Abstract
The hyperthermophilic archaeon, Pyrococcus furiosus, was grown on maltose near its optimal growth temperature, 95°C, and at the lower end of the temperature range for significant growth, 72°C. In addition, cultures were shocked by rapidly dropping the temperature from 95 to 72°C. This resulted in a 5-h lag phase, during which time little growth occurred. Transcriptional analyses using whole-genome DNA microarrays representing 2,065 open reading frames (ORFs) in the P. furiosus genome showed that cells undergo three very different responses at 72°C: an early shock (1 to 2 h), a late shock (5 h), and an adapted response (occurring after many generations at 72°C). Each response involved the up-regulation in the expression of more than 30 ORFs unique to that response. These included proteins involved in translation, solute transport, amino acid biosynthesis, and tungsten and intermediary carbon metabolism, as well as numerous conserved-hypothetical and/or membrane-associated proteins. Two major membrane proteins were evident after one-dimensional sodium dodecyl sulfate-gel analysis of cold-adapted cells, and staining revealed them to be glycoproteins. Their cold-induced expression evident from the DNA microarray analysis was confirmed by quantitative PCR. Termed CipA (PF0190) and CipB (PF1408), both appear to be solute-binding proteins. While the archaea do not contain members of the bacterial cold shock protein (Csp) family, they all contain homologs of CipA and CipB. These proteins are also related phylogenetically to some cold-responsive genes recently identified in certain bacteria. The Cip proteins may represent a general prokaryotic-type cold response mechanism that is present even in hyperthermophilic archaea.
How bacteria respond to temperatures significantly below those optimal for growth has been well characterized (9). A decrease in temperature results in a temporary halt in protein synthesis, but a small subset of so-called cold shock proteins are induced during an acclimation phase (24). After a lag phase of several hours, general protein synthesis and cell growth resume at a much slower rate, and production of most of the cold shock proteins decreases to a basal level. The first major cold shock protein to be characterized was CspA of Escherichia coli (21). This organism contains eight CspA homologs (CspB to -I), although only four (CspA, -B, -G, and -I) are cold inducible (54). They are thought to function as mRNA chaperones that prevent the formation of inhibitory secondary structures, which are stabilized at the lower temperatures. Other cold-inducible proteins in E. coli include initiation factor 2 (IF2) (7, 13), ribosomal binding factor A (RbfA) (22), and DEAD-box RNA helicase (4, 23, 37), all of which associate with the ribosome and are believed to play a role in protein synthesis. Ribosomal function therefore appears to be compromised at lower temperatures, and several new proteins are required to allow protein synthesis to resume. Homologs of the CspA family, IF2 and RbfA are found in a wide range of gram-positive (Bacillus subtilis, Listeria monocytogenes) and gram-negative (Salmonella enterica serovar Typhimurium, Yersinia pestis) bacteria, as well as psychrophilic (Pseudomonas fragi, Arthrobacter globiformis), thermophilic (Bacillus caldolyticus), and even hyperthermophilic (Thermotoga maritima, Aquifex aeolicus) species (http://www.tigr.org).
In contrast to bacteria, little is known about how members of the archaeal domain respond to suboptimal growth temperatures. The genome of the psychrotrophic methanogen Methanococcoides burtonii contains a gene (deaD) encoding a DEAD-box RNA helicase, and this was shown to be cold inducible (31). However, this is the only evidence for a link between the archaeal and bacterial cold shock responses. Homologs of the canonical cold shock proteins CspA and RbfA are not present in any of the 16 genome sequences available for mesophilic and thermophilic members of the archaea (http://www.tigr.org). Therefore, if these organisms possess a cold shock response, it is novel and distinct from the previously characterized cold shock response of bacteria. Of particular interest is the cold shock response of hyperthermophilic archaea. These are defined as organisms with an optimal growth temperature of at least 80°C (51). So far, only one protein implicated in such a response has been studied. Prolyl isomerase was induced when a culture of Thermococcus sp. strain KS-1 growing at its optimal growth temperature of 85°C (14) was shifted to 60°C (17, 18). The enzyme has been proposed to function in the archaea by interacting with the hydrophobic regions of newly synthesized proteins to aid in folding, analogous to the “trigger factor” in E. coli which has also been implicated in the bacterial cold shock response (18).
A genome-wide approach to studying the response of hyperthermophilic archaea to suboptimal growth temperatures has not yet been reported. Herein we describe the results of such a study using DNA microarrays to measure transcription profiles in response to cold stress of all 2,065 open reading frames (ORFs) that have been annotated in the genome of Pyrococcus furiosus (42), an anaerobic heterotroph that grows optimally at 100°C (11). This organism utilizes complex carbohydrates and peptides as carbon and energy sources and produces hydrogen gas, acetate, and organic acids as metabolic end products. It was first isolated from a shallow marine hydrothermal vent in 1986 (11). In this niche, the vent fluids are expelled into oxygenated seawater at ambient temperature (20°C), conditions under which P. furiosus can survive (26). Growth resumes when the organism is subsequently exposed to temperatures above 70°C under anaerobic conditions. It would therefore seem likely that P. furiosus has mechanisms to help it survive temporary exposure to both oxygen and lower temperatures, as proposed earlier (20). The results presented here show that the organism has three very different cellular responses to the suboptimal growth temperature of 72°C. An early shock response occurs immediately upon a decrease in temperature, and this is followed by a late shock response. A different suite of proteins is induced in each phase, and they are not the same as those that are up-regulated in cells adapted to the suboptimal temperature.
MATERIALS AND METHODS
Growth conditions and RNA extraction.
P. furiosus (DSM 3638) was routinely grown with maltose as the primary carbon source in a 20-liter custom fermentor (1). The medium was the same as that previously reported (1), except that the yeast extract concentration was 0.2% (wt/vol). There were two types of growth experiments utilized in this study, termed shock and adapted growth. For the adapted growth experiments, cultures were grown at 95 and 72°C. The two cell types used for RNA isolation were grown at their respective temperatures after at least three successive transfers using batch cultures grown at that temperature. In the shock experiments, cultures growing at 95°C were rapidly cooled to 72°C when they reached a cell density of ∼3 × 107 cells/ml by pumping them through a glass cooling coil maintained at 20°C. The complete culture (15 liters) was cooled from 95 to 72°C within 15 min. To prepare cell extracts from adapted cultures, cells were harvested in late log phase (∼2 × 108 cells/ml) and fractionated as previously described (48). For shocked cultures, cells were harvested 2 or 5 h after the shock was introduced and fractionated as in the adapted cultures. To obtain RNA for the microarray analyses, samples (2 liters) were rapidly removed from the fermentor and cooled to 4°C. Total RNA was extracted using acid-phenol (48) and stored at −80°C until needed.
DNA microarray analyses.
The design and construction of DNA microarrays containing all of the 2,065 ORFs in the annotated genome of P. furiosus (http://comb5-156.umbi.umd.edu/genemate/), preparation of cDNA from the RNA samples, and hybridization experiments were all performed as previously described (48). Fluorescently labeled cDNA was prepared using the ARES DNA labeling kit (Molecular Probes, Eugene, Oreg.). The resulting amine-modified cDNA was purified using a QIAquick PCR purification kit (QIAGEN, Valencia, Calif.) according to the manufacturer's instructions except that the wash buffer was replaced with 75% (vol/vol) ethanol and the cDNA was eluted with 45 μl of distilled water and dried under vacuum. The amine-modified cDNA was labeled with Alexa dye 488, 546, 594, or 647 (Molecular Probes) according to the manufacturer's instructions. The labeled cDNA was purified using the QIAGEN kit and dried under vacuum. Differentially labeled cDNA derived from P. furiosus cells grown at different growth temperatures (95 and 72°C) or cold-shocked cells harvested at specific times (0, 1, 2, or 5 h after a culture was shifted to 72°C) were pooled and hybridized to the DNA microarray, and the fluorescence intensities for the Alexa dyes were measured as described previously (48). For the adapted experiments, each log2 value shown represents an average of four hybridization experiments performed in duplicate using cDNA derived from four different cultures of P. furiosus (two grown at 95°C, two grown at 72°C). Each log2 value from the shock experiments represents an average of two hybridization experiments performed in duplicate using cDNA derived from two different cultures of P. furiosus. Standard deviations for the adapted and shock data sets are included. Individual t test procedures were conducted to identify the significantly expressed ORFs, and Holm's step-down P value adjustment procedure was performed (16) to give modified P values.
Quantitative real-time PCR.
RNA was isolated as described above and further purified using the Absolutely RNA clean up kit (Stratagene, La Jolla, Calif.). cDNA was then prepared as described previously (48) with the exception that aminoallyl dUTP was replaced by the TTP derivative. The regulated genes PF0190 and PF1408 were selected for study, and the nonregulated gene PF0018, which encodes a subunit of DNA polymerase, was selected as a control. Primers for the genes were designed using the program Array Designer version 1.16 (Premier Biosoft International, Palo Alto, Calif.). All quantitative PCR (qPCR) experiments were carried out using an Mx3000P instrument (Stratagene) with SYBR Green as a reporter dye. Each experiment used RNA isolated from the four P. furiosus adapted cultures used for the microarray experiments (two grown at 95°C and two grown at 72°C), and each was repeated three times. The comparative cycle threshold method was used to analyze the resulting data as described by the manufacturer (Bulletin 2, Applied Biosystems).
Enzyme assays.
All assays were carried out at 85°C using cell extracts that were prepared as described previously (1). Acetolactate synthase activity was measured by the formation of acetoin (55). Aminoacyl-releasing enzyme (AARE) activity was measured by the release of p-nitroaniline from a tripeptide substrate (19). The activities of 2-ketoglutarate ferredoxin (Fd) oxidoreductase (KGOR) and pyruvate Fd oxidoreductase were measured as described previously (47).
Other methods.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed on the cytosolic and membrane fractions by using 15% polyacrylamide with the method of Laemmli (30). Protein concentrations were determined by the Bradford method (6) using bovine serum albumin as the standard. The Invitrogen (Carlsbad, Calif.) benchmark protein ladder, ranging from 10 to 220 kDa, was used for molecular mass estimation. Unless otherwise specified, gels were stained with Coomassie brilliant blue R-250 (Fisher Biotech, Fair Lawn, N.J.). To identify proteins, bands were cut out of the SDS-PAGE gel and subjected to in-gel trypsin digestion as described previously (49). The resulting sample was subjected to matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) using an Applied Biosystems 4700 Proteomics Analyzer at the University of Georgia Proteomics Resource Facility (Athens, Ga.). Glycoproteins were identified by staining with Pro-Q Emerald 300 (Molecular Probes) after separating them using an SDS-15% PAGE gel. The total glycoprotein content of the extract was also determined using a glycoprotein carbohydrate estimation kit (Pierce, Rockford, Ill.). The detection of potential signal sequences and the number of transmembrane domains in P. furiosus ORFs were calculated as described previously (15). Operons are defined as adjacent ORFs (on the same strand) in the P. furiosus genome that are less than 16 nucleotides apart and are potentially coregulated and cotranscribed, as described elsewhere (http://www.tigr.org). Amino acid sequences were aligned using Vector NTI AlignX software (suite 9.0.0; Invitrogen). Phylogenetic trees were constructed using MEGA3 software (29) employing the neighbor-joining technique (45). The p-distance substitution model was used to estimate distance values, and bootstrap values (10) were determined using 1,000 replicates of the data.
RESULTS AND DISCUSSION
Effect of temperature on growth.
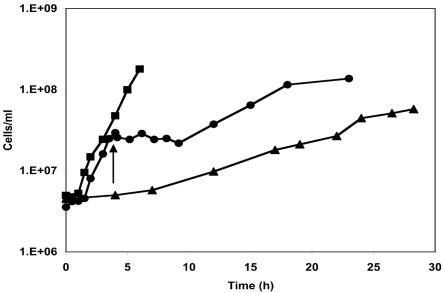
P. furiosus growing with maltose as the primary carbon source has a generation time of 1 h at 95°C (11), but this increases to about 5 h at 72°C, which is near the minimum growth temperature (Fig. 1). In addition, P. furiosus exhibits a cold shock response when the temperature of a growing culture is shifted from 95 to 72°C. This results in a lag phase of about 5 h, during which time there is little if any growth, reminiscent of that seen in bacteria after cold shock (Fig. 1). After this acclimation phase, growth of P. furiosus resumes at a rate similar to that seen in cultures grown continuously at 72°C (Fig. 1). The organism therefore exhibits two responses when exposed to temperatures that support a minimal growth rate. The cold shock response occurs when the temperature of a culture is suddenly shifted to the lower growth temperature with no associated growth, while the cold-adapted response is exhibited by cultures that are actively growing at the lower temperature. The goals of the present study were, therefore, to determine to what extent these two cell types, cold shocked and cold adapted, differed from cells grown at 95°C under standard conditions. This was initially assessed at the transcriptional level using DNA microarrays that represented all 2,065 ORFs annotated (42) in the P. furiosus genome.
FIG. 1.
Growth of P. furiosus at 95 and 72°C. Cells were grown at 95°C (closed squares), at 72°C (closed triangles), or at 95°C for 3.5 h (cell density of ∼3 × 107 cells/ml) and then shocked (indicated by the arrow) by rapidly decreasing the temperature to 72°C (closed circles).
Genome-wide responses to a suboptimal growth temperature.
In the cold shock experiment, the temperature of a log-phase culture was rapidly decreased from 95 to 72°C, and cells were removed at different times (1, 2, and 5 h postshock) from the same culture to prepare RNA samples for transcriptional profiling, and these were compared with an RNA sample prepared from cells harvested just prior to the temperature change (time zero). The four RNA samples were then differentially labeled and hybridized to the same DNA microarray. This experimental approach minimizes both biological and experimental variation. In the cold-adapted experiment, RNA expression profiles were compared in two cultures of P. furiosus grown independently at the two temperatures (95 and 72°C).
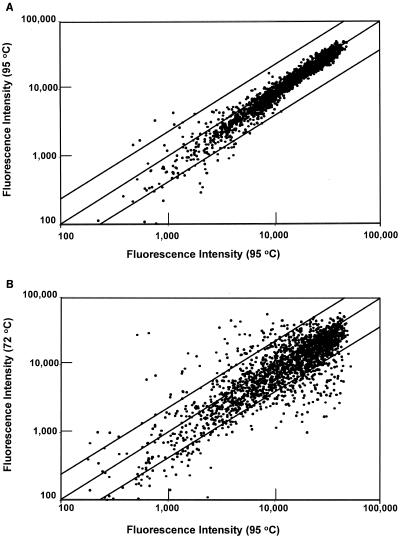
The results of a control experiment using RNA samples prepared from two different cultures of P. furiosus grown under identical conditions at 95°C are shown in Fig. 2A. The fluorescence signal intensities vary over more than a 103 range, and ORFs with intensities less than 2,000 arbitrary units (or twice the detection limit) are considered not to be expressed at a significant level. As expected, the low-intensity signals show a high standard deviation, due to background fluorescence, while the more-highly expressed ORFs lie close to the diagonal. As shown in Fig. 2B, the results of a cold-adapted experiment comparing RNA samples from cells that were grown at 95 and at 72°C revealed a dramatic change in gene expression on a genome-wide basis. Of the 2,065 ORFs analyzed, 245 exhibited statistically significant up- or down-regulation, with P values of <0.01 and at least a 2.5-fold change in expression. Most of them (189 of 245) were down-regulated at the lower growth temperature. Similar microarray analyses were performed using RNA samples obtained 1, 2, and 5 h after switching a culture from 95 to 72°C, and the shock response also resulted in a significant down-regulation of gene expression (after 1, 2, and 5 h, there were 171, 69, and 152 ORFs, respectively, down-regulated by >2.5-fold; P < 0.01). Hence, compared to growth at 95°C, much less mRNA was present for certain cellular processes at 72°C. Remarkably, on the other hand, there are a number of ORFs whose expression is significantly up-regulated in response to the temperature decrease, and these were the focus of the present work. The products of these ORFs are assumed to be involved in the processes by which the cells become acclimatized to 72°C. After 1, 2, and 5 h of shock at 72°C, and in cells adapted to 72°C, there were 49, 35, 30, and 59 ORFs, respectively, that were significantly up-regulated (≥2.5-fold; P < 0.01). Some properties of these ORFs are listed in Tables 1 and 2 and are depicted diagrammatically in Fig. 3. Complete genome-wide data sets for all growth conditions are available at the website http://adams.bmb.uga.edu/pubs/sup252.pdf.
FIG. 2.
Relative fluorescence intensities of DNA microarrays. (A) Comparison of cDNAs derived from two independent cultures of cells grown at 95°C. (B) Comparison of cDNAs derived from two independent cultures of cells grown at 95°C and at 72°C. The upper and lower diagonal lines indicate 2.5-fold changes in the signal intensities. See text for details.
TABLE 1.
ORFs whose expression is up-regulated 1, 2, and 5 h after changing the temperature from 95 to 72°C
| ORF | Descriptiona | TMDsb | Mean fluorescence intensity ratio atc:
|
||
|---|---|---|---|---|---|
| 1 h | 2 h | 5 h | |||
| Threonine biosynthesis | |||||
| PF0029 | [Hypothetical protein] | 0 | 2.2 ± 0.6 (4.6) | 1.8 ± 0.8 (3.5) | 1.4 ± 0.6 (2.7) |
| PF0030 | [Conserved hypothetical protein] | 0 | 2.3 ± 0.7 (4.8) | 1.9 ± 0.6 (3.7) | |
| PF0031 | [Threonine synthase] | 0 | 2.3 ± 0.7 (4.9) | ||
| PF0085 | [DNA helicase] | 0 | 1.6 ± 0.5 (3.1) | ||
| PF0094 | Protein disulfide oxidoreductase (3) | 0 | 1.9 ± 0.6 (3.8) | ||
| PF0101 | [Conserved hypothetical protein] | 0 | 2.3 ± 0.7 (4.9) | ||
| PF0115 | [ATP-dependent 26S protease, regulatory] | 0 | 1.5 ± 0.3 (2.7) | ||
| PF0190 | [Conserved hypothetical protein] | 2 | 1.7 ± 0.7 (3.2) | ||
| Glutamate biosynthesis | |||||
| PF0204 | [Glutamine amidotransferase] | 0 | 1.6 ± 0.5 (3.1) | 2.0 ± 0.5 (4.0) | |
| PF0205 | [Glutamate synthase, alpha] | 0 | 1.4 ± 0.5 (2.6) | ||
| PF0262 | [MFS transporter] | 11 | 1.3 ± 0.4 (2.6) | ||
| PF0264 | [Histidyl-tRNA synthetase] | 0 | 1.6 ± 0.4 (3.0) | ||
| PF0276 | [Oxidoreductase] | 0 | 2.0 ± 0.4 (4.0) | 1.6 ± 0.5 (3.0) | 1.4 ± 0.5 (2.7) |
| PF0295 | [N-type ATP pyrophosphatase] | 0 | 1.4 ± 0.5 (2.7) | ||
| PF0312 | ADP-dependent glucokinase (27) | 0 | 1.4 ± 0.5 (2.6) | ||
| PF0318 | [Acylaminoacyl releasing enzyme] | 0 | 2.0 ± 0.4 (4.0) | 2.2 ± 0.4 (4.5) | 1.9 ± 0.7 (3.8) |
| Unknown | |||||
| PF0324 | [Conserved hypothetical protein] | 1 | 2.4 ± 0.9 (5.2) | 4.6 ± 2.4 (23.8)d | |
| PF0325 | [Conserved hypothetical protein] | 1 | 2.3 ± 0.6 (4.8) | 4.4 ± 1.1 (21.3) | |
| PF0326 | [Conserved hypothetical protein] | 3 | 2.3 ± 0.9 (4.9) | ||
| PF0481 | [Translation initiation factor eIF-2, beta] | 0 | 1.3 ± 0.4 (2.5) | ||
| PF0651 | [Conserved hypothetical protein] | 1 | 3.1 ± 0.6 (8.3) | 2.9 ± 0.9 (7.2) | |
| PF0718 | [Conserved hypothetical protein] | 5 | 2.0 ± 0.4 (4.1) | ||
| PF0721 | [Conserved hypothetical protein] | 0 | 1.4 ± 0.3 (2.7) | 1.6 ± 0.5 (3.0) | |
| PF0822 | [Conserved hypothetical protein] | 0 | 1.3 ± 0.2 (2.5) | ||
| PF0859 | [MttC/TatD homolog] | 0 | 1.8 ± 0.4 (3.4) | 1.4 ± 0.5 (2.6) | |
| PF0929 | [Conserved hypothetical protein] | 0 | 1.8 ± 0.5 (3.5) | 1.6 ± 0.4 (3.1) | |
| Branched-chain amino acid biosynthesis | |||||
| PF0934 | [Conserved hypothetical protein] | 0 | 2.5 ± 0.5 (5.6) | ||
| PF0935 | [Acetolactate synthase] | 0 | 2.5 ± 1.0 (5.6) | ||
| PF0939 | [3-Isopropylmalate dehydratase, small] | 0 | 1.8 ± 0.7 (3.4) | ||
| PF0940 | [3-Isopropylmalate dehydrogenase] | 0 | 2.5 ± 1.0 (5.7) | ||
| PF1026 | [Malic enzyme] | 0 | 1.3 ± 0.4 (2.5) | ||
| Serine/threonine biosynthesis | |||||
| PF1053 | [Aspartokinase II, alpha] | 0 | 1.4 ± 0.4 (2.6) | 1.6 ± 0.5 (3.1) | |
| PF1054 | [Homoserine kinase] | 0 | 1.7 ± 0.6 (3.2) | 2.3 ± 0.7 (5.0) | |
| PF1055 | [Threonine synthase] | 0 | 1.1 ± 0.3 (2.2) | ||
| PF1056 | [Aspartate-semialdehyde dehydrogenase] | 1 | 1.4 ± 0.4 (2.6) | 1.7 ± 0.4 (3.3) | |
| PF1062 | [Nuclease repair enzyme] | 0 | 2.9 ± 0.9 (7.7) | ||
| PF1104 | [Homoserine dehydrogenase] | 0 | 1.3 ± 0.2 (2.5) | ||
| PF1137 | [Translation initiation factor IF-2] | 0 | 1.6 ± 0.5 (3.0) | 1.5 ± 0.5 (2.8) | |
| Methionine biosynthesis | |||||
| PF1266 | [Cystathionine gamma-lyase] | 0 | 1.3 ± 0.5 (2.5) | ||
| PF1267 | [Conserved hypothetical protein] | 0 | 1.8 ± 0.5 (2.3) | ||
| PF1268 | [Conserved hypothetical protein] | 0 | 1.8 ± 0.7 (3.4) | ||
| PF1270 | [Conserved hypothetical protein] | 0 | 1.9 ± 0.9 (3.8)d | ||
| PF1344 | [Maleate cis-trans isomerase] | 0 | 1.4 ± 0.4 (2.6) | ||
| PF1348 | [Hypothetical protein] | 0 | 2.9 ± 1.1 (7.4) | ||
| PF1408 | [Dipeptide-binding protein] | 1 | 1.7 ± 0.7 (3.2) | ||
| Unknown | |||||
| PF1454 | [Conserved hypothetical protein] | 0 | 1.5 ± 0.6 (2.7) | ||
| PF1455 | [Hypothetical protein] | 0 | 1.9 ± 0.3 (3.7) | ||
| Tungstoenzyme-5 | |||||
| PF1479 | [Oxidoreductase, Fe-S subunit] | 0 | 2.3 ± 0.8 (4.9) | 2.5 ± 0.5 (5.6) | |
| PF1480 | [Formaldehyde:Fd oxidoreductase, WOR-5] | 0 | 2.3 ± 0.4 (5.0) | 2.4 ± 0.4 (5.1) | 2.1 ± 0.7 (4.2) |
| Carbohydrate metabolism | |||||
| PF1535 | [Alpha-glucan phosphorylase] | 0 | 1.2 ± 0.1 (2.3) | 1.2 ± 0.4 (2.3) | |
| PF1536 | [Short-chain dehydrogenase] | 0 | 1.0 ± 0.2 (2.0) | 1.1 ± 0.4 (2.1) | |
| PF1537 | [Conserved hypothetical protein] | 5 | 1.2 ± 0.4 (2.3) | 1.5 ± 0.3 (2.8) | |
| PF1538 | [Amidohydrolase] | 0 | 1.4 ± 0.4 (2.7) | 1.8 ± 0.3 (3.5) | |
| PF1654 | [ABC transporter, ATP binding] | 6 | 1.9 ± 0.1 (3.7) | 1.9 ± 0.5 (3.8) | |
| 2-Keto acid ferredoxin oxidoreductases | |||||
| PF1767 | KGOR, delta (47) | 0 | 2.3 ± 0.4 (4.9) | 2.5 ± 0.5 (5.6) | |
| PF1768 | KGOR, alpha (47) | 0 | 2.3 ± 0.5 (4.9) | 2.4 ± 0.5 (5.2) | |
| PF1769 | KGOR, beta (47) | 0 | 1.9 ± 0.6 (3.7) | 2.2 ± 0.5 (4.7) | |
| PF1770 | KGOR, gamma (47) | 0 | 1.9 ± 0.7 (3.7) | 2.1 ± 0.4 (4.3) | |
| PF1771 | [2-Keto acid:Fd oxidoreductase, alpha] | 0 | 2.0 ± 0.5 (4.0) | 2.1 ± 0.4 (4.4) | |
| PF1772 | [2-Keto acid:Fd oxidoreductase, beta] | 0 | 1.9 ± 0.6 (3.6) | 1.9 ± 0.5 (3.8) | |
| PF1773 | [2-Keto acid:Fd oxidoreductase, gamma] | 0 | 1.8 ± 0.6 (3.6) | 2.1 ± 0.5 (4.3) | |
| Unknown | |||||
| PF1907 | [Conserved hypothetical protein] | 1 | 1.2 ± 0.3 (2.3) | 1.6 ± 0.3 (3.1) | 1.2 ± 0.3 (2.3) |
| PF1908 | [Conserved hypothetical protein] | 1 | 1.2 ± 0.4 (2.2) | 1.1 ± 0.3 (2.2) | |
| PF1956 | Fructose-1,6-biphosphate aldolase (50) | 0 | 2.2 ± 0.3 (4.5) | 1.8 ± 0.3 (3.5) | |
| Sugar metabolism | |||||
| PF1959 | Phosphoglycerate mutase (52) | 0 | 1.1 ± 0.2 (2.2) | ||
| PF1960 | [Aldose reductase] | 0 | 2.4 ± 0.8 (5.2) | 2.4 ± 0.6 (5.3) | |
| DNA synthesis | |||||
| PF1971 | Anaerobic ribonucleotide reductase (41) | 0 | 1.3 ± 0.6 (2.5)d | 1.3 ± 0.7 (2.4)d | |
| PF1972 | [Radical SAM enzyme] | 0 | 2.0 ± 0.6 (3.9) | 1.6 ± 0.4 (3.0) | |
| PF1973 | [Conserved hypothetical protein] | 1 | 2.1 ± 0.6 (4.3) | 2.5 ± 0.4 (5.5) | 1.8 ± 0.7 (3.4) |
| PF1975 | [Conserved hypothetical protein] | 1 | 1.5 ± 0.6 (2.8) | ||
| Metal ion transport | |||||
| PF2036 | [Magnesium and cobalt transporter] | 2 | 1.3 ± 0.3 (2.4) | ||
| PF2037 | [Hypothetical protein] | 0 | 1.5 ± 0.6 (2.8) | ||
The ORF description is derived either from the annotation from the website http://combo5-156.umbi.umd.edu/genemate/ (given within brackets), from the indicated reference where there are experimental data to support the ORF assignment specifically in P. furiosus (given without brackets), or where a particular ORF is discussed in the text. Potential operons and their functions are shown in bold.
TMDs refers to the number of predicted transmembrane domains in the protein encoded by that ORF.
The fluorescence intensity ratio (at 72 versus 95°C) for each ORF after the indicated time period (1, 2, or 5 h), is expressed as the log2±standard deviation value so that the standard deviation can be given. The fold change in intensity is given in parentheses. The regulation is statistically significant (P < 0.01) for all ORFs unless indicated otherwise.
Statistics: the 5-h value for PF0324, P = 0.061; the 5-h value for PF1270, P = 0.017; the 1- and 2-h values for PF1971, P = 0.025 and 0.067, respectively.
TABLE 2.
ORFs whose expression is up-regulated in cells adapted to 72°C compared to cells adapted to 95°C
| ORF | Description | TMDs | Mean (±SD) fluoresence intensity ratio
|
|
|---|---|---|---|---|
| 5 ha | Adapteda | |||
| PF0094 | Protein disulfide oxidoreductase (3) | 0 | 1.4 ± 0.7 (2.7) | |
| PF0101 | [Conserved hypothetical protein] | 0 | 2.5 ± 1.3 (5.5) | |
| PF0190 | [Conserved hypothetical protein] | 2 | 1.7 ± 0.7 (3.2) | 2.9 ± 0.6 (7.4) |
| PF0286 | [Conserved hypothetical protein] | 0 | 1.8 ± 0.6 (3.5) | |
| PF0296c | [Cobalamin biosynthesis protein] | 4 | 1.4 ± 0.8 (2.7) | |
| PF0297 | [Conserved hypothetical protein] | 0 | 1.9 ± 1.0 (3.6) | |
| PF0298 | [Conserved hypothetical protein] | 0 | 1.1 ± 0.4 (2.2) | |
| PF0318 | [Acylaminoacyl releasing enzyme] | 0 | 1.9 ± 0.7 (3.8) | 1.6 ± 0.6 (3.1) |
| PF0323 | [Conserved hypothetical protein] | 1 | 2.4 ± 1.3 (5.3) | |
| Unknown | ||||
| PF0324 | [Conserved hypothetical protein] | 1 | 4.6 ± 2.4 (23.8)b | 5.8 ± 2.1 (57.4) |
| PF0325 | [Conserved hypothetical protein] | 1 | 4.4 ± 1.1 (21.3) | 5.3 ± 2.1 (39.0) |
| PF0327 | [Conserved hypothetical protein] | 1 | 2.4 ± 1.9 (5.3) | |
| PF0371 | [Conserved hypothetical protein] | 12 | 1.4 ± 0.9 (2.6) | |
| PF0428 | [Alanyl-tRNA, C terminus] | 0 | 1.4 ± 0.7 (2.7) | |
| PF0429 | [Putative proline permease] | 13 | 3.9 ± 0.9 (14.6) | |
| PF0516 | [Conserved hypothetical protein] | 1 | 1.6 ± 0.6 (3.1) | |
| PF0665 | [Conserved hypothetical protein] | 4 | 1.6 ± 0.6 (2.9) | |
| PF0708 | [Conserved hypothetical protein] | 11 | 1.5 ± 0.3 (2.9) | |
| PF0709 | [Hypothetical protein] | 0 | 1.9 ± 0.7 (3.7) | |
| PF0718 | [Conserved hypothetical protein] | 5 | 2.0 ± 0.7 (3.9) | |
| PF0719 | [Hypothetical protein] | 0 | 2.5 ± 1.4 (5.5) | |
| PF0727 | [Hypothetical protein] | 0 | 2.1 ± 0.6 (4.4) | |
| PF0934 | [Conserved hypothetical protein] | 0 | 2.5 ± 0.5 (5.6) | 2.8 ± 0.8 (6.9) |
| Branched chain amino acid biosynthesis | ||||
| PF0935 | [Acetolactate synthase] | 0 | 2.5 ± 1.0 (5.6) | 3.3 ± 2.2 (9.9) |
| PF0936 | [Ketol-acid reductoisomerase] | 0 | 2.2 ± 1.4 (4.5) | |
| PF0943 | [Conserved hypothetical protein] | 0 | 2.0 ± 1.3 (3.9) | |
| PF1025 | [Conserved hypothetical protein] | 0 | 2.1 ± 0.5 (4.2) | |
| PF1062 | [Conserved hypothetical protein] | 0 | 2.6 ± 1.0 (6.2) | |
| Unknown | ||||
| PF1072 | [Conserved hypothetical protein] | 0 | 3.7 ± 0.6 (12.7) | |
| PF1073 | [Conserved hypothetical protein] | 4 | 3.3 ± 0.8 (9.8) | |
| PF1074 | [Conserved hypothetical protein] | 0 | 1.5 ± 0.6 (2.8) | 3.5 ± 0.8 (11.3) |
| Gyrase modulator | ||||
| PF1076 | [tldD protein homolog] | 0 | 2.0 ± 0.4 (4.1) | |
| PF1077 | [Protein interaction modulator] | 0 | 2.4 ± 0.5 (5.3) | |
| PF1100 | [Conserved hypothetical protein] | 1 | 1.8 ± 0.8 (3.5) | |
| PF1247 | [Conserved hypothetical protein] | 6 | 1.5 ± 0.4 (2.8) | |
| PF1270 | [Conserved hypothetical protein] | 0 | 1.9 ± 0.9 (3.8)b | 1.9 ± 0.7 (3.8) |
| Unknown | ||||
| PF1344 | [Maleate cis-trans isomerase] | 0 | 1.4 ± 0.4 (2.6) | 1.5 ± 0.4 (2.8) |
| PF1345 | [Arylsulfatase] | 0 | 1.6 ± 0.3 (3.0) | |
| PF1346 | [Pheromone shutdown protein] | 0 | 1.9 ± 0.6 (3.7) | |
| PF1347 | [Hypothetical protein] | 0 | 1.3 ± 0.6 (2.5) | |
| PF1408 | [Probable dipeptide-binding protein] | 1 | 1.7 ± 0.7 (3.2) | 2.2 ± 0.5 (4.7) |
| PF1418 | [Conserved hypothetical protein] | 0 | 1.6 ± 0.7 (2.9) | |
| PF1460 | [Conserved hypothetical protein] | 0 | 2.1 ± 1.2 (4.4) | |
| Vitamin biosynthesis | ||||
| PF1528 | [Imidazoleglycerol Pi synthase] | 0 | 2.4 ± 1.6 (5.2) | |
| PF1529 | [Ethylene-inducible protein homolog] | 0 | 1.8 ± 0.6 (3.5) | |
| PF1670 | [Alkaline serine protease] | 1 | 1.4 ± 0.9 (2.6) | |
| PF1677 | [Conserved hypothetical protein] | 6 | 2.8 ± 1.4 (6.8) | |
| Branched-chain amino acid biosynthesis | ||||
| PF1678 | [2-Isopropyl malate synthase] | 0 | 3.2 ± 1.1 (9.4) | |
| PF1679 | [3-Isopropyl malate dehydratase I] | 1 | 3.1 ± 1.4 (8.8) | |
| Aromatic amino acid biosynthesis | ||||
| PF1701 | [Chorismate mutase] | 0 | 3.9 ± 2.3 (14.5) | |
| PF1702 | [Aspartate aminotransferase] | 0 | 2.8 ± 0.9 (7.1) | |
| PF1703 | [Prephenate dehydrogenase] | 0 | 3.1 ± 0.8 (8.5) | |
| PF1711 | [Indole-3-glycerol phosphate synthase] | 0 | 1.6 ± 0.8 (3.0) | |
| PF1767 | KGOR, delta (47) | 0 | 1.8 ± 0.8 (3.4) | 1.5 ± 0.8 (2.8) |
| PF1944 | [Conserved hypothetical protein] | 0 | 1.9 ± 0.6 (3.8) | |
| Tungstoenzyme-4 | ||||
| PF1960 | [Aldehyde/aldose reductase] | 0 | 1.8 ± 0.8 (3.6) | |
| PF1961 | WOR4, W-oxidoreductase (44) | 0 | 1.9 ± 0.5 (3.8) | |
| PF1973 | [Conserved hypothetical protein] | 1 | 1.8 ± 0.7 (3.4) | 1.8 ± 0.7 (3.6) |
| PF2032 | [Conserved hypothetical protein] | 11 | 1.5 ± 0.7 (2.9) | |
See Table 1 and the text for details.
Statistics for the 5-h values: PF0324, P = 0.061; PF 1270, P = 0.017. For all other ORFs the P values are <0.010.
Potential operons are shown in bold where the intergenic distances are less than 16 nucleotides.
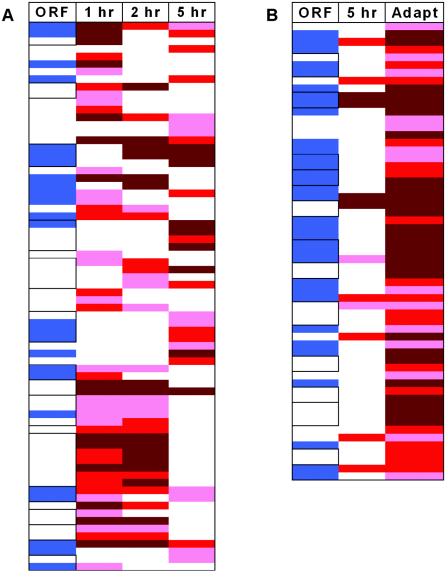
FIG. 3.
Summary of ORFs whose expression is up-regulated by cold shock (A) and by cold adaptation (B). Panel A is a representation of Table 1 and shows the patterns of ORFs that are up-regulated 1, 2, or 5 h after cold shock. ORFs that are annotated as conserved-hypothetical proteins are shown in blue, and those that are part of potential operons are boxed. Those ORFs up-regulated after 1, 2, or 5 h after cold shock are in red, where a light, medium, or dark intensity indicates a more-than-2.5-, 3.0-, or 4.0-fold increase in expression, respectively. Panel B is a representation of Table 2 and shows the pattern of ORFs up-regulated by 5-h cold shock and by cold adaptation. “Adapt” represents all ORFs up-regulated in cells grown at 72°C, and whether the same ORF was also up-regulated after 5 h after cold shock is indicated. ORFs that are annotated as conserved-hypothetical proteins are shown in blue. The intensity of the red color represents the same as it does in panel A. The data used for panels A and B were taken directly from Tables 1 and 2, respectively.
From even a cursory look at Fig. 3 it is clear that P. furiosus has, in fact, three different responses to living at 72°C. Virtually all of the 49 ORFs up-regulated 1 h postshock were also among the 35 ORFs up-regulated after 2 h, but very few of these were included with the 30 ORFs up-regulated at 5 h postshock (Fig. 3A; Table 1). Similarly, and most surprisingly, virtually none of the 59 ORFs that were up-regulated in the cold-adapted cells (grown at 72°C) was up-regulated after 1, 2, or even 5 h after the temperature was decreased from 95 to 72°C (Fig. 3B; Table 2). It therefore appears that there are both early (1 to 2 h) and late (5 h) cold shock responses, in addition to the cold adaptation response, and that these three cellular responses involve very different proteins. However, these proteins do have some properties in common. As indicated in Fig. 3, a significant number are of unknown function (the conserved-hypothetical) and/or are adjacent to each other on the P. furiosus genome and appear to be encoded by operons (http://www.tigr.org). For example, of the 59 ORFs that were up-regulated in the cold-adapted experiment, 28 of them are part of 12 potential operons, and of the 72 ORFs up-regulated during both the early and late shock responses, 43 ORFs are part of 14 potential operons. Such apparent coregulation of adjacent genes tends to support the notion that they have related functions. Moreover, of the 22 total putative cold-responsive operons identified, 9 contain exclusively or predominantly conserved-hypothetical proteins. This is not surprising, as the genome sequences of hyperthermophilic archaea available lack homologs to cold shock proteins previously identified in bacteria.
The early shock response.
The early cold shock response seen within 1 to 2 h of shifting to 72°C is characterized by the up-regulation of a large number of conserved-hypothetical proteins (17 of 55) and of ORFs whose products appear to be involved in the translation process, in amino acid and primary carbohydrate metabolism, in oxidoreductase-type reactions, and in solute transport (Table 1). Those involved in translation include a histidinyl tRNA synthetase (PF0264), an initiation factor (e-IF2-β, PF0481), and what appears to be an unusual version of an initiation-elongation factor (EF-2-like, PF1137). The P. furiosus genome contains homologs of the latter two proteins (PF1349 and PF2012), and these are not affected by the temperature change. P. furiosus may therefore possess initiation and elongation factors that are specifically required for and presumably enhance translation at suboptimal temperatures. There is also tantalizing evidence that DNA biosynthesis may be affected by the temperature decrease. PF1971 encodes anaerobic ribonucleotide reductase, and this is up-regulated (2.5-fold) in the early shock response (at 1 and 2 h). This appears to be coordinately regulated with the adjacent ORF, PF1972, which shows similarity to radical S-adenosylmethionine proteins and is up-regulated almost fourfold. P. furiosus also contains a conventional adenosyl-cobalamin-dependent ribonucleotide reductase (PF0440) (41), the expression of which is down-regulated in adapted cells (by 9.1-fold), although not in cold-shocked cells. Potentially, there is a switch to the anaerobic enzyme for DNA synthesis in response to a drop in temperature.
The early response to cold shock also involves modulation of primary carbon metabolism. The two glycolytic enzymes fructose-1,6-bisphosphate aldolase (PF1956; 4.5-fold; 1 h) and phosphoglycerate mutase (PF1959; 2.2-fold; 1 h) are both up-regulated (as is a third, glucokinase [PF0312], after 5 h). What is surprising is that these are not the primary points of regulation in the glycolytic pathway in P. furiosus (53), which are glucose-6-phosphate isomerase (PF0196), phosphofructokinase (PF1784), and glyceraldehyde-3-phosphate oxidoreductase (PF0464). Expression of the latter genes does not change during the shock response, but presumably the cold response requires additional phosphorylated intermediates and the up-regulated enzymes (PF1956 and PF1959) would otherwise be rate limiting at 72°C.
Perhaps the most dramatic response in the early phase of the shock response is the up-regulation (≥4-fold) of an operon containing seven ORFs (PF1767 to -1773) that encode two 2-ketoacid, Fd-dependent oxidoreductases. One is termed KGOR. This preferentially oxidizes 2-ketoglutarate to succinyl coenzyme A (CoA) and is encoded by four ORFs (PF1767 to -1770) (35). The remaining three ORFs (PF1771 to -1773) are proposed to encode an as-yet-uncharacterized enzyme of this type, XOR, which presumably converts another type of 2-ketoacid to its CoA derivative. Like the succinyl CoA produced by KGOR, this is presumably utilized for biosynthetic purposes. In support of this notion, KGOR is also up-regulated in peptide-grown cells (46). As shown in Table 3, the specific activity of KGOR in cell extracts of P. furiosus was about twofold higher in cells after 2 h at 72°C, compared to cells grown at 95°C. In contrast, the specific activity of the related enzyme pyruvate Fd oxidoreductase (28) was unaffected by the cold response, in accord with the microarray results for its ORFs (data not shown).
TABLE 3.
Activities and relative expression levels of key cold-responsive enzymes
| ORF | Enzymea | Sp actb (U/mg)
|
|||
|---|---|---|---|---|---|
| 95°C (adapted) | 2 h at 72°C (shock) | 5 h at 72°C (shock) | 72°C (adapted) | ||
| PF0318 | [Aminoacyl releasing enzyme] | 0.09 ± 0.02 | 0.26 ± 0.03 | 0.28 ± 0.02 | 0.42 ± 0.07 |
| PF0935 | [Acetolactate synthase] | 0.002 ± 0.001 | 0.004 ± 0.001 | 0.010 ± 0.001 | 0.016 ± 0.003 |
| PF0965-0967, 0971 | POR (47) | 4.52 ± 0.40 | 4.51 ± 0.04 | 3.78 ± 0.39 | 5.06 ± 0.42 |
| PF1767-1770 | KGOR (47) | 0.15 ± 0.01 | 0.28 ± 0.01 | 0.12 ± 0.00 | 0.13 ± 0.03 |
See Table 1 and the text for details. Abbreviations: POR, pyruvate Fd oxidoreductase; KGOR, 2-ketoglutarate Fd oxidoreductase.
Units are defined as micromole of product formed per minute per milligram of protein in cell-free extracts of P. furiosus cells grown under the indicated conditions. Assays were conducted as described in Materials and Methods.
The early cold shock response phase is also characterized by an increase in protein degradation, with an up-regulation of the proteosome (PF0115) and an AARE (PF0318). In the assay developed for AARE in a related Pyrococcus species (19, 39), the specific activity of AARE increased approximately threefold in cell extracts of cold-shocked P. furiosus cells, in agreement with the microarray data (Table 3). These proteases could be degrading nonessential proteins or misfolded essential proteins in the cell upon cold shock to rapidly provide amino acids for protein synthesis. Additional links between carbon and nitrogen metabolism come from the up-regulation of two 4-ORF operons, one (PF1535 to PF1538) encoding a sugar phosphorylase and an amidotransferase and one (PF0204 to PF0205) encoding an Fd-dependent glutamate synthase. A 3-ORF operon (PF0029 to PF0031) encoding a putative threonine synthase is also up-regulated within 1 h (Table 1), although its function is unclear, as the canonical threonine/serine biosynthetic pathway (PF1053 to PF1056) is subsequently up-regulated in the 2-to-5-h time frame.
Other ORFs up-regulated in the early shock response fall into the categories of membrane transporters (PF0262, PF1654, and PF2036) and oxidoreductase-type proteins (PF0094, PF0276, PF1480, and PF1960). PF2036 is a CorA homolog and likely transports magnesium ions, but the solutes bound by the other proteins are not known. The oxidoreductase-type proteins include protein disulfide oxidoreductase (PF0094) (40), which may facilitate protein folding at the lower temperature, and PF1480, which is proposed to encode the fifth and only uncharacterized member of the so-called AOR family of tungsten-containing, Fd-dependent oxidoreductases found in P. furiosus (43). The other members of the family are the glycolytic enzyme glyceraldehyde-3-phosphate oxidoreductase (PF0464) (38), aldehyde Fd oxidoreductase (AOR; PF0346) (8), formaldehyde Fd oxidoreductase (PF1203) (44), and aldehyde Fd oxidoreductase 4 (WOR-4; PF1961) (43), none of which is regulated by the cold (data not shown). The fifth member, WOR-5 (PF1480), appears to be involved in the early cold shock response. Moreover, for the first time, a partner protein is implicated for a member of the AOR family. The adjacent, coregulated ORF, PF1479, encodes a protein that contains 16 cysteine residues, many as motifs that could bind multiple iron-sulfur clusters. This suggests a role in electron transfer, although the biochemical function of the cold-induced WOR-5 system is obviously unknown.
The late shock response.
The expression levels of virtually all of the annotated ORFs discussed above return to near their preshock levels at the end of the acclimation phase, or by 5 h after the switch to 72°C. The notable exceptions are the putative tungstoprotein WOR-5 (PF1480) and AARE (PF0318), as well as several ORFs encoding conserved hypothetical proteins (PF0029, PF0030, PF0721, PF1907, PF1908, and PF1973). In contrast, the late cold shock response (5 h) is characterized by the up-regulation of multiple ORFs involved in the biosynthesis of branched-chain amino acids (PF0934 to PF0940) and methionine (PF1266 to PF1270), none of which are up-regulated even after 2 h, and of the operon involved in serine/threonine biosynthesis (PF1053 to -1056; partially up-regulated after 2 h). The up-regulation of one of the ORFs involved in the biosynthesis of branched-chain amino acids, PF0935, which encodes acetolactate synthase, was investigated in an enzymatic assay. As shown in Table 3, the specific activity increased fivefold after 5 h postshock, in agreement with the microarray data. Other ORFs up-regulated after 5 h (but not 2 h) are annotated to encode DNA helicase (PF0085), pyrophosphatase (PF0295), and a dipeptide-binding protein (PF1408), although the precise role of each is not clear. Several conserved hypothetical proteins (PF0190 and PF1348) are also up-regulated only after 5 h. Indeed, an operon (PF0324 to PF0326) encoding three conserved-hypothetical proteins is the most dramatically up-regulated of all ORFs involved in the late cold shock response (Table 1). The main response at 5 h after the cold shock therefore seems to be an increase in the biosynthesis of certain amino acids that utilize the activated C-3 and C-4 intermediates generated in the first 2 h from the primary carbon source maltose, as well as the up-regulation of a number of proteins of unknown function.
The cold growth or adapted response.
As shown in Table 2 and Fig. 3B, very few of the ORFs that are up-regulated during the late cold shock response (after 5 h) remain so once the cells are fully adapted to growth at the lower temperature (after many generation times) when grown continuously at 72°C. Thus, the complete switch to the expression levels of the adapted state must take place much later than what appears to be the end of the acclimation phase as judged by the growth curve (Fig. 1). Additional experiments will be required to ascertain when the acclimation phase is complete at the transcriptional level. The few annotated ORFs that are up-regulated in both late shock (5 h) and in the adapted cells include those encoding AARE (PF0318) and acetolactate synthase (PF0935). As shown in Table 3, the specific activities of both enzymes are at least fivefold higher in the adapted cells, while the value for KGOR is not affected by adaptation to 72°C, results that are consistent with the array data. On the other hand, there are a number of ORFs encoding conserved-hypothetical proteins that are up-regulated in the late shock phase that either remain at a similar level in the adapted cells (PF0934, PF1270, and PF1973) or increase even further (PF0190 and PF1074). The latter include two adjacent ORFs (PF0324 and PF0325) up-regulated by >20-fold after 5 h and by >39-fold in the adapted cells. Indeed, the response of the adapted cells is dominated by conserved-hypothetical proteins with 34 of 59 up-regulated ORFs falling in this category. There are also a significant number of ORFs encoding proteins predicted to contain transmembrane domains (21 of 59) (Table 2). It should be noted that while no conclusions can be drawn about the function of conserved-hypothetical proteins, several of the annotated ORFs are only given general, nondescriptive names (Table 2). For example, the 3-ORF operon (PF1344 to PF1346) that is up-regulated encodes a putative Asp/Glu racemase, a lactamase-type esterase, and a regulatory protein, but these descriptions shed no light on its biological role in P. furiosus.
On the other hand, some function can be assigned to many of the up-regulated ORFs that are annotated. In fact, the adaptation to growth at 72°C seems to involve many of the same processes as the early and late shock responses, but different proteins are involved. This includes ORFs involved in aromatic amino acid biosynthesis (PF1701 to PF1703), carbohydrate metabolism (aldose reductase [PF1960]), proteolysis (serine protease [PF1670]), transport (PF0371, PF0429, and PF1408), and protein synthesis (amino acyl tRNA synthetase [PF0428]). One of the most interesting examples of the same theme but with different proteins concerns tungsten. The dramatic up-regulation of WOR-5 (PF1480) seen in the shock response is not seen in the adapted response. Rather, WOR-4 (PF1961) is up-regulated in the adapted response. WOR-4 is known to be expressed at a significant level in cells grown at 95°C (43), and why it should be up-regulated in cold-adapted cells (perhaps replacing WOR-5) is not at all clear.
There are also ORFs encoding new processes that are up-regulated in adapted cells. These include proteins involved in vitamin biosynthesis (pyridoxine or B6 [PF1528 to -1529] and cobalamin [PF0296]) and in regulating DNA gyrase activity (PF1076 and PF1077) (2, 36). The latter is particularly intriguing, as an enzyme known as reverse gyrase is the only enzyme unique to hyperthermophiles (both archaea and bacteria). It is the only topoisomerase that positively supercoils DNA and is thought to be essential to life at extreme temperatures (12). The notion that less supercoiling is needed at suboptimal growth temperatures and a specific inhibitor of the gyrase is required would support that idea. Additional evidence comes from studies of other hyperthermophilic archaea, which have shown that there is a change in DNA topology in response to suboptimal growth temperatures, with an increase in negative supercoiling (32-34).
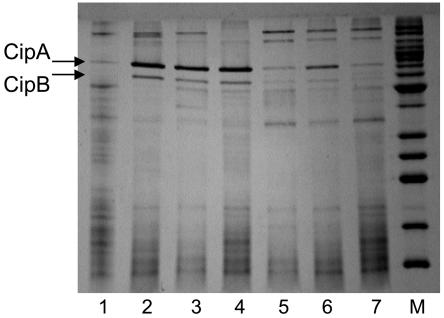
Changes in the proteome content of membranes.
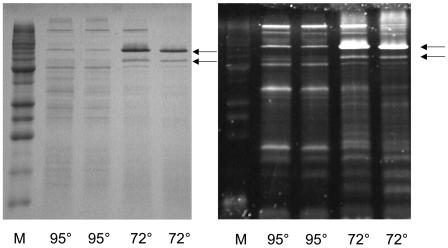
In order to assess changes in the proteome, rather than the transcriptome, cytoplasmic and membrane fractions were prepared from P. furiosus cells obtained in the early and late shock responses, as well as in the adapted response, and these were analyzed by SDS-PAGE. Although no consistent changes were observed in the protein profiles of the cytoplasmic extracts (data not shown), two major cold-induced proteins were readily evident in the membrane fractions of cold-shocked cells. Moreover, both appeared to increase in concentration throughout the shock phase and to accumulate to high concentrations in the adapted cells (Fig. 4). These two proteins were given the names CipA and CipB (for cold induced protein) and, using MALDI-TOF MS analysis of the bands after in-gel trypsin digestion, these were identified as PF0190 (a conserved hypothetical protein) and PF1408 (annotated as a probable dipeptide-binding protein), respectively. As shown in Table 2, PF0190 is up-regulated by 3.2- and 7.4-fold, and PF1408 is up-regulated 3.2- and 4.7-fold, after 5 h postshock and in the adapted cells, respectively, which is generally consistent with the amount of protein seen on the SDS-PAGE gels. In order to validate the results from the DNA microarray data, real-time qPCR was used to assess the expression of PF0190 and PF1408. The RNA preparations examined were the same as those used for the arrays. The qPCR analyses showed that PF0190 and PF1408 were up-regulated in 72°C-adapted cells, relative to those grown at 95°C, by factors of 26.0 ± 9.3 and 5.8 ± 1.8, respectively. These data compare well with the values from the arrays (Table 2).
FIG. 4.
SDS-PAGE analysis of membrane fractions of cold-shocked and cold-adapted cells. Results for SDS-PAGE analysis (15%) of the salt-washed (4.0 M NaCl) membrane fractions of P. furiosus cells grown at various temperatures are shown. Lanes: 1, 95°C; 2 to 4, 72°C (from three different cultures grown independently); 5, grown at 95°C and then shocked at 72°C for 2 h; 6, grown at 95°C and then shocked at 72°C for 5 h; 7, 95°C. M, molecular weight markers. Lanes 1 to 7 each contained 7 μg of protein. The positions of the bands representing CipA and CipB are indicated.
Cytoplasmic and membrane fractions from cells grown at either 95 or 72°C were also subjected after initial separation by SDS-PAGE to a glycoprotein stain, which detects carbohydrate groups that can be oxidized by periodate. With the cytosolic fractions, there was no clear staining of any bands and there were no differences between the two cell types, indicating that there are few if any glycoproteins in the cytosol of P. furiosus cells grown at either temperature. However, the membrane fractions gave dramatically different results. First, as shown in Fig. 5B, the membrane fraction from cells grown at 95°C showed five major glycoproteins with apparent masses of 18, 35, 52, 75, and 100 kDa. Second, in the cold-adapted cells, the lower molecular mass proteins (18, 35, and 52 kDa) decreased in intensity and the 100-kDa band remained the same, while the band at 75 kDa dramatically increased in intensity and a new band appeared near 65 kDa. The bands near 65 and 75 kDa were cut out of the gel, subjected to in-gel trypsin digestion, analyzed by MALDI-TOF MS, and identified as CipA (PF0190) and CipB (PF1408), respectively. Thus, the two major proteins induced by cold shock and adaptation in the membranes of P. furiosus are glycoproteins. Attempts to identify the 14-, 40-, 50-, and 100-kDa glycoproteins by tryptic-MS analyses have so far been unsuccessful. Despite the apparent increase in glycoprotein content according to SDS-PAGE, the overall glycoprotein contents of the membrane fractions of cells grown at 95 and 72°C remained virtually the same by the periodate detection method.
FIG. 5.
SDS-PAGE analysis of membrane fractions of cold-adapted cells stained for carbohydrates. Results are shown for the SDS-PAGE (15%) analysis of the salt-washed (4.0 M NaCl) membrane fractions of P. furiosus cells grown at the indicated temperature (72 or 95°C). M, marker proteins. The gels were stained for protein (with Coomassie brilliant blue) (left) or for carbohydrate (with Emerald 300 Q glycoprotein stain) (right). The arrows indicate the positions of the bands representing CipA (upper) and CipB (lower).
CipA (PF0190) and CipB (PF1408) are large proteins (94.7 and 79.3 kDa, respectively), and both are predicted to be membrane associated with transmembrane domains and signal sequences, consistent with biochemical data. CipB (PF1408) is annotated as a dipeptide-binding protein and by the InterPro (http://www.ebi.ac.uk/interpro/) analysis belongs to the solute-binding protein family (IPR000914). Although CipA is annotated as a conserved hypothetical protein and is null by the InterPro analysis, by BLAST analysis it also appears to be a member of the same solute-binding protein family, showing 10% identity (22% similarity) to CipB. Interestingly, CipA and CipB are each upstream of a cluster of four ORFs (PF0191 to PF0194 for CipA and PF1409 to PF1412 for CipB). The two nearest ORFs in each cluster are annotated as dipeptide transport system permease proteins, while the more distant two ORFs are annotated as dipeptide transport ATP-binding proteins (Fig. 6). While the Cip ORFs and their adjacent 4-ORF clusters clearly encode an ABC-type transporter system, the microarray data indicate that of all 10 ORFs, only CipA and CipB are up-regulated in response to the cold stress. In fact, all of the other ORFs in these clusters are actually down-regulated in response to cold stress (up to 6.7-fold).
FIG. 6.
Genome organization for CipA and CipB gene clusters. The arrangement of CipA and CipB with respect to downstream genes is indicated. The arrangement of analogous gene clusters in L. monocytogenes (Lmo2196 to -2192), B. subtilis (Bsu1144 to -1148), S. pyogenes (SPs0221 to -0225), and S. enterica serovar Typhimurium (STM1746 to -1742) are shown for comparison. The ORFs and intergenic distances are drawn approximately to scale (the bar indicates 100 nucleotides). The information on the bacterial genes was modified from reference 4, and the sequences were taken from the website http://comb5-156.umbi.umd.edu/genemate/.
The oligopeptide-binding protein OppA of L. monocytogenes is also included in the same solute-binding family as CipB (12% identity, 23% similarity). Interestingly, OppA was recently shown to be up-regulated in response to cold shock (5). Moreover, it and the two Cip proteins are part of the same gene arrangement, where the solute-binding protein (OppA [PF0190 or PF1408]) is located far upstream (100 to 300 nucleotides) of a 4-ORF cluster that encodes two permeases and two ATP-binding proteins (Fig. 6). The two 4-ORF clusters in P. furiosus show much higher sequence similarity to their corresponding partners in L. monocytogenes than do CipA and CipB to OppA. For example, for PF0191 to PF0194, the sequence similarities range from 33 to 59% (19 to 44% identity). In addition, not only is OppA required for growth of L. monocytogenes at suboptimal growth temperatures (5°C), it is expressed using its own promoter and is not coexpressed with the rest of the operon (5), as seems to be the case with CipA and CipB of P. furiosus. Similar operons are found in B. subtilis, Salmonella enterica serovar Typhimurium, and Streptococcus pyogenes (Fig. 6). Interestingly, expression of the Cip homolog in B. subtilis was not affected at 18°C (25), although a lower temperature might be required, as up-regulation of the expression of OppA was seen after cold shock at 4°C in L. monocytogenes.
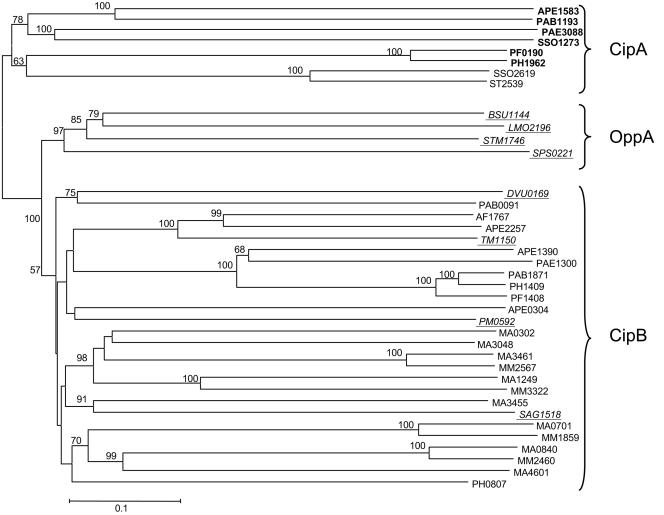
The cold-responsive relationship between CipA and CipB of P. furiosus and OppA of L. monocytogenes is extremely significant, because it is the first definitive overlap between the hyperthermophilic archaeal and bacterial cold shock response. None of the previously defined bacterial cold shock proteins, such as the CspA family, CsdA, or RbfA, are present in the genomes of any sequenced hyperthermophilic archaea. The phylogenetic relationship between CipA, CipB, and OppA are shown in Fig. 7. Close homologs CipA and CipB are present throughout the archaea, although CipB seems to be absent from methanogens and the CipB homologs in Sulfolobus species seem to be more closely related to their CipA homologs. On the other hand, the OppA protein family is found only in the bacterial domain, and they encompass well over a hundred sequences in the database (only a representative sample is shown in Fig. 7). However, four of these are more closely related to the CipB proteins than they are to OppA, including that of the hyperthermophilic bacterium, T. maritima (Fig. 7).
FIG. 7.
Phylogeny of CipA and CipB. CipA (PF0190) and CipB (PF1408) are shown in boxes. Genes from bacterial sources are underlined, while all others are from archaea. Genes that are homologs of CipA (bold type), CipB (plain), and OppA (underlined) are so indicated. The bar indicates a branch length equivalent to 0.1 changes per amino acid, and bootstrap values greater than 50% are indicated at the appropriate branch points. Abbreviations: AF, Archaeoglobus fulgidus; APE, Aeropyrum pernix; Bsu, B. subtilis; DVU, Desulfovibrio vulgaris; lmo, L. monocytogenes; MA, Methanosarcina acetivorans; MM, Methanosarcina mazei; PAB, Pyrococcus abysii; PAE, Pyrobaculum aerophylum; PH, Pyroccous horikoshii; PF, P. furiosus; PM, Pasteurella multocida; SAG, Streptococcus agalactiae; SPs, S. pyogenes; SSO, Sulfolobus solfataricus; ST, Sulfolobus tokodaii; STM, S. enterica serovar Typhimurium; TM, T. maritima.
The hyperthermophilic archaea therefore appear to utilize, at least in part, the same types of proteins to respond to a drop in temperature from 95 to 72°C that bacteria, at least as represented by L. monocytogenes, use when the growth temperature drops from 30 to 5°C. Other than these solute-binding proteins, however, the archaeal response, or responses in the case of P. furiosus, is clearly very different from that previously seen in any bacterium. Although the present work does not provide insight into specific mechanisms of how the organism deals with cold stress, the results do establish that there are three distinct responses, and for each they provide a framework for future studies. These studies are currently directed towards the characterization of the novel cold-responsive glycoproteins and of key proteins involved in the other responses, in particular those involving carbohydrate and amino acid metabolism, translation and replication, and the intriguing role of tungsten.
Acknowledgments
This research was funded by grants from the National Institutes of Health (GM 60329), the National Science Foundation (MCB 0129841 and BES-0317911), and the Department of Energy (FG05-95ER20175).
We thank Frank E. Jenney, Jr., for many helpful discussions and Farris Poole for bioinformatic analyses.
REFERENCES
- 1.Adams, M. W. W., J. F. Holden, A. L. Menon, G. J. Schut, A. M. Grunden, C. Hou, A. M. Hutchins, F. E. Jenney, C. Kim, K. S. Ma, G. L. Pan, R. Roy, R. Sapra, S. V. Story, and M. F. J. M. Verhagen. 2001. Key role for sulfur in peptide metabolism and in regulation of three hydrogenases in the hyperthermophilic archaeon Pyrococcus furiosus. J. Bacteriol. 183:716-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allali, N., H. Afif, M. Couturier, and L. Van Melderen. 2002. The highly conserved TldD and TldE proteins of Escherichia coli are involved in microcin B17 processing and in CcdA degradation. J. Bacteriol. 184:3224-3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartolucci, S., D. De Pascale, and M. Rossi. 2001. Protein disulfide oxidoreductase from Pyrococcus furiosus: biochemical properties. Methods Enzymol. 334:62-73. [DOI] [PubMed] [Google Scholar]
- 4.Beran, R. K., and R. W. Simons. 2001. Cold-temperature induction of Escherichia coli polynucleotide phosphorylase occurs by reversal of its autoregulation. Mol. Microbiol. 39:112-125. [DOI] [PubMed] [Google Scholar]
- 5.Borezee, E., E. Pellegrini, and P. Berche. 2000. OppA of Listeria monocytogenes, an oligopeptide-binding protein required for bacterial growth at low temperature and involved in intracellular survival. Infect. Immun. 68:7069-7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradford, M. M. 1976. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 7.Caldas, T., S. Laalami, and G. Richarme. 2000. Chaperone properties of bacterial elongation factor EF-G and initiation factor IF2. J. Biol. Chem. 275:855-860. [DOI] [PubMed] [Google Scholar]
- 8.Chan, M. K., S. Mukund, A. Kletzin, M. W. W. Adams, and D. C. Rees. 1995. Structure of a hyperthermophilic tungstopterin enzyme, aldehyde ferredoxin oxidoreductase. Science 267:1463-1469. [DOI] [PubMed] [Google Scholar]
- 9.Ermolenko, D. N., and G. I. Makhatadze. 2002. Bacterial cold-shock proteins. Cell Mol. Life Sci. 59:1902-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Felsenstein, J. 1996. Inferring phylogenies from protein sequences by parsimony, distance and likelihood methods. Methods Enzymol. 266:418-427. [DOI] [PubMed] [Google Scholar]
- 11.Fiala, G., and K. O. Stetter. 1986. Pyrococcus furiosus sp. nov. represents a novel genus of marine heterotrophic archaebacteria growing optimally at 100°C. Arch. Microbiol. 145:56-61. [Google Scholar]
- 12.Forterre, P. 2002. A hot story from comparative genomics: reverse gyrase is the only hyperthermophile-specific protein. Trends Genet. 18:236-238. [DOI] [PubMed] [Google Scholar]
- 13.Gualerzi, C. O., A. Maria Giuliodori, and C. L. Pon. 2003. Transcriptional and posttranscriptional control of cold shock genes. J. Mol. Biol. 331:527-539. [DOI] [PubMed] [Google Scholar]
- 14.Hoaki, T., M. Nishijima, M. Kato, K. Adachi, S. Mizobuchi, N. Hanzawa, and T. Maruyama. 1994. Growth requirements of hyperthermophilic sulfur-dependent heterotrophic archaea isolated from a shallow submarine geothermal system with reference to their essential amino-acids Appl. Environ. Microbiol. 60:2898-2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holden, J. F., F. L. Poole, S. L. Tollaksen, C. S. Giometti, H. Lim, J. R. Yates, and M. W. W. Adams. 2001. Identification of membrane proteins in the hyperthermophilic archaeon Pyrococcus furiosus using proteomics and prediction programs. Comp. Funct. Genom. 2:275-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holm, S. 1979. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 6:65-70. [Google Scholar]
- 17.Ideno, A., and T. Maruyama. 2002. Expression of long- and short-type FK506 binding proteins in hyperthermophilic archaea. Gene 292:57-63. [DOI] [PubMed] [Google Scholar]
- 18.Ideno, A., T. Yoshida, T. Iida, M. Furutani, and T. Maruyama. 2001. FK506-binding protein of the hyperthermophilic archaeum, Thermococcus sp. KS-1, a cold-shock-inducible peptidyl-prolyl cis-trans isomerase with activities to trap and refold denatured proteins. Biochem. J. 357:465-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishikawa, K., H. Ishida, Y. Koyama, Y. Kawarabayasi, J. Kawahara, E. Matsui, and I. Matsui. 1998. Acylamino acid-releasing enzyme from the thermophilic archaeon Pyrococcus horikoshii. J. Biol. Chem. 273:17726-17731. [DOI] [PubMed] [Google Scholar]
- 20.Jenney, F. E., M. F. J. M. Verhagen, X. Y. Cui, and M. W. W. Adams. 1999. Anaerobic microbes: oxygen detoxification without superoxide dismutase. Science 286:306-309. [DOI] [PubMed] [Google Scholar]
- 21.Jiang, W. N., Y. Hou, and M. Inouye. 1997. CspA, the major cold-shock protein of Escherichia coli, is an RNA chaperone. J. Biol. Chem. 272:196-202. [DOI] [PubMed] [Google Scholar]
- 22.Jones, P. G., and M. Inouye. 1996. RbfA, a 30S ribosomal binding factor, is a cold-shock protein whose absence triggers the cold-shock response. Mol. Microbiol. 21:1207-1218. [DOI] [PubMed] [Google Scholar]
- 23.Jones, P. G., M. Mitta, Y. Kim, W. Jiang, and M. Inouye. 1996. Cold shock induces a major ribosomal-associated protein that unwinds double-stranded RNA in Escherichia coli. Proc. Natl. Acad. Sci. USA 93:76-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones, P. G., R. A. Vanbogelen, and F. C. Neidhardt. 1987. Induction of proteins in response to low temperature in Escherichia coli. J. Bacteriol. 169:2092-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaan, T., G. Homuth, U. Mader, J. Bandow, and T. Schweder. 2002. Genome-wide transcriptional profiling of the Bacillus subtilis cold-shock response. Microbiology 148:3441-3455. [DOI] [PubMed] [Google Scholar]
- 26.Kelly, R. M., and M. W. W. Adams. 1994. Metabolism in hyperthermophilic microorganisms. Antonie Leeuwenhoek 66:247-270. [DOI] [PubMed] [Google Scholar]
- 27.Kengen, S. W. M., J. E. Tuininga, F. A. M. deBok, A. J. M. Stams, and W. M. deVos. 1995. Purification and characterization of a novel ADP-dependent glucokinase from the hyperthermophilic archaeon Pyrococcus furiosus. J. Biol. Chem. 270:30453-30457. [DOI] [PubMed] [Google Scholar]
- 28.Kletzin, A., and M. W. W. Adams. 1996. Molecular and phylogenetic characterization of pyruvate and 2-ketoisovalerate ferredoxin oxidoreductases from Pyrococcus furiosus and pyruvate ferredoxin oxidoreductase from Thermotoga maritima. J. Bacteriol. 178:248-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar, S., K. Tamura, and M. Nei. 2004. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinform. 5:150-163. [DOI] [PubMed] [Google Scholar]
- 30.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 227:680-685. [DOI] [PubMed] [Google Scholar]
- 31.Lim, J., T. Thomas, and R. Cavicchioli. 2000. Low temperature regulated DEAD-box RNA helicase from the Antarctic archaeon, Methanococcoides burtonii. J. Mol. Biol. 297:553-567. [DOI] [PubMed] [Google Scholar]
- 32.Lopez-Garcia, P., and P. Forterre. 1999. Control of DNA topology during thermal stress in hyperthermophilic archaea: DNA topoisomerase levels, activities and induced thermotolerance during heat and cold shock in Sulfolobus. Mol. Microbiol. 33:766-777. [DOI] [PubMed] [Google Scholar]
- 33.Lopez-Garcia, P., and P. Forterre. 2000. DNA topology and the thermal stress response, a tale from mesophiles and hyperthermophiles. Bioessays 22:738-746. [DOI] [PubMed] [Google Scholar]
- 34.Lopez-Garcia, P., and P. Forterre. 1997. DNA topology in hyperthermophilic archaea: reference states and their variation with growth phase, growth temperature, and temperature stresses. Mol. Microbiol. 23:1267-1269. [DOI] [PubMed] [Google Scholar]
- 35.Mai, X. H., and M. W. W. Adams. 1996. Characterization of a fourth type of 2-keto acid-oxidizing enzyme from a hyperthermophilic archaeon: 2-ketoglutarate ferredoxin oxidoreductase from Thermococcus litoralis. J. Bacteriol. 178:5890-5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maruyama, T., and M. Furutani. 2000. Archaeal peptidyl prolyl cis-trans isomerases (PPIases). Front. Biosci. 5:D821-D836. [DOI] [PubMed] [Google Scholar]
- 37.Moll, I., S. Grill, A. Grundling, and U. Blasi. 2002. Effects of ribosomal proteins S1, S2 and the DeaD/CsdA DEAD-box helicase on translation of leaderless and canonical mRNAs in Escherichia coli. Mol. Microbiol. 44:1387-1396. [DOI] [PubMed] [Google Scholar]
- 38.Mukund, S., and M. W. W. Adams. 1995. Glyceraldehyde-3-phosphate ferredoxin oxidoreductase, a novel tungsten-containing enzyme with a potential glycolytic role in the hyperthermophilic archaeon Pyrococcus furiosus. J. Biol. Chem. 270:8389-8392. [DOI] [PubMed] [Google Scholar]
- 39.Onoe, S., S. Ando, M. Ataka, and K. Ishikawa. 2002. Active site of deblocking aminopeptidase from Pyrococcus horikoshii. Biochem. Biophys. Res. Commun. 290:994-997. [DOI] [PubMed] [Google Scholar]
- 40.Ren, B., G. Tibbelin, D. de Pascale, M. Rossi, S. Bartolucci, and R. Ladenstein. 1998. A protein disulfide oxidoreductase from the archaeon Pyrococcus furiosus contains two thioredoxin fold units. Nat. Struct. Biol. 5:602-611. [DOI] [PubMed] [Google Scholar]
- 41.Riera, J., F. T. Robb, R. Weiss, and M. Fontecave. 1997. Ribonucleotide reductase in the archaeon Pyrococcus furiosus: a critical enzyme in the evolution of DNA genomes? Proc. Natl. Acad. Sci. USA 94:475-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robb, F. T., D. L. Maeder, J. R. Brown, J. DiRuggiero, M. D. Stump, R. K. Yeh, R. B. Weiss, and D. M. Dunn. 2001. Genomic sequence of hyperthermophile, Pyrococcus furiosus: implications for physiology and enzymology. Methods Enzymol. 330:134-157. [DOI] [PubMed] [Google Scholar]
- 43.Roy, R., and M. W. W. Adams. 2002. Characterization of a fourth tungsten-containing enzyme from the hyperthermophilic archaeon Pyrococcus furiosus. J. Bacteriol. 184:6952-6956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roy, R., S. Mukund, G. J. Schut, D. M. Dunn, R. Weiss, and M. W. W. Adams. 1999. Purification and molecular characterization of the tungsten-containing formaldehyde ferredoxin oxidoreductase from the hyperthermophilic archaean Pyrococcus furiosus: the third of a putative five-member tungstoenzyme family. J. Bacteriol. 181:1171-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 46.Schut, G. J., S. D. Brehm, S. Datta, and M. W. W. Adams. 2003. Whole-genome DNA microarray analysis of a hyperthermophile and an archaeon: Pyrococcus furiosus grown on carbohydrates or peptides. J. Bacteriol. 185:3935-3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schut, G. J., A. L. Menon, and M. W. W. Adams. 2001. 2-Keto acid oxidoreductases from Pyrococcus furiosus and Thermococcus litoralis. Methods Enzymol. 331:144-158. [DOI] [PubMed] [Google Scholar]
- 48.Schut, G. J., J. Z. Zhou, and M. W. W. Adams. 2001. DNA microarray analysis of the hyperthermophilic archaeon Pyrococcus furiosus: evidence for a new type of sulfur-reducing enzyme complex. J. Bacteriol. 183:7027-7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shevchenko, A., M. Wilm, O. Vorm, and M. Mann. 1996. Mass spectrometric sequencing of proteins from silver stained polyacrylamide gels. Anal. Chem. 68:850-858. [DOI] [PubMed] [Google Scholar]
- 50.Siebers, B., H. Brinkmann, C. Dorr, B. Tjaden, H. Lilie, J. van der Oost, and C. H. Verhees. 2001. Archaeal fructose-1,6-bisphosphate aldolases constitute a new family of archaeal type class I aldolase. J. Biol. Chem. 276:28710-28718. [DOI] [PubMed] [Google Scholar]
- 51.Stetter, K. 1996. Hyperthermophilic prokaryotes. FEMS Microbiol. Rev. 18:149-158. [Google Scholar]
- 52.Van Der Oost, J., M. Huynen, and C. Verhees. 2002. Molecular characterization of phosphoglycerate mutase in archaea. FEMS Microbiol. Lett. 212:111-120. [DOI] [PubMed] [Google Scholar]
- 53.Verhees, C. H., S. W. M. Kengen, J. E. Tuininga, G. J. Schut, M. W. W. Adams, W. M. De Vos, and J. Van der Oost. 2003. The unique features of glycolytic pathways in archaea. Biochem. J. 375:231-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang, N., K. Yamanaka, and M. Inouye. 1999. CspI, the ninth member of the CspA family of Escherichia coli, is induced upon cold shock. J. Bacteriol. 181:1603-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xing, R. Y., and W. B. Whitman. 1987. Sulfometuron methyl-sensitive and methyl-resistant acetolactate synthases of the archaebacteria Methanococcus spp. J. Bacteriol. 169:4486-4492. [DOI] [PMC free article] [PubMed] [Google Scholar]