Abstract
Background.
Herpes simplex virus type 2 (HSV-2; herpes) exacerbates human immunodeficiency virus type 1 (HIV) by unclear mechanisms. These studies tested the impact of HSV-2 on systemic T-cells and HIV reservoirs.
Methods.
Peripheral blood mononuclear cells from HIV-infected women on antiretroviral therapy who were HSV-2 seropositive or seronegative and HIV-uninfected controls were analyzed by flow cytometry. Cell-associated HIV DNA and RNA were quantified in the absence or presence of activating stimuli, recombinant interleukin 32γ (IL-32γ), and a RUNX1 inhibitor. RNA was assessed by nanostring.
Results.
CD4, but not CD8, T-cell phenotypes differed in HIV+/HSV-2+ versus HIV+/HSV-2− (overall P = .002) with increased frequency of CCR5+, CXCR4+, PD-1+, and CD69+ and decreased frequency of CCR10+ and CCR6+ T-cells. The changes were associated with higher HIV DNA. Paradoxically, IL-32, a proinflammatory cytokine, was lower in subpopulations of CD4+ T-cells in HSV-2+ versus HSV-2− women. Recombinant IL-32γ blocked HIV reactivation in CD4+ T-cells and was associated with an increase in RUNX1 expression; the blockade was overcome by a RUNX1 inhibitor.
Conclusions.
Herpes is associated with phenotypic changes in CD4+ T-cells, including a decrease in IL-32, which may contribute to increased HIV reservoirs. Blocking IL-32 may facilitate HIV reactivation to improve shock and kill strategies.
Keywords: Herpes simplex virus, human immunodeficiency virus, HIV reservoirs, IL-32, CD4+, T cells.
The human immunodeficiency virus (HIV) epidemic is fueled by coinfections, which may promote HIV acquisition, replication, and the establishment or expansion of viral reservoirs. One of the most significant coinfections is herpes simplex virus type 2 (HSV-2) [1, 2]. Herpes simplex virus type 2–seropositive (HSV-2+) individuals have an increased risk of HIV acquisition, and dually-infected (HIV+/HSV-2+) individuals are more likely to transmit HIV to their partner(s) or from mother to child [2–6]. The high prevalence of HSV-2 in sub–Saharan Africa has been suggested to contribute more than other biological and behavioral factors to HIV transmission [7–9].
The major burden of HSV-2 is linked to frequent recurrences, most of which are unrecognized. Herpes simplex virus type 2 shedding was detected on a median of 25% of days in HIV-uninfected (HIV−) individuals and is more common in HIV+ persons, leading to the recognition of HSV-2 as a persistent, rather than latent, pathogen [10–13]. Herpes simplex virus type 2 recurrences may facilitate HIV acquisition or transmission through disruption of epithelial barrier and/or induction of an inflammatory response with an increase in HIV-1 target cells at sites of HSV-2 reactivation [14, 15]. However, these mechanisms do not account for the effects of HSV-2 on HIV plasma viral loads (PVLs) and disease progression. Plasma viral load levels in HIV+/HSV-2+ women before antiretroviral therapy (ART) were 0.20–0.55 log10 copies/mL higher than in HSV-2–seronegative (HSV-2−) women [5, 6, 16, 17]. Thus, in addition to an increase in immune cells at the site of HSV reactivation, HSV-2 may have a more global effect on systemic immunity.
Persistent pathogens may impact HIV infection by multiple mechanisms [18, 19]; however, few studies have examined the effects of HSV-2 on systemic immunity and how this may modulate HIV infection [20–24]. A study of 46 HIV-uninfected (HIV−) women (54% HSV-2+) found that HSV-2 was associated with increased expression of CD38/HLA-DR and integrin α4β7 by peripheral blood CD4+ T cells [22], whereas a study of recently HIV-infected adults did not detect differences in CD4+ T cell activation or differentiation comparing HSV-2+ with HSV-2− [25].
Interleukin 32 (IL-32), an intracellular cytokine expressed in multiple cell types [26], has recently been linked to HIV infection. However, studies to determine its effects have yielded contradictory results. In a monocytic cell line, IL-32 interacted with protein kinase Cε and STAT3 to induce interleukin 6 (IL-6) [27] and activated NF-κB and MAPK, leading to its designation as inflammatory [28.]. Interleukin 32 was upregulated in gut lymphatic tissue in HIV+ compared with HIV− controls in a microarray study [29]. Interleukin 32 induced expression of immunosuppressive molecules indoleamine 2,3-dioxygenase and Ig-like transcript 4, leading the authors to speculate that IL-32 impaired host defense to promote HIV replication [29]. Dinarello used small interfering RNA to silence IL-32 in a chronically HIV-infected pro-monocyte cell line (U1) or peripheral blood mononuclear cells (PBMCs) and found decreased tumor necrosis factor (TNF), IL-6, and interferon γ (IFNγ) but, paradoxically, increased HIV p24 [30]. Blockade of IFN receptors further increased p24, which suggests that IL-32 exerted an antiviral effect by increasing IFN [30]. No studies have examined the impact of HSV-2 on IL-32.
The current studies were designed to test the hypothesis that HSV-2 induces changes in peripheral blood T-cell populations, characterized by an increase in activated CD4+ and/or CD8+ T cells and increased IL-32 expression, which could promote HIV reactivation in HIV+/HSV-2+ women. We took advantage of a biorepository of PBMCs and compared CD4+ and CD8+ T cell phenotypes in HIV+ women who were also HSV-2+ or HSV-2− and explored the impact of observed changes on HIV reactivation and potential viral reservoirs.
METHODS
Ethics Statement
The study was conducted according to the Declaration of Helsinki and was approved by the Albert Einstein College of Medicine and Yale University Institutional Review Boards. All participants provided written informed consent.
Participants and Coinfection Diagnostics
Periperheral blood mononuclear cells were available from a sample biorepository from women who participated in a study of mucosal effects of HIV and HSV-2 (n = 50) and the Bronx Women’s Interagency HIV Study (WIHS; n = 41). For the former study, women were selected based on a history of prior genital herpes. Bronx WIHS participants were included if they were also human leukocyte antigen (HLA)-A2 positive. None of the participants had active clinical herpes or other signs of sexually transmitted infections at the study visit. The OraQuick ADVANCE Rapid HIV-1/2 Antibody test and HerpeSelect HSV-1 and HSV-2 serum antibody assay were performed to determine HIV and HSV serostatus if not previously documented. Epstein-Barr virus (EBV) and cytomegalovirus (CMV) serostatus were determined using Zeus Scientific ELISA IgG kits.
Plasma HIV-1 RNA levels were quantified using the Abbott m2000 HIV-1 RealTime System with a lower limit of quantification of 40 copies/mL. Plasma CMV and EBV DNA was quantified by real-time polymerase chain reaction (PCR); probes targeted UL54 and UL55 for CMV and EBER and EBNA for EBV. Peripheral blood mononuclear cells were isolated by density gradient centrifugation using Ficoll-Paque PLUS. Frozen samples were used for phenotyping, and freshly collected samples were used for HIV reactivation.
Flow Cytometry
Cells were resuspended in phosphate-buffered saline, stained with LIVE/DEAD marker, washed with FACS Buffer (phosphate-buffered saline, 3% fetal bovine serum, 0.02% sodium azide), and stained with indicated antibodies (Supplementary Methods). HLA-A2 tetramers were obtained from MBL International and were allophycocyanin (APC) labeled. Cells were analyzed on a Fortessa flow cytometer. Electronic gates were placed on CD4+ and CD8+ T cells to determine the percentage of T-cell subsets and the mean fluorescence intensity of IL-32 staining.
HIV Cell-Associated DNA and RNA Quantification
Cellular RNA and genomic DNA were isolated simultaneously using the AllPrep DNA/RNA mini kit from 0.7–2.1 × 106 cells. Alternatively, RNA isolation was performed using the Absolutely RNA miniprep kit. Total HIV cell-associated DNA copy number was interpolated from a standard curve generated using DNA isolated from U1 cells [31] combined with uninfected PBMCs at varying ratios. Samples were measured in duplicate and normalized to input DNA concentration. Human immunodeficiency virus primers and probe targeted a conserved region of the long terminal repeat (LTR) (Supplementary Methods) [32]. Targets were amplified in 10-µL reactions in a QuantStudio 7 Flex Real-Time PCR System, and data were analyzed using QuantStudio software. Samples below the limit of quantification (3.34 HIV copies/million cells) were assigned a value of 1.67 copies/million cells.
Cell-associated RNA was quantified by interpolation from a standard curve generated with known amounts of genomic HIV RNA extracted from the laboratory HIV-1 isolate NL4-3 and quantified using the RealTime HIV-1 assay (Abbott Laboratories). RNA isolated from CD4+ T-cell samples (100 ng) was reverse-transcribed in parallel with standards using the High-Capacity cDNA Reverse Transcription Kit with RNase Inhibitor, and HIV LTR was detected as described above. The limit of quantification was 10 copies/µg RNA; samples below were assigned 5 copies/µg RNA.
Gene Expression Analysis
Total RNA was extracted, cDNA synthesized, and reverse-transcription PCR (RT-PCR) amplification performed as described above using primer/probe sets as detailed in the Supplementary Methods. Quantification was normalized against the number of RPLPO transcripts in the same RNA extracts, and relative gene expression was calculated using the 2^(- ΔΔCt) method [33].
RNA NanoString Analysis
CD4+ T cells were cultured for 24 hours with phytohemagglutinin (PHA) ± recombinant IL-32γ (rIL-32γ; 100 ng/mL). Cells were then resuspended in lysis buffer. Lysate corresponding to 10000 cells (4 µL) was used to load the Human Immunology nCounter gene expression panel slides. Expression of 579 human immune cell genes, including 16 housekeeping genes, was measured. Genes were filtered if their expression was <20 counts in >20% of the samples, and data were normalized based on expression of housekeeping genes [34].
Human Immunodeficiency Virus Reactivation
CD4+ T cells were isolated from PBMCs by negative selection using the EasySe Human CD4+ T Cell Enrichment Kit and cultured in Roswell Park Memorial Institute (RPMI) medium supplemented with 10% fetal bovine serum, 100 U/mL penicillin, 100 µg/mL streptomycin, and 50 U/mL interleukin 2 (complete medium), unless stated otherwise. CD4+ T cells from HIV+ women were exposed to complete medium supplemented with TNF (100 U/mL), PHA (5 µg/mL) or phorbol 12-myristate 13-acetate (PMA) (2nM) for 24 hours ± rIL-32γ. Cells were washed 3 times with serum-free medium and cultured for 7 days ± rhIL-32γ and the RUNX1 inhibitor Ro5-3335 (5 µM). Cells and supernatant were collected by centrifugation (300 g) for quantification of HIV cell-associated RNA and secreted cytokines and chemokines, respectively.
Multiplex Analysis of Cytokines and Chemokines
Interleukin 6, IL-8, TNF, IFN-γ, granulocyte macrophage colony-stimulating factor (GM-CSF), macrophage inflammatory protein (MIP)-1α, MIP-1β, and regulated on activation, normal T cell expressed and secreted (RANTES) were measured in culture supernatants by Luminex100 with beads from Chemicon International and analyzed using StarStation. Concentrations below the lower limit of detection were set at the midpoint between zero and the lower limit of detection.
Statistical Analysis
A cross-sectional analysis of cell subsets in CD4+ and CD8+ T cells was performed with a general linear model using HIV and/or HSV-2 status as fixed effects. In some analyses, age and HSV-1 serostatus were used as covariates. One and two-way analyses of variance (ANOVAs) were performed to compare groups and to study the effects of HIV-1 and/or HSV-2 infection on T cells and IL-32 expression; Spearman correlation coefficients were calculated to assess associations between HIV DNA and T-cell markers.
RESULTS
Changes in CD4+ but not CD8+ T Cells Associated With HSV-2
Demographic and clinical characteristics of the 49 HIV+/HSV-2+ and 15 HIV+/HSV− women are shown in Table 1. Most were on ART and had low or undetectable HIV PVLs. The HIV+/HSV-2+ women were older, had been diagnosed with HIV longer, and were more frequently CMV-seropositive compared with the HIV+/HSV-2− women, but other clinical parameters including HSV-1 and EBV serostatus were similar.
Table 1.
Demographic and Clinical Characteristics of Human Immunodeficiency Virus–Positive Women Dichotomozied by Herpes Simplex Virus Type 2 Serostatus
| Variable | HIV + /HSV-2 – (n = 15) | HIV + /HSV-2 + (n = 49) | P value a |
| Age, y, median [IQR] | 38 [24–45.5] | 51 [46–55] | <.001 |
| Race: White | 3 | 0 | .78 |
| Black | 5 | 27 | |
| Hispanic | 7 | 20 | |
| Other | 0 | 2 | |
| Years since HIV diagnosis, median [IQR] | 20 [12–25] | 25 [21.5–31] | .03 |
| HSV-1 seropositive | 9/15 (60%) | 28/49 (57%) | .84 |
| CMV seropositive | 6/13 (46%) | 39/44 (89%) | .003 |
| EBV seropositive | 12/13 (92%) | 43/44 (98%) | .87 |
| Plasma CMV DNA detected [IU/ml in positive sample] | 0/13 (0%) | 1/44 (2%) [<96 IU/mL] | .58 |
| Plasma EBV DNA detected, no. (%), [IU/mL in positive samples] | 3/13 (23%) [<49 × 2, 56 IU/mL] | 8/44 (18%) [<49 × 3, 60, 66,76, 88, 129 IU/mL] | .69 |
| HIV plasma viral load >40 copies/mL | 4/15 (27%) | 23/49 (47%) | .16 |
| HIV plasma viral load, log10 copies/mL, median [IQR] | 1.60 [1.3–2.0] | 1.68 [1.3–3.45] | .34 |
| CD4+ cells/mm3, median [IQR] | 638 [386688] | 470 [338642] | .34 |
| Combination antiretroviral therapy | 12/15 (80%) | 45/49 (92%) | .20 |
| Current HSV antiviral therapy (acyclovir) | 5/49 (10%) |
Abbreviations: CMV, cytomegalovirus; EBV, Epstein-Barr virus; HIV, human immunodeficiency virus; HSV, herpes simplex virus; IQR, interquartile range.
aFisher’s exact test used except for age, years since HIV diagnosis, and CD4+ cell count, which were compared by Student’s t test.
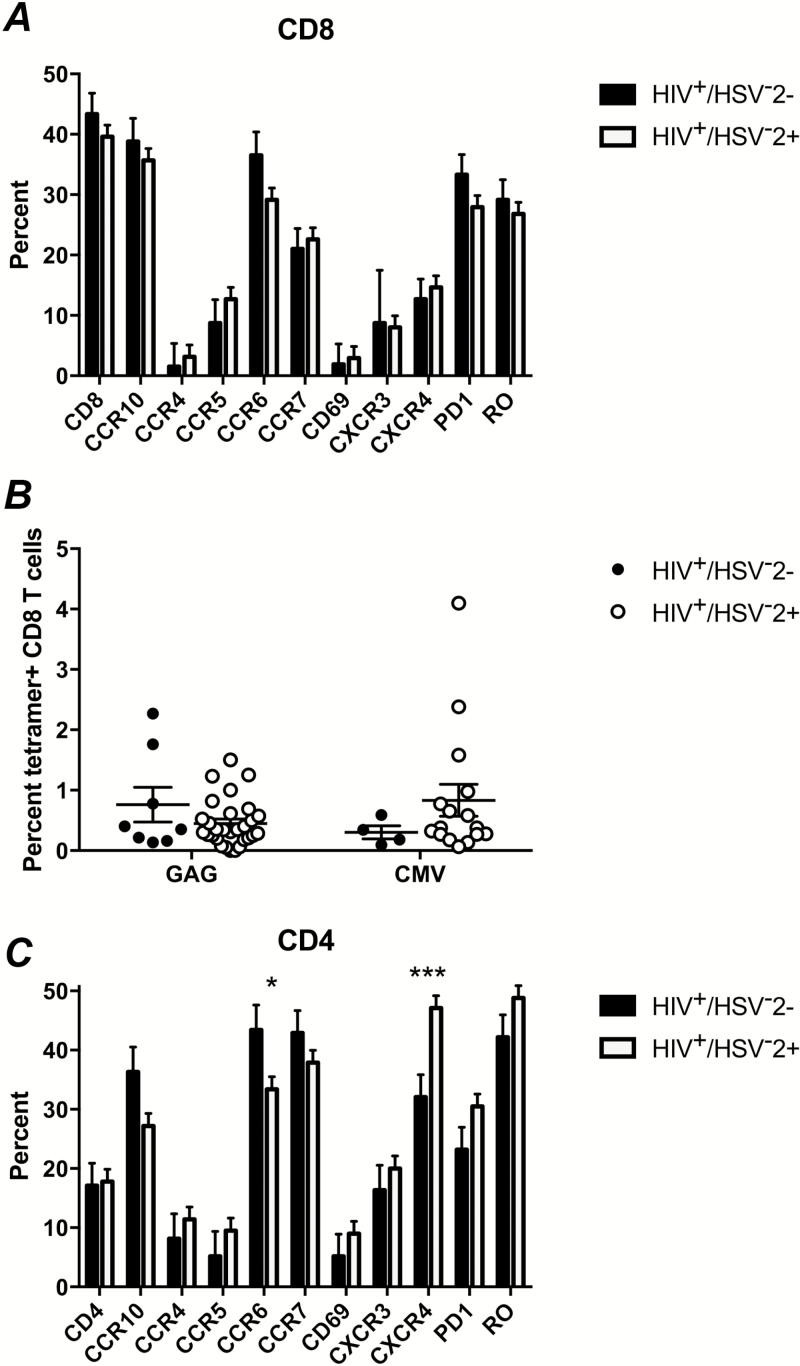
We first asked whether HSV-2 coinfection affected CD8+ T cells because of the primary role of these cells in killing infected cellular targets. We did not detect significant differences in the frequency of CD8+ T cells or in expression of 10 different cell surface markers individually or in a cross-sectional analysis comparing group effects in HIV+/HSV-2+ versus HIV+/HSV− women (P = .65) (Figure 1A). We also did not find differences in the frequency of HIV Gag or CMV-specific CD8+ T cells, measured by Class I major histocompatibility complex (MHC) tetramer staining in the HLA-A2-positive women (Figure 1B).
Figure 1.
Phenotype of CD8+ T cells and CD4+ T cells in human immunodeficiency virus (HIV)–seropositive women who are seropositive or seronegative for herpes simplex virus type 2 (HSV-2). Percentage of CD8+ (A) and CD4+ (C) T cells among PBMC and, within each of these populations, the frequency of cells expressing the indicated cell-surface markers by cross-sectional analysis comparing group effects among HIV+/HSV-2− (n = 15) and HIV+/HSV-2+ (n = 49) women. The data represent the percentage of cells (+SEM). There was a significant difference in the frequency of cells expressing indicated markers among the CD4+ (P = .002) but not CD8+ (P = .65) cells. The asterisks indicate significant differences in CXCR4+ (P = .005) and CCR6+ (P = .04) cells from the model. B, The frequency of HIV Gag or cytomegalovirus (CMV)–specific CD8+ T cells by class I major histocompatibility complex (MHC) tetramer staining among human leukocyte antigen (HLA)-A2+subjects (n = 8 HIV+/HSV-2− and n = 29 HIV+/HSV-2+). Each symbol represents an individual subject.
However, we did find effects of HSV-2 in CD4+ T-cell subsets. The HIV+/HSV-2− women had a similar frequency of CD4+ T cells as the HIV+/HSV-2+ women (Figure 1C), but there were increases in CXCR4 (P = .005) and PD-1 (P = .09) and decreases in CCR6 (P = .04) and CCR10 (P = .05). The changes did not correlate with age, and the differences persisted when HSV-1 serostatus was used as a covariate. No significant differences in CD4+ T cell subsets were identified when women were dichotomized by CMV serostatus (not shown).
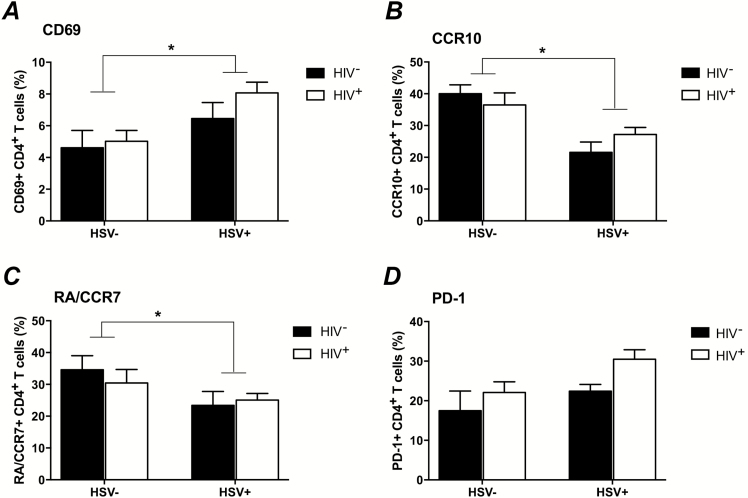
To further understand the impact of HSV-2 on CD4+ T cells, we extended the studies to HIV− women. Peripheral blood mononuclear cells were available from 18 HIV−/HSV-2+ and 10 HIV−/HSV-2− controls (median = 43 [interquartile range = 29.5–48.3] and 46.5 [interquartile range = 37.8–57.3] years, respectively). The effect of HSV-2 was significant for naive (RACCR7+; P = .04), CCR10+ (P = .008), and CD69+ (P = .02), and of borderline significance for PD-1+ cells (P = .06) (Figure 2A–D). There was also a borderline effect of HSV-2 on CCR6+ (P = .08) and CXCR4+ (P = .09) (not shown).
Figure 2.
Differences in CD4+ T cells in herpes simplex virus type 2 (HSV-2) and human immunodeficiency virus (HIV)–infected and –uninfected women. The percentage of live CD4+ T cells expressing the indicated markers in 4 cohorts of women were compared by 2-way analysis of variance (HIV−/HSV-2−: n = 10; HIV−/HSV-2+: n = 18; HIV+/HSV-2−: n = 15; and HIV+/HSV-2+: n = 49). There was a significant effect of HSV-2 infection on the frequency of CD69+ (P = .02) (A), CCR10+ (P = .008) (B), and naive CD45RA+/CCR7+ (P = .04) (C) T cells. The P value comparing PD-1 expression among HSV-2+ versus HSV-2− women was .06 (D).
Association of HSV-2 Coinfection With HIV DNA
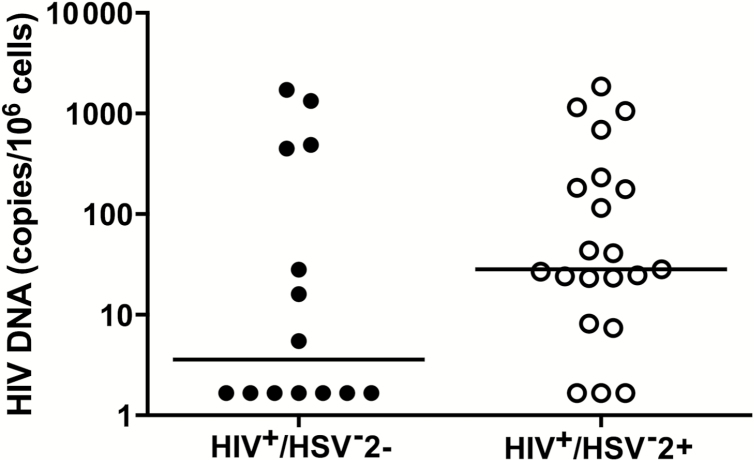
Human immunodeficiency virus replicates in activated CD4+ T cells, and thus the increased frequency of cells expressing CD69 and PD-1 suggested that coinfection might facilitate HIV replication and increase potential HIV reservoirs. We found an increase in the proportion of women with cell-associated HIV DNA in CD4+ cells when comparing HIV+/HSV-2+ and HIV+/HSV-2− (n = 18/21 vs 7/14; P = .05, chi-square) (Figure 3). The total cell-associated DNA correlated significantly and positively with surface expression of CCR5 (Spearman r = 0.45; P = .02) and CCR4 (r = 0.47, P = .01) and negatively with the frequency of CD4+ T cells (r = −0.41; P = .02). There was also a trend toward a positive correlation with CD69 (r = 0.34; P = .06) and PD-1 (r = 0.31; P = .08), but no correlation with PVL, consistent with the effects of ART.
Figure 3.
Human immunodeficiency virus (HIV) cell-associated DNA in HIV-infected women. Total cell-associated HIV DNA in CD4+ cells of study participants (HIV+/HSV-2−: n = 14; HIV+/HSV-2+: n = 21) was determined by real-time quantitative polymerase chain reaction. Each sample was assessed in duplicate, and results were normalized to cellular input. Data are presented for each subject, and the line indicates the median. Samples below the limit of quantification were assigned 1.67 copies/million cells. (P = .05, Fisher’s exact test comparing detectable viral loads in the 2 groups).
Lower Interleukin 32 in CD4+ T Cells From Herpes Simplex Virus Type 2–Positive Women
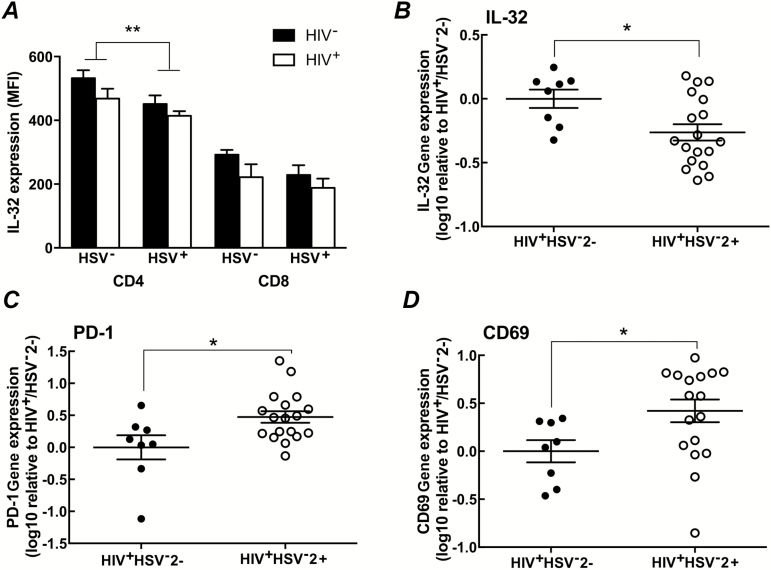
Because of the previous description of IL-32 as inflammatory, we hypothesized that IL-32 levels would be higher in CD4+ T cells isolated from HSV-2+ versus HSV-2− women and examined IL-32 expression by intracellular cytokine staining in the subset of women in whom cells were available. Paradoxically, we found that IL-32 expression was lower in CD4+ T cells in HSV-2+ versus HSV-2− women (P = .007), but not in CD8+ T cells (P = .13) (Figure 4A). Note that the overall frequency of IL-32+ CD4+ T cells was high in all groups, ranging from 62.2% ± 5.11% in HIV+/HSV-2+ women to 76.2% ± 6.53% in HIV-/HSV-2− women. Interleukin 32 gene expression was also reduced in the CD4+ T cells when measured by RT-PCR. In contrast, there was a significant increase in PD-1 and CD69 gene expression in HIV+/HSV-2+ women (n = 18) relative to HIV+/HSV-2− women (n = 8; all P < .05) (Figure 4B).
Figure 4.
Herpes simplex virus type 2 (HSV-2) infection was associated with a decrease in interleukin 32 (IL-32) expression. A, Interleukin 32 expression was assessed by intracellular cytokine staining in CD4+ and CD8+ T cells isolated from women in all 4 groups (HIV−/HSV-2−: n = 9; HIV−/HSV-2+: n = 8; HIV+/HSV-2−: n = 9; HIV+/HSV-2+: n = 14) and compared using a 2-way analysis of variance. Results are presented as mean fluorescence intensity (MFI) + SEM; ** P = .007 comparing HSV-2+ versus HSV-2− subjects. B–D, RNA was extracted from CD4+ T cells, converted to complementary DNA, and analyzed by real-time quantitative polymerase chain reaction for IL-32, PD-1, and CD69 expression. Each symbol represents the gene expression of an individual subject relative to the HIV+/HSV-2− mean, and the line indicates the mean for the group; *P < .05, by unpaired t test, comparing HIV+/HSV-2+ versus HIV+/HSV-2−.
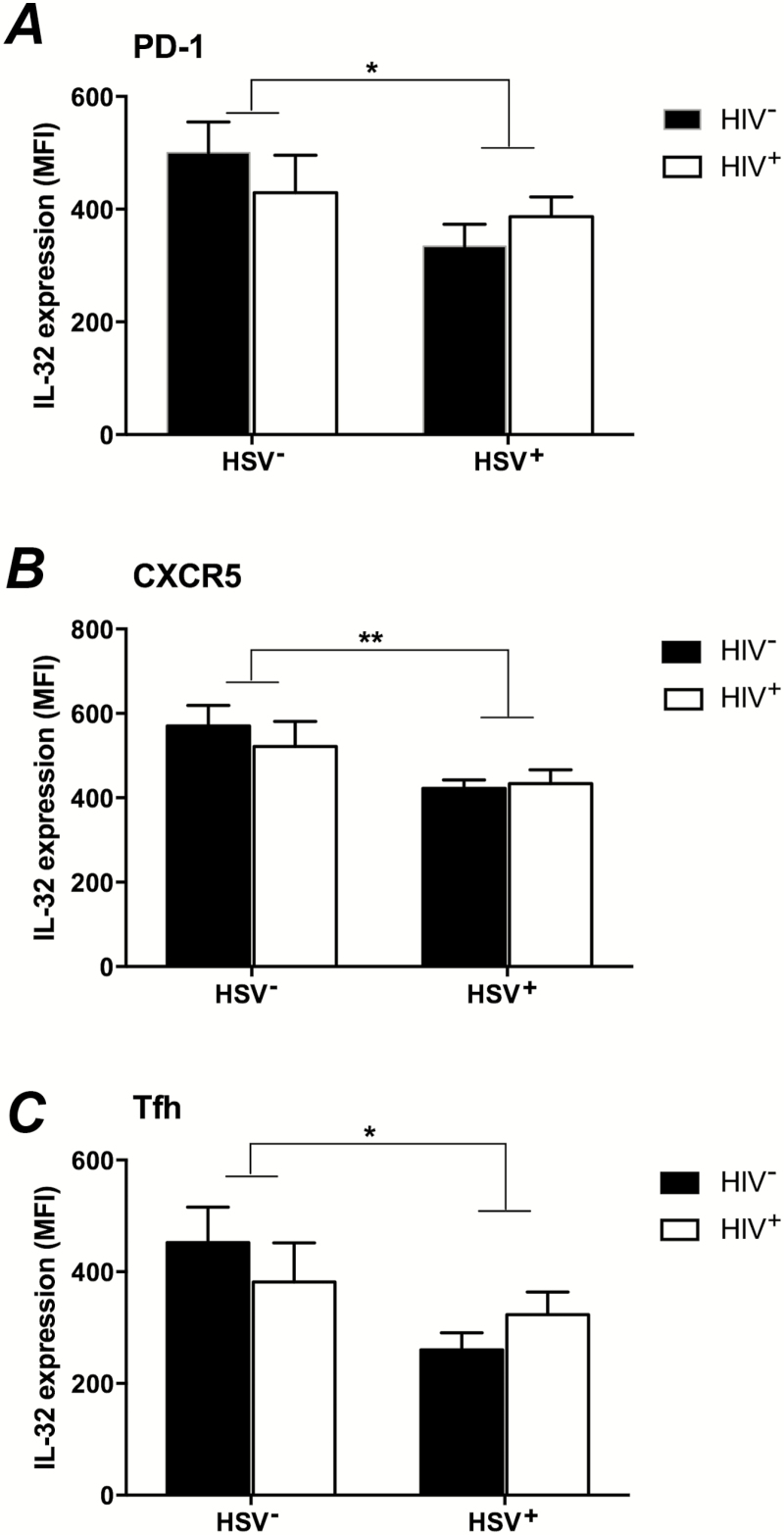
We examined which CD4+ T cell subsets had reduced levels of IL-32 and found decreased IL-32 expression in PD-1+ T cells (P = .046) (Figure 5A), which prompted us to determine whether the affected cells were T follicular helper (Tfh) cells (CXCR5+, PD-1+) (Figure 5B) (representative histograms, see Supplementary Figure 1). Recent studies have shown that Tfh cells are increased in HIV-infected patients and may constitute the largest viral reservoir, particularly in the lymph node and peripheral blood [35]. We found an increase in the frequency of Tfh in the peripheral blood of HIV+ versus HIV− women (P = .02), but HSV-2 infection did not change the frequency of Tfh (not shown). However, HSV-2 was associated with decreased IL-32 expression in CXCR5+CD4+ cells (P = .013) (Figure 5B) and among Tfh cells (P = .03) (Figure 5C).
Figure 5.
Interleukin 32 (IL-32) expression in CD4+ T-cell subsets. Interleukin 32 (IL-32) expression was assessed by intracellular cytokine staining in PD-1+ (A), CXCR5+ (B), and T follicular helper cells (Tfh; identified as CD4+PD-1+CXCR5+) (C) T cells in women in the 4 study groups. (HIV−/HSV-2−: n = 9; HIV−/HSV-2+: n = 8; HIV+/HSV-2−: n = 9; HIV+/HSV-2+: n = 14). The mean fluorescence intensity (MFI) in HSV-2+ versus HSV-2− women was compared by 2-way analysis of variance. (*PD-1: P = .046; **CXCR5: P = .009; *Tfh: P = .03).
Interleukin 32 Blocked Human Immunodeficiency Virus Reactivation
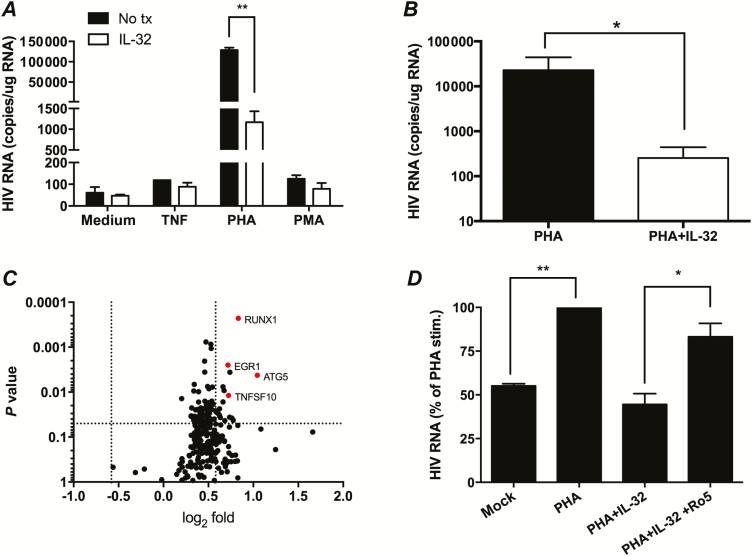
We then studied the effects of IL-32 on HIV reactivation. We cultured CD4+ T cells (approximately 1–2 million) from HIV+ women (PVL <40 copies/mL) with inducers of HIV reactivation in the absence or presence of rIL-32γ. Human immunodeficiency virus reactivation was observed in response to PHA and/or TNF in 6 of 24 women, but the addition of rIL-32γ consistently reduced HIV RNA levels. Representative results from 1 HIV+/HSV-2+ woman and average IL-32-driven reduction after PHA stimulation for the 6 women are shown (Figure 6A and 6B). The IL-32–mediated reduction in HIV reactivation was also reflected in decreased levels of HIV p24 in culture supernatants (data not shown).
Figure 6.
Effects of interleukin 32 (IL-32) on human immunodeficiency virus (HIV) reactivation, RUNX1 gene expression, and blockade of HIV reactivation by addition of RUNX1 inhibitor. A, CD4+ T cells from a representative HIV+/herpes simplex virus type 2–positive (HSV-2+) subject were exposed to medium or medium supplemented with tumor necrosis factor (TNF; 100 U/mL), phytohemagglutinin (PHA) (5 µg/mL), or phorbol 12-myristate 13-acetate (PMA) (2nM) for 24 hours in the absence or presence of recombinant human IL-32γ (100 ng/mL). Cells were washed, and fresh medium with or without IL-32γ was added. Seven days after stimulation, HIV cell-associated RNA was quantified; results are presented as copies per microgram RNA and are means + SEM of duplicate wells (**P = .002, t test). B, Average reduction in HIV RNA transcription following PHA stimulation and IL-32 treatment (n = 6; P = .03, paired t test). C, CD4+ T cells were cultured with PHA or PHA plus recombinant IL-32γ. After 24 hours, RNA was prepared from the cells, and gene expression was analyzed by nanostring. The individual symbols show the 238 genes with detectable signals after filtering as the log2 fold change (PHA+rIL-32γγ/PHA) versus P value (by paired t test). The dotted lines represent 1.5-fold change in gene expression and a P value of .05. The red symbols indicate top genes that were differentially expressed. D, CD4+ T cells from 2 additional women were treated with medium, medium supplemented with PHA, PHA plus recombinant IL-32γ, or PHA plus recombinant IL-32γ, and (after removal of PHA) recombinant IL-32γ plus Ro5-3335 (5 μM). Results are presented relative to HIV RNA detected in PHA-treated cells 7 days after stimulation (*P < .05 and **P < .01, analysis of variance).
Impact of Interleukin 32 on Gene Expression and Role of RUNX1
The concentrations of IFNγ and other cytokines increased following treatment of cells with PHA (relative to mock-treated cells), but there were no significant differences in cytokines (Supplementary Figure 2) or in gene expression of IFN-stimulated genes OAS1, MX1, MX2, IFI44, and IFIT or CD69 when cells were cultured with PHA in the presence of rIL-32γ (data not shown). These findings indicate that, in contrast with what was reported for U1 cells [30], IFNγ does not mediate the IL-32 blockade of HIV reactivation in CD4+ T cells. To identify potential mechanisms contributing to the inhibitory effects of IL-32, we analyzed gene expression in cells from HIV+/HSV-2+ women that were activated with PHA for 24 hours in the presence or absence of rIL-32 by nanostring in a paired comparison (n = 3 subjects) (Figure 6C). Among the 238 genes for which a detectable signal was found, we identified significant changes (P < .05 by paired t test with fold-change of at least 1.5) in 18 genes (Supplementary Table 2) including EGR1, a transcriptional regulator; ATG5, which is involved in autophagy; TNFSF10, which can trigger caspases and the MAPK8/JNK pathway; CHUK, an inhibitor of NF-κB, and RUNX1, a transcriptional regulator. After Bonferroni correction, RUNX1 was significantly increased 3.43-fold (P = .0002 by paired t test). RUNX1 has been reported to repress HIV transcription by binding DNA sequences within the HIV-1 LTR [36]. To verify the role of RUNX1 in response to IL-32, we stimulated CD4+ T cells from 2 additional HIV+/HSV-2+ women with PHA and IL-32 in the absence or presence of Ro5-3335, a small molecule inhibitor of RUNX1. We recapitulated the data presented in Figures 6A and 6B, with IL-32 blocking the PHA-mediated increase in HIV RNA detection, but addition of Ro5-3335 reduced the IL-32–mediated suppression (Figure 6D).
DISCUSSION
Epidemiological studies have consistently identified HSV-2 as a major factor fueling the HIV epidemic. Prior studies have focused primarily on the effects of HSV-2 locally in the skin or mucosa and the impact of preexisting HSV-2 on HIV acquisition. The results presented here add a new dimension to the potential mechanisms contributing to the HIV–HSV-2 syndemic and demonstrate that in women with longstanding HIV (>20 years), who were well controlled on ART, HSV-2 is associated with changes in peripheral blood CD4+ T-cell populations, including decreased expression of IL-32 that may impact HIV reservoirs.
These new findings suggest a model in which HSV-2 reactivation, which is more frequent and may be more sustained in the setting of HIV, might promote the expansion of HIV reservoirs. As suggested by biopsy studies, HSV reactivation triggers a local inflammatory response characterized by the recruitment and activation of immune cells, including CD4+ T cells, which may migrate to the skin or mucosa [14]. We speculate that the increased frequency of HIV coreceptor (CCR5 and CXCR4) and activation marker (CD69 and PD-1) expression by recruited T cells, coupled with a decrease in IL-32 expression, may facilitate HIV replication, leading to expansion of CD4+ T-cell HIV reservoirs.
We initially hypothesized that IL-32, classified as an inflammatory cytokine, would be increased in HSV-2 coinfection, and the paradoxical findings of decreased IL-32 RNA and protein in CD4+ T cells from HSV-2+ women were unanticipated. However, the lower levels may contribute to the transient increase in HIV replication in HIV+/HSV-2+ patients who are on ART. This notion is supported by the observation that the addition of rIL-32γ blocked PHA-induced HIV reactivation in CD4+ T cells isolated from women on ART with well-controlled PVLs. Prior studies implicated an increase in IFNγ as mediating the anti-HIV effect of IL-32 [30], but we did not detect differences in IFNγ (protein or RNA) when CD4 T cells were treated with PHA compared with cells treated with the combination of PHA and rIL-32γ. Instead, we observed increased expression of several transcriptional regulators (RUNX1, EGR1, and CHUK) and genes involved in autophagy and apoptosis (ATG5 and TNFSF10) when CD4+ T cells were activated with PHA in the presence compared with the absence of rIL-32γ.
We focused on RUNX1 because, in addition to being differentially expressed, the HIV LTR contains several RUNX1 binding sites. Overexpression of RUNX1 blocks HIV transcription, and pharmacologic blockade of RUNX1 synergized with the histone deacetylase inhibitor SAHA to activate HIV transcription [36]. Consistent with these findings, our studies demonstrate that addition of the RUNX1 inhibitor, Ro5-3335, partially abrogated the IL-32 blockade of HIV transcription. These results suggest that IL-32 blocks HIV reactivation, at least in part, by increasing RUNX1 expression. Other mechanisms, such as the increase in CHUK, an inhibitor of NF-κB, may also contribute to the blockade of HIV reactivation.
This is the first study to identify a link between HSV, changes in the peripheral immune compartment, and IL-32 expression. It was recently shown that IL-32 is upregulated following EBV infection and plays a role in latency by trapping protein kinase Cδ in the cytoplasm of EBV-infected B cells [37]. In contrast with EBV, which targets B cells, HSV does not productively infect or establish latency in T cells but has been shown to interfere with T-cell function through several immune evasion strategies. Possibly the downregulation of IL-32 in CD4 T cells by HSV-2 interferes with effective T-cell killing, providing another immune evasion strategy that could facilitate HSV persistence.
Human immunodeficiency virus eradication strategies are being designed to induce viral transcription (“shock”), which would render cells susceptible to “kill” by antivirals or cytolytic T cells. Consistent latency reversal, however, has been difficult to achieve. The findings here would suggest that IL-32 blockade and/or RUNX1 inhibition may synergize with strategies to reactivate HIV, although the overall immunological impact of targeting these pathways requires further study.
There are several limitations to our studies, including the use of total CD4+ T cells for HIV reactivation studies and the fact that we did not have access to large volumes of blood to perform viral outgrowth assays to accurately measure the latent reservoir. However, cell-associated HIV DNA correlates well with viral outgrowth assay [38]. We do not know the local concentration of IL-32 that latently infected cells may be exposed to in vivo and recognize that the concentration of rIL-32 added may be supraphysiologic. However, this approach enabled us to identify transcriptional regulators that contribute to the maintenance of HIV reservoirs. We also could not fully address potential confounders, including other persistent infections such as CMV, as too few of the HIIV+/HSV-2+ women were CMV-seronegative. Longitudinal studies with larger cohorts and greater cell numbers are needed to more precisely define how HSV-2 alters the phenotype of the CD4+ cells and the potential HIV reservoir and to identify how IL-32 impacts HIV reactivation.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We thank Jenny Stanwix for assistance with recruitment and John Nico, Brian Starkman, and Jamie Freiermuth for technical assistance.
Financial support. This work was supported by the National Institutes of Health (NIH: grants U01AI035004 and R01AI065309) and a Department of Pediatrics Career Development Award, Albert Einstein College of Medicine (Pedro Mesquita). This work was also supported by NIH/National Center for Advancing Translational Science (NCATS) Einstein-Montefiore CTSA Grant Number UL1TR00193 and at Yale: CTSA grant UL1TR000142. Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID) with additional cofunding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NDA), and the National Institute on Mental Health (NIMH). Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute on Alcohol Abuse and Alcoholism (NIAAAA), the National Institute on Deafness and other Communication Disorders (NDCD), and the NIH Office of Research on Women’s Health. WIHS data collection is also supported by UL1-TR000004 (UCSF CTSA) and UL1-TR000454 (Atlanta CTSA).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Abu-Raddad LJ, Magaret AS, Celum C, et al. Genital herpes has played a more important role than any other sexually transmitted infection in driving HIV prevalence in Africa. PLoS One 2008; 3:e2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Corey L. Synergistic copathogens–HIV-1 and HSV-2. N Engl J Med 2007; 356:854–6. [DOI] [PubMed] [Google Scholar]
- 3. Drake AL, John-Stewart GC, Wald A, et al. Herpes simplex virus type 2 and risk of intrapartum human immunodeficiency virus transmission. Obstet Gynecol 2007; 109:403–9. [DOI] [PubMed] [Google Scholar]
- 4. Schacker T, Zeh J, Hu H, Shaughnessy M, Corey L. Changes in plasma human immunodeficiency virus type 1 RNA associated with herpes simplex virus reactivation and suppression. J Infect Dis 2002; 186:1718–25. [DOI] [PubMed] [Google Scholar]
- 5. Gray RH, Li X, Wawer MJ, et al. Determinants of HIV-1 load in subjects with early and later HIV infections, in a general-population cohort of Rakai, Uganda. J Infect Dis 2004; 189:1209–15. [DOI] [PubMed] [Google Scholar]
- 6. Mole L, Ripich S, Margolis D, Holodniy M. The impact of active herpes simplex virus infection on human immunodeficiency virus load. J Infect Dis 1997; 176:766–70. [DOI] [PubMed] [Google Scholar]
- 7. Chen L, Jha P, Stirling B, et al. Sexual risk factors for HIV infection in early and advanced HIV epidemics in sub-Saharan Africa: systematic overview of 68 epidemiological studies. PLoS One 2007; 2:e1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS 2006; 20:73–83. [DOI] [PubMed] [Google Scholar]
- 9. Looker KJ, Magaret AS, Turner KM, Vickerman P, Gottlieb SL, Newman LM. Global estimates of prevalent and incident herpes simplex virus type 2 infections in 2012. PLoS One 2015; 10:e114989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mostad SB, Kreiss JK, Ryncarz AJ, et al. Cervical shedding of herpes simplex virus in human immunodeficiency virus-infected women: effects of hormonal contraception, pregnancy, and vitamin A deficiency. J Infect Dis 2000; 181:58–63. [DOI] [PubMed] [Google Scholar]
- 11. Posavad CM, Wald A, Kuntz S, et al. Frequent reactivation of herpes simplex virus among HIV-1-infected patients treated with highly active antiretroviral therapy. J Infect Dis 2004; 190:693–6. [DOI] [PubMed] [Google Scholar]
- 12. Mark KE, Wald A, Magaret AS, et al. Rapidly cleared episodes of herpes simplex virus reactivation in immunocompetent adults. J Infect Dis 2008; 198:1141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schiffer JT, Corey L. Rapid host immune response and viral dynamics in herpes simplex virus-2 infection. Nat Med 2013; 19:280–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhu J, Hladik F, Woodward A, et al. Persistence of HIV-1 receptor-positive cells after HSV-2 reactivation is a potential mechanism for increased HIV-1 acquisition. Nat Med 2009; 15:886–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Peng T, Zhu J, Phasouk K, Koelle DM, Wald A, Corey L. An effector phenotype of CD8+ T cells at the junction epithelium during clinical quiescence of herpes simplex virus 2 infection. J Virol 2012; 86:10587–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Duffus WA, Mermin J, Bunnell R, et al. Chronic herpes simplex virus type-2 infection and HIV viral load. Int J STD AIDS 2005; 16:733–5. [DOI] [PubMed] [Google Scholar]
- 17. Serwadda D, Gray RH, Sewankambo NK, et al. Human immunodeficiency virus acquisition associated with genital ulcer disease and herpes simplex virus type 2 infection: a nested case-control study in Rakai, Uganda. J Infect Dis 2003; 188:1492–7. [DOI] [PubMed] [Google Scholar]
- 18. Stelekati E, Wherry EJ. Chronic bystander infections and immunity to unrelated antigens. Cell Host Microbe 2012; 12:458–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jamieson AM, Pasman L, Yu S, et al. Role of tissue protection in lethal respiratory viral-bacterial coinfection. Science 2013; 340:1230–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Long BR, Erickson AE, Chapman JM, et al. Increased number and function of natural killer cells in human immunodeficiency virus 1-positive subjects co-infected with herpes simplex virus 2. Immunology 2010; 129:186–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sheth PM, Sunderji S, Shin LY, et al. Coinfection with herpes simplex virus type 2 is associated with reduced HIV-specific T cell responses and systemic immune activation. J Infect Dis 2008; 197:1394–401. [DOI] [PubMed] [Google Scholar]
- 22. Shannon B, Yi TJ, Thomas-Pavanel J, et al. Impact of asymptomatic herpes simplex virus type 2 infection on mucosal homing and immune cell subsets in the blood and female genital tract. J Immunol 2014; 192:5074–82. [DOI] [PubMed] [Google Scholar]
- 23. Tan DH, Raboud JM, Kaul R, et al. Herpes simplex virus type 2 coinfection does not accelerate CD4 count decline in untreated HIV infection. Clin Infect Dis 2013; 57:448–57. [DOI] [PubMed] [Google Scholar]
- 24. Tan DH, Raboud JM, Szadkowski L, et al. Herpes simplex virus type 2 serostatus is not associated with inflammatory or metabolic markers in antiretroviral therapy-treated HIV. AIDS Res Hum Retroviruses 2015; 31:276–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Barbour JD, Sauer MM, Sharp ER, et al. HIV-1/HSV-2 co-infected adults in early HIV-1 infection have elevated CD4+ T cell counts. PLoS One 2007; 2:e1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Joosten LA, Heinhuis B, Netea MG, Dinarello CA. Novel insights into the biology of interleukin-32. Cell Mol Life Sci 2013; 70:3883–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kang JW, Park YS, Lee DH, et al. Intracellular interaction of interleukin (IL)-32alpha with protein kinase Cepsilon (PKCepsilon) and STAT3 protein augments IL-6 production in THP-1 promonocytic cells. J Biol Chem 2012; 287:35556–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim SH, Han SY, Azam T, Yoon DY, Dinarello CA. Interleukin-32: a cytokine and inducer of TNFalpha. Immunity 2005; 22:131–42. [DOI] [PubMed] [Google Scholar]
- 29. Smith AJ, Toledo CM, Wietgrefe SW, et al. The immunosuppressive role of IL-32 in lymphatic tissue during HIV-1 infection. J Immunol 2011; 186:6576–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nold MF, Nold-Petry CA, Pott GB, et al. Endogenous IL-32 controls cytokine and HIV-1 production. J Immunol 2008; 181:557–65. [DOI] [PubMed] [Google Scholar]
- 31. Folks TM, Justement J, Kinter A, Dinarello CA, Fauci AS. Cytokine-induced expression of HIV-1 in a chronically infected promonocyte cell line. Science 1987; 238:800–2. [DOI] [PubMed] [Google Scholar]
- 32. Greenhead P, Hayes P, Watts PS, Laing KG, Griffin GE, Shattock RJ. Parameters of human immunodeficiency virus infection of human cervical tissue and inhibition by vaginal virucides. J Virol 2000; 74:5577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 2008; 3:1101–8. [DOI] [PubMed] [Google Scholar]
- 34. Tooley JE, Vudattu N, Choi J, et al. Changes in T-cell subsets identify responders to FcR-nonbinding anti-CD3 mAb (teplizumab) in patients with type 1 diabetes. Eur J Immunol 2016; 46:230–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Banga R, Procopio FA, Noto A, et al. PD-1(+) and follicular helper T cells are responsible for persistent HIV-1 transcription in treated aviremic individuals. Nat Med 2016; 22:754–61. [DOI] [PubMed] [Google Scholar]
- 36. Klase Z, Yedavalli VS, Houzet L, et al. Activation of HIV-1 from latent infection via synergy of RUNX1 inhibitor Ro5-3335 and SAHA. PLoS Pathog 2014; 10:e1003997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lai KY, Chou YC, Lin JH, et al. Maintenance of Epstein-Barr virus latent status by a novel mechanism, latent membrane protein 1-induced interleukin-32, via the protein kinase cδ pathway. J Virol 2015; 89:5968–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Eriksson S, Graf EH, Dahl V, et al. Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLoS Pathog 2013; 9:e1003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.