Abstract
Purpose
To investigate the relationship between visual function and severity of early age-related macular degeneration (AMD) and activity of neovascular (nv-) AMD.
Methods
The following data was collected from 66 eyes of 66 subjects with early AMD and 47 eyes of 47 subjects with active nv-AMD: corrected distance visual acuity (CDVA); contrast sensitivity (CS); glare disability (GD); and retinotopic ocular sensitivity (ROS) of the central 5° of the retina, by microperimetry. Fundus photographic grading of early AMD was performed in a masked fashion. Mean foveal thickness (MFT) was measured by spectral domain optical coherence tomography in patients with nv-AMD.
Results
In subjects with early AMD, there was an inverse and statistically significant relationship between measures of ROS within the central 5° of retina (including fixation) and severity of early AMD (p=0.01). In eyes with active nv-AMD, an inverse and statistically significant relationship was observed between measures of MFT and measures of ROS at the central 5° of retina (r=-0.34; p=0.02). No other measures, including CDVA, were significantly related to severity of early AMD, or to MFT in nv-AMD.
Conclusion
Although ROS was cross-sectionally associated with disease severity, and inversely related to MFT, an important determinant of need-to-treat in cases of nv-AMD, further research is required to determine the appropriateness of ROS for monitoring early and active neovascular forms of this disease.
Keywords: Age-related macular degeneration, Visual function, Microperimetry, Retinotopic ocular sensitivity
Introduction
Age-related macular degeneration (AMD), a degenerative condition of the macula affecting individuals fifty years or older in most cases, is the leading cause of blind registration in the developed world [1]. Early AMD is characterised by large soft drusen and/or pigmentary changes, whereas the late form of AMD can be classed as atrophic or neovascular, the latter amenable to treatment with serial injections of anti-VEGF therapy. AMD often affects central vision, and in its advanced form has an adverse impact on an individual’s quality of life, as daily tasks such as reading, driving or recognising faces are impaired [2].
A diagnosis of AMD is determined by fundoscopic appearance of the macula, and is graded according to the morphological findings apparent on fundus photography. Several methods of grading AMD across the spectrum of early and late disease have been devised, and the grade of AMD reflects the severity of the condition, and increasing grade of early AMD is associated with increased risk of developing the late and visually consequential forms of the disease [3,4]. Optical coherence tomography (OCT) findings, although valuable, have not as yet been incorporated into any validated grading scheme, and fundus fluorescein angiography (FFA) remains a clinical tool that informs the decision-making process regarding treatment in cases and suspected cases of neovascular AMD (nv-AMD).
Beyond clinical signs and morphological findings, evaluation of the impact of disease on visual function is an important component of the clinical assessment of patients with AMD, and represents the ultimate subjective outcome measure for any proposed treatment.
In cases of early AMD and in cases of late AMD not involving the fovea, measures of corrected distance visual acuity (CDVA) may not provide a measure of daily visual experience, or of disease severity [4]. One limitation of CDVA rests on the fact that it measures the angular resolution limits of the eye at high contrast only. Therefore, CDVA does not capture changes in colour, contrast or other aspects of visual perception, all of which are subject to influence by environmental (e.g. lighting) and neurophysiological (e.g., state of retinal adaptation) factors. It has been shown that reliance on measures of visual acuity represent an under-appreciation of the functional visual difficulty experienced by a patient with early AMD, [4] as well as in other ophthalmic pathologies, such as glaucoma, cataract and diabetic retinopathy [5,6].
Early AMD is typically associated with a decrease in CDVA of two letters or fewer when compared to eyes without early disease, [7] and this observation should be viewed in the context that the test-retest variability of measures of CDVA can be up to as much as two lines of letters on a logMAR chart [8]. Late AMD, on the other hand, is associated with a more profound adverse impact on CDVA (approximately seven lines of letters), but only when signs of advanced AMD involve the central subfields of the macula [7]. Although there is no statistically significant difference in CDVA between subjects with nv-AMD and subjects with geographic atrophy (GA), [7] a wider range of CDVA has been demonstrated in eyes with GA, in spite of comparable areas of macular tissue involved by the atrophic changes, although, unsurprisingly and intuitively, foveal involvement was the key predictor of CDVA in cases of GA [9]. It has also been shown that, for eyes exhibiting comparable measures of CDVA, eyes with GA have worse visual function, particularly for dark adaptation and reading speed, than eyes with only drusen [10]. Similarly, lesion size in subfoveal nv-AMD fails to explain the wide variations in CDVA in such eyes [11]. In brief, therefore, CDVA is not a sensitive psychophysical measure that reflects severity of disease or subjective visual experience in cases of early AMD, or when atrophic or neovascular AMD does not affect the fovea. The purpose of this study was to explore psychophysical measures of visual function that might be used clinically in addition to CDVA in cases of early or nv-AMD, and to identify one that might be incorporated into the clinical setting.
Methods
Subjects
Baseline data were collected from 66 eyes (of 66 subjects) with early AMD and 47 eyes of 47 subjects with active nv-AMD.
The inclusion criteria for early AMD subjects were: early AMD, graded at the Ocular Epidemiology Group (University of Wisconsin, Madison, USA) in at least one eye (the study eye); CDVA of ≥ 20/40 in the study eye; no visually consequential ocular morbidity other than early AMD. Of note, in subjects who exhibited early AMD in both eyes, the eye with better CDVA was selected for the study.
Inclusion criteria for nv-AMD were: active nv-AMD in at least one eye (the study eye), confirmed by FFA and OCT; CDVA of ≥ 20/100 (to ensure participants could complete the series of visually demanding functional tests required); no visually consequential ocular pathology other than nv-AMD in the proposed study eye. In cases where both eyes had active nv-AMD, the eye with the better CDVA was selected for the study.
Visual function
The following psychophysical methods were used to assess visual function:
Corrected distance visual acuity
Corrected distance visual acuity was measured for the study eye monocularly, and with the patient’s best subjective refraction, using the logMAR chart (Test Chart 2000 PRO™; Thomson Software Solutions, Hertfordshire, England) and Early Treatment of Diabetic Retinopathy Study (ETDRS) lettersets, at a testing distance of 4 m.
Contrast sensitivity and glare disability
Contrast Sensitivity (CS) was measured using the sine wave grating-based Functional Vision Analyser (FVA)™ (Stereo Optical Co. Inc, Chicago, USA). Testing was performed under mesopic (3 candelas per square metre [cd/m2]) and photopic (85 cd/m2) conditions. This test was repeated in a similar manner, and under the same lighting conditions, but in the presence of an inbuilt circumferential LED (light emitting diode) glare source (1 lux for mesopic and 10 lux for photopic glare testing) to assess glare disability (GD) [12].
Retinotopic ocular sensitivity
Retinotopic ocular sensitivity (ROS) was measured by microperimetry (Microperimeter MP 1; Nidek Technologies Srl, Albignasego, Italy). ROS was measured monocularly at a constant room illuminance of 1.5 lux, according to a previously defined protocol [13]. The study eye was pharmacologically dilated with one drop of guttae Tropicamide BP 1% w/v minims® (Chauvin Pharmaceuticals Ltd, Ashton Road, 75 Harold Hill, Routond, Essex, RM3 8SL, UK) fifteen minutes prior to the test. The other eye was covered for the duration of the test, and the eye tracking function was used throughout. ROS was calculated for nine stimulus locations across the central 5° of the retina, including fixation. An integrated infrared fundus camera (1392 × 1038-pixel resolution; 45° field of view) allowed real-time fundus imaging on a monitor. The fixation target and stimuli were projected onto the retina by a liquid crystal color monitor. White monochromatic background illumination was set at 4 apostilbs (asb; 1.27 cd/m2). Stimulus intensity could be varied on a 1 (0.1 log)–step scale from 0 to 20 dB, where 0 dB represented the brightest luminance of 400 asb (127 cd/m2). A standard Goldmann III stimulus size was used, with a presentation duration of 200 ms. A 4-2 staircase test strategy was used. Light stimuli were randomly presented during the examination as in standard static perimetry.
Disease status
Early AMD
AMD was graded using colour stereoscopic 30 degree fundus photographs, that were obtained using a ZeissVisuCam® (Carl ZeissMeditec AG, Jena, Germany) fundus camera, and were graded at the University of Wisconsin, Madison, USA, using the Wisconsin Age-Related Maculopathy Grading System. A detailed description of all grading procedures and definitions has been previously described [14,15]. Overall findings were reported on an 11-step AREDS AMD-severity scale.
Neovascular AMD
FFA was performed in all cases in order for a diagnosis of nv-AMD to be established or refuted. Spectral domain OCT was performed using a Topcon 3D OCT-1000® (version 3.0, Mark I; Topcon Corporation, Tokyo, Japan). Centration was manually controlled by a single operator, and unreliable data were excluded in the analysis. The central 1 mm mean foveal thickness (MFT) was obtained from typical ETDRS macular thickness maps [16]. The central foveal thickness was defined as the distance between the inner and outer boundaries of the scanned image, identified using a validated internal algorithm, and did not include any fluid under the retinal pigment epithelium (RPE).
Statistical analysis
One way analysis of variance, with Tukey post-hoc analysis, was used to investigate the relationship between AMD severity and measures of visual function in cases of early AMD. Pearson correlations were used to investigate bivariate relationships between measures of foveal thickness and measures of visual function in cases of nv-AMD. Assuming a 5% level of significance and a two-tailed test, a sample of 66 has power of 0.92 and a sample of 47 has power of 0.8, for detecting a correlation of 0.4.
Ethical approval for these studies was granted by the Dublin Institute of Technology Ethics Committee, and by the Waterford Regional Hospital Ethics Committee. Informed consent was secured from each subject, and the research was conducted in accordance with the principles of the Declaration of Helsinki. We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research.
Results
Visual function and AMD status
A: Early AMD
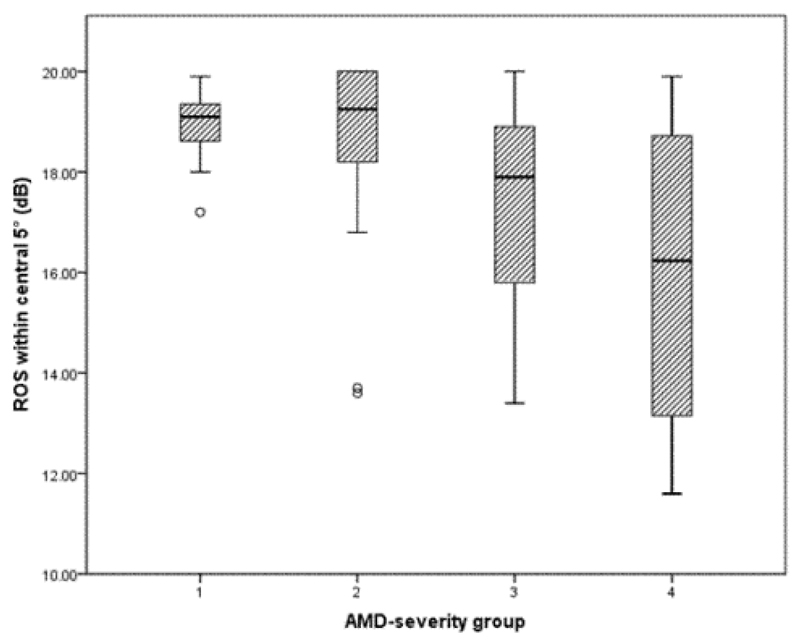
The eight grades of early AMD were collapsed (to facilitate statistical analysis) as follows: Group 1=grades 1 and 2 (n=12); Group 2=grades 3 and 4 (n=25); Group 3=grades 5 and 6 (n=18); Group 4=grades 7 and 8 (n=11). There was a statistically significant and inverse relationship between measures of ROS within the central 5° of retina (including fixation) and severity of early AMD (p=0.01) Figure 1.
Figure 1.
The relationship between severity of early AMD and ROS within the central 5° of the retina, including fixation. AMD: Age-Related Macular Degeneration; ROS: Retinotopic Ocular Sensitivity. AMD severity group defined as: group 1=grades 1 and 2; group 2=grades 3 and 4; group 3=grades 5 and 6; group 4=grades 7 and 8.
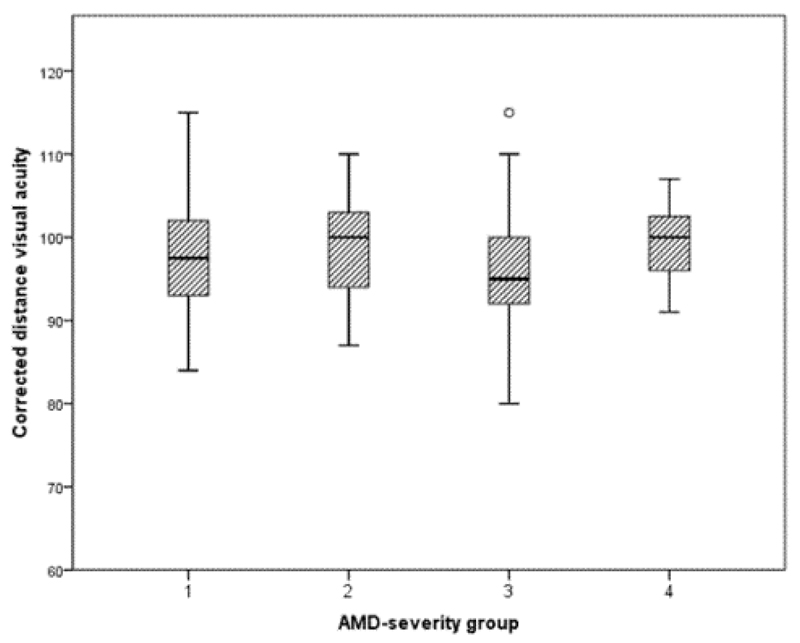
Tukey post-hoc analysis revealed that Group 4 exhibited significantly lower ROS values than Groups 1 and 2 (p<0.05 for each), but revealed no other significant differences. No other parameters of visual function, including CDVA (Figure 2), were significantly related to severity of early AMD.
Figure 2.
The relationship between severity of early AMD and CDVA. AMD: Age-Related Macular Degeneration; AMD severity group defined as: group 1=grades 1 and 2; group=grades 3 and 4; group 3=grades 5 and 6; group 4=grades 7 and 8.
B: nv-AMD
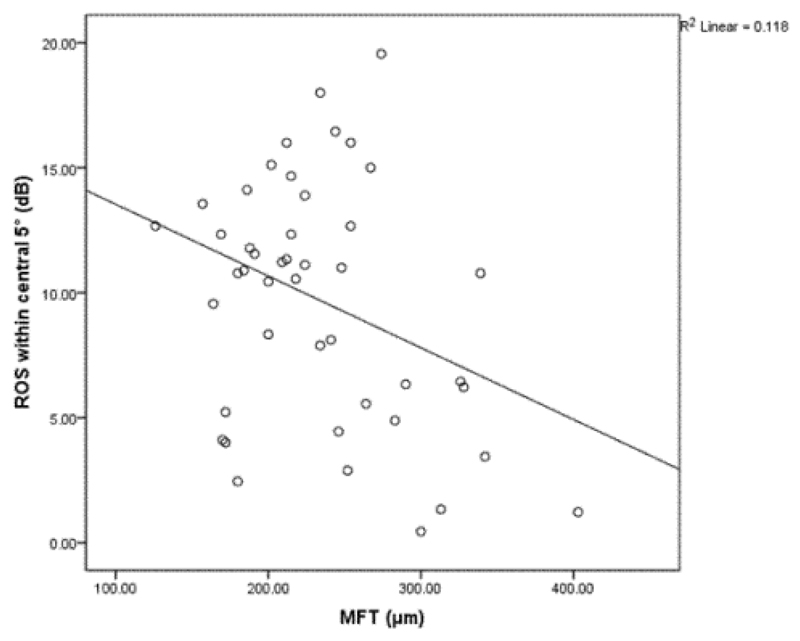
A statistically significant and inverse correlation between measures of MFT and measures of ROS within the central 5° of retina, including fixation, is reported (r=-0.34; p=0.02); see Figure 3 for a graphical representation of the relationship) in patients with active nv-AMD. No other measure of visual function (including CDVA [r=-0.247; p=0.094]) was significantly related to MFT (p>0.05, for all) in eyes with nv-AMD.
Figure 3.
The relationship between mean foveal thickness and retinotopic ocular sensitivity within the central 5° of the retina, including fixation, in subjects with neovascular age-related macular degeneration. MFT: Mean Foveal Thickness; Db: Decibels; ROS: Retinotopic Ocular Sensitivity
Discussion
This study was designed to investigate the relationship, if any, between psychophysical visual function and disease severity in eyes with early AMD, or MFT in eyes with active nv-AMD.
CDVA was not associated with disease severity in eyes with early AMD. ROS was the only measure of psychophysical function tested that was associated with severity of early AMD, based on the AREDS AMD-severity scale [15]. ROS was significantly lower in cases with the most severe form of early AMD (Group 4) when compared to less advanced forms of early AMD (Groups 1 and 2).
ROS was also related to activity of nv-AMD, reflected in the inverse relationship observed between this parameter of visual function and MFT, where no significant relationships were observed between MFT and any other tests of psychophysical function. As OCT-determined MFT represents an important component of treatment and retreatment regimes in nv-AMD, the observed relationship suggests that ROS may be more suited than CDVA to monitor visual function in patients with active neovascular disease.
There was no statistically significant association between measures of CS or GD and severity of early AMD or activity of nv-AMD in this study. The results here are in conflict, therefore, with those of other studies which suggest that CS should be included as an important measure of visual function in early AMD, [17] and possibly as an indicator of disease progression and treatment efficacy in nv-AMD [18–20]. Although there is a paucity of evidence in relation to GD and AMD, improvements in GD have been recently reported in response to anti-VEGF therapy in patients with the neovascular form of the condition [19]. The observed disparity between our findings and those of previous investigators in relation to CS and GD may relate to differences in study design and methodology, including baseline CDVA (reasonable baseline CDVA was an inclusion criterion in the current study), test used to determine CS and GD, or variation in sample size.
Although CDVA remains the most commonly used test of foveal function in AMD, it may be unsuited to monitoring the functional changes in early AMD associated with the increasing involvement of large soft drusen or pigmentary abnormalities and treatment efficacy in cases of nv-AMD for a variety of reasons. First, functional losses associated with AMD progression can promote eccentric fixation patterns that mask the depth of visual loss when only CDVA is measured, [21] as foveal fixation is maintained in only a small percentage of individuals with reduced CDVA, [22] which may account, at least in part, for the under-appreciation of visual impairment experienced by patients with AMD when using CDVA [23]. Second, CDVA provides an assessment of photopic, and therefore exclusively cone-mediated, visual function. It is known, however, that rod function is more severely impaired than cone function in early AMD [24,25]. Third, CDVA probes only a small portion of an individual’s spatial vision, restricted to high frequency aspects only, and is thus incapable of detecting clinically important functional changes at moderate and low spatial frequencies [26]. The combined effect of these limitations is such that CDVA does not provide a comprehensive measure of visual experience, and is unlikely to capture fully, the functional and structural changes which affect photoreceptors, the RPE, Bruch’s membrane and the choriocapillaris in eyes with AMD.
Further, microperimetry allows investigators to accurately assess the relationship between functional and structural changes associated with disease and disease treatment, reflected in the findings reported here. No study, to our knowledge, has reported on the relationship between ROS (or CS or GD) with respect to severity of early AMD, using a validated system such as the Wisconsin age-related maculopathy grading scale. However, a range of studies have looked at the relationship between morphological features at the macula and ROS in cases of AMD. Lower ROS in subjects with early AMD, when compared with age-matched controls, has been reported by previous investigators, while ROS over individual druse has been shown to be significantly lower than adjacent retinal areas without underlying druse [27]. For example, it has been shown that in subjects with early AMD, ROS is reduced in areas overlying drusen and/or pigmentary abnormalities, despite the presence of good CDVA (20/20), and this observed reduction in sensitivity was even greater when both types of lesions were present [28].
An association appears to exist between ROS and central retinal thickness in patients with nv-AMD, prompting the view that ROS may be a more appropriate means to assess central visual function than conventional CDVA in persons with nv-AMD [29]. In this respect, our findings are consistent with previous observations of a significant and inverse relationship between improvements in ROS (within the central 10° of fixation) and changes in foveal thickness, but where significant improvements in CDVA were not observed, following photodynamic therapy in patients with nv-AMD [30]. RPE lesion area (area of disease defined as the site presenting a significant deviation in RPE contour at the location of the sub-retinal disease) also relates inversely to central ROS (but not to CDVA) in patients with nv-AMD undergoing anti-VEGF therapy [31].
Although the study was appropriately powered from a statistical perspective, the small sample size does represent a limitation of our study. In particular, the low numbers of subjects across each grade of early AMD required that the eight grades be grouped for the purposes of statistical analysis. Statistically significant relationships did emerge, however, which could prove stronger in a larger sample. The failure to measure (or correct for) features relating to implanted or crystalline lenses represents a further limitation of this study given the potential impact of variable lenticular light scatter and transmission on visual function. The cross-sectional nature of the study also limits the interpretation of our findings, and, despite the relationships that emerged in our analysis, they provide no real insight into the prognostic and long term monitoring capacity of ROS relative to conventional techniques. These shortcomings need to be addressed in any further attempt to evaluate the potential role of alternatives to CDVA for AMD patients in the clinical setting.
In summary, and although we have shown a cross-sectional relationship between ROS and severity of early AMD, and between this parameter of visual function and MFT in cases of nv-AMD, there remains a need to show longitudinally that ROS is a sensitive and clinically meaningful measure, and one that is independent of CDVA, before it can be used in clinical practice as a prognostic indicator for patients with early AMD. Nevertheless, our findings do suggest that this is an area of research that should not be ignored.
Acknowledgement
This study was supported by a grant from Novartis Pharma AG.
References
- 1.Bressler NM. Age-related macular degeneration is the leading cause of blindness…. JAMA. 2004;291:1900–1901. doi: 10.1001/jama.291.15.1900. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell J, Bradley C. Quality of life in age-related macular degeneration: a review of the literature. Health Qual Life Outcomes. 2006;4:97. doi: 10.1186/1477-7525-4-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group. Martin DF, Maguire MG, Fine SL, Ying GS, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119:1388–1398. doi: 10.1016/j.ophtha.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dubuc S, Wittich W, Gomolin JE, Kapusta M, Overbury O. Beyond visual acuity: functional outcome and patient satisfaction following treatment for age-related macular degeneration. Can J Ophthalmol. 2009;44:680–685. doi: 10.3129/i09-163. [DOI] [PubMed] [Google Scholar]
- 5.Broman AT, Munoz B, Rodriguez J, Sanchez R, Quigley HA, et al. The impact of visual impairment and eye disease on vision-related quality of life in a Mexican-American population: proyecto VER. Invest Ophthalmol Vis Sci. 2002;43:3393–3398. [PubMed] [Google Scholar]
- 6.Charalampidou S, Nolan J, Loughman J, Stack J, Higgins G, et al. Psychophysical impact and optical and morphological characteristics of symptomatic non-advanced cataract. Eye (Lond) 2011;25:1147–1154. doi: 10.1038/eye.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein R, Wang Q, Klein BE, Moss SE, Meuer SM. The relationship of age-related maculopathy, cataract, and glaucoma to visual acuity. Invest Ophthalmol Vis Sci. 1995;36:182–191. [PubMed] [Google Scholar]
- 8.Rosser DA, Cousens SN, Murdoch IE, Fitzke FW, Laidlaw DA. How sensitive to clinical change are ETDRS logMAR visual acuity measurements? Invest Ophthalmol Vis Sci. 2003;44:3278–3281. doi: 10.1167/iovs.02-1100. [DOI] [PubMed] [Google Scholar]
- 9.Sarks JP, Sarks SH, Killingsworth MC. Evolution of geographic atrophy of the retinal pigment epithelium. Eye (Lond) 1988;2:552–577. doi: 10.1038/eye.1988.106. [DOI] [PubMed] [Google Scholar]
- 10.Sunness JS, Rubin GS, Applegate CA, Bressler NM, Marsh MJ, et al. Visual function abnormalities and prognosis in eyes with age-related geographic atrophy of the macula and good visual acuity. Ophthalmology. 1997;104:1677–1691. doi: 10.1016/s0161-6420(97)30079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Visual outcome after laser photocoagulation for subfoveal choroidal neovascularization secondary to age-related macular degeneration The influence of initial lesion size and initial visual acuity. Macular Photocoagulation Study Group. Arch Ophthalmol. 112:480–488. doi: 10.1001/archopht.1994.01090160056023. [DOI] [PubMed] [Google Scholar]
- 12.Hohberger B, Laemmer R, Adler W, Juenemann AG, Horn FK. Measuring contrast sensitivity in normal subjects with OPTEC 6500: influence of age and glare. Graefes Arch Clin Exp Ophthalmol. 2007;245:1805–1814. doi: 10.1007/s00417-007-0662-x. [DOI] [PubMed] [Google Scholar]
- 13.Rohrschneider K, Springer C, Bültmann S, Völcker HE. Microperimetry--comparison between the micro perimeter 1 and scanning laser ophthalmoscope--fundus perimetry. Am J Ophthalmol. 2005;139:125–134. doi: 10.1016/j.ajo.2004.08.060. [DOI] [PubMed] [Google Scholar]
- 14.Klein R, Davis MD, Magli YL, Segal P, Klein BE, et al. The Wisconsin age-related maculopathy grading system. Ophthalmology. 1991;98:1128–1134. doi: 10.1016/s0161-6420(91)32186-9. [DOI] [PubMed] [Google Scholar]
- 15.Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study Severity Scale for Age-Related Macular Degeneration. AREDS Report No. 17. Arch Ophthalmol. 2005;123:1484–1498. doi: 10.1001/archopht.123.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Massin P, Vicaut E, Haouchine B, Erginay A, Paques M, et al. Reproducibility of retinal mapping using optical coherence tomography. Arch Ophthalmol. 2001;119:1135–1142. doi: 10.1001/archopht.119.8.1135. [DOI] [PubMed] [Google Scholar]
- 17.Kleiner RC, Enger C, Alexander MF, Fine SL. Contrast sensitivity in age-related macular degeneration. Arch Ophthalmol. 1988;106:55–57. doi: 10.1001/archopht.1988.01060130061028. [DOI] [PubMed] [Google Scholar]
- 18.Fletcher DC, Schuchard RA. Visual function in patients with choroidal neovascularization resulting from age-related macular degeneration: the importance of looking beyond visual acuity. Optom Vis Sci. 2006;83:178–189. doi: 10.1097/01.opx.0000204510.08026.7f. [DOI] [PubMed] [Google Scholar]
- 19.Sabour-Pickett S, Loughman J, Nolan JM, Stack J, Pesudovs K, et al. Visual performance in patients with neovascular age-related macular degeneration undergoing treatment with intravitreal ranibizumab. J Ophthalmol. 2013 doi: 10.1155/2013/268438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monés J, Rubin GS. Contrast sensitivity as an outcome measure in patients with subfoveal choroidal neovascularisation due to age-related macular degeneration. Eye (Lond) 2005;19:1142–1150. doi: 10.1038/sj.eye.6701717. [DOI] [PubMed] [Google Scholar]
- 21.Midena E, Radin PP, Pilotto E, Ghirlando A, Convento E, et al. Fixation pattern and macular sensitivity in eyes with subfoveal choroidal neovascularization secondary to age-related macular degeneration. A microperimetry study. Seminars in Ophthalmology. 2004;19:55–61. doi: 10.1080/08820530490882896. [DOI] [PubMed] [Google Scholar]
- 22.Mathew R, Pearce E, Sivaprasad S. Determinants of fixation in eyes with neovascular age-related macular degeneration treated with intravitreal ranibizumab. Am J Ophthalmol. 2012;153:490–496. doi: 10.1016/j.ajo.2011.08.034. [DOI] [PubMed] [Google Scholar]
- 23.McClure ME, Hart PM, Jackson AJ, Stevenson MR, Chakravarthy U. Macular degeneration: do conventional measurements of impaired visual function equate with visual disability? Br J Ophthalmol. 2000;84:244–250. doi: 10.1136/bjo.84.3.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scholl HP, Bellmann C, Dandekar SS, Bird AC, Fitzke FW. Photopic and scotopic fine matrix mapping of retinal areas of increased fundus autofluorescence in patients with age-related maculopathy. Invest Ophthalmol Vis Sci. 2004;45:574–583. doi: 10.1167/iovs.03-0495. [DOI] [PubMed] [Google Scholar]
- 25.Chen JC, Fitzke FW, Pauleikhoff D, Bird AC. Functional loss in age-related Bruch's membrane change with choroidal perfusion defect. Invest Ophthalmol Vis Sci. 1992;33:334–340. [PubMed] [Google Scholar]
- 26.Thibos LN, Bradley A. New methods for discriminating neural and optical losses of vision. Optom Vis Sci. 1993;70:279–287. doi: 10.1097/00006324-199304000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Hartmann KI, Bartsch DU, Cheng L, Kim JS, Gomez ML, Klein H, et al. Scanning laser ophthalmoscope imaging stabilized microperimetry in dry age-related macular degeneration. Retina. 2011;31:1323–1331. doi: 10.1097/IAE.0b013e31820a6850. [DOI] [PubMed] [Google Scholar]
- 28.Midena E, Vujosevic S, Convento E, Manfre' A, Cavarzeran F, et al. Microperimetry and fundus autofluorescence in patients with early age-related macular degeneration. Br J Ophthalmol. 2007;91:1499–1503. doi: 10.1136/bjo.2007.119685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sulzbacher F, Kiss C, Kaider A, Eisenkoelbl S, Munk M, et al. Correlation of SD-OCT features and retinal sensitivity in neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2012;53:6448–6455. doi: 10.1167/iovs.11-9162. [DOI] [PubMed] [Google Scholar]
- 30.Okada K, Kubota-Taniai M, Kitahashi M, Baba T, Mitamura Y, et al. Changes in visual function and thickness of macula after photodynamic therapy for age-related macular degeneration. Clin Ophthalmol. 2009;3:483–488. doi: 10.2147/opth.s6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kiss CG, Geitzenauer W, Simader C, Gregori G, Schmidt-Erfurth U. Evaluation of ranibizumab-induced changes in high-resolution optical coherence tomographic retinal morphology and their impact on visual function. Invest Ophthalmol Vis Sci. 2009;50:2376–2383. doi: 10.1167/iovs.08-2017. [DOI] [PubMed] [Google Scholar]