ABSTRACT
ULK1 (unc-51 like autophagy activating kinase 1) is well known to be required to initiate the macroautophagy/autophagy process, and thus activation of ULK1-modulating autophagy/autophagy-associated cell death (ACD) may be a possible therapeutic strategy in triple negative breast cancer (TNBC). Here, our integrated The Cancer Genome Atlas (TCGA) data set, tissue microarray-based analyses and multiple biologic evaluations together demonstrate a new small-molecule activator of ULK1 for better understanding of how ULK1, the mammalian homolog of yeast Atg1, as a potential drug target can regulate ACD by the ULK complex (ULK1-ATG13-RB1CC1/FIP200-ATG101), as well as other possible ULK1 interactors, including ATF3, RAD21 and CASP3/caspase3 in TNBC. Moreover, such new inspiring findings may help us discover that this activator of ULK1 (LYN-1604) with its anti-tumor activity and ACD-modulating mechanisms can be further exploited as a small-molecule candidate drug for future TNBC therapy.
KEYWORDS: autophagy, autophagy-associated cell death (ACD), triple negative breast cancer (TNBC), ULK1 activator, UNC-51-like kinase 1 (ULK1)
Triple negative breast cancer (TNBC) is well known to be an aggressive type characterized by the absence of well-defined molecular targets, including ESR (estrogen receptor), PGR (progesterone receptor), and ERBB2/HER-2 (erb-b2 receptor tyrosine kinase 2). Typically, TNBCs have a relatively poor outcome due to the inherently invasive and metastatic clinical behavior, and a lack of effective targeted therapies represents a huge challenge. Thus, the discovery of a novel targeted small-molecule drug has been emerging as the focus for the current TNBC therapeutics.
Autophagy is a highly conserved cellular self-digestion process by which cellular components are targeted to lysosomes for their degradation. Defects of the autophagic machinery, such as those imposed by the heterozygous deletion of BECN1/Beclin 1, can lead to malignant transformation, especially in breast cancer. ULK1 has been widely reported as the autophagic initiator that may decide subsequent tumor cell fate. In the autophagic process, ULK1 is a component of the ULK complex (ULK1-ATG13-RB1CC1-ATG101) that is essential for autophagy induction in different types of human cancers, such as TNBC.
Recently, accumulating evidence has revealed that downregulation of ULK1 is often found in most breast cancer tissues, including TNBC. In this study, we found that ULK1 is downregulated in breast cancer tissue samples from TCGA and tissue microarray-based analyses, especially in TNBC, suggesting that ULK1 may be a novel anti-TNBC target. Thus, these results indicate that activating ULK1 would be a promising TNBC therapeutic strategy.
To design an activator of ULK1, we integrated in silico high-throughput screening and chemical synthesis to acquire a series of candidate activators of ULK1. After several rounds of kinase activity and anti-proliferative activity screening, we eventually discovered a potent and targeted activator of ULK1 (LYN-1604) that was able to induce autophagy-associated cell death (ACD) via the ULK complex (Fig. 1A). Additionally, we determined by site-directed mutagenesis and biochemical assays that 3 amino acid residues (LYS50, LEU53 and TYR89) were key to the activation site of LYN-1604 and ULK1 (Fig. 1B). To further explore LYN-1604-induced ACD mechanisms, we constructed a ULK1-regulated protein-protein interaction network and found some potential ULK1 interactors such as ATF3, RAD21 and CASP3 by performing comparative microarray analysis under different conditions of overexpression and siRNA of ULK1 in MDA-MB-231 cells. Intriguingly, we demonstrated that LYN-1604 induces ACD involved with ATF3, RAD21 and CASP3, accompanied by apoptosis (Fig. 1C). As mentioned above, discovery of a small-molecule activator of ULK1 and elucidation of its regulatory mechanisms of ACD in TNBC are summarized.
Figure 1.
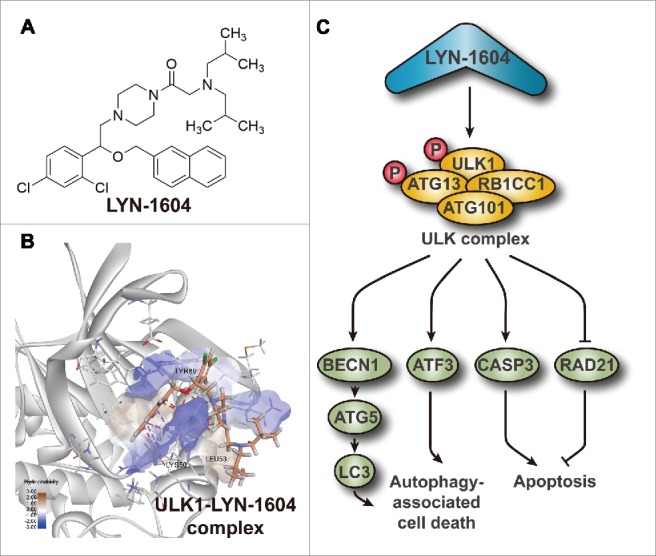
Schematic model of discovery of a small-molecule activator (LYN-1604) of ULK1 that can induce autophagy-associated cell death pathways, associated with apoptosis in TNBC cells. (A) Chemical structure of the ULK1 activator LYN-1604. (B) ULK1-LYN-1604 complex with some key amino acid residues in their activation site. (C) Molecular mechanisms of LYN-1604-induced ACD in TNBC, associated with apoptosis.
Moreover, we found that LYN-1604 had a good therapeutic potential by targeting ULK1-modulating ACD of TNBC in vivo. Undoubtedly, ULK1 can be regarded as a promising therapeutic target of TNBC, which is a specific and potent target for TNBC rather than other types of breast cancer. Together, these results will shed light on exploiting this novel activator of ULK1 for further utilization as a candidate small-molecule drug.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Grant Nos. 81673455, 81602953, 81602627 and 81473091).


