, it has been demonstrated that mild overexpression of ftsZ84 provides complementation of its lethal cell division defect (44). Therefore, suppressor and complementation studies are most likely to identify previously characterized mechanisms by which ftsZ expression levels can be modified rather than novel interactions with accessory proteins (6, 7, 15, 20, 42, 46, 57). Even so, analysis of the ftsZ84 allele has contributed significantly to our knowledge of FtsZ function. For example, we know that the high GTPase activity of FtsZ detected in vitro (17, 37, 47) is not essential for the in vivo function of FtsZ (28). This is because the ftsZ84 allele, which confers significantly reduced GTPase activity in vitro (17, 47), resulting in a less dynamic Z ring in vivo (54), can nevertheless support essentially normal cell division at the permissive temperature (2).
We therefore chose to isolate new temperature-sensitive (Ts) ftsZ mutants with the aim of identifying alleles with different properties than ftsZ84, which may be of use in genetic dissection of FtsZ function. Using P1-mediated, localized mutagenesis, we isolated six temperature-sensitive ftsZ mutant strains of E. coli; five of the mutations result in different single amino acid changes in the FtsZ protein. In this paper we describe characterization of the new mutants, including DNA sequencing, determination of FtsZ localization patterns at permissive and restrictive temperatures, and polymerization and GTPase assays of purified mutant proteins (FtsZ6460 and FtsZ9124) which have polymerization defects in vivo at the permissive temperature. We also present data on the stability of the new FtsZ proteins and the results of tests of the ability of each allele, when overexpressed, to complement its own lethality at 42°C.
MATERIALS AND METHODS
Bacterial strains and media.
All strains used were derivatives of E. coli K-12. W3110 (prototroph) from laboratory stocks was used as the wild-type strain. Strain YYC100 carries a transposon (zac284::Tn10) downstream of ftsZ and was obtained from Mary Berlyn at the E. coli Genetic Stock Centre. Strain YYC100 itself was found to be temperature sensitive for growth at 42°C. The zac-284::Tn10 was therefore moved from strain YYC100 into MG1655 by P1 transduction. Only 2% of the transductants were temperature resistant, suggesting that the temperature-sensitive mutation in YYC100 is closely linked to the Tn10. A temperature-resistant transductant of P1(YYC100) × MG1655 (designated MGzac284::Tn10) was chosen as the donor strain for the P1 mutagenesis procedure. Strain BL21(λDE3)/pLysS was used for overexpression of ftsZ alleles with T7 promoter expression vectors. The media used were Oxoid nutrient agar, LB (Luria-Bertani medium containing 10 g of Oxoid tryptone per liter, 5 g of yeast extract per liter, and 10 g of NaCl per liter), and LBNS (LB without added NaCl). Oxoid bacteriological agar no. 1 was added to a final concentration of 1.5% when needed.
Isolation of ftsZ(Ts) mutants.
We used P1-mediated localized mutagenesis as described previously (24) to target mutagenesis to the chromosomal region close to the ftsZ gene. A P1 lysate prepared from strain MGzac284::Tn10 was mutagenized with hydroxylamine and then used to transduce strain W3110 to tetracycline resistance (Tetr). Tetr transductants were screened for temperature-sensitive growth by patching them onto nutrient agar (Oxoid) plates at 30 and 42°C. Temperaure-sensitive isolates were examined by phase-contrast microscopy, and the isolates showing an fts phenotype, characterized by the formation of long aseptate filaments at 42°C, were transformed with plasmid pT7-3Z (see below) to test for complementation. The mutants which were complemented by pT7-3Z at 42°C were designated ftsZ(Ts).
Construction of plasmids.
Plasmid pT7-3Z was constructed by cloning a 1.8-kbp HindIII/ClaI fragment (containing the 3′ end of ftsA, all of ftsZ, and the 5′ end of envA) into the HindIII/ClaI sites in the polylinker of pT7-3 (a gift from Stan Tabor, Harvard Medical School). Plasmid pT7-3Z provides low-level expression of ftsZ (see below) from an endogenous promoter(s) in the distal portion of ftsA, as well as inducible, high levels of ftsZ expression when it is transformed into strain BL21(λDE3)/pLysS. Plasmids pT7-3Z and pKD126 (14, 41) were altered to encode mutant FtsZ proteins by site-directed mutagenesis by using a QuikChange kit (Stratagene) according to the manufacturer's instructions. The ftsZ genes from each of the resultant plasmids (pT73Z-972, pT73Z-2066, pT73Z-2863, pT73Z-6460, pT73Z-9124, pKD126-6460, and pKD126-9124) were sequenced to confirm the presence of the intended mutation and to rule out secondary mutations. The pKD126-6460 plasmid was unstable, since multiple attempts to amplify DNA from an original small-scale preparation resulted in the appearance of new mutations in the ftsZ gene. Hence, pT73Z-6460 was used for overexpression of FtsZ6460 for purification (Table 1).
TABLE 1.
In vitro properties of FtsZ6460 and FtsZ9124 compared to those of wild-type FtsZ
| Protein | GTPase activity
|
Polymerizationi with:
|
||||
|---|---|---|---|---|---|---|
| GTP (light scattering)a | Calciumb
|
DEAE (electron microscopy)c | ||||
| mol/mol/min | % of wild type | Light scattering | Electron microscopy | |||
| Wild typed,f | 2.8 | Yes | Yes | ND | ND | |
| Wild typed,g | 1.1 | Yes | ND | Yes | Yes | |
| Wild typee,f | 1.9 | Yes | Yes | Yes | Yes | |
| Wild typee,g | 2 | Yes | ND | ND | ND | |
| Wild typed,f,h | 1.4 | Yes | Yes | ND | ND | |
| Wild typee,f,h | 1.4 | Yes | Yes | ND | ND | |
| FtsZ6460d,f,h | 0.44 | 31.4 | No | No | No | No |
| FtsZ6460e,f,h | 0.59 | 42.1 | No | No | No | No |
| FtsZ9124d,f | 0.67 | 23.9 | No | No | No | No |
| FtsZ9124d,g | 0.41 | 37.3 | No | No | ND | ND |
| FtsZ9124e,f | 0.26 | 13.7 | No | No | ND | ND |
| FtsZ9124e,g | 0.45 | 22.5 | No | No | No | No |
The mixture contained 8.3 μM protein (mutants were also tested with 16.6 μM protein) plus 0.2 to 0.5 mM GTP.
The mixture contained 8.3 or 16.6 μM protein plus 0.1 mM GTP and 10 mM Ca2+.
The mixture contained 8.3 μM protein plus 0.1 mM GTP and 0.6 mg of DEAE-dextran per ml.
20 to 30% NH3SO4 precipitate.
30% NH3SO4 precipitate.
Expressed at 30°C.
Expressed at 37°C.
Expressed from pT7-37.
Yes, polymerization detected; No, no polymerization detected; ND, not done.
Quantification of FtsZ levels.
Since pT7-3Z was used in complementation experiments, we quantified the low-level expression of ftsZ provided by this pBR322-derived plasmid as follows. Samples from exponentially growing cultures of W3110(pBR322) and W3110(pT7-3Z), which had identical doubling times, at A600 of 0.2 and 0.4 were added to sodium dodecyl sulfate (SDS) sample buffer. A sample similarly taken from an overnight culture of W3110(pBR322) was used to prepare a standard curve for FtsZ protein concentration by serial dilution. Samples from the standard curve were subjected to SDS-polyacrylamide gel electrophoresis (PAGE) alongside test samples on a 10% acrylamide gel and then electrotransferred to nitrocellulose. Immunoblotting was carried out by using affinity-purified sheep anti-FtsZ primary antibodies (Small E and SG [Addinall, unpublished data]), followed by donkey anti-sheep immunoglobulin G (a generous gift from V. Allan, University of Manchester) conjugated to horseradish peroxidase secondary antibodies (both diluted 1/20,000 in Tris-buffered saline [20 mM Tris-HCl, 150 mM NaCl; pH 7.7] containing 1% Triton X-100 and 1% skim milk powder). The signal was detected in alkaline phosphatase buffer (100 mM Tris-HCl [pH 9.5], 100 mM NaCl, 5 mM MgCl2) containing 330 μg of nitroblue tetrazolium per ml and 165 μg of 5-bromo-4-chloro-3-indolylphosphate per ml. The final membrane was scanned, and a densitometric analysis was carried out (AIDA 200, version 2.0). Test samples were compared to the standard curve for the FtsZ signal to obtain a value in arbitrary units, and then the ratio of W3110(pT7-3Z) to W3110(pBR322) was calculated from these values. At an A600 of 0.2 the ratio of FtsZ content in W3100(pT7-3Z) to FtsZ content in W3100(pBR322) was 2.0, and at an A600 of 0.4 the ratio was 1.9. We therefore concluded that pT7-3Z approximately doubles the FtsZ concentration in the W3110 background. The levels of mutant FtsZ proteins expressed from pT7-3Z derivatives and the relative levels of mutant FtsZ proteins encoded chromosomally (42°C versus 30°C) were determined similarly. In each case, the sample volumes were corrected based on the optical density at 600 nm.
Immunofluorescence localization of FtsZ.
Localization of the FtsZ protein in fixed cells was performed as described previously (2), except that the primary and secondary antibodies were mouse monoclonal anti-FtsZ antibody (F168-12 at a 1/1,000 dilution; a generous gift from N. Nanninga [18]) and goat anti-mouse immunoglobulin G antibody conjugated to ALEXA 488 (1/500 to 1/200 dilution; Molecular Probes), respectively. Log-phase cultures at 30°C or 75 min after a shift to 42°C were prepared for immunofluorescence as described previously (2). Slides were examined by using a Leica DMRXA microscope with a ×100 Plan fluotar objective and filter sets suitable for 4′,6′-diamidino-2-phenylindole (DAPI), fluorescein isothiocyanate, and Texas Red. Images were initially captured with a Coolsnap FX charge-coupled device camera (Roper Scientific) by using the MetaVue software (Universal Imaging) and then were processed with Adobe Photoshop.
Expression and purification of FtsZ proteins.
The FtsZ9124 protein was expressed from pKD126-9124, as previously described for wild-type FtsZ (41, 52). FtsZ6460 was expressed by induction of a culture of BL21(λDE3)/pLysS containing plasmid pT73Z-6460 at an A600 of 0.3 with 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 2 h. FtsZ9124 was initially purified as previously described for wild-type FtsZ protein (52). It was found that a substantial amount of the FtsZ9124 protein was lost during the 20% ammonium sulfate precipitation step; therefore, a second preparation in which the 20% ammonium sulfate precipitate was not discarded was produced. FtsZ6460 was purified by both methods described above (i.e., with and without the 20% ammonium sulfate step). Wild-type FtsZ was prepared to directly mimic each combination of expression system and purification method outlined above in order to allow fair comparisons of the biochemical properties of wild-type and mutant proteins. The concentration of FtsZ protein was measured by the Bio-Rad protein assay by using bovine serum albumin as a standard and a conversion factor of 0.82 as described previously (27).
Light-scattering assay.
Right-angle light scattering was measured as previously described (52) by using a Cary Eclipse fluorescence spectrophotometer with both the excitation and emission wavelengths set at 350 nm and at a constant temperature of 30°C (provided by a thermostat-controlled cuvette holder). Unless stated otherwise, a 125-μl reaction mixture consisting of 50 mM morpholineethanesulfonic acid (MES)-NaOH (pH 6.5), 10 mM MgCl2, 50 mM KCl, 0.25 mM GTP, and 8.3 μM FtsZ protein (wild type or mutant) was initially prepared without the GTP. This mixture was transferred to a prewarmed cuvette, and once a consistent baseline level of light scattering was obtained, 10 μl of 3.13 mM GTP was added, the sample was mixed thoroughly, and light scattering was monitored thereafter. Polymerization was deemed to have occurred (Table 1) if there was an increase in light scattering (52) which then returned to baseline levels upon GTP hydrolysis. In practice, no light scattering was observed for any of the mutant FtsZ preparations (data not shown).
Electron microscopy.
Negative-stain electron microscopy was performed as described previously (52). Samples were taken from polymerization reaction mixtures before and 1 and 10 min after GTP addition. This was done in the presence of either 10 mM calcium or 6 mg of DEAE-dextran per ml, as described previously (28). Polymerization was deemed to have occurred if any linear, curved, bundled, sheet- or tube-shaped polymers were observed on at least two squares of a grid. In practice, no polymeric structures were observed on multiple grid squares from both times for any of the mutant protein preparations tested (Table 1 and data not shown).
GTP binding assay.
Reaction mixtures (10 μl) containing 50 pmol of wild-type FtsZ, FtsZ9124, and FtsZ6460 proteins (we used preparations in which the 20% ammonium sulfate precipitate was discarded) and 20 μCi of [α32P]GTP in binding buffer (50 mM Tris-HCl [pH 7.9], 50 mM KCl, 1 mM EDTA) were incubated for 10 min on ice. The reaction mixtures were subjected to irradiation at 254 nm for 10 min (UVIlink CL508 cross-linker; Uvitec) on Parafilm on top of an ice-cold metal block and then added to 10 μl of 10 mM GTP in binding buffer on ice. Ten microliters of 0.1% Surfact-Amps X-100 [Pierce & Warriner (UK) Ltd.] and then 300 μl of 20% tricarboxylic acid were added to each mixture, which was mixed vigorously and then incubated on ice for 30 min. Precipitated protein was pelleted by centrifugation in a microcentrifuge for 10 min, and the supernatant was discarded. The pellet was then washed with 300 μl of 10% of tricarboxylic acid, followed by 300 μl of 80% acetone. The pellet was air dried briefly and then resuspended in 1× SDS-PAGE sample buffer containing 5 mM GTP. After heating at 65°C for 15 min, samples were separated by SDS-PAGE on a 10% acrylamide gel, which was then stained with Coomassie blue. Cross-linking of radioactive GTP to proteins was assessed by autoradiography.
GTPase assays.
GTPase activity was measured by thin-layer chromatography by using [γ-32P]GTP (Amersham) as described previously (52). The GTPase activity of mutant FtsZ proteins was expressed both in moles of GTP hydrolyzed per mole of protein per minute and as a percentage of the activity of wild-type FtsZ purified under similar conditions (Table 1).
RESULTS
Isolation and identification of new ftsZ temperature-sensitive alleles.
Of 10,000 individual Tetr transductants isolated, approximately 1% were temperature sensitive for growth at 42°C on nutrient agar plates. This result is consistent with previous results obtained when a similar approach was used (24). Although each strain was identified as sensitive for growth at 42°C on nutrient agar and in nutrient broth, we found that the fts phenotypes were most severe and reproducible on LBNS (data not shown). Six of the temperature-sensitive isolates had an fts phenotype and were complemented by the ftsZ plasmid pT7-3Z. Mutant ftsZ(Ts) alleles were amplified by PCR from chromosomal DNA, and this was followed by sequencing of PCR products cloned into M13. To eliminate errors due to amplification or sequencing, ftsZ alleles were amplified and sequenced again. Each of the six ftsZ(Ts) mutants was found to have a single base change in the ftsZ coding region, resulting in a single amino acid change. The mutation alleles were designated ftsZ972, ftsZ2066, ftsZ2863, ftsZ4227, ftsZ6460, and ftsZ9124 after the numbers of the original isolates. Although isolated independently, ftsZ2066 and ftsZ4227 are identical, and therefore we do not present further data for ftsZ4227 below.
We described the locations of the mutations with reference to the three-dimensional structure of Methanococcus jannaschii FtsZ1 (26) by using primary sequence and secondary structure alignments of the sequence and the E. coli FtsZ sequence (data not shown). The ftsZ972, ftsZ2066, and ftsZ6460 mutations are in the amino-terminal domain of the FtsZ core region (55), and ftsZ2863 is in the carboxy-terminal domain of the core region; ftsZ9124 is essentially at the junction between the two domains.
The ftsZ6460 allele encodes a glycine-to-serine change at residue 109 (G109S), and therefore the glycine-rich motif which is directly involved in GTP binding and hydrolysis is disrupted. This residue is invariant in 228 full-length FtsZ sequences from bacteria, archaea, and eukaryotes (55), indicating its importance for FtsZ function. It is not surprising, therefore, that ftsZ6460 resulted in the most severe phenotype of all five new mutations described here (see below). The A129T mutation encoded by ftsZ972 falls in strand 5. This is a well-conserved residue since of 228 full-length FtsZ sequences, 15 have serine at this position and all others have either glycine or alanine (55). The ftsZ2066 mutation encodes V157M and lies in a turn between helix 4 and strand 6. This is a well-conserved residue in bacterial FtsZ sequences (55). The ftsZ9124 mutation encodes P203L and is of interest due to its position between helix 5 of the amino-terminal domain and helix C1, the first α-helix of the carboxy-terminal domain. While not particularly well conserved, proline is by far the most frequent residue at this position in FtsZ sequences (55). The ftsZ2863 mutation (A239V) is the only one of the six mutations which falls in an α-helix (namely, helix C2) of the carboxy-terminal domain encoding the FtsZ core region. This residue is extremely well conserved, and only four bacterial FtsZs have amino acids other than alanine at this position (55).
Four of the mutant alleles imparted classic cell division mutant phenotypes, in that cells grew as short rods at the permissive temperature of 30°C but exhibited uniform extensive filamentation at 42°C in liquid media (Fig. 1). The exception was the ftsZ6460 allele, which imparted a moderate filamentation phenotype at 30°C and extensive, lethal filamentation at 42°C, suggesting that the ftsZ6460 mutation severely damages the function of the FtsZ protein, even at 30°C.
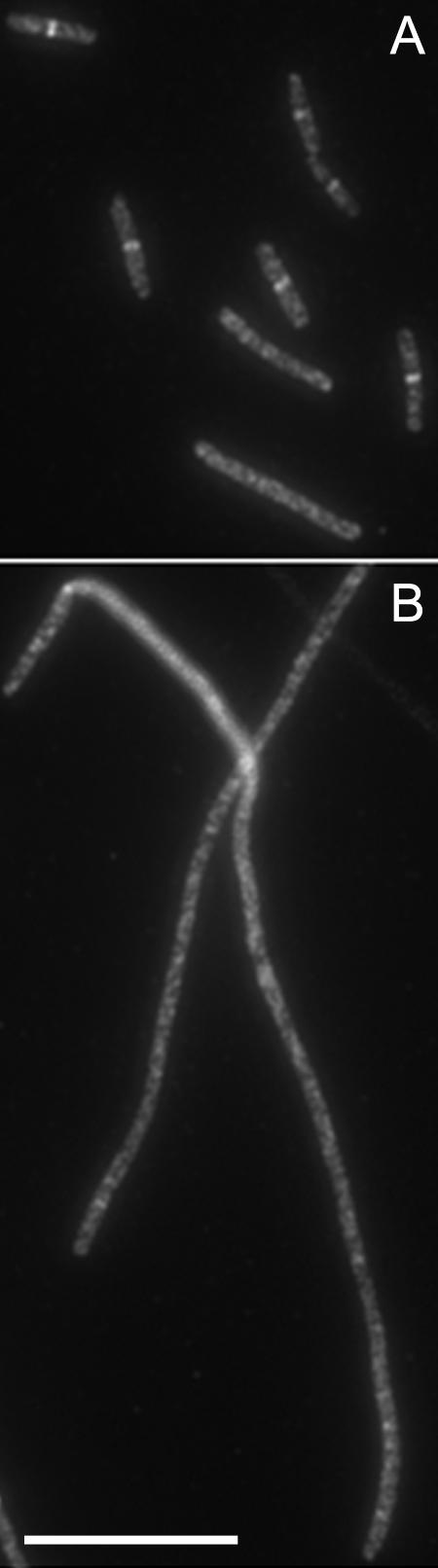
FIG. 1.
Temperature-sensitive phenotype of the ftsZ2863(Ts) mutant strain: immunolocalization of FtsZ at 30°C (A) and 42°C (B). Results similar to those shown in panel A were obtained with ftsZ972and ftsZ2066 at 30°C, and results similar to those shown in panel B were obtained with all strains at 42°C (data not shown). Scale bar = 10 μm.
FtsZ localization in novel ftsZ(Ts) mutants.
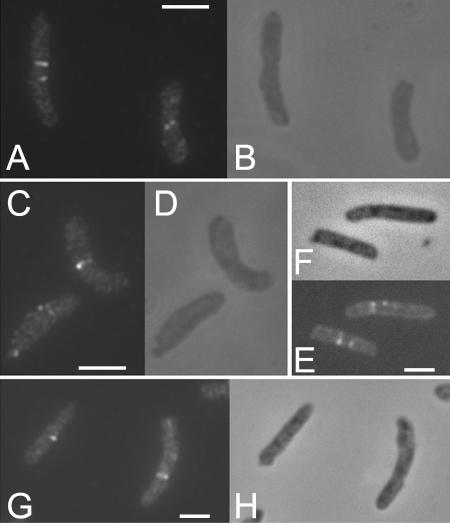
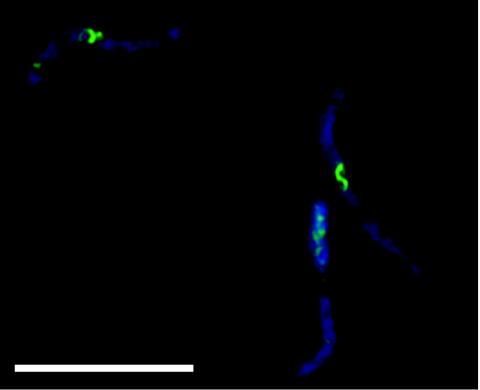
Strains carrying the ftsZ972, ftsZ2066, ftsZ9124, and ftsZ2863 alleles of the FtsZ gene exhibited patterns of FtsZ localization very similar to that observed with the strain carrying the ftsZ84 allele (1, 2); that is, most cells had a single central Z ring at 30°C, and a proportion of cells (approximately 30% in the case of the ftsZ2863 mutant shown in Fig. 1) had no Z rings, which is consistent with previous observations of partial defects in fts mutants at permissive temperatures (4). In all mutants, more than 98% of the cells completely lacked Z rings at 42°C (Fig. 1). Closer examination of ftsZ9124 cells at the permissive temperature revealed a small but consistent proportion of cells with aberrant FtsZ structures. These structures included slanted rings, double spots, single spots, and half (incomplete) rings (Fig. 2). Therefore, the ftsZ9124 allele causes subtle defects in the formation of FtsZ rings at the permissive temperature. In strains carrying the ftsZ6460 allele, again Z rings were absent at the nonpermissive temperature (data not shown). However, at 30°C this mutation caused cells to be filamentous, although many cells with apparently normal, single Z rings were observed. In addition, we observed cells (approximately 40%) (data not shown) exhibiting spiral FtsZ structures associated with bends or kinks in the cells (Fig. 3), very similar to the phenotype observed with the ftsZ26 mutation (5). Therefore, the ftsZ6460 allele confers serious defects in the formation of Z rings at the permissive temperature.
FIG. 2.
Mislocalization of FtsZ9124: representative images showing immunolocalization of FtsZ (A, C, E, and G) alongside phase-contrast images (B, D, F, and H). The images show examples of unusual FtsZ localization, including two adjacent Z structures (A [left cell] and E), slanted Z structures (A [right cell]), and single Z spots (C [left cell] and G [left cell]). Scale bars = 2 μm.
FIG. 3.
Mislocalization of FtsZ6460: FtsZ spiral formation at 30°C in strains expressing the FtsZ6460 allele. In the composite pseudocolored image DAPI staining is indicated by blue and FtsZ immunolocalization is indicated by green. Scale bar = 10 μm.
In vivo activity of FtsZ(Ts) proteins.
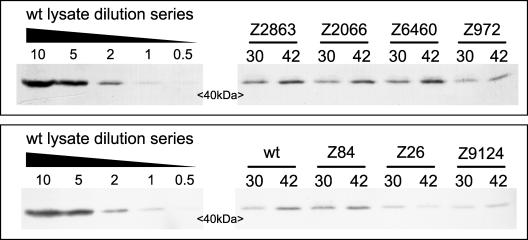
To assess whether the novel mutant FtsZ proteins simply had reduced activity in vivo at the nonpermissive temperature (as has been demonstrated for FtsZ84 [44]), we tested the abilities of plasmids pT73Z-972, pT73Z-2066, pT73Z-2863, pT73Z-6460, and pT73Z-9124 to complement their respective temperature-sensitive isolates on LBNS agar at 42°C. Plating efficiencies were measured by comparing viability counts (36) at 30 and 42°C. Mutant strains with no plasmid or carrying pBR322 served as controls. The data (Table 2) demonstrated that none of the new ftsZ(Ts) alleles offered full complementation when it was overexpressed from the respective plasmid, although in the case of ftsZ2066 (and to a small extent in the case of ftsZ2863), partial complementation was observed. The level of FtsZ varied depending on the allele expressed, and plasmid pT73Z-84 resulted in an FtsZ level that was similar to that obtained with pT7-3Z, which was sufficient for full complementation of the ftsZ84 mutant (Table 2). In order to achieve levels of expression of mutant proteins higher than those obtained with pT7-3Z, we constructed derivatives of the inducible ftsZ overexpression vector pKD126 carrying the ftsZ972, ftsZ6460, and ftsZ9124 alleles. The pKD126-6460 construct was inherently unstable, with mutations accumulating readily in the gene upon propagation (see Materials and Methods) (data not shown). This finding and the deleterious effects of expressing ftsZ6460, even at the permissive temperature, argue strongly that further overexpression of this protein would not rescue its temperature-sensitive properties. Both the pKD126-972 and pKD126-9124 constructs were stably amplified in wild-type cloning strains; however, they gave significantly reduced efficiency compared to equal amounts of pKD126 DNA when they were transformed into the respective mutant strains under permissive conditions (data not shown). Consistent with this, only a few colonies were obtained when pKD126-972 and pKD126-9124 were transformed into their respective mutant strains and plated at 42°C on LBNS, whereas when pKD126 was used at the same concentration, many hundreds of viable transformants were obtained (Table 2 and data not shown). Again, this suggests strongly that overexpression of ftsZ972 and ftsZ9124 at levels comparable to the level observed for the wild type in pKD126 does not complement the respective alleles at 42°C. We tested the stability of mutant FtsZ alleles at the nonpermissive temperature, and this analysis revealed that the inability of the new FtsZ proteins to self-complement is not due to inherent instability (Fig. 4).
TABLE 2.
Plating efficiencies of new ftsZ(Ts) mutants
| Strain | Plasmid | Efficiency of plating ina:
|
FtsZ level | |
|---|---|---|---|---|
| LBNS | LB | |||
| W3110 | pBR322 | 1b | ||
| W3110 | pT7-3Z | 2c | ||
| 972 | 1.0 × 10−6 | 0.95 | 1.1a | |
| 972 | pBR322 | 3.9 × 10−6 | ||
| 972 | pT7-3Z | 0.8 | ||
| 972 | pT73Z-972 | 1.9 × 10−6 | ||
| W3110 | pT73Z-972 | 1.2c | ||
| 2066 | 7.9 × 10−6 | 0.99 | 1.4a | |
| 2066 | pBR322 | 4.1 × 10−6 | ||
| 2066 | pT7-3Z | 0.95 | ||
| 2066 | pT73Z-2066 | 0.064 | ||
| W3110 | pT73Z-2066 | 1.8c | ||
| 2863 | 4.0 × 10−6 | 1.1 | 1.25a | |
| 2863 | pBR322 | 1.5 × 10−6 | ||
| 2863 | pT7-3Z | 1.3 | ||
| 2863 | pT73Z-2863 | 1.7 × 10−4 | ||
| W3110 | pT73Z-2863 | 1.4c | ||
| 6460 | 2.0 × 10−7 | 0.001 | 1.4a | |
| 6460 | pBR322 | 3.0 × 10−5 | ||
| 6460 | pT7-3Z | 0.6 | ||
| 6460 | pT73Z-6460 | 1.5 × 10−5 | ||
| W3110 | pT73Z-6460 | 1.4c | ||
| 9124 | 4.6 × 10−6 | 1.5 | 1.25a | |
| 9124 | pBR322 | <2.3 × 10−7 | ||
| 9124 | pT7-3Z | 0.96 | ||
| 9124 | pT73Z-9124 | 5.7 × 10−6 | ||
| W3110 | pT73Z-9124 | 1.3c | ||
| 84 | 8 × 10−5d | 0.8 | 1.1a | |
| 84 | pT7-3Z | 1d | 1.2 | |
| 84 | pT73Z-84 | 0.9d | 1.1 | |
| W3110 | pT73Z-84 | 1.8c | ||
At 42°C relative to 30°C.
The value for this experiment was arbitrarily defined as 1.
At 30°C relative to W3110/pBR322.
Determined by using LB with 0.2% NaCl.
FIG. 4.
Stability of FtsZ mutant proteins under nonpermissive conditions. Lysates from ftsZ mutant strains (allele numbers are indicated) and W3110 (wt) were obtained at 30°C and 60 min after a shift to 42°C in LBNS. Loading was equalized relative to the optical densities at 600 nm of the cultures. The relative levels of FtsZ were calculated by comparison to a dilution series for a W3110 lysate run on the same gel. Pencil lines indicating marker positions were cropped from each blot image after all image processing. With the exception of the ftsZ26 mutation (included as an extra control), the levels of mutant proteins were slightly higher at 42°C than at 30°C. The ratios of the level at 42°C to the level at 30°C are shown in Table 2.
In vitro activity of FtsZ6460 and FtsZ9124.
Since the Z-ring localization patterns of FtsZ6460 and FtsZ9124 at 30°C show aberrant structures and the ftsZ6460 allele confers a moderate division block at the permissive temperature, we decided to purify these two mutant proteins and investigate their abilities to polymerize and hydrolyze GTP in vitro. We noticed that a considerable proportion of the FtsZ9124 protein was present in the initial 20% ammonium sulfate precipitate (designed to remove inactive FtsZ protein [27]). We therefore repeated the purification procedure, this time omitting the 20% ammonium sulfate precipitation, which resulted in a better yield of protein (data not shown) and provided two different preparations of FtsZ9124 to compare. We also purified FtsZ6460 and wild-type FtsZ by both these alternative methods. In addition, wild-type and FtsZ9124 proteins were induced at both 30 and 37°C (see Materials and Methods) (Table 1).
We initially tested for GTP-dependent polymerization of FtsZ proteins using right-angle light scattering (38). While wild-type FtsZ polymerized efficiently upon addition of GTP to the polymerization reaction mixture, regardless of the purification protocol used, no preparations of either FtsZ6460 or FtsZ9124 were able to polymerize under the same conditions (Table 1). We therefore performed light-scattering assays for polymerization in the presence of 10 mM calcium, conditions which enhance the polymerization signal by promoting polymer bundling and reducing polymer turnover (34, 38, 51, 52, 60). Again, no polymerization was detected from either of the mutant proteins, whereas wild-type preparations readily formed polymers under the same conditions (Table 1).
In order to test whether polymers too small for detection by light scattering were being formed by the mutant proteins, we observed polymerization mixtures by negative stain electron microscopy in the presence and absence of calcium or DEAE-dextran. Once again, polymers from FtsZ9124 or FtsZ6460 were not observed under conditions in which wild-type FtsZ produced polymers and polymer bundles (10 mM calcium) and polymer tubes (DEAE-dextran) (Table 1 and data not shown). We concluded that neither FtsZ6460 nor FtsZ9124 is capable of GTP-induced polymerization in vitro at 30°C.
Initially, we noted that preparations of both FtsZ6460 and FtsZ9124 had measurable GTPase activity (Table 1). Compared to the activity of wild-type FtsZ purified under similar conditions, these activities were significantly reduced (13 to 42% of the activity of the wild-type protein purified under similar conditions). Binding assays with purified FtsZ6460 and FtsZ9124 with wild-type FtsZ as a control demonstrated that both mutant proteins were able to bind GTP (data not shown) but at lower levels than the wild type. The only protein which exhibited GTP binding in the FtsZ9124 preparation was FtsZ9124 itself. In contrast, a major contaminating GTP binding protein with a molecular mass of approximately 20 kDa was present in the FtsZ6460 preparation. We concluded, therefore, that the GTPase activity detected from FtsZ9124 was genuine, whereas the GTPase activity of FtsZ6460 preparations was due at least in part to a contaminant.
DISCUSSION
The FtsZ protein continues to be the focus of much research because it is a key player in bacterial cell division. The fact that this protein is a predecessor to eukaryotic tubulin and a model molecule for similar dynamic proteins has led to numerous studies of its structure-function relationships. We adopted a classical genetic approach through the isolation of temperature-sensitive mutations in ftsZ in vivo using P1-mediated, localized mutagenesis. Although only two temperature-sensitive alleles of ftsZ (namely, ftsZ84 and ftsZ26) have been reported previously, we were able to isolate five new temperature-sensitive alleles that had a single point mutation in each allele. Temperature-sensitive mutants that had an fts phenotype but were not complemented by plasmid pT7-3Z were obtained but were not studied further. These mutants presumably carried mutations in one or more of the other division genes in the dcw cluster at 2 min on the E. coli chromosome.
Each of the mutations which we describe here is located at conserved residues in the FtsZ-encoding core region. Although none of these residues has been targeted for specific mutagenesis in recent studies (28, 58), we can in some cases compare the new mutations to previously characterized alterations of adjacent amino acids. The ftsZ6460 allele (G109S) is particularly interesting in this respect. The glycine at position 109 is completely conserved in all available FtsZ sequences (55) and makes contact with the guanine nucleotide (26). The extreme phenotype encoded by the ftsZ6460 mutation in vivo and the lack of polymerization (and GTPase) activity of purified FtsZ6460 are therefore not surprising. In fact, ftsZ6460 imparts a phenotype intermediate between the phenotypes imparted by two adjacent, previously characterized mutant alleles. The ftsZ3 mutation (T108A) results in a protein which is nonfunctional for division in vivo and lacks both GTP binding and GTPase activities in vitro (14, 40). In contrast, E. coli cells with the ftsZ84 mutation (which also changes a glycine that is in close contact with the guanine nucleotide to serine; G105S) are essentially normal at the permissive temperature (2, 54). The ftsZ84 mutation reduces the GTPase activity substantially, but FtsZ84 can still polymerize in vitro with the aid of DEAE-dextran (28). In contrast, FtsZ6460 shows no detectable ability to polymerize in vitro (Table 1). It should be noted that overexpression of FtsZ6460 is extremely deleterious to E. coli cells (see Results).
Site-directed mutagenesis of neighboring residues of both ftsZ2066 and ftsZ2863 has been characterized previously. The D158A, E238A (28, 53), and D158N (56) mutations resulted in FtsZ proteins with properties very similar to those of the wild type, in contrast to the mutants described here, which are temperature sensitive in vivo. The ftsZ9124 allele encodes P203L at the junction between the amino- and carboxy-terminal domains of FtsZ (26) and as such may have a significant effect of the overall structure of the protein. However, this mutation is also adjacent to the synergy loop (19), which is critical for the GTPase activity of FtsZ. Mutations in this loop reduce GTPase activity significantly but have variable effects on polymerization ability (28, 51, 56).
The ftsZ6460 phenotype at the permissive temperature is very similar to the ftsZ26 phenotype. Each mutant produces both normal and aberrant Z rings. Interestingly, the aberrant Z rings are often able to constrict, but this (together with some aborted constriction events) leads to misshapen cells (5, 8). At present, there are no published biochemical data on the FtsZ26 protein, but our data suggest that it may be less stable at the nonpermissive temperature (Fig. 4).
The subtle ftsZ9124 phenotype at the permissive temperature is reminiscent of partial rings observed in FtsZ84 mutant cells recovering from a temperature shift (2) and likely represents either slow or aborted Z-ring formation in the cells. Although the partial structures were observed in multiple experiments with ftsZ9124 in both the W3110 and C600 backgrounds (data not shown), we cannot completely discount the possibility that FtsZ9124 rings might be more fragile than wild-type rings and hence more likely to be disrupted by the immunofluorescence procedure.
Observation of BL21(λDE3)/pLysS carrying each of the pT73Z derivatives (the wild type and all of the new mutants) revealed the presence of small numbers of minicells (data not shown). This indicates that each of the mutant proteins is able to combine with wild-type FtsZ to drive extra divisions, when it is overexpressed to a small extent (Table 2). In addition, the fact that each mutant strain divides at the permissive temperature indicates that mutant proteins are capable of GTP binding, GTP hydrolysis, and polymerization in vivo. The discrepancy between in vivo and in vitro properties of proteins such as FtsZ6460 suggests that other factors (e.g., accessory proteins, macromolecular crowding) maximize the FtsZ function in vivo. However, in the light of current models of FtsZ polymerization (48, 50), we surmised that FtsZ9124 at least is capable of dimerization and possibly some small degree of polymerization in vitro.
The behavior of the FtsZ proteins in vivo is summarized in Table 2. While ftsZ2066 and ftsZ2863 showed partial self-complementation when they were overexpressed, ftsZ972, ftsZ6460, and ftsZ9124 failed to do this. Attempts to complement the ftsZ972, ftsZ6460, and ftsZ9124 alleles with derivatives of pKD126 were unsuccessful. In contrast, we were able to demonstrate clearly the ability of ftsZ84 to self-complement fully when it was present at double the normal level in the cell (Table 2). Furthermore, it is evident that the temperature-sensitive behavior of all the FtsZ mutant proteins is due to loss of function and not to instability at 42°C (Fig. 4).
We demonstrated, therefore, that in combination with predictive and selective strategies (8, 15, 23, 28, 31, 51, 56, 58, 59), an essentially random mutagenesis approach is productive even when the three-dimensional structure of a well-characterized protein is known. The new ftsZ alleles, particularly ftsZ972, ftsZ6460, and ftsZ9124, provide invaluable tools in the search for unique functional suppressors when classical genetics are used, especially since the overexpression of these alleles appears to be deleterious. We expect that further characterization of the novel mutants, as well as suppressor studies, should enhance our knowledge of FtsZ function in vivo.
Acknowledgments
This work was supported by funds from the MRC (United Kingdom), The Welcome Trust (grant 059935/Z/99/Z) (United Kingdom), The Nuffield Foundation (United Kingdom), and the University Research Board (American University of Beirut, Beirut, Lebanon).
REFERENCES
- 1.Addinall, S. G., E. Bi, and J. Lutkenhaus. 1996. FtsZ ring formation in fts mutants. J. Bacteriol. 178:3877-3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Addinall, S. G., C. Cao, and J. Lutkenhaus. 1997. Temperature shift experiments with an ftsZ84(Ts) strain reveal rapid dynamics of FtsZ localization and indicate that the Z ring is required throughout septation and cannot reoccupy division sites once constriction has initiated. J. Bacteriol. 179:4277-4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Addinall, S. G., and B. Holland. 2002. The tubulin ancestor, FtsZ, draughtsman, designer and driving force for bacterial cytokinesis. J. Mol. Biol. 318:219-236. [DOI] [PubMed] [Google Scholar]
- 4.Addinall, S. G., and J. Lutkenhaus. 1996. FtsA is localized to the septum in an FtsZ-dependent manner. J. Bacteriol. 178:7167-7172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Addinall, S. G., and J. Lutkenhaus. 1996. FtsZ-spirals and -arcs determine the shape of the invaginating septa in some mutants of Escherichia coli. Mol. Microbiol. 22:231-237. [DOI] [PubMed] [Google Scholar]
- 6.Aldea, M., T. Garrido, J. Pla, and M. Vicente. 1990. Division genes in Escherichia coli are expressed coordinately to cell septum requirements by gearbox promoters. EMBO J. 9:3787-3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aldea, M., C. Hernandez-Chico, A. G. de la Campa, S. R. Kushner, and M. Vicente. 1988. Identification, cloning, and expression of bolA, an ftsZ-dependent morphogene of Escherichia coli. J. Bacteriol. 170:5169-5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bi, E., and J. Lutkenhaus. 1992. Isolation and characterization of ftsZ alleles that affect septal morphology. J. Bacteriol. 174:5414-5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bi, E. F., and J. Lutkenhaus. 1991. FtsZ ring structure associated with division in Escherichia coli. Nature 354:161-164. [DOI] [PubMed] [Google Scholar]
- 10.Boyle, D. S., M. M. Khattar, S. G. Addinall, J. Lutkenhaus, and W. D. Donachie. 1997. ftsW is an essential cell-division gene in Escherichia coli. Mol. Microbiol. 24:1263-1273. [DOI] [PubMed] [Google Scholar]
- 11.Bramhill, D. 1997. Bacterial cell division. Annu. Rev. Cell Dev. Biol. 13:395-424. [DOI] [PubMed] [Google Scholar]
- 12.Buddelmeijer, N., N. Judson, D. Boyd, J. J. Mekalanos, and J. Beckwith. 2002. YgbQ, a cell division protein in Escherichia coli and Vibrio cholerae, localizes in codependent fashion with FtsL to the division site. Proc. Natl. Acad. Sci. USA 99:6316-6321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen, J. C., and J. Beckwith. 2001. FtsQ, FtsL and FtsI require FtsK, but not FtsN, for co-localization with FtsZ during Escherichia coli cell division. Mol. Microbiol. 42:395-413. [DOI] [PubMed] [Google Scholar]
- 14.Dai, K., A. Mukherjee, Y. Xu, and J. Lutkenhaus. 1994. Mutations in ftsZ that confer resistance to SulA affect the interaction of FtsZ with GTP. J. Bacteriol. 176:130-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D'Ari, R. 1997. The Escherichia coli cell cycle, cell division and ppGpp: regulation and mechanisms. Folia Microbiol. 42:161-164. [DOI] [PubMed] [Google Scholar]
- 16.Datta, P., A. Dasgupta, S. Bhakta, and J. Basu. 2002. Interaction between FtsZ and FtsW of Mycobacterium tuberculosis. J. Biol. Chem. 277:24983-24987. [DOI] [PubMed] [Google Scholar]
- 17.de Boer, P., R. Crossley, and L. Rothfield. 1992. The essential bacterial cell-division protein FtsZ is a GTPase. Nature 359:254-256. [DOI] [PubMed] [Google Scholar]
- 18.Den Blaauwen, T., N. Buddelmeijer, M. E. Aarsman, C. M. Hameete, and N. Nanninga. 1999. Timing of FtsZ assembly in Escherichia coli. J. Bacteriol. 181:5167-5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erickson, H. P. 1998. Atomic structures of tubulin and FtsZ. Trends Cell Biol. 8:133-137. [DOI] [PubMed] [Google Scholar]
- 20.Gervais, F. G., P. Phoenix, and G. R. Drapeau. 1992. The rcsB gene, a positive regulator of colanic acid biosynthesis in Escherichia coli, is also an activator of ftsZ expression. J. Bacteriol. 174:3964-3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hale, C. A., and P. A. de Boer. 1997. Direct binding of FtsZ to ZipA, an essential component of the septal ring structure that mediates cell division in E. coli. Cell 88:175-185. [DOI] [PubMed] [Google Scholar]
- 22.Hale, C. A., and P. A. J. de Boer. 2002. ZipA is required for recruitment of FtsK, FtsQ, FtsL, and FtsN to the septal ring in Escherichia coli. J. Bacteriol. 184:2552-2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haney, S. A., E. Glasfeld, C. Hale, D. Keeney, Z. Z. He, and P. de Boer. 2001. Genetic analysis of the Escherichia coli FtsZ · ZipA interaction in the yeast two-hybrid system—characterization of FtsZ residues essential for the interactions with ZipA and with FtsA. J. Biol. Chem. 276:11980-11987. [DOI] [PubMed] [Google Scholar]
- 24.Khattar, M. M., K. J. Begg, and W. D. Donachie. 1994. Identification of FtsW and characterization of a new ftsW division mutant of Escherichia coli. J. Bacteriol. 176:7140-7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu, Z., A. Mukherjee, and J. Lutkenhaus. 1999. Recruitment of ZipA to the division site by interaction with FtsZ. Mol. Microbiol. 31:1853-1861. [DOI] [PubMed] [Google Scholar]
- 26.Lowe, J., and L. A. Amos. 1998. Crystal structure of the bacterial cell-division protein FtsZ. Nature 391:203-206. [DOI] [PubMed] [Google Scholar]
- 27.Lu, C., and H. P. Erickson. 1998. Purification and assembly of FtsZ. Methods Enzymol. 298:305-313. [DOI] [PubMed] [Google Scholar]
- 28.Lu, C., J. Stricker, and H. P. Erickson. 2001. Site-specific mutations of FtsZ—effects on GTPase and in vitro assembly. BMC Microbiol. 1:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lutkenhaus, J., and S. G. Addinall. 1997. Bacterial cell division and the Z ring. Annu. Rev. Biochem. 66:93-116. [DOI] [PubMed] [Google Scholar]
- 30.Ma, X., D. W. Ehrhardt, and W. Margolin. 1996. Colocalization of cell division proteins FtsZ and FtsA to cytoskeletal structures in living Escherichia coli cells by using green fluorescent protein. Proc. Natl. Acad. Sci. USA 93:12998-13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma, X., and W. Margolin. 1999. Genetic and functional analyses of the conserved C-terminal core domain of Escherichia coli FtsZ. J. Bacteriol. 181:7531-7544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma, X., Q. Sun, R. Wang, G. Singh, E. L. Jonietz, and W. Margolin. 1997. Interactions between heterologous FtsA and FtsZ proteins at the FtsZ ring. J. Bacteriol. 179:6788-6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Margolin, W. 2000. Themes and variations in prokaryotic cell division. FEMS Microbiol. Rev. 24:531-548. [DOI] [PubMed] [Google Scholar]
- 34.Marrington, R., E. Small, A. Rodger, T. R. Dafforn, and S. G. Addinall. 2004. FtsZ fibre bundling is triggered by a conformational change in bound GTP. J. Biol. Chem. 279:48821-48829. [DOI] [PubMed] [Google Scholar]
- 35.Mercer, K. L. N., and D. S. Weiss. 2002. The Escherichia coli cell division protein FtsW is required to recruit its cognate transpeptidase, FtsI (PBP3), to the division site. J. Bacteriol. 184:904-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miles, A. A., and S. S. Misra. 1938. The estimation of the bactericidal power of the blood. J. Hyg. 38:732-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mukherjee, A., K. Dai, and J. Lutkenhaus. 1993. Escherichia coli cell division protein FtsZ is a guanine nucleotide binding protein. Proc. Natl. Acad. Sci. USA 90:1053-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mukherjee, A., and J. Lutkenhaus. 1999. Analysis of FtsZ assembly by light scattering and determination of the role of divalent metal cations. J. Bacteriol. 181:823-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mukherjee, A., and J. Lutkenhaus. 1998. Dynamic assembly of FtsZ regulated by GTP hydrolysis. EMBO J. 17:462-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mukherjee, A., and J. Lutkenhaus. 1994. Guanine nucleotide-dependent assembly of FtsZ into filaments. J. Bacteriol. 176:2754-2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mukherjee, A., and J. Lutkenhaus. 1998. Purification, assembly, and localization of FtsZ. Methods Enzymol. 298:296-305. [DOI] [PubMed] [Google Scholar]
- 42.Navarro, F., A. Robin, R. D'Ari, and D. Joseleau-Petit. 1998. Analysis of the effect of ppGpp on the ftsQAZ operon in Escherichia coli. Mol. Microbiol. 29:815-823. [DOI] [PubMed] [Google Scholar]
- 43.Nogales, E., S. G. Wolf, and K. H. Downing. 1998. Structure of the alpha beta tubulin dimer by electron crystallography. Nature 391:199-203. [DOI] [PubMed] [Google Scholar]
- 44.Phoenix, P., and G. R. Drapeau. 1988. Cell division control in Escherichia coli K-12: some properties of the ftsZ84 mutation and suppression of this mutation by the product of a newly identified gene. J. Bacteriol. 170:4338-4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pichoff, S., and J. Lutkenhaus. 2002. Unique and overlapping roles for ZipA and FtsA in septal ring assembly in Escherichia coli. EMBO J. 21:685-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Powell, B. S., and D. L. Court. 1998. Control of ftsZ expression, cell division, and glutamine metabolism in Luria-Bertani medium by the alarmone ppGpp in Escherichia coli. J. Bacteriol. 180:1053-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.RayChaudhuri, D., and J. T. Park. 1992. Escherichia coli cell-division gene ftsZ encodes a novel GTP-binding protein. Nature 359:251-254. [DOI] [PubMed] [Google Scholar]
- 48.Romberg, L., and P. A. Levin. 2003. Assembly dynamics of the bacterial cell division protein FtsZ: poised at the edge of stability. Annu. Rev. Microbiol. 57:125-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rothfield, L. I., and S. S. Justice. 1997. Bacterial cell division: the cycle of the ring. Cell 88:581-584. [DOI] [PubMed] [Google Scholar]
- 50.Scheffers, D., and A. J. Driessen. 2001. The polymerization mechanism of the bacterial cell division protein FtsZ. FEBS Lett. 506:6-10. [DOI] [PubMed] [Google Scholar]
- 51.Scheffers, D. J., J. G. de Wit, T. den Blaauwen, and A. J. Driessen. 2002. GTP hydrolysis of cell division protein FtsZ: evidence that the active site is formed by the association of monomers. Biochemistry 41:521-529. [DOI] [PubMed] [Google Scholar]
- 52.Small, E., and S. G. Addinall. 2003. Dynamic FtsZ polymerization is sensitive to the GTP to GDP ratio and can be maintained at steady state using a GTP-regeneration system. Microbiology 149:2235-2242. [DOI] [PubMed] [Google Scholar]
- 53.Stricker, J., and H. P. Erickson. 2003. In vivo characterization of Escherichia coli ftsZ mutants: effects on Z-ring structure and function. J. Bacteriol. 185:4796-4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stricker, J., P. Maddox, E. D. Salmon, and H. P. Erickson. 2002. Rapid assembly dynamics of the Escherichia coli FtsZ-ring demonstrated by fluorescence recovery after photobleaching. Proc. Natl. Acad. Sci. USA 99:3171-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vaughan, S., B. Wickstead, K. Gull, and S. G. Addinall. 2004. Molecular evolution of FtsZ protein sequences encoded within the genomes of Archaea, Bacteria and Eukaryota. J. Mol. Evol. 58:19-29. [DOI] [PubMed] [Google Scholar]
- 56.Wang, X., J. Huang, A. Mukherjee, C. Cao, and J. Lutkenhaus. 1997. Analysis of the interaction of FtsZ with itself, GTP, and FtsA. J. Bacteriol. 179:5551-5559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang, X. D., P. A. de Boer, and L. I. Rothfield. 1991. A factor that positively regulates cell division by activating transcription of the major cluster of essential cell division genes of Escherichia coli. EMBO J. 10:3363-3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang, Y., B. D. Jones, and Y. V. Brun. 2001. A set of ftsZ mutants blocked at different stages of cell division in Caulobacter. Mol. Microbiol. 40:347-360. [DOI] [PubMed] [Google Scholar]
- 59.Yan, K., K. H. Pearce, and D. J. Payne. 2000. A conserved residue at the extreme C-terminus of FtsZ is critical for the FtsA-FtsZ interaction in Staphylococcus aureus. Biochem. Biophys. Res. Commun. 270:387-392. [DOI] [PubMed] [Google Scholar]
- 60.Yu, X. C., and W. Margolin. 1997. Ca2+-mediated GTP-dependent dynamic assembly of bacterial cell division protein FtsZ into asters and polymer networks in vitro. EMBO J. 16:5455-5463. [DOI] [PMC free article] [PubMed] [Google Scholar]