Abstract
Anodal transcranial direct current stimulation (tDCS) can boost the effects of motor training and facilitate plasticity in the healthy brain. Motor rehabilitation depends on learning and plasticity, and motor learning can occur after stroke. Here, we tested whether brain stimulation using anodal tDCS added to motor training could improve rehabilitation outcomes in patients after stroke. We performed a randomized, controlled trial in 24 patients at least 6 months after a first unilateral stroke not directly involving the primary motor cortex. Patients received either anodal tDCS (n=11) or sham treatment (n=13) paired with daily motor training for 9 days. We observed improvements that persisted for at least 3 months post-intervention after anodal tDCS but not sham treatment on the Action Research Arm Test (ARAT) and Wolf Motor Function Test (WMFT) but not on the Fugl-Meyer upper extremity score (UEFM). Functional MRI showed increased activity during movement of the affected hand in the ipsilesional motor and premotor cortex in the anodal tDCS group compared to the sham treatment group. Structural MRI revealed intervention-related increases in gray matter volume in cortical areas including ipsilesional motor and premotor cortex after anodal tDCS but not sham treatment. The addition of ipsilesional anodal tDCS to a 9-day motor training program improved long-term clinical outcomes relative to sham treatment in patients after stroke.
Introduction
Stroke is a leading cause of severe long-term disability. Spontaneous recovery typically plateaus within three to six months, but rehabilitation can be effective at improving motor outcome, even in the chronic phase. Motor rehabilitation approaches vary widely, but there is increasing support for programs that encourage active movement and use neuroscience principles of motor learning to facilitate progression (1). Although greater intensity and duration of training leads to greater recovery (2), delivery of one-to-one training is time consuming and expensive. Thus, there is increasing interest in adjunct therapies to enhance responses (3).
Rehabilitation-mediated recovery depends largely on processes of learning and plasticity (4, 5), so manipulations that promote plasticity might be expected to enhance rehabilitation outcomes. For example, anodal transcranial direct current stimulation (tDCS) to the motor cortex is known to enhance excitability (6), reduce local inhibition (7), and facilitate motor learning (8, 9) in healthy individuals. We therefore predicted that application of anodal tDCS to ipsilesional motor cortex, when paired with motor training, would enhance rehabilitation outcomes after stroke by facilitating brain plasticity.
Although the effects of serial sessions using other tDCS configurations have been reported (10–12), there is limited evidence on the use of ipsilesional anodal tDCS. Two recent systematic reviews (13, 14) provide tentative support for the use of ipsilesional anodal tDCS in chronic stroke, but studies have not used serial sessions of anodal tDCS concurrent with motor training. We therefore performed a double-blind randomised controlled trial of anodal tDCS as an adjunct to a daily motor training program, the graded repetitive arm supplementary programme (GRASP) (15), delivered over 9 consecutive working days. In addition, serial multi-modal MRI was undertaken in order to test whether an effective intervention was associated with increased activation of the ispilesional hemisphere and associated structural changes. Long-term follow-up clinical and imaging assessments tested the persistence of observed changes in motor function.
Results
We conducted a stratified, double-blind, sham-controlled, parallel-group study designed to test the effects of anodal tDCS as an adjunct to motor training in chronic stroke patients (at least one month post single stroke affecting motor function). Of 1191 patients assessed for eligibility, 26 were randomized to receive either anodal tDCS or sham treatment, and 24 completed the intervention (fig. S1). Details of stroke patients who completed the intervention are given in Table 1 and location of lesions is shown in figure S2. Patient sex, age at intervention, time post stroke, lesion side, type and volume did not differ between the anodal tDCS and sham groups (Table 1). No lesions included primary motor cortex (fig. S2).
Table 1. Details of patients in the sham (top) and anodal tDCS (bottom) groups.
| Subject Number | Sex | Age at intervention (years) |
Time post-stroke (months) |
Lesion location side/typ |
Lesion volume mm3 |
Muscle response | |
|---|---|---|---|---|---|---|---|
| Sham | 1 | F | 76 | 47 | L/S | 3294 | Y |
| 3 | M | 67 | 47 | R/S | 431 | Y | |
| 5 | M | 56 | 29 | R/C | 30149 | Y | |
| 6 | M | 55 | 92 | L/S | 26160 | Y | |
| 8 | M | 64 | 19 | R/C | - | N | |
| 11 | F | 79 | 68 | L/S | 14822 | N | |
| 13 | M | 72 | 31 | R/C | 91237 | Y | |
| 14 | M | 78 | 141 | R/C | 45736 | Y | |
| 18 | M | 64 | 39 | R/S | 40167 | Y | |
| 19 | M | 68 | 97 | R/S | 3751 | Y | |
| 20 | M | 69 | 99 | R/S | 63866 | Y | |
| 22 | F | 44 | 9 | R/S | 56010 | N | |
| 23 | F | 77 | 18 | L/S | 10548 | Y | |
| 66.8 | 56.6 | 4L/9R | 32181 | 3N/10Y | |||
| (10.4) | (39.8) | (28232) | |||||
| Anodal | 2 | M | 74 | 44 | R/C | 366389 | Y |
| 4 | M | 58 | 72 | L/S | 1418 | Y | |
| 7 | F | 45 | 54 | R/C | 21955 | Y | |
| 9 | M | 65 | 52 | R/S | 108420 | Y | |
| 10 | M | 71 | 44 | R/S | 3448 | Y | |
| 12 | M | 72 | 109 | R/S | 71101 | Y | |
| 15 | M | 58 | 105 | R/S | 34305 | Y | |
| 16 | F | 52 | 17 | R/S | 5824 | N | |
| 17 | M | 70 | 41 | R/S | 735 | Y | |
| 21 | M | 53 | 6 | L/S | 11061 | N | |
| 24 | F | 37 | 19 | L/S | 6593 | N | |
| 59.5 | 51.2 | 3L/8R | 57386 | 3N/8Y | |||
| (12.1) | (33.4) | 2C/9S | (108027) | ||||
Group means (SD) or counts are given for each variable. No variable differed significantly between groups. M, male; F, female; L, left; R, right; C, cortical; S,subcortical; Muscle response Y/N refers to whether an observable muscle twitch from the affected hand could be evoked (Y) or not (N) after transcranial magnetic stimulation (TMS) of the ipsilesional motor cortex.
Clinical assessments and MRI were carried out at multiple time points before and after the intervention period (fig. S3). Neuroimaging measures were also taken before and after the intervention. During the intervention period participants conducted daily, supervised one-hour sessions of the Graded Repetitive Arm Supplementary Program (GRASP) (15) over 9 days. For the first 20 minutes of each session tDCS electrodes were positioned on the participant’s scalp to deliver either brain stimulation via tDCS or sham treatment.
Improved clinical test scores in the anodal tDCS group compared to sham group
Clinical outcomes were assessed at multiple timepoints following the intervention (fig. S3) using three clinical measures: Upper Extremity Fugl-Meyer Assessment (UEFM) (16), Action Research Arm Test (ARAT) (17) and Wolf Motor Function Test (WMFT) (18). Mean scores for each group and each timepoint on these clinical measures are shown in Table 2.
Table 2. Mean scores for functional assessment measures for anodal tDCS and sham treatment groups.
| Anodal tDCS Mean (SD) |
Sham Mean (SD) | |
|---|---|---|
| Baseline UEFM | 38.90 (15.89) | 36.42 (17.38) |
| Day 10 UEFM | 50.36 (11.16) | 45.53 (14.62) |
| 1 Week UEFM | 48.91 (11.90) | 45.92 (15.52) |
| 1 Month UEFM | 49.73 (12.67) | 46.46 (14.35) |
| 3 Month UEFM | 48.18 (14.35) | 43.15 (16.29) |
| Baseline ARAT | 20.27 (17.37) | 26.27 (20.17) |
| Day 10 ARAT | 29.91 (21.54) | 32.54 (21.54) |
| 1 Week ARAT | 30.45 (20.67) | 33.08 (21.84) |
| 1 Month ARAT | 30.27 (21.91) | 31.92 (20.64) |
| 3 Month ARAT | 30.45 (20.92) | 31.31 (21.84) |
| Baseline WMFT | 37.91 (20.21) | 39.65 (25.39) |
| Day 10 WMFT | 47.18 (17.46) | 48.00 (23.42) |
| 1 Week WMFT | 49.45 (20.30) | 48.92 (24.44) |
| 1 Month WMFT | 49.18 (19.08) | 46.54 (23.12) |
| 3 Month WMFT | 48.36 (18.19) | 43.09 (23.78) |
In all assessments, higher scores indicate better performance. The UEFM ranges from 0-66, ARAT ranges from 0-57, and WMFT ranges from 0-75. Results are shown as mean ± SD.
To test whether clinical scores increased in the anodal tDCS group compared to the sham group, each clinical score was subjected to multiple regression using the general linear model. The model variables were group (sham and anodal tDCS) and individual participant baseline scores for the respective clinical measure. By including baseline scores in the model we ensured that variability in starting scores was taken into account. Plots of clinical scores for each group and for each of the post-intervention timepoints are shown in Figure 1.
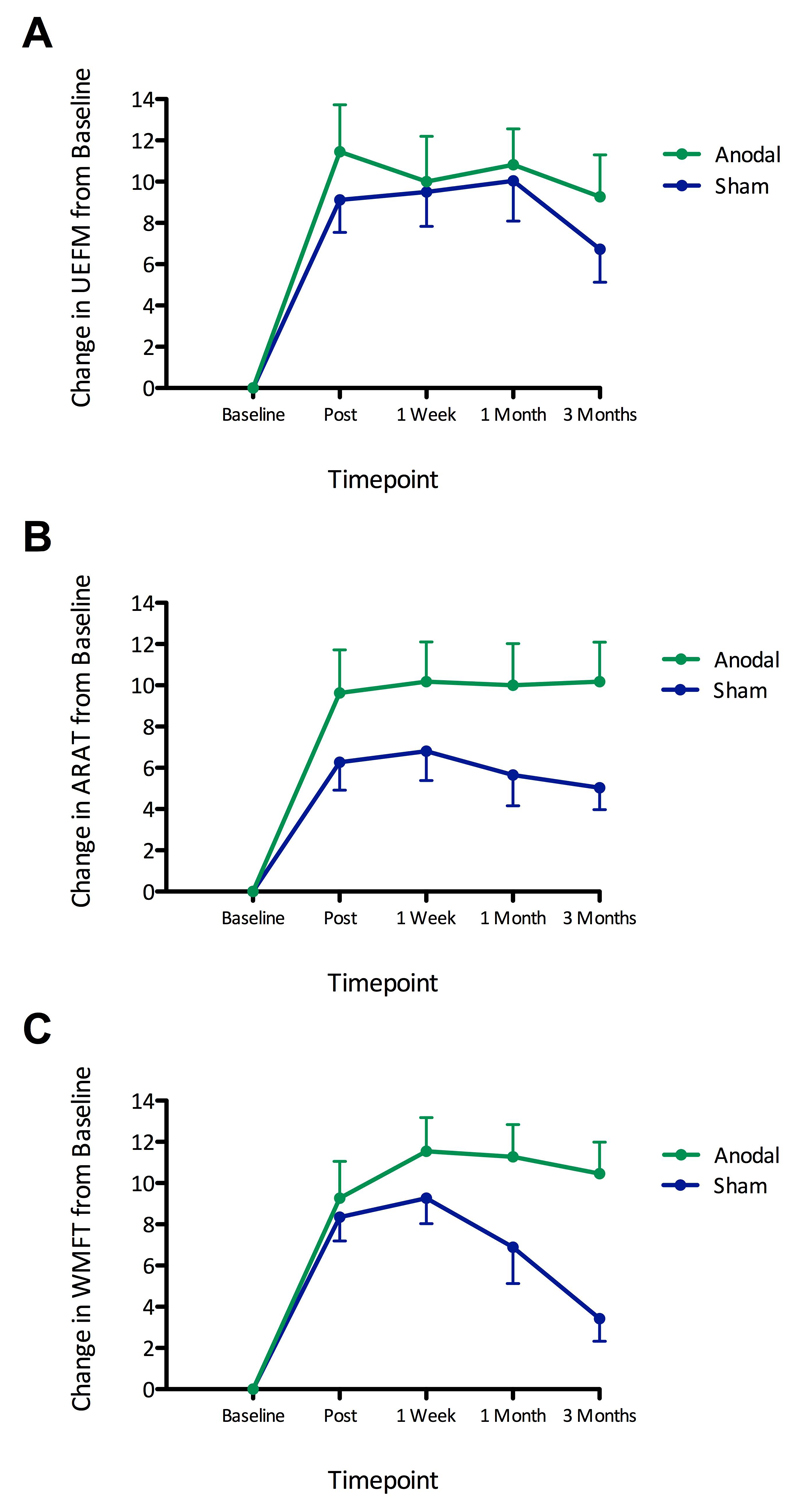
Figure 1. Increased clinical scores in the anodal tDCS group compared to the sham treatment group.
We assessed UEFM, ARAT, and WMFT clinical scale ratings before and at multiple time points after rehabilitation and either anodal tDCS or sham treatment. Shown are changes in scores from baseline for UEFM (A), ARAT (B), and WMFT (C) for anodal tDCS (green, n=11) and sham treatment (blue, n=13) groups, for the four post-intervention timepoints, after regressing out the respective baselines. Error bars represent standard errors of the mean. Fisher’s combined probability tests showed greater scores in the anodal tDCS group compared to the sham treatment group combined across all tests and all timepoints (p=0.008). Considering each test separately, greater scores were found in the anodal tDCS group for both ARAT (p=0.031) and WMFT (p=0.037) but not for UEFM (p=0.329). For the timepoint of primary interest, the 3 month follow-up, Fisher’s combined probability test showed greater scores for the anodal tDCS group compared to the sham treatment group when combining across all tests (p=0.004). Considering each test separately, greater scores were found in the anodal tDCS group at the 3-month follow up for both ARAT (p=0.045) and WMFT (p=0.001), but not for UEFM (p=0.550). All p-values were corrected for multiple comparisons across measures/timepoints as appropriate. Changes in clinical scores from baseline in the anodal tDCS and sham treatment groups are shown separately in Figure S4.
To test for any effect of anodal tDCS on clinical outcomes, we first ran a single global test combining all three clinical measures and all four post-intervention timepoints, and tested for a difference between the anodal tDCS and sham group using Fisher’s combined probability test, assessed with permutations. This showed greater scores in the anodal tDCS group than the sham group [Fisher’s χ2(24)=71.00, p=0.008]. Next, we ran the same test for each clinical measure separately, pooling across all post intervention timepoints. We found higher scores for the anodal tDCS group compared to the sham group for both ARAT [χ2(8)=28.88, p=0.031, corrected for the 3 measures considered], and WMFT [χ2(8)=27.91, corrected p=0.037] but not for UEFM [χ2(8)=14.22, corrected p=0.329].
The timepoint of primary interest was the 3 month followup. At this timepoint, pooling across all clinical measures revealed greater scores in the anodal tDCS group compared to the sham group [χ2(6)=29.06, p=0.004, correcting for 4 timepoints]. At the 3month followup, the mean absolute difference in the change in clinical scores from baseline between anodal tDCS and sham groups was 2.898 for UEFM [95% CI: -2.136 to 7.932; t(21)=1.20; p=0.550)] 5.763 for ARAT [95% CI: 1.560 to 9.966; t(21)=2.85, p=0.045], and 6.871 for WMFT [95% CI: 3.411 to 10.331; t(21)=4.13; p=0.001; these p-values were corrected for family-wise error rate across all tests, i.e., 4 timepoints, 3 clinical scores]. At earlier timepoints and pooling across clinical measures using Fisher’s test, we observed similar trends but these failed to reach significance after correction for the 4 timepoints [immediately post: χ2(6)=13.23, corrected p=0.195; 1 week: χ2(6)=12.05, corrected p=0.248; 1 month: χ2(6)=16.66, corrected p=0.092].
Increased activity and gray matter volume in ipsilesional motor areas in the anodal tDCS compared to sham treatment group
MRI data were unavailable for patient 08 (due to claustrophobia) and patient 23 (due to scheduling constraints), both of whom were in the sham group. Neuroimaging analysis was therefore based on 11 participants per group. We considered fMRI measures of activity during passive movement of the affected hand, voxel-based morphometry (VBM) measures of gray matter volume, and fractional anisotropy (FA) asymmetry measures of the corticospinal tract. For all voxel-wise analyses, images from patients with right hemispheric strokes were flipped about the midline after registration to standard space so that all lesions appeared on the left side.
We first tested for correlations between clinical sores and imaging measures at baseline. No correlations were found for gray matter volume or movement-related fMRI activity. However, baseline asymmetry of corticospinal tract FA correlated negatively with all baseline clinical scores (Spearman’s test: ARAT ρ=-0.77, p< 0.001; UEFM ρ=-0.79, p<0.001; WMFT ρ=-0.78, p<0.001).
We next tested whether baseline imaging measures correlated with subsequent change in clinical scores due to the intervention. No significant relationships were found for fMRI or VBM measures. FA asymmetry correlated with subsequent behavioral improvements for UEFM (ρ=0.51, p=0.015) such that greater corticospinal tract asymmetry at baseline was associated with greater intervention-mediated behavioral improvements. However, this relationship did not survive co-varying out the baseline UEFM score (r=-0.67, p=0.8).
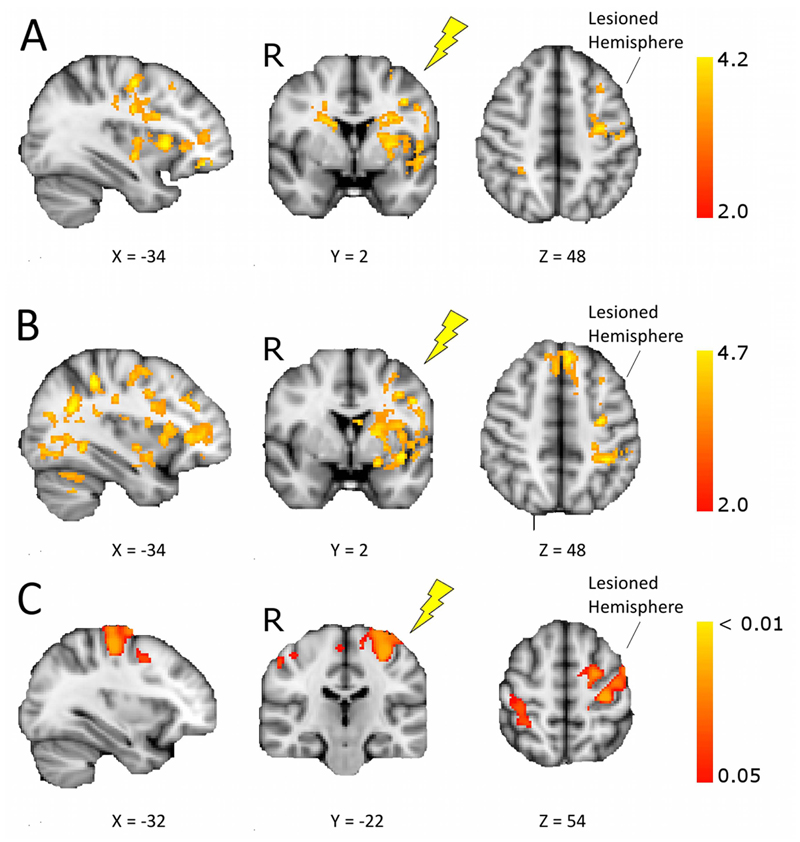
Comparing changes in imaging measures from pre to post intervention between groups revealed greater increases in the anodal tDCS group compared to the sham group for fMRI activity and gray matter VBM but not for FA asymmetry. Specifically, larger increases in movement-related fMRI activation were found in the anodal tDCS group compared to the sham group (cluster p<0.05, corrected), in regions including ipsilesional motor areas, both immediately after the intervention (fig. 2A; Table S1) and at one month follow up (fig. 2B; Table S1). These fMRI results were similar after controlling for variation in gray matter (fig. S5). For gray matter VBM, greater increases were found immediately after the intervention in the anodal tDCS group relative to the sham group in ipsilesional premotor cortex, primary motor cortex and the contralesional postcentral gyrus (fig. 2C). Within this cluster, direction of mean change in gray matter volume was positive for the anodal tDCS group (0.0169±0.021) and negative for the sham group (-0.0210±0.023). However, when we tested across the whole brain for changes in gray matter volume from baseline in each group separately we did not find any significant clusters. Therefore, while our results show a greater increase in gray matter in the anodal tDCS group compared to the sham group, we did not have evidence regarding the direction of gray matter change in the absence of tDCS. No significant correlations were found between changes in clinical scores and changes in fMRI or VBM measures for any of the clusters. FA asymmetry values did not change after motor training (RM-ANOVA, main effect of time, and group x time interaction: p>=0.5).
Figure 2. Increased fMRI activity and gray matter volume in the anodal tDCS group compared to the sham treatment group.
Shown are changes in fMRI activation and gray matter volume before and after intervention in stroke patients receiving motor rehabilitation plus anodal tDCS or sham treatment (post>pre, anodal>sham, voxel-wise General Linear Model, p<0.05 corrected). Images are in radiological convention (R – right), and all lesions appear on the left, the target for tDCS (yellow flash). The X or Y coordinates for each brain slice are given below the slice. (A) Brain regions showing increases in fMRI activity during affected hand movement from baseline to immediately post-intervention for the anodal tDCS group versus the sham treatment group. (B) Regions showing greater increases in movement-related fMRI activity for anodal tDCS versus sham group from baseline to one month follow-up. See table S1 for the location and Z statistic of peak voxels from fMRI analysis. (C) Brain regions showing increases in gray matter density as assessed by voxel-based morphometry (VBM), from baseline to immediately post-intervention for the anodal tDCS group versus the sham treatment group.
Discussion
We report long-term improvements in upper limb ability in patients receiving repeated sessions of anodal tDCS to the ipslesional motor cortex compared to the sham-treated group when tDCS was paired with motor training. We also found that these clinical improvements were associated with increased activation of ipsilesional motor cortical areas.
Previous proof-of-principle studies have shown that single sessions of anodal tDCS to the ipsilesional motor cortex temporarily improved motor function (19). For tDCS to have clinical relevance, however, it is critical that it be tested for long-term benefit. In healthy volunteers, repeated sessions of anodal tDCS paired with motor training provided long-lasting behavioral improvements (20). In chronic stroke patients, benefits have been found immediately or at one week after repeated sessions using other electrode configurations (10–12). One small study of sub-acute stroke patients found benefits of cathodal tDCS to contralesional motor cortex compared to sham treatment at six months of follow-up, but only a trend for beneficial long-term outcomes after anodal tDCS (21). However, it is unclear whether cathodal tDCS to the contralesional hemisphere is suitable for all patients as some patients may depend on contralesional activation for movement of the affected hand (22, 23). We therefore investigated the long-term effects of anodal tDCS on patients undergoing motor rehabilitation.
Clinical outcomes from a rehabilitation intervention can be manifest at different levels, including the domains of body function, activity, or participation. Focusing on a single domain may risk missing effects on other domains (24, 25). To maximise our chances of detecting effects that could then be followed up in future targeted trials, we considered outcomes using three clinical scales in the domains of body function (UEFM) and activity (ARAT, WMFT). While the GRASP program itself reduced impairment and improved activity (i.e., UEFM, ARAT and WMFT improved in both group), tDCS only modulated activity improvements. Our findings therefore suggest that anodal tDCS to the ipsilesional hemisphere may exert its effects by enhancing activity and reducing functional limitations, rather than by changing the impairment.
Improvements in activity may be mediated not only by reductions in impairment, but also through motor learning achieved through repetitive training on specific motor tasks. In line with others (27–29), our rationale for combining training strategies with adjunctive approaches depends on the fact that motor training programs such as GRASP involve motor learning and motor learning is possible in stroke patients (30, 31). Anodal tDCS applied to motor cortex has been shown to improve motor learning in healthy subjects (20) in part through local disinhibition (7).
Improvements in activity may be considered as ‘compensation’ as distinct from ‘true recovery’, which can only occur if impairment is reduced and original pathways for movement are restored (4, 27, 32, 33). This distinction is theoretically useful for understanding the level through which tDCS exerted its effects and is important for conceptualising a theory-based rationale for how best to employ tDCS in stroke rehabilitation. However, even ‘compensation’ can be useful to the patient if it allows them to perform movement tasks more effectively than before.
If anodal tDCS exerts its beneficial effects by enhancing learning, it is important to establish whether patients simply improve performance of specific trained movements or whether improvements can be generalised to non-trained items (28). There was some overlap between specific items included in the GRASP training and elements of the ARAT/WMFT assessment, and so tDCS may in part have exerted its effects through boosting task-specific training. However, the specific movements trained in the GRASP program differ among patients depending on the level at which they enter the program, which was variable in this trial (number of patients at level1/level 2/level 3 were: anodal tDCS 5/2/4 patients; sham, 4/3/6 patients). Further, there are items included in ARAT/WMFT which do not feature in GRASP. For example, adjusting a trained pinch grip to accommodate a different shaped object requires at least some degree of generalisation. It will be important for future studies to design training and testing regimens in order to fully characterise the extent to which task-specific training versus generalisation can be boosted by tDCS.
To test whether changes in brain structure and function could explain any observed clinical variation, we assessed neuroimaging measures. At baseline, worse clinical scores were associated with greater asymmetry of cortico-spinal tract microstructure, whereas no correlations were found between baseline clinical scores and fMRI or gray matter volume. We tested whether baseline neuroimaging measures predicted response to motor training, as shown previously (34). This is a clinically important question as such measures could potentially be used to target interventions at those patients who are most likely to benefit. Although we found correlations between baseline measures of corticospinal tract integrity and response to the intervention, these relationships did not increase predictive power above what could be explained by baseline clinical scores alone. Studies in larger and more variable patient groups will be required to assess the added value of such measures for clinical decision making.
We found greater increases in fMRI activation and gray matter volume in ipsilesional motor cortical areas in the anodal tDCS group compared to the sham group. Similar fMRI changes have been reported after a single session of anodal tDCS (35), and for serial sessions of other electrode configurations (11, 12). A previous study reported an increase in gray matter with rehabilitative training alone (36), whereas here, although we found changes in gray matter between the two groups, within-group comparisons did not reveal clear evidence for a significant change over time for either group. We found no change in diffusion MRI measures of white matter microstructure with training between the groups, unlike a previous study showing new motor learning in healthy individuals (37).
Together with our previous observations of short-term training interventions (38), these findings suggest that the condition of residual brain pathways, as measured by diffusion MRI, may place some constraints on motor ability (as reflected by baseline correlations between FA asymmetry and clinical sores). In spite of this, further gains in function may be possible, and are more strongly associated with altered activation of motor cortical areas and gray matter structural changes, rather than with changes in white matter connectivity.
There are some limitations to this trial. First, although our sample size compares favourably to other studies of anodal tDCS in chronic stroke, a larger sample would provide greater power for identifying predictors of response. Second, the assessing researcher, who was blind to stimulation condition, also delivered the training. It is possible that knowledge of how participants had performed during the training session could have influenced their assessment of outcomes. Although we do not believe this would unblind the assessor, it could potentially inflate any differences between participants that began to be apparent during the training sessions. In addition, the treating assessor, who operated the tDCS equipment, was not blind to stimulation conditions. Although this experimenter played no role in the training, and was not present at assessment sessions, it is possible that their behavior may have influenced the patient or the training researcher during training sessions. Future studies could make use of tDCS equipment that allows for stimulation protocols to be pre-programmed using a code in order to ensure that all those present are blind to stimulation conditions. Furthermore, because our hypothesis concerned the use of anodal tDCS as an adjunct to motor training we included motor training in both arms of our study. Because we did not include an arm without motor training or tDCS we cannot comment about the effect of the motor training itself, although this has been studied previously (15). Finally, as we did not test any healthy controls, our data do not provide evidence on whether the changes seen here would also be seen in healthy individuals.
Future larger studies are required to assess whether patient characteristics, such as baseline clinical scores or brain measures, could be used to stratify patients for maximal benefit. In addition, while our study was well tolerated, future trials using a less-intensive training regimen, perhaps consisting of 2-3 sessions per week, will be important to translate these findings into a clinical setting.
Materials and Methods
Study design
This was a stratified, double blind, sham-controlled, parallel-group study designed to test the effects of anodal tDCS as an adjunct to motor training in chronic stroke patients. Clinical tests were used to assess upper limb function before and after motor training paired with either real or sham tDCS. Neuroimaging measures were also taken before and after the intervention. The study is registered with clinicaltrials.gov (NCT01414582) and was conducted in accordance with CONSORT guidelines. No replication was performed. The study was conducted in the United Kingdom and all training and testing sessions took place within the John Radcliffe Hospital, Oxford. We aimed to recruit patients who were at least six months post a single unilateral ischemic or hemorrhagic stroke affecting motor function in the contralesional hand. Of 1191 patients assessed for eligibility, 26 were randomized to receive either anodal or sham tDCS, and 24 completed the intervention (fig. S1, Table 1); Patient sex, age at intervention, time post stroke, lesion side, type and volume did not differ between the anodal and sham groups (Table 1). Location of lesions is shown in figure S2; no lesions were in the primary motor cortex.
All those who took part gave written informed consent to participate in accordance with local Research Ethics Committee approval after the nature and possible consequences of the study was explained. No participant had any history, signs or symptoms of any other neurological condition, nor did they have dysphasia that limited communication. Exclusion criteria were: previous stroke or stroke affecting the primary motor cortex; inability to provide informed consent due to severe language or cognitive impairment; contraindications for tDCS. Participants with contraindications for MRI did not undergo MRI scans.
Clinical assessments and MRI were carried out at multiple time-points before and after the intervention period (fig. S3). During the intervention period participants conducted daily, supervised one-hour sessions of the Graded Repetitive Arm Supplementary Program (GRASP) (15) over 9 days. For the first 20 minutes of each session tDCS electrodes were positioned on the participant’s scalp to deliver anodal tDCS or sham treatment.
Randomisation and masking
Following the first baseline assessment, patients were randomised to the anodal or sham stimulation group. A random number generator was used to assign conditions in blocks of 4, stratified by starting level on the motor training program (see below). Randomisation was performed by a researcher (HJB) who was not involved in any baseline assessments and allocation was communicated to one other researcher (UA). Motor training was carried out by a researcher blind to stimulation conditions (assessing researcher: CA, with occasional cover by another blinded researcher, CS). All clinical assessments were scored by a researcher blind to stimulation conditions (CA). tDCS was delivered by a researcher who was aware of the stimulation conditions (treating researcher: UA, with occasional cover by HJB) and who was not involved in any clinical assessments or motor training.
Intervention: Motor training and tDCS
Participants conducted daily, supervised one-hour sessions of the Graded Repetitive Arm Supplementary Program (GRASP) (15), over 9 days (Monday-Friday; Monday-Thursday). Patients began the program at one of three different starting levels, depending on initial assessment of their motor abilities by a physiotherapist. There was a good spread of starting levels across patients and our randomisation procedure ensured that these were fairly distributed across both stimulation groups (number of patients within each group starting at level1/level 2/level 3: anodal: 5/2/4; sham: 4/3/6). Progression was achieved by increasing repetitions and changing the weight or sizes of objects used. Motor training was supervised by the assessing researcher, who was blind to stimulation conditions. At the start of each session, this same researcher positioned two 5x7cm electrodes, encased in saline-soaked sponges, on the participant’s scalp, one centred over ipsilesional primary motor cortex (5cm lateral to Cz: C3) and the other over the contralateral supraorbital ridge. The electrodes connected to a direct current stimulator (Eldith GmbH; Germany), which was controlled by the treating researcher. For anodal stimulation the current was ramped up over ten seconds, held at a constant 1mA for 20 minutes and then ramped down over ten seconds. For sham stimulation, the current was ramped up over ten seconds and then immediately switched off. Stimulation commenced at the same time as the motor training protocol. After 20 minutes the electrodes were removed, and motor training continued for a further 40 minutes.
Clinical assessments
Clinical outcomes were assessed at multiple timepoints following the intervention (fig. S3) by a researcher blind to stimulation conditions (CA). The clinical outcome measures were the Upper Extremity Fugl-Meyer Assessment (UEFM) (16), Action Research Arm Test (ARAT) (17) and Wolf Motor Function Test (WMFT) (18).
Statistical analysis of clinical assessments
Statistical analysis of clinical scores was carried out using MATLAB R2013b. To test our primary hypothesis that greater clinical scores would be found in the anodal tDCS group, each score was subjected to a multiple regression using the General Linear Model (GLM). The model variables were group (sham treatment and anodal tDCS) and the baseline for the respective score. We implemented the Non-Parametric Combination (39, 40) to allow for combined tests that interrogate aggregate effects of anodal tDCS on scores and timepoints (see supplementary methods). We first ran a single global test, pooling the clinical scores and the post-intervention timepoints using the Fisher’s combined probability test. To assess which clinical measures were showing any effect, we next repeated the procedure for each measure, pooling all post-intervention timepoints, and computing family-wise error rate-corrected p-values for each Fisher’s χ2 combined statistic. Next, as we were primarily interested in long-lasting clinical effects, we ran a similar Non-Parametric Combination strategy using Fisher’s test on data from the 3-month follow up, pooling the 3 clinical measures, and computing p-values family-wise error rate-corrected across the four post-intervention timepoints. Finally, for each clinical measure, we reported the mean absolute difference and parametric confidence intervals (95%) between anodal tDCS and sham groups at the 3-month follow up, and tested the significance of this difference.
To assess the timecourse and persistence of clinical gains within each group, we additionally ran paired t-tests between baseline scores (for ARAT, UEFM and WMFT) and scores at each post-intervention timepoint (immediate, 1 week, 1 month, 3 months) as well as between the immediate post-intervention timepoint and all subsequent timepoints (1 week, 1 month, 3 months). Permutation testing was run for each group separately (anodal tDCS, sham) to generate p-values, correcting for the 3 clinical scores and 7 timepoint pairings assessed for each group.
MRI Acquisition and Statistical Analysis
MRI was performed on a 3T Verio scanner (Siemens, Erlangen, Germany) and included T1-weighted MRI (MPRAGE, voxel size=1mm isotropic, TR=2040ms, TE=4.7ms, flip angle=8°), and diffusion MRI (voxel size= 2mm isotropic; TR=9600ms; TE= 87ms; 2 repeats of 60 directions, b-value of 1000 s/mm2; and 8 volumes without diffusion weighting, b-value=0 s/mm2). During functional MRI (TR=2410ms, TE=30ms, flip angle=90°, voxel size=3x3x3mm, 44 axial slices), passive flexion-extension of the stroke-affected hand was performed manually by a researcher, cued by a 1Hz auditory cue. Movement and rest blocks alternated in 30 second periods.
Pre-processing and statistical analyses of MRI data was carried out using tools from the FMRIB Software Library (www.fmrib.ox.ac.uk/fsl) (41) (see Supplementary methods).
In brief, a standard FSL-VBM pipeline was used to preprocess data prior to analysis of longitudinal changes in grey matter. Images were then analysed using permutation-based testing to test for statistical differences between timepoints and groups and for correlations with clinical scores. Threshold-free cluster enhancement was used to determine significance at p<0.05, corrected for family-wise error. For clusters showing significant effects, mean voxel-wise estimates of grey matter were extracted for each subject for correlation with other variables.
Task fMRI data were pre-processed and analysed using FEAT (see Supplementary Methods). A boxcar regressor modelling the 30-second task and rest blocks was used to create first-level statistical maps for each patient at each timepoint. Higher-level, mixed effects analyses were then run using FLAME to compare activation maps across groups and timepoints and to test for correlations with clinical scores. Z statistic images were thresholded with an initial cluster-forming threshold of Z = 2.0, and a corrected cluster extent threshold of p < 0.01. For clusters showing significant effects, mean voxel-wise contrasts of parameter estimates were extracted for each subject for correlation with other variables.
Diffusion data were pre-processed using FMRIB’s Diffusion Toolbox (FDT). For each patient and timepoint, mean voxel-wise values of fractional anisotropy (FA) were extracted from within standard-space corticospinal tract (CST) regions of interest. Asymmetry of the corticospinal tracts was calculated as: (Contralesional CST FA – Ipsilesional CST FA) / (Contralesional CST FA + Ipsilesional CST FA), with larger asymmetry reflecting relatively lower FA values in the ipsilesional CST. Statistical analysis of diffusion MRI measures of FA asymmetry in the corticospinal tract was carried out using SPSS (Statistical Package for the Social Sciences, Version 22). Measures were compared between groups and timepoints using repeated measures ANOVA, with p<0.05 considered significant.
Correlations between imaging variables and clinical scores were carried out using SPSS. Correlations were calculated using Pearson’s or Spearman’s correlation, depending on whether or not tests of normality were significant. A significance threshold of p<0.017 (two-tailed) was used to correct for the three clinical scores considered.
Supplementary Material
One Sentence Summary.
Anodal tDCS applied to the ipsilesional motor cortex of patients with chronic stroke enhances the beneficial effects of motor rehabilitation.
Editor’s Summary. Stimulating motor recovery in stroke.
Rehabilitation of movement after stroke requires repeated practice and involves learning and brain changes. We tested whether delivering brain stimulation during a 9 day course of hand and arm training improved movement in patients after stroke. We found greater improvements in movement in patients who received real compared to sham (placebo) brain stimulation. Better scores in patients who received real stimulation were still present 3 months after training ended. Our results suggest that brain stimulation could be added to rehabilitative training to improve outcomes in stroke patients.
Acknowledgments
We thank Charlotte Winward for providing baseline functional assessments of participants. Funding: Supported by the Dunhill Medical Trust, Oxford NIHR Biomedical Research Centre, Wellcome Trust, Medical Research Council and The People Programme (Marie Curie Actions) of the European Union's Seventh Framework Programme FP7/2007-2013/ under REA grant agreement no. PITN-GA-2011-290011.
Footnotes
Author contributions: HJB, CS and UK designed the study. CA, UA carried out the majority of data acquisition and LW, CS, and HJB contributed to data acquisition. CA, UA, NF, CS, AMW and HJB were involved in analysis of data. CA, UA, CS and HJB were involved in interpretation of data. HJB, CA and UA drafted the manuscript. CA, UA, AMW, LW, NF, UK, CS and HJB revised the manuscript and approved the final version.
Competing interests: All authors declare that they have no competing interests.
Data and materials availability: Clinical scores (after regression of baseline) and MATLAB code used to analyze clinical scores are available in the supplementary materials. Raw clinical scores can be obtained on request from H.J-B.
References
- 1.Sunderland A, Tinson DJ, Bradley EL, Fletcher D, Langton HR, Wade DT. Enhanced physical therapy improves recovery of arm function after stroke. A randomised controlled trial. J Neurol Neurosurg Psychiatry. 1992;55:530. doi: 10.1136/jnnp.55.7.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwakkel G, Wagenaar RC, Twisk JW, Lankhorst GJ, Koetsier JC. Intensity of leg and arm training after primary middle-cerebral-artery stroke: a randomised trial. Lancet. 1999;354:191. doi: 10.1016/S0140-6736(98)09477-X. [DOI] [PubMed] [Google Scholar]
- 3.Stagg C, Johansen-Berg H. In: Stroke Rehabilitation: Insights from Neuroscience and Imaging. Carey LM, editor. Oxford University Press; Oxford: 2011. [Google Scholar]
- 4.Krakauer JW. Motor learning: its relevance to stroke recovery and neurorehabilitation. Curr Opin Neurol. 2006;19:84–90. doi: 10.1097/01.wco.0000200544.29915.cc. [DOI] [PubMed] [Google Scholar]
- 5.Matthews PM, Johansen-Berg H, Reddy H. Non-invasive mapping of brain functions and brain recovery: Applying lessions from cognitive neuroscience to neurorehabilitation. Restor Neurol Neurosci. 2004;20:1–16. [PubMed] [Google Scholar]
- 6.Nitsche M, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stagg CJ, Best JG, Stephenson MC, O'Shea J, Wylezinska M, Kincses ZT, Morris PG, Matthews PM, Johansen-Berg H. Polarity-Sensitive Modulation of Cortical Neurotransmitters by Transcranial Stimulation. J Neurosci. 2009;29:5202–5206. doi: 10.1523/JNEUROSCI.4432-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stagg CJ, Jayaram G, Pastor D, Kincses ZT, Matthews PM, Johansen-Berg H. Polarity and timing-dependent effects of transcranial direct current stimulation in explicit motor learning. Neuropsychologia. 2011;49:800–804. doi: 10.1016/j.neuropsychologia.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nitsche M, Schauenburg A, Lang N, Liebetanz D, Exner C, Paulus W, Tergau F. Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. J Cogn Neurosci. 2003;15:619–626. doi: 10.1162/089892903321662994. [DOI] [PubMed] [Google Scholar]
- 10.Boggio P, Nunes A, Rigonatti S, Nitsche M, Pascual-Leone A, Fregni F. Repeated sessions of noninvasive brain DC stimulation is associated with motor function improvement in stroke patients. Restor Neurol Neurosci. 2007;25:123–129. [PubMed] [Google Scholar]
- 11.Nair DG, Renga V, Lindenberg R, Zhu L, Schlaug G. Optimizing recovery potential through simultaneous occupational therapy and non-invasive brain-stimulation using tDCS. Restor Neurol Neurosci. 2011;29:411–420. doi: 10.3233/RNN-2011-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindenberg R, Renga V, Zhu LL, Nair D, Schlaug G. Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology. 2010;75:2176–2184. doi: 10.1212/WNL.0b013e318202013a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bastani A, Jaberzadeh S. Clinical Neurophysiology. Clin Neurophysiol. 2012;123:644–657. doi: 10.1016/j.clinph.2011.08.029. [DOI] [PubMed] [Google Scholar]
- 14.Butler A, Shuster M, O'Hara E, Hurley K, Middlebrooks D, Guilkey K. A meta-analysis of the efficacy of anodal transcranial direct current stimulation for upper limb motor recovery in stroke survivors. J Hand Ther. 2013;26:162–171. doi: 10.1016/j.jht.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Harris JE, Eng JJ, Miller WC, Dawson AS. A self-administered Graded Repetitive Arm Supplementary Program (GRASP) improves arm function during inpatient stroke rehabilitation: a multi-site randomized controlled trial. Stroke. 2009;40:2123–2128. doi: 10.1161/STROKEAHA.108.544585. [DOI] [PubMed] [Google Scholar]
- 16.Fugl-Meyer AR, Jääskö L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. Scand J Rehab Med. 1975;7:13–31. [PubMed] [Google Scholar]
- 17.Lyle RC. A performance test for assessment of upper limb function in physical rehabilitation treatment and research. Int J Rehabil Res. 1981;4:483–492. doi: 10.1097/00004356-198112000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Wolf SL, Catlin PA, Ellis M, Archer AL, Morgan B, Piacentino A. Assessing Wolf motor function test as outcome measure for research in patients after stroke. Stroke. 2001;32:1635–1639. doi: 10.1161/01.str.32.7.1635. [DOI] [PubMed] [Google Scholar]
- 19.Hummel FC, Cohen LG. Non-invasive brain stimulation: a new strategy to improve neurorehabilitation after stroke? Lancet Neurol. 2006;5:708–712. doi: 10.1016/S1474-4422(06)70525-7. [DOI] [PubMed] [Google Scholar]
- 20.Reis J, Schambra H, Cohen LG, Buch ER, Fritsch B, Zarahn E, Celnik P, Krakauer J. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci. 2009;106:1590–1595. doi: 10.1073/pnas.0805413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim D-Y, Lim J-Y, Kang EK, You DS, Oh M-K, Oh B-M, Paik N-J. Effect of transcranial direct current stimulation on motor recovery in patients with subacute stroke. Am J Phys Med Rehabil. 2010;89:879–886. doi: 10.1097/PHM.0b013e3181f70aa7. [DOI] [PubMed] [Google Scholar]
- 22.Johansen-Berg H, Rushworth M, Bogdanovic M, Kischka U, Matthews P. The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci U S A. 2002;99:14518–14523. doi: 10.1073/pnas.222536799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lotze M, Markert J, Sauseng P, Hoppe J, Plewnia C, Gerloff C. The role of multiple contralesional motor areas for complex hand movements after internal capsular lesion. J Neurosci. 2006;26:6096–6102. doi: 10.1523/JNEUROSCI.4564-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsieh YW, Wu CY, Lin KC, Chang YF, Chen CL, Liu JS. Responsiveness and Validity of Three Outcome Measures of Motor Function After Stroke Rehabilitation. Stroke. 2009;40:1386–1391. doi: 10.1161/STROKEAHA.108.530584. [DOI] [PubMed] [Google Scholar]
- 25.Lin JH, Hsu MJ, Sheu CF, Wu TS, Lin RT, Chen CH, Hsieh C-L. Psychometric Comparisons of 4 Measures for Assessing Upper-Extremity Function in People With Stroke. Phys Ther. 2009;89:840–850. doi: 10.2522/ptj.20080285. [DOI] [PubMed] [Google Scholar]
- 26.Pollock A, Farmer SE, Brady MC, Langhorne P, Mead GE, Mehrholz J, van Wijck F. Interventions for improving upper limb function after stroke. Cochrane database of systematic reviews (Online) 2014;11:CD010820–CD010820. doi: 10.1002/14651858.CD010820.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeiler SR, Krakauer JW. The interaction between training and plasticity in the poststroke brain. Curr Opin Neurol. 2013;26:609–616. doi: 10.1097/WCO.0000000000000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kitago T, Goldsmith J, Harran M, Kane L, Berard J, Huang S, Ryan SL, Mazzoni P, Krakauer JW, Huang VS. Robotic therapy for chronic stroke: general recovery of impairment or improved task-specific skill? J Neurophysiol. 2015;114:1885–1894. doi: 10.1152/jn.00336.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krakauer JW. In: Oxford Textbook of Neurorehabilitation. Dietz V, Ward N, editors. Oxford University Press; [Google Scholar]
- 30.Borich MR, Brown KE, Boyd LA. Motor Skill Learning Is Associated With Diffusion Characteristics of White Matter in Individuals With Chronic Stroke. J Neurol Phys Ther. 2013 doi: 10.1097/NPT.0b013e3182a3d353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boyd LA, Quaney BM, Pohl PS, Winstein CJ. Learning Implicitly: Effects of Task and Severity After Stroke. Neurorehabil Neural Repair. 2007;21:444–454. doi: 10.1177/1545968307300438. [DOI] [PubMed] [Google Scholar]
- 32.Buma F, Kwakkel G, Ramsey N. Understanding upper limb recovery after stroke. Rest Neurol Neurosci. 2013;31:707–722. doi: 10.3233/RNN-130332. [DOI] [PubMed] [Google Scholar]
- 33.Levin M, Kleim J, Wolf S. What Do Motor “Recovery” and “Compensation” Mean in Patients Following Stroke? Neurorehabil Neural Repair. 2008 doi: 10.1177/1545968308328727. [DOI] [PubMed] [Google Scholar]
- 34.Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2007;130:170–180. doi: 10.1093/brain/awl333. [DOI] [PubMed] [Google Scholar]
- 35.Stagg CJ, Bachtiar V, O'Shea J, Allman C, Bosnell RA, Kischka U, Matthews PM, Johansen-Berg H. Cortical activation changes underlying stimulation-induced behavioural gains in chronic stroke. Brain. 2012;135:276–284. doi: 10.1093/brain/awr313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gauthier LV, Taub E, Perkins C, Ortmann M, Mark VW, Uswatte G. Remodeling the brain: plastic structural brain changes produced by different motor therapies after stroke. Stroke. 2008;39:1520–1525. doi: 10.1161/STROKEAHA.107.502229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scholz J, Klein MC, Behrens TEJ, Johansen-Berg H. Training induces changes in white-matter architecture. Nat Neurosci. 2009;12:1370–1371. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bosnell RA, Kincses ZT, Stagg CJ, Tomassini V, Kischka U, Jbabdi S, Woolrich MW, Andersson J, Matthews PM, Johansen-Berg H. Motor Practice Promotes Increased Activity in Brain Regions Structurally Disconnected After Subcortical Stroke. Neurorehabil Neural Repair. 2011;25:607–616. doi: 10.1177/1545968311405675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pesarin F, Salmaso L. Permutation Tests for Complex Data: Theory, Applications and Software. John Wiley and Sons; West Sussex: 2010. [Google Scholar]
- 40.Winkler AM, Webster MA, Brooks JCW, Tracey I, Smith S, Nichols TE. Non-Parametric Combination and related permutation tests for neuroimaging. Hum Brain Map. doi: 10.1002/hbm.23115. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith SM, Jenkinson M, Woolrich M, Beckmann C, Behrens T, Johansen-Berg H, Bannister P, De Luca M, Drobnjak I, Flitney D, Niazy R, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 42.Shapiro SS, Wilk MB. An analysis of variance test for normality (complete samples) Biometrika. 1965:591–611. [Google Scholar]
- 43.Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. NeuroImage. 2014;92:381–397. doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Douaud G, Smith S, Jenkinson M, Behrens T, Johansen-Berg H, Vickers J, James S, Voets N, Watkins K, Matthews PM, James A. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain. 2007;130:2375–2386. doi: 10.1093/brain/awm184. [DOI] [PubMed] [Google Scholar]
- 45.Jenkinson M, Bannister P, Brady J, Smith S. Improved optimisation for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 46.Beckmann C, Smith S. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans on Medical Imaging. 2004;23:137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- 47.Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. NeuroImage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- 48.Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. NeuroImage. 2009;48:63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Behrens TEJ, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magnetic Resonance in Medicine. 2003;50:1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- 50.Behrens TEJ, Johansen-Berg H, Jbabdi S, Rushworth MFS, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? NeuroImage. 2007;34:144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.