Abstract
STUDY QUESTION
Do peroxiredoxins (PRDXs) control reactive oxygen species (ROS) levels during human sperm capacitation?
SUMMARY ANSWER
PRDXs are necessary to control the levels of ROS generated during capacitation allowing spermatozoa to achieve fertilizing ability.
WHAT IS KNOWN ALREADY
Sperm capacitation is an oxidative event that requires low and controlled amounts of ROS to trigger phosphorylation events. PRDXs are antioxidant enzymes that not only act as scavengers but also control ROS action in somatic cells. Spermatozoa from infertile men have lower levels of PRDXs (particularly of PRDX6), which are thiol-oxidized and therefore inactive.
STUDY DESIGN, SIZE, DURATION
Semen samples were obtained from a cohort of 20 healthy nonsmoker volunteers aged 22–30 years old over a period of 1 year.
PARTICIPANTS/MATERIALS, SETTINGS, METHODS
Sperm from healthy donors was capacitated with fetal cord serum ultrafiltrate (FCSu) in the absence or presence of thiostrepton (TSP), inhibitor of 2-Cys PRDXs or 1-Hexadecyl-3-(trifluoroethyl)-sn-glycero-2-phosphomethanol lithium (MJ33), inhibitor of calcium independent-phospholipase A2 (Ca2+-iPLA2) activity of PRDX6, added at different times of incubation. Capacitation was also induced by the dibutyryl cAMP+3-isobuty1-1-methylxanthine system. Sperm viability and motility were determined by the hypo-osmotic swelling test and computer-assisted semen analysis system, respectively. Capacitation was determined by the ability of spermatozoa to undergo the acrosome reaction triggered by lysophosphatidylcholine. Percentages of acrosome reaction were obtained using the FITC-conjugated Pisum sativum agglutinin assay. Phosphorylation of tyrosine residues and of protein kinase A (PKA) substrates were determined by sodium dodecyl sulfate polyacrylamide gel electrophoresis immunoblotting with specific antibodies. Actin polymerization was determined by phalloidin labeling.
MAIN RESULTS AND THE ROLE OF CHANCE
TSP and MJ33 prevented sperm capacitation and its associated actin polymerization in spermatozoa incubated with 10% FCSu (capacitation inducer) compared to non-capacitated controls (P < 0.05) without altering sperm viability. PKA substrates and tyrosine phosphorylations were prevented in FCSu-treated spermatozoa in a differential fashion depending on the type and the time of addition of the inhibitor used compared to non-capacitated controls (P < 0.05). TSP and MJ33 promoted an increase of lipid peroxidation in spermatozoa (P < 0.01) and these levels were higher in those spermatozoa incubated with the inhibitors and FCSu compared to those capacitated spermatozoa incubated without the inhibitors (P < 0.0001). Inhibition of 2-Cys PRDXs by TSP generated an oxidative stress in spermatozoa, affecting their viability compared to controls (P < 0.05). This oxidative stress was prevented by nuclephile D-penicillamine (PEN). MJ33 also promoted an increase of lipid peroxidation and impaired sperm viability compared to non-treated controls (P < 0.05) but its effect was not circumvented by PEN, suggesting that not only peroxidase but also Ca2+-iPLA2 activity of PRDX6 are necessary to guarantee viability in human spermatozoa.
LARGE SCALE DATA
Not applicable.
LIMITATIONS REASONS FOR CAUTION
We focused on the global effect of PRDXs inhibitors on human sperm capacitation and in two of its associated phosphorylation events. Thus, other phosphorylation events and mechanisms necessary for capacitation may also be affected.
WIDER IMPLICATIONS OF THE FINDINGS
PRDXs are the major antioxidant system in ejaculated spermatozoa and are necessary to allow spermatozoon to achieve fertilizing ability (capacitation and acrosome reaction).
STUDY FUNDING/COMPETING INTEREST(S)
This research was supported by Canadian Institutes of Health Research (MOP 133661) and the Fonds de Recherché en Santé Quebec (FRSQS #22151) to C.O. The authors have nothing to disclose.
Keywords: spermatozoa, redox signaling, 2-Cys peroxiredoxins, calcium independent phospholipase A2 activity, oxidative stress
Introduction
Sperm capacitation is a series of biochemical and morphological changes needed for the spermatozoon to acquire fertilizing ability (Florman and Ducibella, 2006). One of the early events that occur during sperm capacitation is the production of low levels of reactive oxygen species (ROS) (de Lamirande and O'Flaherty, 2012). These ROS are molecular messengers that trigger a series of phosphorylation events necessary for the spermatozoon to accomplish capacitation (O'Flaherty et al., 2006a,b). This series of phosphorylation events initiated by ROS signaling can be divided into three time points: early, middle and late (O'Flaherty et al., 2006a; de Lamirande and O'Flaherty, 2012). Early events occur within the first 30 min of initiation of capacitation and are stimulated by ROS produced at the level of cell membrane. These events include the early activation of adenylyl cyclase and of protein kinase A (PKA) (Lefievre et al., 2002) that will phosphorylate proteins carrying the Arg-X-X-Ser motif (PKA substrates) (O'Flaherty et al., 2004). These phosphorylated proteins are needed to activate the late tyrosine phosphorylation that occurs after 2 h of the beginning of capacitation (Leclerc et al., 1996).
Although low levels of ROS are needed for sperm capacitation, the establishment of oxidative stress, an imbalance between the production of ROS and the antioxidant defenses, which produces a net increase of ROS levels, is associated with male infertility (Tremellen, 2008). The oxidative stress impairs spermatozoa's motility and ability to undergo capacitation (Morielli and O'Flaherty, 2015) and to bind to the oolema (Aitken et al., 1998), thus impeding the fertilization of the oocyte by the spermatozoon.
Peroxiredoxins (PRDXs) are ancestral sulfhydryl-dependent, selenium- and heme-free peroxidases highly expressed in practically all living species, from prokaryotes to eukaryotes (Rhee et al., 2005). They scavenge hydroperoxides (e.g. hydrogen peroxide (H2O2) and organic hydroperoxides) and peroxynitrite (ONOO−) (Zhang et al., 1997; Peshenko and Shichi, 2001; Dubuisson et al., 2004). They can be classified according to the cysteine (Cys) residues present in their active site: 2-Cys PRDXs including PRDX1 to 5, and 1-Cys PRDX represented by PRDX6 (Rhee et al., 2005). Interestingly, PRDX6 is a bifunctional enzyme with peroxidase and phospholipase A2 activities (Chen et al., 2000). Moreover, PRDXs are important modulators of ROS signaling (Wood et al., 2003).
PRDXs play a crucial role in the protection of human spermatozoa against oxidative stress (O'Flaherty and de Souza, 2011; Gong et al., 2012; O'Flaherty, 2014a). Low amounts and/or inactivation of PRDX1 and PRDX6 result in increased levels of sperm lipid peroxidation, DNA damage and impairment of sperm motility, associated with male infertility (Gong et al., 2012). Preliminary results from our laboratory suggested that inhibitors of PRDXs prevented sperm capacitation in humans (O'Flaherty, 2015), but it is still unknown how PRDXs regulate the redox signaling involved in the process and what pathways are modulated. We hypothesize that inactivation of PRDXs will result in the inhibition of PKA-dependent phosphorylation that leads to the late tyrosine phosphorylation and capacitation of human spermatozoa. Therefore, the aim of this study was to determine whether PRDXs are necessary to sustain actin polymerization and PKA substrates and tyrosine phosphorylations that take place during human sperm capacitation.
Materials and Methods
Materials
Percoll was obtained from GE Healthcare (Baie d'Urfe, QC, Canada). 4,4-difluoro-5-(4-phenyl-1,3-butadienyl)-4-bora-3a,4a-diaza-s-indacene-3-undecanoic acid (BODIPY 581/591 C11) was purchased from Life Technologies (Burlington, ON, Canada). The mouse monoclonal anti-phosphotyrosine (clone 4G10) was purchased from Upstate Biotechnology, Inc. (Lake Placid, NY, USA) and the polyclonal antibody raised against the phosphorylated Arg-X-X-Serine/Threonine motif (anti-phospho PKA substrates antibody) was purchased from Cell Signaling Technology (Beverly, MA, USA). Fetal cord blood was collected at the birthing centre of the Royal Victoria Hospital (Montreal, QC, Canada) to produce the fetal cord serum ultrafiltrate (FCSu), as described previously (de Lamirande and Gagnon, 1995; O'Flaherty and de Lamirande, 2004). The monoclonal anti-α-tubulin antibody, thiostrepton (TSP) (from Streptomyces azureus), MJ33 and penicillamine (PEN) were purchased from Sigma-Aldrich (Milwaukee, WI, USA). Donkey anti-rabbit IgG and goat anti-mouse IgG antibodies (both conjugated with horse-radish peroxidase) were provided by Cederlane Laboratories Ltd (Hornby, Canada). Nitrocellulose membranes (pore size, 0.22 µm) were purchased from Osmonics, Inc. (Westborough, MA, USA) and the enhanced chemiluminescence kit Lumi-Light from Roche Molecular Biochemicals (Laval, QC Canada). Radiographic films (obtained from Fuji; Minami-Ashigara, Japan) were used for immunodetection of blotted proteins. Other chemicals used were of at least reagent grade.
Sperm preparation and capacitation
Semen samples were obtained from a cohort of 20 healthy nonsmoker volunteers aged 22–30 years old, who remained sexually abstinent for 72 h prior to the collection. This study was approved by the Ethics Committee of the McGill University Health Centre and complies with the suggested guidance for human semen studies, as previously published (Sanchez-Pozo et al., 2013; Bjorndahl et al., 2016). Donors were recruited and provided a semen sample at the Royal Victoria Hospital, Montreal, QC, Canada. The exclusion criteria were the presence of illness or whether they were taking any medication. These criteria were used during the entire period of the study. Donors live in the Montreal area and were healthy men and healthy students attending the local universities. Those donors included in the study provide semen samples regularly and sperm motility in each sample was analyzed using a computer-assisted semen analysis system (CASA) (Sperm vision HR software v1.01, Penetrating Innovations, Ingersoll, ON, Canada) and sperm concentration, by hemacytometry using an improved Neubauer chamber, by well-trained observers. Sperm analysis was performed in duplicate, at two times: after 30 min of liquefaction at 37°C (raw sample) and after the Percoll selection. Only normozoospermic samples according to World Health Organization guidelines were used (World Health Organization, 2010). Liquefied semen samples were placed over a four-layer Percoll gradient (95-65-40-20%) and centrifuged for 30 min at 2300 × g at 20°C (de Lamirande and Gagnon, 1995; O'Flaherty et al., 2004). This step was useful in separating the abnormal sperm cells and seminal plasma, without increasing ROS levels (Iwasaki and Gagnon, 1992; Zini et al., 1993; Plante et al., 1994). Highly motile spermatozoa recovered from the 95% layer and the 65–95% interface were diluted to either 10 or 50 × 106 cells/ml in Biggers, Whitten and Whittingham medium (BWW, pH 8.0) (Biggers et al., 1971), and used for experimentation.
Sperm samples were incubated in BWW in the presence of different concentrations (0–20 µM) of TSP or MJ33, inhibitor of 2-Cys PRDXs or Ca2+-iPLA2 activity of PRDX6, respectively, in the presence or absence of 10% FCSu or 1 mM dibutyryl cAMP (dbcAMP) + 0.1 mM 3-isobutyl-1-methylxanthine (IBMX) as capacitation inducers (O'Flaherty et al., 2004). In order to determine the role of PRDXs in the modulation of phosphorylation of PKA substrates and of tyrosine residues during capacitation, inhibitors were added at different times, as each pathway would be activated at different times. TSP or MJ33 were added at 0, 15, 30, 60 and 120 min after the addition of FCSu or dbcAMP+IBMX and sperm were incubated at 37°C for 30 min (to detect phosphorylation of PKA substrates) or for 4 h (for detection of phosphorylation of tyrosine residues) (O'Flaherty et al., 2004, 2006b). Controls without FCSu (non-capacitating spermatozoa) were run in parallel. In order to determine the level of capacitation, non-capacitated and capacitated spermatozoa were incubated, after the end of the 3.5-h incubation, with 2.5 μM lysophosphatidylcholine (LPC) in BWW for 30 min at 37°C to induce the acrosome reaction. Spermatozoa were labeled with FITC-conjugated Pisum sativum agglutinin and the percentage of reacted spermatozoa was recorded by analyzing 200 cells per slide in duplicate (Morielli and O'Flaherty, 2015). In order to test the effect of PRDXs inhibitors on sperm motility, we incubated spermatozoa in BWW in the presence or absence of 10 μM of TSP or MJ33, with or without 2 mM PEN, for 4 h at 37°C. None of the inhibitors affected any motility parameter (Supplementary data, Table S1).
Sperm motility and viability analyses
We tested the potential toxic effect of the presence of PRDXs inhibitors in the capacitating medium. Spermatozoa were incubated with or without TSP or MJ33 at 5 and 10 μM at 37°C for 4 h. Sperm motility was then analyzed by CASA as mentioned above. To determine sperm viability, sperm were centrifuged for 5 min at 600 × g at 20°C. The supernatant was removed and replaced with a hypo-osmotic solution at 37°C (WHO, 2010). Sperm suspensions were incubated for 30 min at 37°C. Then, sperm samples were centrifuged for 5 min at 1000 × g. After removing the supernatant the pellet was fixed with 2.5% glutaraldehyde; the fixed cells were smeared onto superfrost plus slides, and viability was assessed by using the bright field of a Carl Zeiss (Germany) Axiophot microscope at 1000× magnification, to observe the presence or absence of tail curl (WHO, 2010). Only sperm with a visible tail curl were considered viable and at least 200 cells were counted for each treatment, in duplicate.
Next, we determined whether the inhibition of PRDXs will impact on the viability of spermatozoa when they are treated with 2 mM H2O2, that promotes an oxidative stress and thus increases the levels of lipid peroxidation (O'Flaherty and de Souza, 2011) and of protein oxidation (Morielli and O'Flaherty, 2015) resulting in impairment of sperm function. Spermatozoa were incubated in BWW in the presence or absence of 2 mM H2O2 with or without 10 μM TSP or MJ33 at 37°C for 4 h. Sperm aliquots with H2O2 and PRDXs were supplemented previously with 2 mM PEN, a nucleophilic compound that protects spermatozoa against oxidative stress (Aitken et al., 2012). Sperm viability was then assessed as described above.
Lipid peroxidation determination
Lipid peroxidation levels were determined by flow cytometry using a BODIPY 581/591 C11 probe (Aitken et al., 2007) with modifications. Spermatozoa were incubated in BWW medium with or without 10% FCSu in the presence or absence of PRDXs inhibitors at 37°C for 4 h. Then, spermatozoa were washed and incubated with 10 μM BODIPY 581/591 C11 in PBS for 30 min at 37°C. A positive control was prepared by incubating a sperm aliquot with 40 μM ferrous sulfate (FeSO4) for 4 h at 37°C. A minimum of 10 000 of BODIPY 581/591 C11-labeled spermatozoa were analyzed for each sample using a MACSQuant Analyzer flow cytometer (Miltenyi Biotec, Inc., Auburn, CA, USA) equipped with an argon laser (488 nm) and 585/625 nm filter. Levels of lipid peroxidation were expressed as relative intensity of green fluorescence/red+green fluorescence. Data were transformed to log (x) for statistical analysis.
Phosphorylation of tyrosine residues and PKA substrates
Tyrosine phosphorylation, a recognized marker of sperm capacitation (Leclerc et al., 1996) and phosphorylation of PKA substrates (O'Flaherty et al., 2004) were determined in non- and capacitated spermatozoa using specific antibodies. After incubation, sperm proteins were supplemented with a sample buffer containing 100 mM dithiothreitol, boiled for 5 min at 100°C and centrifuged, and the supernatant loaded on 12% polyacrylamide gels, electrophoresed and electrotransferred on to nitrocellulose membranes using a transfer buffer (192 mM glycine and 25 mM Tris, pH 8.3) containing 20% methanol. The membranes were then blocked via a 30-min incubation in 5% skim milk dissolved in 2 mM Tris (pH 7.8)-buffered saline and 0.1% tween 20 (TTBS). After two quick washes with TTBS at room temperature, the membranes were incubated with anti-phosphotyrosine antibody (1:10 000, v/v) for 1 h at room temperature or anti-phospho Arg-X-X-Ser/Thr motif antibody (1:1000,v/v) overnight at 4°C (O'Flaherty et al., 2004, 2006b). Then, the membranes were washed with TTBS at room temperature and incubated with the respective horse-radish peroxidase conjugated goat anti-mouse IgG or donkey anti-rabbit IgG antibodies for 1 h at room temperature. Positive immunoreactive bands were detected using chemiluminescence (Lumi-light; Roche Molecular Biochemicals, Laval, QC, Canada). After detection, membranes were washed with distilled water and silver stained (Jacobson and Karsnas, 1990) or stripped and incubated with anti-α-tubulin to ensure the equal loading of each sample.
Relative intensity of proteins bands was assessed as previously reported using the Un-Scan-It gel software version 5.1 (O'Flaherty et al., 2005; Gong et al., 2012). Each band's intensity was obtained and normalized to the respective intensity of tubulin.
Actin polymerization
To determine the effect of TSP or MJ33 on actin polymerization during capacitation, spermatozoa were labeled with Alexa Fluor 555-conjugated phalloidin. After 3.5 h of capacitation, the sperm suspension was smeared onto Superfrost plus slides (Fisher Scientific, Montreal, QC, Canada), allowed to air dry and fixed in a solution of 2% glutaraldehyde and 0.2% Triton in PBS for 10 min. Sperm were then rehydrated in fresh PBS for 5 min. The slides were incubated overnight at 4°C in the staining buffer of 50 μg/ml LPC and 0.4 μM methanol-Phalloidin-Alexa Fluor 555 in PBS (Noiles et al., 1997; Brener et al., 2003). Then, smears were washed three times with TTBS, mounted with Prolong Antifade with DAPI (Molecular Probes, Eugene, OR, USA) and observed under a Carl Zeiss (Germany) Axiophot microscope at 1000× magnification. Pictures were taken and the intensity of Phallodin-Alexa Fluor 555 was calculated using the ImageJ software v.1.46 (National Institutes of Health, MD, USA). Total fluorescence within the area of the head was measured and background fluoresce was subtracted to give the net sperm head fluorescence (Burgess et al., 2010; Burnett et al., 2011). A minimum of 200 cells per sample were studied. The net fluorescence was then normalized to that of the non-capacitated controls (net fluorescence of a given sample divided by the net fluorescence of non-capacitated control) and results were expressed as relative intensity of F-actin.
Statistical analysis
All graphical data are presented as the mean ± SEM; statistical differences between group means were determined using ANOVA and Bonferroni or Tukey test, Kruskal–Wallis and Dwass–Steel–Chritchlow–Fligner test or Student's t-test followed by Bonferroni's correction, as appropriate, using Sigma Systat 13 (Systat software Inc., San Jose, CA, USA). Shapiro–Wilks and Levene tests were used to determine normality of data distribution and variance homogeneity, respectively. Outliers were identified by the software used and eliminated for the analysis. Difference among samples were significant when the P-value was < 0.05, unless otherwise indicated.
Results
PRDXs inhibitors prevented capacitation and the associated tyrosine phosphorylation or actin polymerization without altering sperm viability
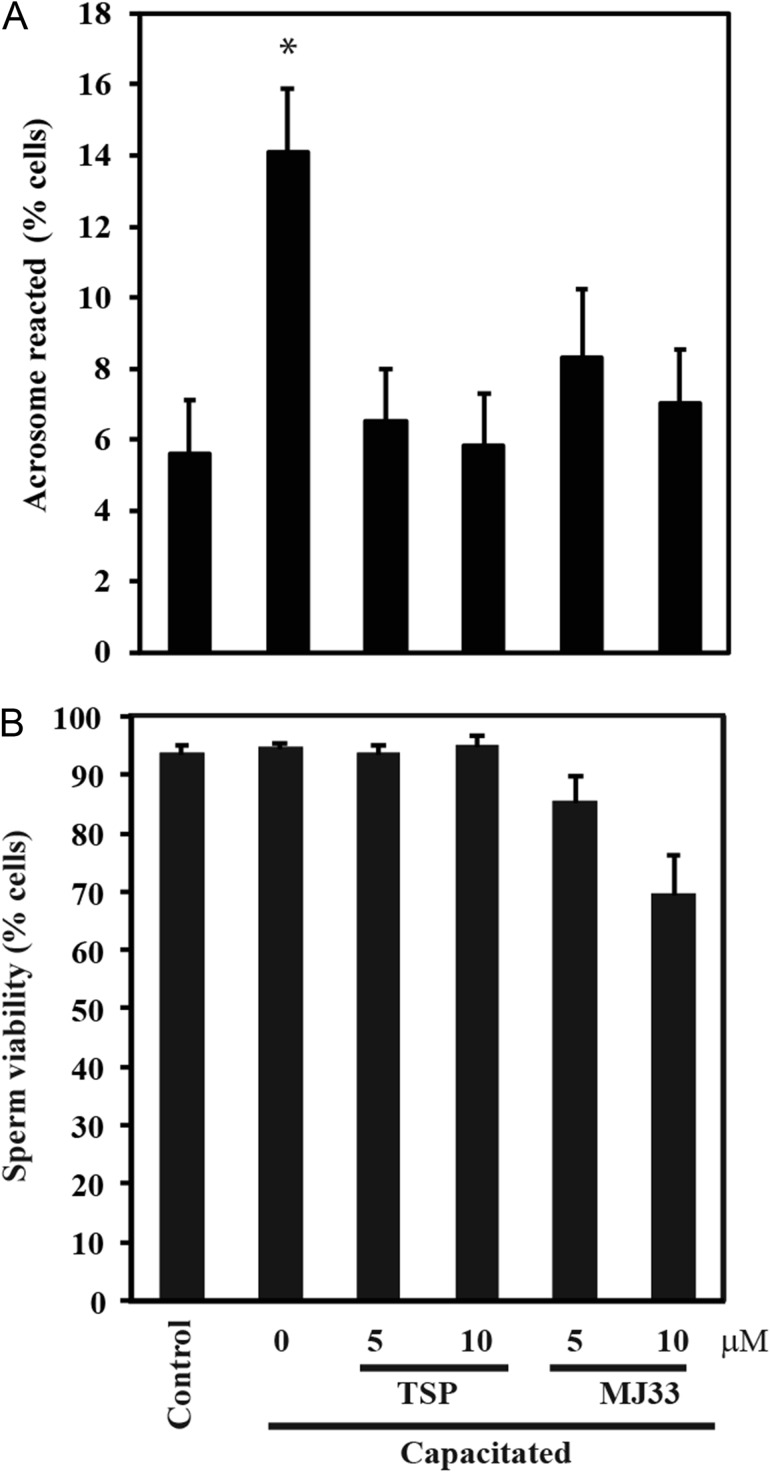
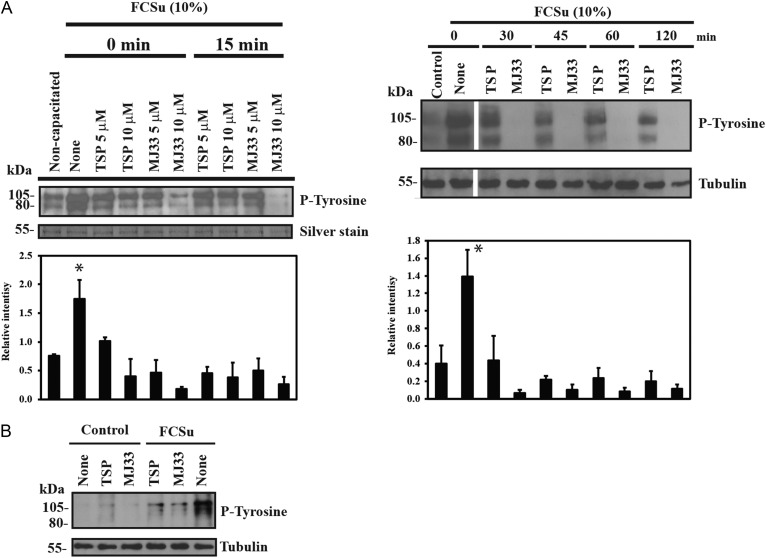
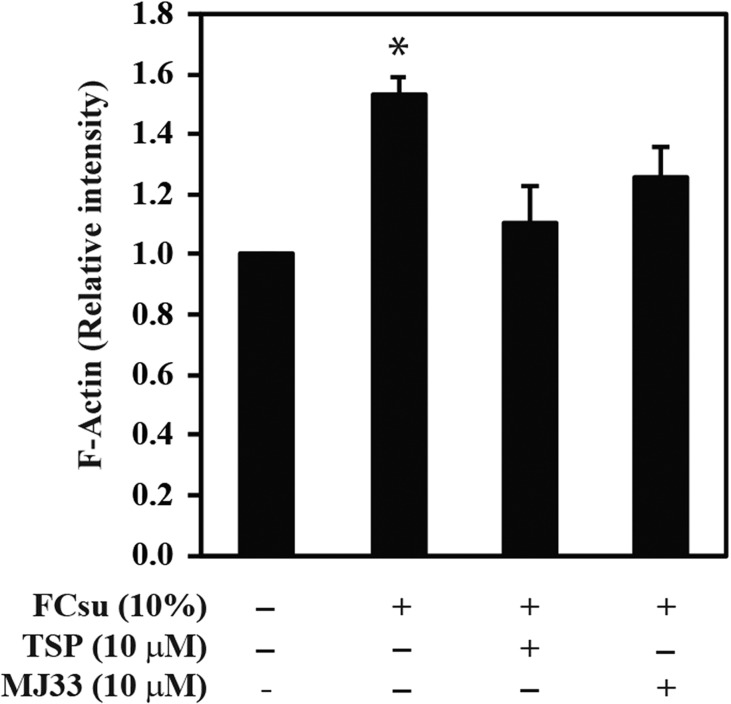
The presence of TSP or MJ33 prevented sperm capacitation without altering sperm viability (Fig. 1) or sperm motility (Supplementary data, Table S1). Similar results were obtained when 10 μM progesterone was used as capacitation inducer (data not shown). The capacitation-associated tyrosine phosphorylation was prevented by TSP or MJ33 (Fig. 2A). When TSP or MJ33 were added at different times (0–120 min) during capacitation, MJ33 promoted a complete reduction of the tyrosine phosphorylation signal at all time points (Fig. 2A). As previously shown (O'Flaherty, 2015), we found no differences in tyrosine phosphorylation in non-capacitated control spermatozoa with or without the PRDXs inhibitors (Fig. 2B). Spermatozoa treated with TSP also promoted lower tyrosine phosphorylation signals than the capacitated samples (Fig. 2). The presence of TSP or MJ33 in the incubation medium prevented the actin polymerization, a process occurring during sperm capacitation (Fig. 3).
Figure 1.
Sperm capacitation and viability in the presence of peroxiredoxin (PRDXs) inhibitors. Human spermatozoa were capacitated in Biggers, Whitten and Whittingham medium (BWW) supplemented with 10% fetal cord serum ultrafiltrate (FCSu) with or without thiostreprton (TSP) or 1-Hexadecyl-3-(trifluoroethyl)-sn-glycero-2-phosphomethanol lithium (MJ33) for 3.5 h at 37°C. (A) Capacitation was determined by the induction of the acrosome reaction with lysophosphatidylcholine for 30 min at 37°C. *P < 0.05 versus all other groups using ANOVA and Bonferroni test (for capacitation), and *P < 0.05 for Kruskal–Wallis and Dwass–Steel–Chritchlow–Fligner test (for viability) for all pairwise comparisons. (B) Sperm viability was determined by the HOST test. Results are presented as mean ± SEM (n = 4 different donors). There were no significant differences among groups.
Figure 2.
PRDXs are involved in the regulation of tyrosine phosphorylation induced by FCSu during human sperm capacitation. (A) Percoll-selected spermatozoa were capacitated with 10% FCSu in BWW medium at 37°C for 4 h in the absence (None) or presence of 5 or 10 μM TSP or MJ33 added at different times of incubation. (B) Effect of 10 μM TSP and MJ33 on spermatozoa incubated with or without FCSu for 4 h at 37°C. Sperm suspensions were supplemented with sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer and solubilized proteins from 0.1 × 106 spermatozoa were loaded in each well, electrophoresed, electrotransferred and immunoblotted using the anti-P-Tyrosine antibody (P-Tyrosine; 1:10 000). Equal loading in each well was determined by silver staining the membranes or re-blotting with anti-α-tubulin antibody (1:10 000). The immunoblots presented are representative of four experiments (n = 4 different donors). One sperm sample was used for all the experimental lanes shown on the immunoblots. Relative intensity of phosphor tyrosine in A was normalized to the relative intensity of α-tubulin of each sample and expressed as mean ± SEM. *P < 0.05 versus all other groups using ANOVA and Bonferroni test (n = 4).
Figure 3.
Capacitation-associated actin polymerization is prevented by PRDXs inhibitors. Spermatozoa were capacitated in BWW supplemented with 10% FCSu with or without 10 μM TSP or MJ33 for 4 h at 37°C. Then, spermatozoa were labeled with phalloidin-FITC conjugate to determine actin polymerization as described in material and methods. Results are presented as mean ± SEM (n = 9 different donors). *P < 0.05 versus all other groups (ANOVA and Bonferroni test).
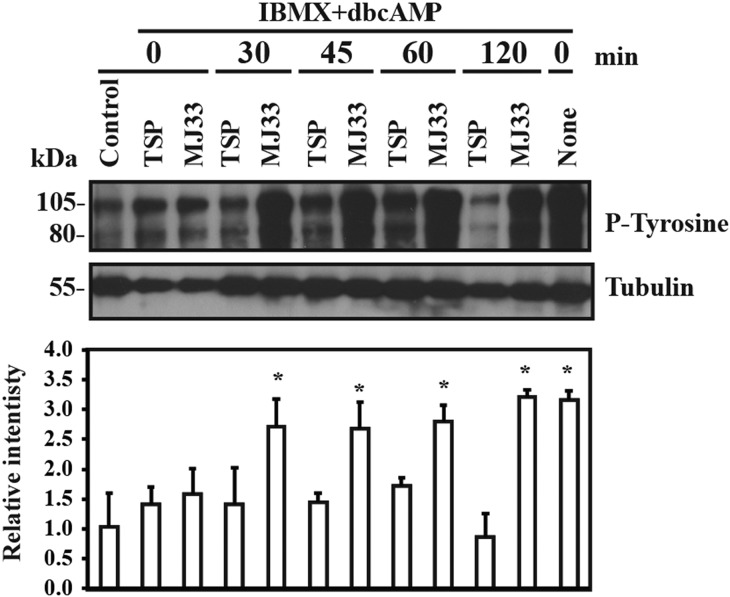
Because the capacitation-associated tyrosine phosphorylation depends on the cAMP/PKA pathway, spermatozoa were incubated with the capacitation inducer IBMX-dbcAMP system (O'Flaherty et al., 2004). When TSP or MJ33 were added at zero time at the beginning of capacitation, spermatozoa displayed reduced levels of tyrosine phosphorylation compared to capacitating spermatozoa without PRDXs inhibitors (Fig. 4). The addition of TSP but not of MJ33 at 30–120 min after the beginning of incubation promoted inhibition of IBMX-dbcAMP-induced tyrosine phosphorylation.
Figure 4.
Capacitation-associated tyrosine phosphorylation induced by 3-isobutyl-1-methylxanthine (IBMX)-dibutyryl cAMP (dbcAMP) is differentially affected by PRDXs inhibitors added at different times of incubation. Percoll-selected human spermatozoa were capacitated with 0.1 mM IBMX+ 1 mM dbcAMP in BWW medium in the absence or presence of 10 μM TSP or MJ33 at 37°C for 4 h. PRDXs inhibitors were added after 0, 30, 45, 60 and 120 min of incubation. Sperm suspensions were supplemented with SDS-PAGE sample buffer and solubilized proteins from 0.1 × 106 spermatozoa were loaded in each well, electrophoresed, electrotransferred and immunoblotted using the anti-P-Tyrosine antibody (1:10 000). Equal loading was determined by using an anti-α-tubulin antibody (1:10 000). The immunoblots presented are representative of four experiments (n = 4 different donors). Relative intensity of protein tyrosine phosphorylation was normalized to the relative intensity of tubulin of each sample and expressed as mean ± SEM. *P < 0.05 versus non-capacitated controls (ANOVA and Bonferroni test; n = 4).
PRDXs inhibitors prevented phosphorylation of PKA substrates during capacitation
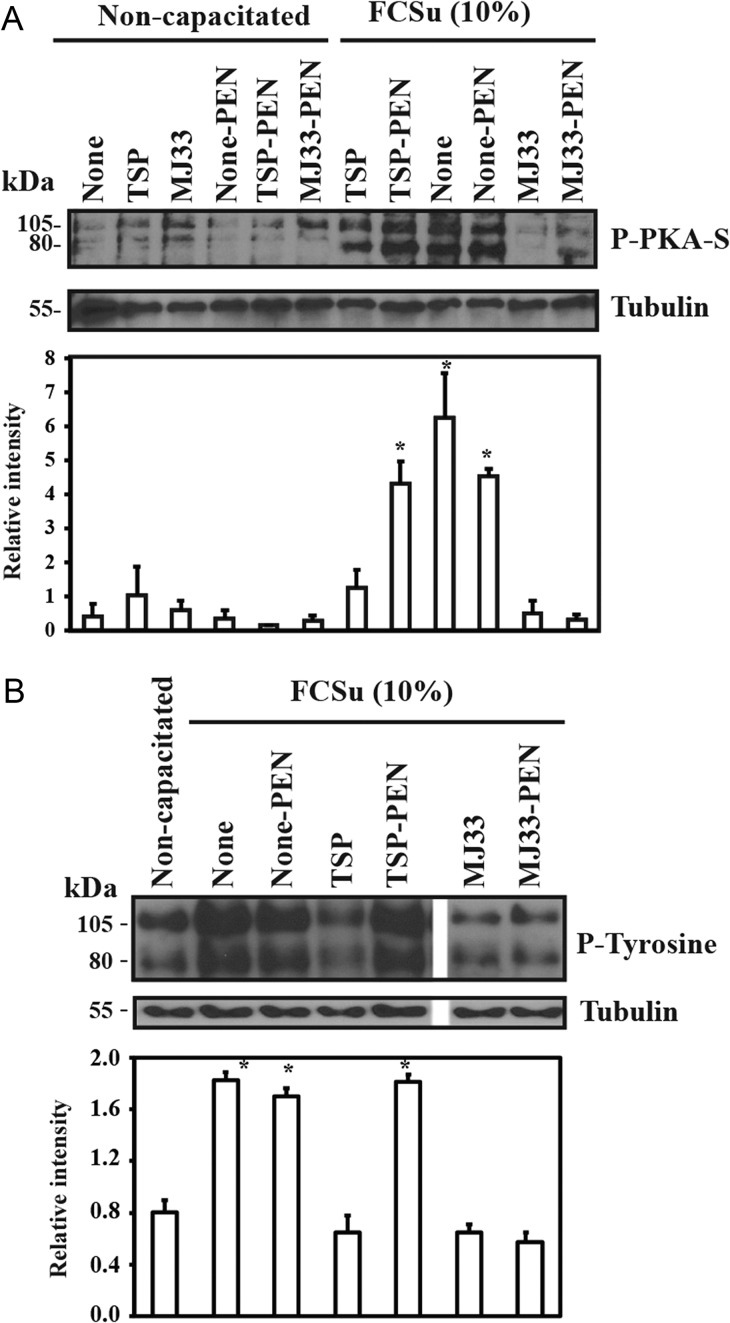
Spermatozoa treated with TSP or MJ33 showed lower levels of phosphorylated PKA substrates (P-PKA-S) than spermatozoa capacitated in the absence of the PRDXs inhibitors present in the incubation medium (Fig. 5A). This inhibition was observed whether the inhibitors were added at zero or at 15 min after the beginning of capacitation (data not shown). Moreover, the levels of P-PKA-S seen in spermatozoa treated with MJ33 were lower than those incubated with TSP in the capacitating medium. The presence of PEN, a compound known to prevent oxidative stress in human spermatozoa (Aitken et al., 2012), prevented the inhibition of PKA-S phosphorylation by TSP but not by MJ33. This protective effect of PEN was observed also for tyrosine phosphorylation when spermatozoa were incubated with TSP but not with MJ33 under capacitating conditions (Fig. 5B).
Figure 5.
Inhibition of PKA substrates and tyrosine phosphorylations by TSP but not by MJ33 was prevented by D-penicillamine (PEN). Percoll-selected human spermatozoa were capacitated with 10% FCSu in BWW medium with or without 10 μM TSP or MJ33 in the presence or absence of 2 mM D-PEN at 37°C for 4 h. Sperm suspensions were supplemented with SDS-PAGE sample buffer and solubilized proteins from 0.5 × 106 spermatozoa were loaded in each well, electrophoresed, electrotransferred and immunoblotted using the (A) anti-P-PKA substrates (P-PKA-S; 1:1000 dilution) or (B) the anti-P-Tyrosine antibody (1:10 000). Equal loading control was determined by using an anti-α-tubulin antibody (1:10 000). The immunoblots are representative of four experiments (n = 4) different donors. Relative intensity of P-Tyr or P-PKA-S was normalized to the relative intensity of tubulin of each sample and expressed as mean ± SEM. *P < 0.05 versus non-capacitated controls or sperm samples capacitated in the presence of MJ33 (n = 4).
Inhibition of PRDXs increased the levels of lipid peroxidation in spermatozoa
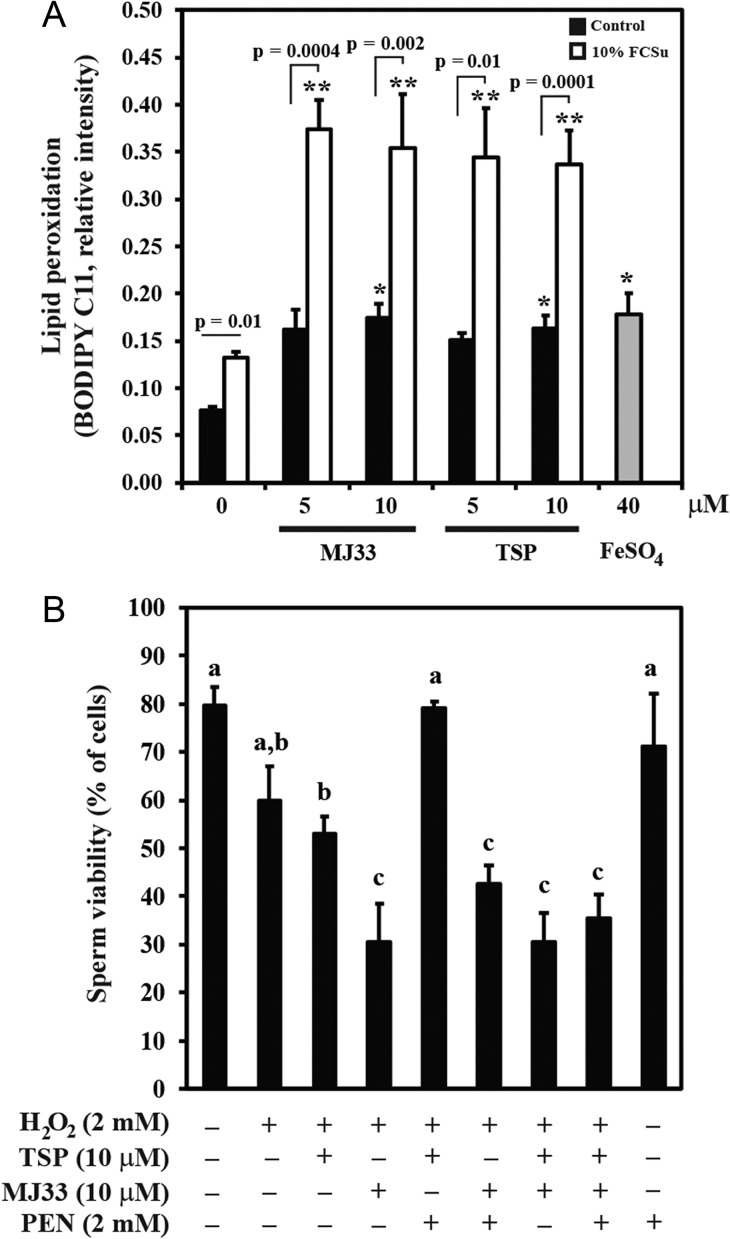
In order to demonstrate that inhibition of PRDXs leads to an oxidative stress, spermatozoa were incubated in the presence of TSP or MJ33 for 4 h and lipid peroxidation was monitored by the BODIPY C11 fluorescence by flow cytometry. We observed increased levels of lipid peroxidation, like those promoted by 40 μM FeSO4 (positive control) in spermatozoa incubated with TSP or MJ33 at the concentrations that inhibit capacitation (Fig. 6A). Spermatozoa capacitated with FCSu alone displayed higher levels of lipid peroxidation than those observed in the controls but lower than those obtained when spermatozoa were incubated with 10 μM MJ33 or TSP alone or the positive control (FeSO4) without affecting sperm viability. These results confirm that these capacitating conditions promote the production of ROS, as was previously demonstrated (de Lamirande and Gagnon, 1995; de Lamirande and O'Flaherty, 2012). Spermatozoa incubated with FCSu and the PRDXs inhibitors displayed the highest levels of lipid peroxidation, regardless the type of inhibitor involved, suggesting the establishment of a stronger oxidative stress.
Figure 6.
PRDXs inhibitors promote an oxidative stress that affects viability and increases lipid peroxidation in human spermatozoa. (A) Percoll-selected spermatozoa were incubated with 10% FCSu or without (control) in the presence or absence of different concentrations of TSP or MJ33 for 4 h at 37°C. Lipid peroxidation was determined by BODIPY C11 fluorescence by flow cytometry. Results are presented as mean ± SEM (n = 8 different donors). *P ≤ 0.01 FCSu-treated spermatozoa versus 10 μM TSP- and MJ33- or FeSo4-treated sperm, **P ≤ 0.006 versus FCSu-treated spermatozoa. All comparisons were using Student's t-test with Bonferroni's correction. (B) Percoll-selected spermatozoa were capacitated with 10% FCSu in BWW medium in the absence or presence of TSP, MJ33 or PEN at 37°C for 4 h. Sperm viability was measured by the HOST test. Results are presented as the mean ± SEM (n = 4 different donors). Different letters denote significant differences (ANOVA and Bonferroni test, all P < 0.05).
Sperm viability was severely compromised when spermatozoa were challenged with hydrogen peroxidase and PRDXs inhibitors
Spermatozoa treated with 2 mM H2O2 displayed a reduction of their viability compared to untreated controls (Fig. 6B). This effect on sperm viability was slightly increased by the presence of TSP in the incubation medium, which was only avoided by the addition of PEN in the incubation medium. Spermatozoa treated with MJ33 displayed a larger decrease in their viability compared to TSP, but in this case, PEN did not show a protective effect. When TSP and MJ33 were present together in the incubation medium, PEN was unable to prevent the decrease of sperm viability; indicating a major protective role of PRDX6 against oxidative stress.
Discussion
In this study, we demonstrated the need for PRDXs to maintain the capacitation process in human spermatozoa. Sperm capacitation is a redox-dependent process that requires a tight regulation of the ROS levels in order to avoid their toxic effects caused by the establishement of an oxidative stress, as we previously hypothesized (O'Flaherty, 2014a, 2015). Indeed, the inactivation of peroxidase activity of 2-Cys PRDXs by TSP prevented the increase of the capacitation-associated tyrosine phosphorylation (Fig. 2) and actin polymerization (Fig. 3) leading to a decrease in the percentage of capacitated spermatozoa (Fig. 1). These effects were due to inactivation of PRDXs and not by altering sperm viability or motility (Fig. 1B and Supplementary data, Table S1). This study is the first to demonstrate a mechanism that controls the levels of ROS to help assure the capacitation of the human spermatozoon.
During the delivery of the redox signaling by ROS during capacitation, 2-Cys PRDXs (PRDX1 to 5) are temporarily inactivated as the thiols in their active site are oxidized by these reactive species (e.g. H2O2, ONOO−). After the signal is transmitted, PRDXs are re-activated by the thioredoxin–thioredoxin reductase system utilizing NADPH as reducing equivalent (O'Flaherty, 2015). The same localization of PRDXs and the TRX/TRD system in the sperm subcellular compartments support this reactivation mechanism (O'Flaherty, 2014a,b). A permanent inactivation of 2-Cys PRDXs or a failure to re-activate their activity will promote a rise of ROS and the consequent oxidative stress that will prevent capacitation (Morielli and O'Flaherty, 2015) and lead to male infertility (Gong et al., 2012).
Thiol oxidation and S-glutathionylation are common redox-dependent modifications of sperm proteins (O'Flaherty and de Souza, 2011; Morielli and O'Flaherty, 2015). When enzymes are either thiol-oxidized or S-glutahionylated, they become inactivated. PKA and PKC, important enzymes necessary for sperm capacitation (Furuya et al., 1993; Lefievre et al., 2002; O'Flaherty et al., 2005), can be inactivated by high levels of ROS (Ward et al., 2000; Humphries et al., 2005), thus explaining the inhibition of capacitation by TSP. Moreover, the addition of TSP to the incubation medium promotes an oxidative stress that can lead to oxidation (thiol and or S-glutathionylation) of actin and subsequent impairment of its polymerization. As mentioned before, TSP will inactivate 2-Cys PRDXs, thus increasing the intracellular levels of ROS and promoting their toxic effects. This was evidenced by the increase in lipid peroxidation observed in spermatozoa incubated with TSP (Fig. 6A). The fact that PEN, a scavenger of ROS, prevented the reduction of phosphorylation of PKA substrates and of tyrosine residues in capacitating spermatozoa (Fig. 5A) and of viability in H2O2-treated spermatozoa incubated in the presence of TSP (Fig. 6B) strongly suggest that the inhibition of PRDXs leads to an oxidative stress that will impair sperm capacitation. Noteworthy, the lack of protection by PEN when MJ33 was present in the incubation medium leading to a reduction in sperm viability indicates that the Ca2+-iPLA2 activity of PRDX6 is essential to prevent oxidative stress damage. This evidence is supported by the increased levels of lipid peroxidation observed in the presence of MJ33 (Fig. 6A) and indicates the need of Ca2+-iPLA2 of PRDX6 to protect the spermatozoon against lipid peroxidation, assuring its normal function.
The higher levels of lipid peroxidation observed when spermatozoa were incubated under capacitating conditions with FCSu in the presence of TSP or MJ33 compared to those treated with each inhibitor alone, clearly indicates the production of ROS during capacitation that cannot be regulated when PRDXs are inhibited (Fig. 6A). Altogether, these findings confirm the need for active 2-Cys PRDXs and of Ca2+-iPLA2 activity of PRDX6 to regulate ROS levels during sperm capacitation.
We observed a lower inhibitory effect of TSP compared to MJ33 on capacitation-associated phosphorylations. This phenomenon could be explained by the fact that TSP targets 2-Cys PRDXs, without affecting PRDX6 activities (both peroxidase and Ca2+-iPLA2 activities). The fact that the phosphorylation events investigated here are partially prevented with TSP (particularly at 5 µM) suggests the presence of an active antioxidant protection by PRDX6. It is known that the peroxidase activity of PRDX6 is essential to prevent oxidative stress-dependent DNA damage and impairment of motility in mouse spermatozoa (Ozkosem et al., 2015, 2016). Moreover, it has been suggested that Ca2+-iPLA2 of PRDX6 is necessary for the removal of lipid peroxides of cell membranes (Fisher et al., 1999); here we demonstrated that lipid peroxidation is increased in spermatozoa incubated with MJ33 at doses known to inhibit capacitation (Fig. 6A). Thus, the inhibitory effects of MJ33 on capacitation could be due to the impossibility of Ca2+-iPLA2 activity of PRDX6 to remove lipid peroxides that are known to impair sperm motility and fertilizing capacity. Indeed, the oxidation of phospholipids due to oxidative stress affects sperm membrane fluidity (Aitken et al., 1993). Spermatozoa lacking PRDX6 show high levels of stress oxidative-dependent damage (high levels of lipid peroxidation and of protein and DNA oxidation) and a reduction of membrane fluidity leading to the inhibition of sperm capacitation (Ozkosem et al., 2016). Furthermore, spermatozoa from infertile men with low amounts of PRDXs and inactive PRDXs have increased levels of lipid peroxidation (Gong et al., 2012); an indication of extensive oxidation of phospholipids, thus the inactivation of PRDXs could be responsible for the impairment of membrane fluidity observed in spermatozoa from infertile men. Supporting the major protective effect of Ca2+-iPLA2 activity of PRDX6 is the fact that the presence of MJ33 (that inhibits this activity) promotes a major reduction of sperm viability compared to TSP, and that this reduction is not prevented when PEN is present in the medium. These results and the fact that capacitation-associated phosphorylation of tyrosine residues and of PKA substrates along with actin polymerization are prevented by MJ33, support the necessity for the peroxidase and Ca2+-iPLA2 activities of PRDX6 to protect the spermatozoon and to allow it to achieve fertilizing ability.
When we capacitated spermatozoa with IBMX-dbcAMP, we observed that MJ33 prevented phosphorylation of tyrosine residues at the beginning of capacitation but not when the inhibitor was added at later times during incubation (Fig. 4). These results suggest that Ca2+-iPLA2 activity of PRDX6 is involved in the IBMX-dbcAMP-dependent mechanism at the early stages of capacitation involving the activation of PKA. The observation that MJ33 prevented the phosphorylation of PKA substrates, an early event of sperm capacitation, accounts for this possibility. These results support the need for Ca2+-iPLA2 activity of PRDX6 regulating mechanisms required in the early stages of capacitation after the activation of PKA took place.
Lipid peroxides are known to inhibit several kinases involved in sperm capacitation such as PKC and extracellular signal-regulated kinase (O'Flaherty et al., 2006b); a high concentration of 4-hydroxy-2,3-nonenal inhibits PKC activity (Pronzato et al., 1990; Chiarpotto et al., 1999) and ERK phosphorylation and thus its activation in rat hepatocytes (Sampey et al., 2007). Lipid peroxides promote the oxidation of the cytoskeleton proteins, such as actin and tubulin, in the brain (Bizzozero et al., 2007). The inhibition of actin polymerization could be due to the inactivation of Ca2+-iPLA2 of PRDX6 by MJ33 that leads to an increase in lipid peroxidation. Thus, PRDX6 is then necessary for removal of lipid peroxides to ensure actin polymerization during human sperm capacitation.
It is known that the remodeling of plasma membrane phospholipids by PLA2 activity occurs during the entire process of capacitation that is needed for the spermatozoon to undergo the acrosome reaction (Flesch and Gadella, 2000). Recently, the isoform beta of the Ca2+-iPLA2 was found in spermatozoa and associated with the acrosome reaction. Ca2+-iPLA2β is not involved in capacitation since its inhibitors S-bromoenol lactone and FKGK18 do not prevent the capacitation-associated tyrosine phosphorylation in mouse spermatozoa (Abi Nahed et al., 2015). On the contrary, we demonstrated that MJ33 does prevent this phosphorylation in spermatozoa incubated under capacitating conditions and thus confirming the need for a novel Ca2+-iPLA2 activity driven by PRDX6 in human sperm capacitation. Certainly, it is known that the generation of lysophospholipids by PLA2 increases the fusogenicity of the sperm plasma membrane that is required to undergo acrosome reaction and to fuse with the oolema during fertilization (Florman and Ducibella, 2006). Noteworthy, a reduction of membrane fluidity and tyrosine phosphorylation was observed in spermatozoa from patients with varicocele or idiopathic infertility (Buffone et al., 2005, 2006). Both conditions are associated with oxidative stress and inactivation of PRDX6 in spermatozoa (Gong et al., 2012). Since the absence of PRDX6 inhibits the increase of membrane fluidity in spermatozoa incubated under capacitating conditions, it is possible that the Ca2+-iPLA2 activity of PRDX6 would be involved in human sperm capacitation as well.
It is known that sperm capacitation is an oxidative event where ROS are produced to trigger and control phosphorylation events (O'Flaherty et al., 2006a, b; de Lamirande and O'Flaherty, 2012). It is evident then that there is a necessity to control the levels of ROS to avoid their toxic effects and PRDXs are the perfect players to accomplish this task as they are differentially localized in subcellular compartment of the spermatozoon (O'Flaherty, 2014a, 2015). Here, we have presented robust evidence for this regulatory role of PRDXs during human sperm capacitation.
In conclusion, our results strongly suggest the need for active PRDXs to control ROS levels and support capacitation in human spermatozoa throughout the entire duration of this process. Moreover, we showed a differential participation of PRDXs depending on the type of PRDX isoform involved, the capacitation inducer and the time during human sperm capacitation. We also demonstrated a major role of PRDX6 in the regulation of capacitation by preventing the toxic effects of oxidative stress. This study enhances our knowledge of the regulation of ROS levels to allow the redox-dependent signaling observed during sperm capacitation.
Supplementary Material
Acknowledgements
We thank the volunteers that participated in this study.
Supplementary data
Supplementary data are available at Molecular Human Reproduction online.
Authors’ roles
D.L., A.R.M., T.M., M.C.F. and C.O. designed the study. D.L., A.R.M., T.M. and M.C.F. carried out the experiments and D.L., A.R.M., M.C.F. and C.O. analyzed the data. D.L., A.R.M. and C.O. wrote the manuscript.
Funding
This research was supported by Canadian Institutes of Health Research (MOP 133661) to C.O. C.O. is the recipient of a Chercheur Boursier Junior 2 Salary Award from the Fonds de la Recherche en Santé du Québec. (22151).
Conflict of interest
None declared.
References
- Abi Nahed R, Martinez G, Escoffier J, Yassine S, Karaouzene T, Hograindleur J, Turk J, Kokotos G, Ray PF, Bottari S et al. Progesterone-induced acrosome exocytosis requires sequential involvement of calcium-independent iPLA2β and group X sPLA2 . J Biol Chem 2015;291:3076–3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken RJ, Harkiss D, Buckingham D. Relationship between iron-catalysed lipid peroxidation potential and human sperm function. J Reprod Fertil 1993;98:257–265. [DOI] [PubMed] [Google Scholar]
- Aitken RJ, Gibb Z, Mitchell LA, Lambourne SR, Connaughton HS, De Iuliis GN. Sperm Motility is lost in vitro as a consequence of mitochondrial free radical production and the generation of electrophilic aldehydes but can be significantly rescued by the presence of nucleophilic thiols. Biol Reprod 2012;87:110. [DOI] [PubMed] [Google Scholar]
- Aitken RJ, Wingate JK, De Iuliis GN, McLaughlin EA. Analysis of lipid peroxidation in human spermatozoa using BODIPY C11. Mol Hum Reprod 2007;13:203–211. [DOI] [PubMed] [Google Scholar]
- Aitken RJ, Gordon E, Harkiss D, Twigg JP, Milne P, Jennings Z, Irvine DS. Relative impact of oxidative stress on the functional competence and genomic integrity of human spermatozoa. Biol Reprod 1998;59:1037–1046. [DOI] [PubMed] [Google Scholar]
- Biggers JD, Whitten WK, Whittngham DG. The culture of mouse embryos in vitro In: Daniel JC (ed).. Methods in Mammalian Embryology. San Francisco, USA: Freeman, 1971:86–116. [Google Scholar]
- Bizzozero OA, Reyes S, Ziegler J, Smerjac S. Lipid peroxidation scavengers prevent the carbonylation of cytoskeletal brain proteins induced by glutathione depletion. Neurochem Res 2007;32:2114–2122. [DOI] [PubMed] [Google Scholar]
- Bjorndahl L, Barratt CLR, Mortimer D, Jouannet P. How to count sperm properly: checklist for acceptability of studies based on human semen analysis. Hum Reprod 2016;31:227–232. [DOI] [PubMed] [Google Scholar]
- Brener E, Rubinstein S, Cohen G, Shternall K, Rivlin J, Breitbart H. Remodeling of the actin cytoskeleton during mammalian sperm capacitation and acrosome reaction. Biol Reprod 2003;68:837–845. [DOI] [PubMed] [Google Scholar]
- Buffone MG, Brugo-Olmedo S, Calamera JC, Verstraeten SV, Urrutia F, Grippo L, Corbetta JP, Doncel GF. Decreased protein tyrosine phosphorylation and membrane fluidity in spermatozoa from infertile men with varicocele. Mol Reprod Dev 2006;73:1591–1599. [DOI] [PubMed] [Google Scholar]
- Buffone MG, Calamera JC, Verstraeten SV, Doncel GF. Capacitation-associated protein tyrosine phosphorylation and membrane fluidity changes are impaired in the spermatozoa of asthenozoospermic patients. Reproduction 2005;129:697–705. [DOI] [PubMed] [Google Scholar]
- Burgess A, Vigneron S, Brioudes E, Labbe JC, Lorca T, Castro A. Loss of human Greatwall results in G2 arrest and multiple mitotic defects due to deregulation of the cyclin B-Cdc2/PP2A balance. Proc Natl Acad Sci U S A 2010;107:12564–12569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett LA, Sugiyama H, Bieber AL, Chandler DE. Egg jelly proteins stimulate directed motility in Xenopus laevis sperm. Mol Reprod Dev 2011;78:450–462. [DOI] [PubMed] [Google Scholar]
- Chen JW, Dodia C, Feinstein SI, Jain MK, Fisher AB. 1-Cys peroxiredoxin, a bifunctional enzyme with glutathione peroxidase and phospholipase A2 activities. J Biol Chem 2000;275:28421–28427. [DOI] [PubMed] [Google Scholar]
- Chiarpotto E, Domenicotti C, Paola D, Vitali A, Nitti M, Pronzato MA, Biasi F, Cottalasso D, Marinari UM, Dragonetti A et al. Regulation of rat hepatocyte protein kinase C beta isoenzymes by the lipid peroxidation product 4-hydroxy-2,3-nonenal: a signaling pathway to modulate vesicular transport of glycoproteins. Hepatology 1999;29:1565–1572. [DOI] [PubMed] [Google Scholar]
- de Lamirande E, O'Flaherty C. Sperm capacitation as an oxidative event In: Aitken J, Alvarez J, Agawarl A (eds).. Studies on Men's Health and Fertility, Oxidative Stress in Applied Basic Research and Clinical Practice. USA: Springer Science, 2012:57–94. [Google Scholar]
- de Lamirande E, Gagnon C. Capacitation-associated production of superoxide anion by human spermatozoa. Free Radic Biol Med 1995;18:487–495. [DOI] [PubMed] [Google Scholar]
- Dubuisson M, Vander Stricht D, Clippe A, Etienne F, Nauser T, Kissner R, Koppenol W, Rees J, Knoops B. Human peroxiredoxin 5 is a peroxynitrite reductase. FEBS Lett 2004;571:161–165. [DOI] [PubMed] [Google Scholar]
- Fisher AB, Dodia C, Manevich Y, Chen JW, Feinstein SI. Phospholipid hydroperoxides are substrates for non-selenium glutathione peroxidase. J Biol Chem 1999;274:21326–21334. [DOI] [PubMed] [Google Scholar]
- Flesch F, Gadella B. Dynamics of the mammalian sperm plasma membrane in the process of fertilization. Biochim Biophys Acta 2000;1469:197–235. [DOI] [PubMed] [Google Scholar]
- Florman HM, Ducibella T. Fertilization in mammals In: Jimmy DN, Tony MP, Donald WP, John RGC, David MdK, JoAnne S, Richards, Paul MW (eds).. Knobil and Neill's Physiology of Reproduction, 3rd edn St Louis, USA: Academic Press, 2006:55–112. [Google Scholar]
- Furuya S, Endo Y, Osumi K, Oba M, Suzuki S. Effects of modulators of protein kinase C on human sperm capacitation. Fertil Steril 1993;59:1285–1290. [PubMed] [Google Scholar]
- Gong S, San Gabriel M, Zini A, Chan P, O'Flaherty C. Low amounts and high thiol oxidation of peroxiredoxins in spermatozoa from infertile men. J Androl 2012;33:1342–1351. [DOI] [PubMed] [Google Scholar]
- Humphries KM, Deal MS, Taylor SS. Enhanced dephosphorylation of cAMP-dependent protein kinase by oxidation and thiol modification. J Biol Chem 2005;280:2750–2758. [DOI] [PubMed] [Google Scholar]
- Iwasaki A, Gagnon C. Formation of reactive oxygen species in spermatozoa of infertile patients. Fertil Steril 1992;57:409–416. [DOI] [PubMed] [Google Scholar]
- Jacobson G, Karsnas P. Important parameters in semi-dry electrophoretic transfer. Electrophoresis 1990;11:46–52. [DOI] [PubMed] [Google Scholar]
- Leclerc P, de Lamirande E, Gagnon C. Cyclic adenosine 3’,5’monophosphate-dependent regulation of protein tyrosine phosphorylation in relation to human sperm capacitation and motility. Biol Reprod 1996;55:684–692. [DOI] [PubMed] [Google Scholar]
- Lefievre L, Jha KN, de Lamirande E, Visconti PE, Gagnon C. Activation of protein kinase A during human sperm capacitation and acrosome reaction. J Androl 2002;23:709–716. [PubMed] [Google Scholar]
- Morielli T, O'Flaherty C. Oxidative stress impairs function and increases redox protein modifications in human spermatozoa. Reproduction 2015;149:113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noiles E, Thompson K, Storey B. Water permeability, Lp, of the mouse sperm plasma membrane and its activation energy are strongly dependent on interaction of the plasma membrane with the sperm cytoskeleton. Cryobiology 1997;35:79–92. [DOI] [PubMed] [Google Scholar]
- O'Flaherty C, de Lamirande E, Gagnon C. Phosphorylation of the Arginine-X-X-(Serine/Threonine) motif in human sperm proteins during capacitation: modulation and protein kinase A dependency. Mol Hum Reprod 2004; 10:355–363. [DOI] [PubMed] [Google Scholar]
- O'Flaherty C, de Lamirande E, Gagnon C. Reactive oxygen species and protein kinases modulate the level of phospho-MEK-like proteins during human sperm capacitation. Biol Reprod 2005;73:94–105. [DOI] [PubMed] [Google Scholar]
- O'Flaherty C, de Lamirande E, Gagnon C. Positive role of reactive oxygen species in mammalian sperm capacitation: triggering and modulation of phosphorylation events. Free Radic Biol Med 2006. a;41:528–540. [DOI] [PubMed] [Google Scholar]
- O'Flaherty C, de Lamirande E, Gagnon C. Reactive oxygen species modulate independent protein phosphorylation pathways during human sperm capacitation. Free Radic Biol Med 2006. b;40:1045–1055. [DOI] [PubMed] [Google Scholar]
- O'Flaherty C. Peroxiredoxins: hidden players in the antioxidant defence of human spermatozoa. Basic Clin Androl 2014. a;24:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Flaherty C. The enzymatic antioxidant system of human spermatozoa. Adv Androl 2014. b;2014:1–15. [Google Scholar]
- O'Flaherty C. Redox regulation of mammalian sperm capacitation. Asian J Androl 2015;17:583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Flaherty C, de Souza AR. Hydrogen peroxide modifies human sperm peroxiredoxins in a dose-dependent manner. Biol Reprod 2011;84:238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkosem B, Feinstein SI, Fisher AB, O'Flaherty C. Advancing age increases sperm chromatin damage and impairs fertility in peroxiredoxin 6 null mice. Redox Biol 2015;5:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkosem B, Feinstein SI, Fisher AB, O'Flaherty C. Absence of Peroxiredoxin 6 Amplifies the Effect of Oxidant Stress on Mobility and SCSA/CMA3 Defined Chromatin Quality and Impairs Fertilizing Ability of Mouse Spermatozoa. Biol Reprod 2016. Mar;94(3):68. doi: 10.1095/biolreprod.115.137646. Epub 2016 Jan 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peshenko IV, Shichi H. Oxidation of active center cysteine of bovine 1-Cys peroxiredoxin to the cysteine sulfenic acid form by peroxide and peroxynitrite. Free Radic Biol Med 2001;31:292–303. [DOI] [PubMed] [Google Scholar]
- Plante M, de Lamirande E, Gagnon C. Reactive oxygen species released by activated neutrophils, but not by deficient spermatozoa, are sufficient to affect normal sperm motility. Fertil Steril 1994;62:387–393. [DOI] [PubMed] [Google Scholar]
- Pronzato MA, Domenicotti C, Biasi F, Chiarpotto E, Cottalasso D, Viotti P, Melloni E, Marinari UM, Poli G. Inactivation of hepatocyte protein kinase C by carbon tetrachloride: involvement of drug's metabolic activation and prooxidant effect. Biochem Biophys Res Commun 1990;171:1353–1360. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Chae HZ, Kim K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med 2005;38:1543–1552. [DOI] [PubMed] [Google Scholar]
- Sampey BP, Carbone DL, Doorn JA, Drechsel DA, Petersen DR. 4-Hydroxy-2-nonenal adduction of extracellular signal-regulated kinase (Erk) and the inhibition of hepatocyte Erk-Est-like protein-1-activating protein-1 signal transduction. Mol Pharmacol 2007;71:871–883. [DOI] [PubMed] [Google Scholar]
- Sanchez-Pozo MC, Mendiola J, Serrano M, Mozas J, Bjorndahl L, Menkveld R, Lewis SEM, Mortimer D, Jorgensen N, Barratt CLR et al. Proposal of guidelines for the appraisal of SEMen QUAlity studies (SEMQUA). Hum Reprod 2013;28:10–21. [DOI] [PubMed] [Google Scholar]
- Tremellen K. Oxidative stress and male infertility: a clinical perspective. Hum Reprod Update 2008;14:243–258. [DOI] [PubMed] [Google Scholar]
- Ward NE, Stewart JR, Ioannides CG, O'Brian CA. Oxidant-induced S-glutathiolation inactivates protein kinase C-alpha (PKC-alpha): a potential mechanism of PKC isozyme regulation. Biochemistry 2000;39:10319–10329. [DOI] [PubMed] [Google Scholar]
- Wood ZA, Poole LB, Karplus PA. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science 2003;300:650–653. [DOI] [PubMed] [Google Scholar]
- World Health Organization WHO Laboratory Manual for the Examination and Processing of Human Semen. Geneva: WHO Press, 2010. [Google Scholar]
- Zhang P, Liu B, Kang SW, Seo MS, Rhee SG, Obeid LM. Thioredoxin peroxidase is a novel inhibitor of apoptosis with a mechanism distinct from that of Bcl-2. J Biol Chem 1997;272:30615–30618. [DOI] [PubMed] [Google Scholar]
- Zini A, de Lamirande E, Gagnon C. Reactive oxygen species in semen of infertile patients: levels of superoxide dismutase- and catalase-like activities in seminal plasma and spermatozoa. Int J Androl 1993;16:183–188. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.