Encroachment of humans into pristine habitats is a major threat to biodiversity. We found that some paleotropical bat species from disturbed forests suffered from chronic stress and an impaired immune system, suggesting that allostatic overload may play a pivotal role in the extinction of wildlife species.
Keywords: Anthropogenic disturbance, bats, body mass, chronic stress, fragmentation, white blood cell count
Abstract
Anthropogenic habitat disturbance is a major threat to biodiversity worldwide. Yet, before population declines are detectable, individuals may suffer from chronic stress and impaired immunity in disturbed habitats, making them more susceptible to pathogens and adverse weather conditions. Here, we tested in a paleotropical forest with ongoing logging and fragmentation, whether habitat disturbance influences the body mass and immunity of bats. We measured and compared body mass, chronic stress (indicated by neutrophil to lymphocyte ratios) and the number of circulating immune cells between several bat species with different roost types living in recovering areas, actively logged forests, and fragmented forests in Sabah, Malaysia. In a cave-roosting species, chronic stress levels were higher in individuals from fragmented habitats compared with conspecifics from actively logged areas. Foliage-roosting species showed a reduced body mass and decrease in total white blood cell counts in actively logged areas and fragmented forests compared with conspecifics living in recovering habitats. Our study highlights that habitat disturbance may have species-specific effects on chronic stress and immunity in bats that are potentially related to the roost type. We identified foliage-roosting species as particularly sensitive to forest habitat deterioration. These species may face a heightened extinction risk in the near future if anthropogenic habitat alterations continue.
Introduction
Habitat degradation, loss and fragmentation are widespread causes of biodiversity loss worldwide (Barnosky et al., 2011; Gibson et al., 2011; Laurance et al., 2014). Considering the pace at which habitats are altered by humans, it is essential to understand how anthropogenic land-use changes affect wildlife species and whether this impact could as well influence human and animal health (Ellis et al., 2012). Encroachment of humans into formerly pristine habitats often leads to reduced wildlife populations and local extinction of species (Newbold et al., 2014; Haddad et al., 2015; Meyer et al., 2016). However, human disturbances also affect wildlife at the individual level (Ellis et al., 2012). Focusing on individual physiology may help us to estimate the consequences of environmental stressors on wildlife before the effects begin to manifest in decreased population sizes (Davis et al., 2008).
Indeed, previous studies have observed chronic stress and an impaired body condition in various wildlife species from disturbed habitats, suggesting that anthropogenic perturbations may become a major stressor for wildlife species (reviewed by Romero, 2004; Ellis et al., 2012). This is because habitat fragmentation may force individuals of mobile taxa to use more than one patch for foraging. By using multiple patches, individuals are thus exposed to a higher predation risk and have to spend more energy and time to meet their food requirement (Hinsley, 2000). Additionally, the quality of the habitat may decline, exacerbating the limitation of food resources (Ellis et al., 2012). Further, habitat deterioration and fragmentation may result in the loss of shelter, and also an increased exposure to abiotic factors such as wind and rain, especially at habitat edges (Ellis et al., 2012).
Although chronic stress caused by natural stressors (e.g. predation pressure) can be adaptive in wildlife populations (Boonstra, 2013), it also has the potential to lead to an impaired body condition and to permanently disrupt the homeostasis of animals (Romero, 2002, 2004; Wingfield, 2005; Wikelski and Cooke, 2006; Romero and Wikelski, 2010; Dickens and Romero, 2013). For example, chronically elevated plasma levels of circulating glucocorticoid hormones may suppress the immune system of animals, decrease their survival, their growth and reproductive function (Dhabhar et al., 1996; Wingfield et al., 1997; Buchanan, 2000; Sapolsky et al., 2000). Thus, animals that are not resilient to anthropogenic perturbations may suffer from chronic stress and, as a consequence, from impaired immunity (Suorsa et al., 2004). For example, Allen et al. (2009) found that bats roosting at human-made bridges experienced physiological stress, which resulted in reduced immune system functioning. An impaired immune system may then result in increased susceptibility, shedding of pathogens and decreased survival rates (Demas et al., 2011).
Bats (Chiroptera) are a promising taxon to study the effects of forest degradation, habitat loss and fragmentation on body condition, chronic stress and immunity, since bats are known to be highly sensitive to the effects of environmental change (Jones et al., 2009; Struebig et al., 2011; Meyer, et al., 2016). Furthermore, more than 100 different viruses have been associated with Chiroptera, including several particularly virulent ones, such as rabies, Severe Acute Respiratory Syndrome (SARS)-like coronavirus, Nipah, Hendra, Ebola and Marburg (Chua et al., 2000; Leroy et al., 2005; Li et al., 2005; Towner et al., 2009; Wibbelt et al., 2010; Wang et al., 2011; Baker et al., 2013; Brook and Dobson, 2015). Bats harbor on average the highest number of viruses than any other taxa per species, probably due to their extraordinary longevity and high levels of interspecific viral transmission (Luis et al., 2013). Surprisingly, bats often do not show clinical signs of viral infections (Wibbelt et al., 2010; Epstein and Field, 2016). Despite their potential role as viral reservoirs, there is almost no information on the immune function of bat species, and an understanding of the ecological and social factors influencing the intra- and interspecific immune differences between taxa is lacking (but see Allen et al., 2009; Schneeberger et al., 2013a).
In our study, we assessed the impact of anthropogenic disturbances on body mass in eight, and on immunological parameters in three forest bat species in Malaysian Borneo, each of which utilize the cluttered forest environment for foraging. In contrast to bat species utilizing forest edges, these forest specialists might suffer from fragmentation in particular because of decreased availability of potential prey insects and roost sites in the forest interior. Other studies in the Paleo- and Neotropics have already shown that feeding activity and abundance of some forest bat species is lower in smaller compared with larger fragments or continuous forests (Meyer et al., 2008; Estrada-Villegas et al., 2010; Struebig et al., 2011). Therefore, we predicted that foliage-roosting bats from actively logged areas and fragmented forest exhibit lower body masses, signs of chronic stress, assessed by the neutrophils to lymphocytes (N/L)-ratio (Davis et al., 2008), and impaired immunity, measured as total white blood cell counts, compared with bats of recovering forest. In cave-roosting bats, we predicted smaller effects due to their substantial larger home range sizes and more permanent roosting sites compared with foliage-roosting species, which may enable cave-roosting bats to cover wider, disturbed and intact areas by flying longer distances from respective roosts to their foraging site (Struebig et al., 2013).
Methods
Study site and species
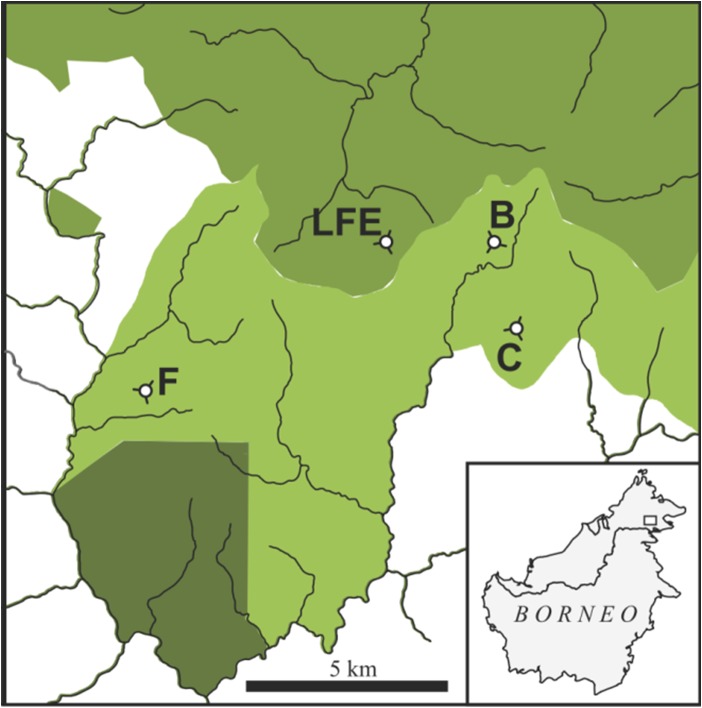
The study was conducted within the SAFE project (Stability of Altered Forest Ecosystems, www.safeproject.net), a 7200 ha landscape fragmentation experiment established in Sabah, Borneo. The SAFE landscape comprises logged over dipterocarp rainforest, some of which is being converted to oil palm plantation, leaving behind a network of disturbed forest fragments. Thus, the landscape represents a land-use transition common across much of Southeast Asia (Fitzherbert et al., 2008; Gaveau et al., 2014; Marlier et al., 2015). All sample locations were situated within 10 km of a research camp at N4.73 E117.60 (Fig. 1). Much of the landscape had been logged twice prior to our study and the coupes allocated for conversion to oil palm had been heavily logged multiple times before (Struebig et al., 2013). At the time of sampling, these areas were experiencing a final harvest prior to conversion and were at the early stages of fragmentation, with large areas devoid of any tree cover, ultimately resulting in ten isolated plots (e.g. B, C, F and LFE). We sampled bats multiple times at various sites across this disturbance gradient during the conversion process: in plot B, C, F and LFE. These plots were located 2–10 km apart from each other, exceeding the mean home range size of foliage-roosting insectivorous bat species (Struebig et al., 2013). LFE is a twice-logged site at which logging operations ceased in the late 1990s leaving time for substantial recovery of a tall dipterocarp forest; and sites B, C and F had experienced additionally logging prior to the onset of the study, and then experienced heavy logging and fragmentation throughout the 2-year study period. We categorized these sites into three disturbance levels according to human logging activity at the time of sampling: (1) Relatively undisturbed (only presence of SAFE-affiliated researchers with minimal impact), hereafter ‘Recovering forest’: LFE, B1 and C1, whereby subscript denotes the order of sampling. (2) Sites that were heavily logged at the time of sampling were categorized as ‘Actively logged forest’: B2, C2 and F1. After logging was completed and thus sites relatively undisturbed, site F (i.e. F2) was categorized as ‘Fragmented’ (for sites B and C logging was still underway at the end of sampling). Data collection took place between July and September in 2014 and 2015, with additional sampling period between March and April in 2015 (Table 1).
Figure 1:
Location of forest sites sampled for bats at the Stability of Altered Forest Ecosystems Project in Sabah, Borneo. Prior to sampling all sites had been logged multiple times, with sites B, C and F (light shading) experiencing much higher extraction rates than site LFE (intermediate shading), and old growth forest in the southwest (dark shading). Sites were sampled for bats multiple times during the final round of logging and subsequent fragmentation of sites B, C and F. Site LFE remained as a control site throughout the study with no further logging or fragmentation activity at or around this locality. The oil palm plantation matrix is shown in white and lines indicate rivers.
Table 1:
Study sites, habitat type and sampling year
| Recovering forest | Actively logged forest | Fragmented forest | |
|---|---|---|---|
| LFE | 2014, 2015 | – | – |
| B | 2014, 2015 | 2015 | – |
| C | 2014, 2015 | 2015 | – |
| F | – | 2014, 2015 | 2015 |
The landscape has a well characterized insectivorous bat fauna, which is known to have experienced a substantial shift in assemblage composition in response to past logging (Struebig et al., 2013). We recorded body measurements of all adult individuals captured, but selected study species from the families Vespertilionidae (subfamily: Kerivoulinae), Hipposideridae and Rhinolophidae, which were sufficiently abundant across the landscape to warrant sufficient sample size. Within the subfamily Kerivoulinae (woolly bats), we studied the following congeneric bats: Kervivoula intermedia, K. papillosa and K. hardwickii. Within the family Hipposideridae (leaf-nosed bats), we focused on the congeneric species Hipposideros cervinus and H. dyacorum, and within the family of Rhinolophidae (horseshoe bats) on Rhinolophus sedulus, R. trifoliatus and R. borneensis. All species of the subfamily Kerivoulinae are thought to roost solitarily in foliage, except for K. papillosa which roosts in tree cavities living in dynamic fission-fusion societies (Payne et al., 1985; Kingston et al., 2006). Hipposideros cervinus, H. dyacorum and R. borneensis form colonies roosting in caves or cave-like structures, whereas R. sedulus and R. trifoliatus are solitary or monogamous, roosting in trees (Payne et al., 1985; Kingston et al., 2006). All species are small, insectivorous bats with body masses ranging between 3 and 16 g (Payne et al., 1985). In 2011/2012, prior to our sampling, bat abundance for our study species was moderate to high in all sites, but richness was lower in the repeatedly logged sites (B, C, F) compared to our recovering twice logged site (LFE, Table 2, Struebig et al., 2013).
Table 2:
Bat capture rates (individuals/harp trap night) at sampling sites prior to the study in 2011/2012 (Struebig et al., 2013) and this study period (2014/2015)
| Prior to conversion (2011/2012) | Recovering forest (2014/2015) | Actively logged forest (2014/2015) | Fragmented forest (2015) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Species/plot | B | C | F | LFE | B | C | LFE | B | C | F | F |
| Hipposideros cervinus | 0.74 | 0.88 | 0.86 | 0.45 | 0.22 | 0.68 | 0.23 | 0.19 | 0.47 | 0.1 | 0.2 |
| Hipposideros dyacorum | 0 | 0.02 | 0.76 | 0.02 | 0 | 0.08 | 0.04 | 0.02 | 0 | 0.25 | 0.43 |
| Kerivoula hardwickii | 0.40 | 0.46 | 0.26 | 0.31 | 0.5 | 0.42 | 0.35 | 0.12 | 0.11 | 0.15 | 0.17 |
| Kerivoula intermedia | 0.71 | 0.29 | 0.07 | 0.26 | 0.77 | 0.45 | 0.54 | 0.31 | 0.19 | 0.3 | 0.36 |
| Kerivoula papillosa | 0.17 | 0 | 0.12 | 0.07 | 0.02 | 0 | 0.07 | 0.02 | 0 | 0.03 | 0.04 |
| Rhinolophus borneensis | 0.12 | 0.24 | 0.17 | 0.10 | 0.02 | 0.1 | 0.04 | 0.05 | 0.06 | 0.05 | 0.16 |
| Rhinolophus sedulus | 0.33 | 0.17 | 0.05 | 0.29 | 0.04 | 0.05 | 0.11 | 0.05 | 0.06 | 0.03 | 0 |
| Rhinolophus trifoliatus | 0.29 | 0.43 | 0.33 | 0.14 | 0.25 | 0.25 | 0.09 | 0.19 | 0.06 | 0.25 | 0.25 |
Bat capture
In the morning hours after sunrise, we set up six harp traps (Museum Zoologicum Bogoriense, Bogor, Indonesia) along established trails in the forest, with a minimum distance of 30–100 m. Harp traps are an established sampling method for capturing forest bats and are particularly effective in the paleotropics (Kingston et al., 2003; Kunz et al., 2009). Between subsequent nights, we moved traps to new positions, resulting in a total of 15–20 positions per site and survey. The total sampling effort over the study period was 321 harp trap nights.
We checked traps at 1900 and at 0700 the following day. Bats were retrieved from harp traps and transported back to the camp in individual cloth bags for processing, with the exception of fruit bats (Pteropodidae), individuals of H. cervinus (known to be highly sensitive towards handling), juveniles, pregnant or lactating females of all species. These individuals were instead processed on site and released as soon as possible at the point of capture, typically within 2 h. Pregnant females were excluded from analyses of body mass because the fetus influences body mass to an unknown extent. We identified species according to Kingston et al. (2006) and Struebig and Sujarno (2006). Juveniles were distinguished from adults by the epiphyseal closure of phalanges (Kunz and Anthony, 1982). We classified the reproductive status of females (non-reproductive, pregnant, lactating or post-lactating) by abdominal palpation and visual inspection of the teats and surrounding area.
We recorded body mass (g) by using a spring balance (Pesola balance, Switzerland, accuracy = 0.25 g) and length of forearm (mm) using a caliper (Wiha Werkzeuge GmbH, Schonach, Germany). Further, we noted if the bat had consumed a large visible amount of prey based on visual inspection of the torso. We marked all adult bats with a uniquely coded forearm band of 2.9 or 4.2 mm, depending on size of the bat (Porzana Limited, East Sussex, UK) as described in Kunz and Weise (2009). Blood samples were collected only from the rhinolophid species as these taxa were larger than Kerivoulinae and Hipposideridae, and thus sufficient blood could be collected without undue harm to the individual. From rhinolophid species we collected a small quantity of whole blood (max. 140 µl, <1 % of the body mass) in a heparinized capillary (Paul Marienfeld GmbH & Co.KG, Lauda-Koenigshofen, Germany) by puncturing the antebrachial vein as described in Voigt and Cruz-Neto (2009). For venipuncture we used sterile needles (Henry Schein Inc., Melville, New York, USA). The blood sample was transferred with a micropipetter (Hirschmann Laborgeräte GmbH & Co.KG, Eberstadt, Germany) to a tube, and a subsample of 3 µl was used to produce a blood smear on glass slides (Carl Roth GmbH & Co.KG, Karlsruhe, Germany). All bats were released at the capture site within 12 h. Although we took measurements for all adult bats captured, only data of the eight focus species are used in analyses as justified above.
Ethics statement
Our study and export of samples was authorized by the scientific committee of the Sabah Biodiversity Center, Sabah, Malaysia (JKM/MBS.1000-2/2 (317); JKM/MBS.1000-2/3 JLD.2 (16); JKM/MBS.1000-2/2 JLD.3 (153)), as well as the SAFE project and complies with the laws of Malaysia and Germany.
White blood cell counts
We stained blood smears with May-Gruenwald's solution (#T863.2, Carl Roth GmbH & Co.KG, Karlsruhe, Germany) and Giemsa (#T862.1, Carl Roth GmbH & Co.KG, Karlsruhe, Germany). Blood smears were analyzed with a microscope under oil immersion at a 100× magnification. Some samples were suboptimal due to adverse weather conditions (e.g. high humidity causing mold) and had to be discarded without analyses. Blood smears were analyzed blindly with respect to the identity of individuals and conducted by the same person (A.S.).
Total white blood cell (TWBC) counts were estimated manually by the mean number of leukocytes per visual field using the total count of leukocytes in 10 fields, or the number of fields that was necessary to examine until a single leukocyte was observed (Schneeberger et al., 2013b). For the differential white blood cell (DWBC) counts, we counted 100 (or 50 in few cases) leukocytes and identified the different types of leukocytes by size, color, shape and cytoplasmic contents. From this, we calculated relative numbers (%) of lymphocytes, neutrophils, eosinophils, monocytes and basophils and also the N/L-ratio, which was used as an estimate for chronic stress (Davis et al., 2008).
Statistical analyses
We performed all statistical analyses using the statistical software R version 3.3.1 (R Core Team, 2016). We conducted two-tailed tests (when applicable) and set the level of significance to α = 0.05. Means and standard errors are given in the format X ± SE, unless stated otherwise.
Due to the large sample size of the dataset for body mass (N = 443), we studied the influence of different predictor variables on the body mass using a parametric method—the linear model. We included the following predictor variables in the linear model: the interaction between species (8 levels) and habitat type (3 levels: recovering forest, actively logged forest or fragmented forest), the standardized deviation from the species-specific mean forearm length (continuous: z-score) to control for intraspecific variation in body size, plot identity (4 levels: B, C, F and LFE), reproductive status and sex (3 levels: male, lactating and non-reproducing females), recent feeding status (2 levels: fed, non-fed), season (2 levels: dry season: March-April, characterized by a mean monthly precipitation of 77 mm; beginning of rainy season: July–September, characterized by a mean monthly precipitation of 170 mm, personal communication from Prof. R. Walsh, Swansea University, UK) and year (2014, 2015). The body mass and the forearm length (as z-scores) were log-transformed before the analysis. We did not consider the interaction between species and the forearm length because within our double logarithmic approach the regression coefficient associated to the forearm length corresponds to an allometric coefficient that has been shown to present little variation between bat species (Norberg, 1981). The total number of parameters considered in the linear model was thus 31. As required, the residuals of our model were homoscedastic and normally distributed. We used the function lm readily available in R to fit our linear model, followed by Anova from the package ‘car’ (Fox and Weisberg, 2011) to assess the significance of predictor variables using marginal (type II) F-tests. If a non-continuous predictor had a significant effect on the response variable, we performed post hoc analyses using the function glht from the package ‘multcomp’ (Hothorn et al., 2008), which performs generalized linear hypotheses testing (GLHT). Because we were interested in the effect of roost type on body mass in different habitats, we grouped the species according to their roosting habit in cave- (H. cervinus, H. dyacorum, R. borneensis) and foliage-roosting species (K. hardwickii, K. intermedia, K. papillosa, R. trifoliatus, R. sedulus) prior to performing post hoc analyses.
Our sample size was sufficient to fulfill the recommended minimal amount of data points for our model to avoid overfitting (i.e. 10 × k; e.g. Harrel, 2001 or 50 + 8 × k; Field, 2005 with k = 31). Our linear model should also not suffer from potential problems arising from multi-collinearity (i.e. correlation between the predictors). We tested this possibility by computing generalized variance inflation factors (specifically, the square of the generalized variance inflation factors scaled for the number of degree of freedom, or [GVIF^(1/(2*Df))]^2) according to Fox and Monette (1992). The maximum value obtained was 3.5 which is below the critical threshold of 4–10 usually mentioned in the literature (reviewed by O'Brien, 2007).
As some pairs of bat species are more related to each other than others in our sample, we also fitted the same linear model as a phylogenetic generalized least squares (PGLS) using the R packages ‘ape’, ‘geiger’ and ‘phytools’ (Paradis et al., 2004; Harmon et al., 2008; Revell, 2012) in order to test for the presence of a phylogenetic signal. We considered the Brownian, the Grafen and the Pagel's lambda correlations structure in the PGLS (Felsenstein, 1985; Grafen, 1989; Martins and Hansen, 1997), and based our phylogenetic information on Khan et al. (2010) and Sazali et al. (2011). Because we did not find evidence for a phylogenetic signal, we only present the linear model in this paper for the reason of simplicity.
Due to small sample sizes, we analyzed differences in TWBC counts and in N/L-ratio between habitat types within rhinolophid species using the non-parametric Kruskal–Wallis-test followed by the Dunn's test for post hoc analyses (R package ‘dunn.test’; Dinno, 2015). We used the Bonferroni method to perform multiple-comparison adjustments during the Dunn's test.
Results
In total, we obtained data from 443 adult individuals of the eight target species (see Table 2). Data on body mass were available for all 443 individuals (see Table 3). For rhinolophid species, we obtained differential and TWBC counts for 85 and 82 individual bats, respectively.
Table 3:
Body masses and roosting ecology of study species in each habitat type (values indicate mean ±SE (g) and sample size in parentheses)
| Species | Roosting ecology | Recovering forest | Actively logged forest | Fragmented forest |
|---|---|---|---|---|
| Rhinolophus trifoliatus | Foliage | 13.8 ± 1.6 (26) | 14.0 ± 1.7 (20) | 13.2 ± 1.5 (10) |
| Hipposideros cervinus | Cave | 9.8 ± 1.4 (52) | 10.4 ± 1.1 (29) | 10.3 ± 1.0 (10) |
| Rhinolophus sedulus | Foliage | 10.9 ± 2.6 (9) | 9.4 ± 0.9 (5) | NA |
| Kerivoula papillosa | Foliage | 9.5 ± 1.5 (6) | 11.5 ± 2.1 (2) | 7.5 ± 0.7 (2) |
| Rhinolophus borneensis | Cave | 8.8 ± 1.1 (7) | 9.3 ± 0.7 (7) | 9.1 ± 1.1 (7) |
| Hipposideros dyacorum | Cave | 7 ± 0.4 (5) | 7.3 ± 1.4 (11) | 6.9 ± 0.9 (21) |
| Kerivoula hardwickii | Foliage | 3.7 ± 0.4 (63) | 3.7 ± 0.4 (16) | 3.2 ± 0.2 (9) |
| Kerivoula intermedia | Foliage | 3.4 ± 0.4 (78) | 3.3 ± 0.4 (32) | 3.0 ± 0.3 (19) |
Species are ordered by size (largest to smallest).
Body mass in relation to habitat type
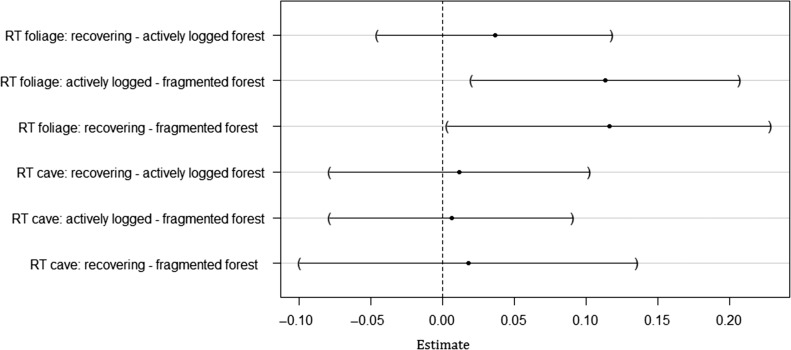
Overall, the linear model for body mass (log) accounted for 97% of the total variation in body mass. Body mass varied significantly depending on the habitat-species combination (Anova, F = 1.86, df1 = 13, df2 = 401, P = 0.033). In cave-roosting bats, there were no significant differences in body mass across habitat types (GLHT, absolute t-values < 0.40, P > 0.99 for all pairwise comparison between habitat types, Fig. 2). In foliage-roosting species, body masses of individual bats were significantly lower in fragmented compared to actively logged forests (GLHT, exp (Estimate) = −1.12 g, t-value = −3.14, P = 0.01) and higher in recovering than in fragmented forest (GLHT, exp (Estimate) = 1.12 g, t-value = 2.67, P = 0.04). Foliage-roosting bats captured in recovering forest were slightly heavier than those captured in actively logged forest, although the difference was not significant (GLHT, exp (Estimate) = 1.04 g, t-value = 1.15, P = 0.70).
Figure 2:
Post hoc pairwise comparisons between predicted body mass (log) for different habitat types within roost type (RT). The figure shows the difference in estimate (dot) and the 95% confidence interval of the difference (line) for each pairwise comparison.
As expected, body mass significantly increased with the z-score of forearm length (log) (Anova, F = 34.35, df1 = 1, df2 = 401, P < 0.001). The location of capture (plot) had a significant effect on body mass (Anova, F = 4.32, df1 = 3, df2 = 401, P = 0.005): In plot B, bats weighed significantly more than in plot LFE (GLHT, Estimate = 0.06, t-value = 3.57, P = 0.002). There were no significant differences in body mass between the other plots (GLHT, all t-values < 2.3, all P > 0.1). Bats that had clearly eaten recently were significantly heavier than bats that did not recently feed before capture (Anova, F = 28.04, df1 = 1, df2 = 401, P < 0.001). Body mass varied with reproductive condition (Anova, F = 5.43, df1 = 2, df2 = 401, P = 0.005): Non-reproducing females weighed significantly less than males (GLHT, Estimate = −0.04, t-value = 3.27, P = 0.003). There were no significant differences in body mass between lactating and non-reproducing females and males, respectively (GLHT, all t-values <0.04, all P > 0.36). In addition, body mass was significantly higher in bats captured during the beginning of the rainy season compared with those being captured during the dry season (Anova, F = 14.42, df1 = 1, df2 = 401, P < 0.001). We did not detect any annual fluctuations in body mass (Anova, F = < 0.01, df1 = 1, df2 = 401, P = 0.99).
Stress and immunological measures in relation to forest categories
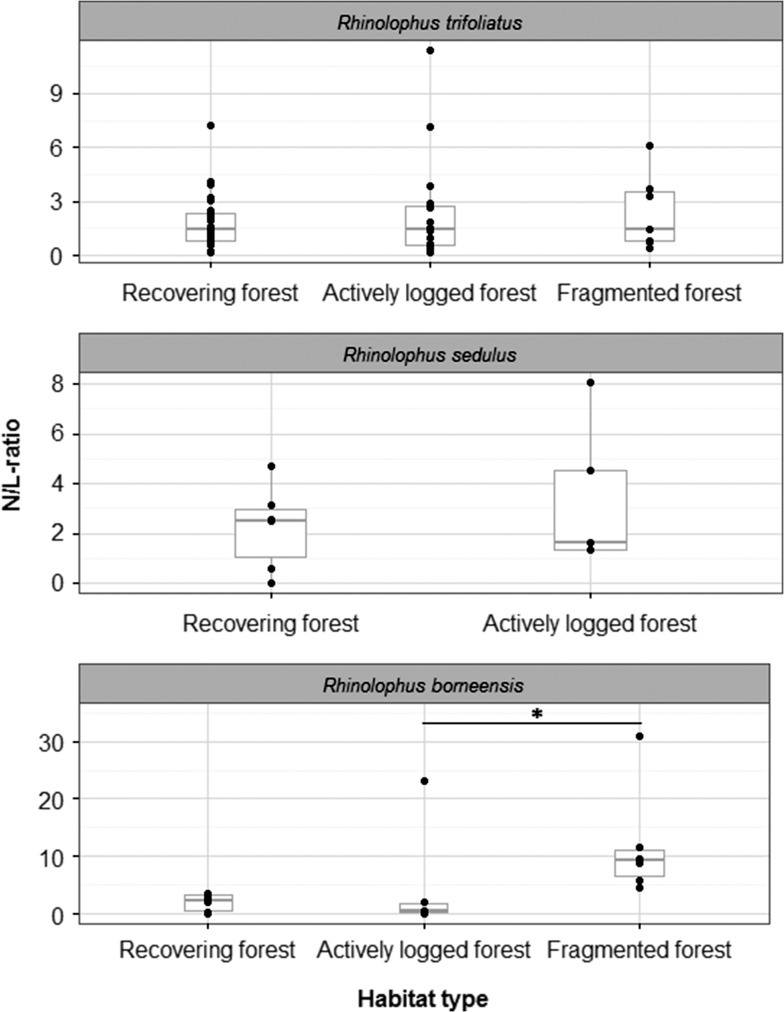
We measured the N/L-ratio (a proxy for chronic stress levels) in three congeneric species. Rhinolophus trifoliatus and R. sedulus had N/L-ratios with relatively low variation of 0.18–11.38 (median 1.48) and 0–8.09 (median 2.48), respectively, whereas R. borneensis showed higher variation in N/L-ratio (0.06–47.5, median 3.36). The overall results of the DWBC counts can be found in Table 4. Kruskal–Wallis (KW)-tests within each species revealed that there was no significant difference in the N/L-ratio between habitat types in R. trifoliatus and R. sedulus (R. trifoliatus: KW chi-squared = 0.19, df = 2, P = 0.91, n = 55; R. sedulus: KW chi-squared = 0.13, df = 1, P = 0.72, n = 11; Fig. 3). In R. borneensis, we found that individuals living in fragmented forest had significantly higher N/L-ratios than conspecifics from actively logged areas (fragmented forest: 11.91 ± 9.69, active logging areas: 5.25 ± 9.24, recovering forest: 8.51 ± 17.25; KW-tests, KW chi-squared = 6.52, df = 2, P = 0.04, n = 19; Dunn's test between fragmented and actively logged forest, Z = −2.46, P = 0.02, Dunn's test between recovering and fragmented forest, Z = −1.86, P = 0.1, Dunn's test between recovering and actively logged forest, Z = 0.7, P = 0.72).
Table 4:
Differential white blood cell counts in Rhinolophus trifoliatus, R. sedulus and R. borneensis. Values indicate mean ± SE (%)
| Species (sample size) | Neutrophils | Lymphocytes | Eosinophils | Monocytes | Basophils |
|---|---|---|---|---|---|
| Rhinolophus trifoliatus (n = 55) | 54.42 ± 19.82 | 41.75 ± 20.05 | 0.42 ± 1.07 | 2.38 ± 2.31 | 0.07 ± 0.26 |
| Rhinolophus sedulus (n = 11) | 61.09 ± 25.31 | 36.73 ± 24.28 | 0.54 ± 1.04 | 1.55 ± 1.63 | 0.09 ± 0.30 |
| Rhinolophus borneensis (n = 19) | 62.05 ± 31.97 | 35.13 ± 31.90 | 1.21 ± 1.51 | 1.39 ± 1.48 | 0.21 ± 0.54 |
Figure 3:
Association between N/L-ratio and habitat type for Rhinolophus borneensis,R. sedulus and R. trifoliatus. The figure shows data points (dots), medians (bold line) and 25–75% percentiles (box). The N/L-ratio does not significantly differ between habitat types in R. sedulus (n = 11) and R. trifoliatus (n = 55). In R. borneensis (n= 19), individuals living in fragmented forest had significantly higher N/L-ratios than those in actively logged forest. For reasons of clearer representation one data point in the dataset of R. borneensis (N/L-ratio = 47.5, habitat type: recovering forest) is not depicted.
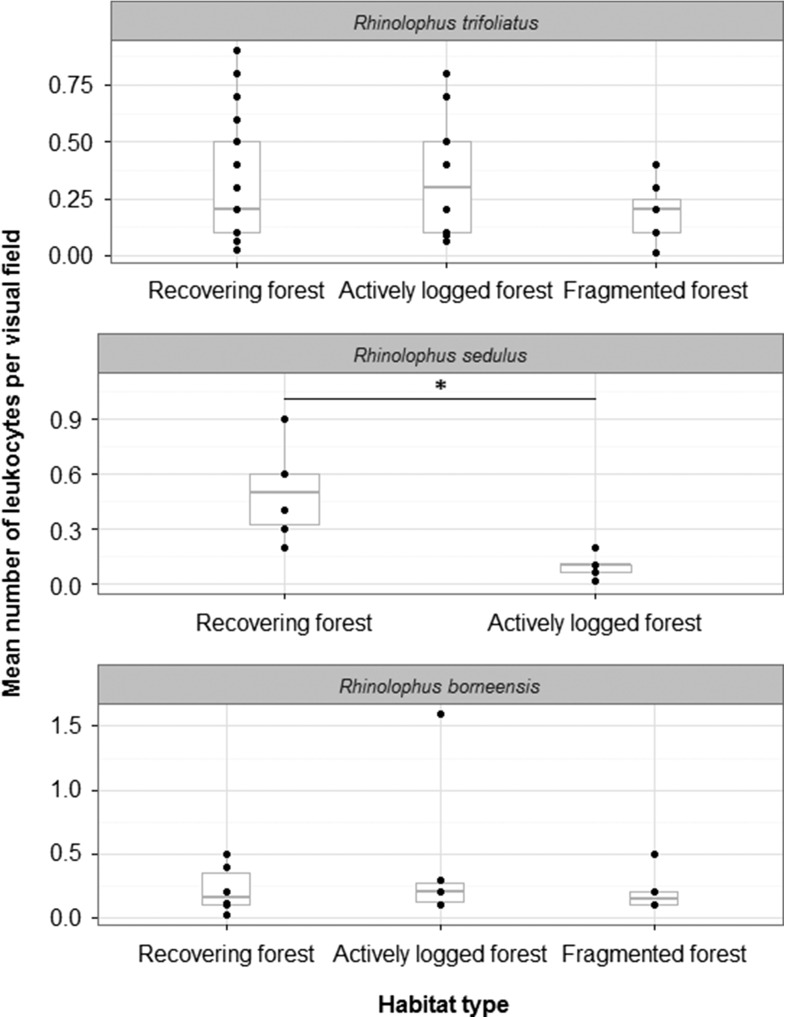
The mean number of leukocytes per optical field was similar for all study species (R. trifoliatus: 0.31 ± 0.2, R. sedulus: 0.32 ± 0.28, R. borneensis: 0.28 ± 0.36). Leukocyte number was not related to habitat type in R. trifoliatus and R. borneensis (KW-tests, R. trifoliatus: KW chi-squared = 1.74, df = 2, P = 0.42, n = 53; R. borneensis: KW chi-squared = 0.38, df = 2, P = 0.83, n = 18; Fig. 4). We found significantly lower numbers of leukocytes in R. sedulus captured in logging areas compared with conspecifics from recovering forests (actively logged forest: 0.1 ± 0.07, recovering forest: 0.5 ± 0.25; Mann–Whitney U test, W = 29.5, P = 0.01, n = 11). Rhinolophus sedulus was absent from fragmented forest.
Figure 4:
Association between mean number of leukocytes per optical field and habitat type for Rhinolophus borneensis, R. sedulus and R. trifoliatus. The figure shows data points (dots), medians (bold line) and 25–75% percentiles (box). There is no significant difference in the number of leukocytes between habitat types in Rhinolophus trifoliatus (n = 53) and R. borneensis (n = 18), while numbers of leukocytes are significantly lower in individuals from actively logged than from recovering forest in R. sedulus (n = 11).
Discussion
This study aimed to evaluate the impact of habitat disturbance on body mass, chronic stress and cellular immunity in forest-dwelling paleotropical bats. Forest disturbance was associated with a reduced individual body mass in foliage-roosting species. In one cave-roosting species (Rhinolophus borneensis), we found elevated N/L-ratios in individuals from fragmented forest compared to individuals from active logging areas, indicating chronic stress. In a foliage-roosting species (R. sedulus), we found reduced white blood cell numbers in actively logged compared to less disturbed recovering forest. Keeping in mind that the biological differences might be small, we may not have been able to detect significant differences due to low sample sizes in some of the study species.
Body mass and habitat alteration
Anthropogenic habitat disturbance had a roost type-specific effect on bat body mass in our study. In foliage-roosting species, body mass of individual bats decreased with increasing disturbance level. In cave-roosting species, the body mass was not significantly associated with habitat type, although trends were similar to foliage-roosting species. Cave-roosting species may benefit from increased edge habitats and decreased abundance of individuals of foliage-roosting species, resulting in reduced competition for food due to their relatively large home range size associated with central-place foraging (Struebig et al., 2013). Confirming our findings, Struebig et al. (2013) found that some cave-roosting species such as H. cervinus were highly abundant across heavily disturbed sites.
The observation that habitat alteration affects body mass supports the findings of other studies, although variation exists across species. Most work in this direction has so far been conducted in birds. For example, in montane white-eyes (Zosterops poliogaster) body mass was lower in individuals from forest fragments compared with conspecifics from continuous forests (Habel et al., 2014). Suorsa and colleagues found that body condition was lower in Eurasian treecreeper (Certhia familiaris) nestlings that were raised in smaller compared to nestlings raised in larger forest patches (Suorsa et al., 2003, 2004). However, body condition did not change with habitat deterioration in Taita thrushes (Turdus helleri; Lens et al., 2002).
In mammals, the effect of habitat disturbance on nutritional status also varies considerably, and the limited number of studies undertaken precludes identification of a general trend. For example, food limitation, measured by the urinary urea nitrogene:creatinine ratio, increased in New England cottontails (Sylvilagus transitionalis) with decreasing home range patch (Villafuerte et al., 1997). However, in wood mice (Apodemus sylvaticus), body condition was not related to the size of fragments in which the animals lived (Diaz et al., 1999). Body masses of agile antechinus (Antechinus agilis) may increase or decrease in individuals from disturbed habitats, depending on sex and season (Johnstone et al., 2010; 2011; 2014).
As bats represent one of the most diverse and species-rich order of mammals, replicating our study in other bats species could help to better understand the impact of habitat alteration on body condition in mammals. According to a broad study on insect abundance at the study site, potential prey for insectivorous bats is reduced at the more disturbed logged sites (Ewers et al., 2015). Thus, it is not surprising, that in our study, habitat deterioration led to a reduced body mass in some bat species, even though bats are much more mobile than most other terrestrial mammals and thus could potentially compensate for a reduction in food availability by visiting alternative, presumably more distant, foraging areas. Indeed, our findings indicate that bat species were differently affected by habitat disturbance depending on their roost preference, which may be caused by varying home range sizes. In foliage-roosting bat species, for example, body mass was lower in actively logged and fragmented forest compared to conspecifics from lesser disturbed recovering forest.
Nutritional stress, reflected by a loss in body mass, can lead to an increased susceptibility to pathogens (Plowright et al., 2008). For example, in female Brazilian free-tailed bats (Tadarida brasiliensis) a low body mass was associated with a higher risk for rabies infection (Turmelle et al., 2010). Further, Lau et al. (2010) found an association of a coronavirus detection rate and low body mass in Chinese horseshoe bats (Rhinolophus sinicus). Thus, foliage-roosting bat species in disturbed habitats might become especially susceptible to viral infections and present increased shedding, potentially leading to zoonotic spillover events when they come into contact with humans or livestock.
Chronic stress in disturbed habitats
In many vertebrates, especially mammals, N/L-ratios are positively correlated with the plasma concentration of circulating stress hormones (Davis et al., 2008). Chronically elevated levels of glucocorticoids shift the numbers of neutrophils and lymphocytes, causing an increase in the N/L-ratio making it a suitable measurement of chronic stress (Davis et al., 2008; Ellis et al., 2012). We used this ratio as a proxy for stress level in our study instead of directly measuring plasma glucocorticoid concentrations because hormone concentrations change within 3–5 min after capture in mammals (Romero, 2004), whereas N/L-ratios remains stable after capture and handling (Davis, 2005; Davis et al., 2008). Further, we were limited in the amount of available plasma given ethical considerations for the study taxa.
In one of our study species, N/L-ratios differed between individuals of different habitat types. Rhinolophus borneensis from fragmented forest showed higher N/L-ratios than conspecifics from actively logged forests. Similarly, in another small mammal, the agile antechinus, Johnstone and colleagues found that the N/L-ratio of individuals living in fragmented areas was elevated throughout most of the year, although not during winter (Johnstone et al., 2012a, 2014). In birds, results are inconsistent. On the one hand, Suorsa et al. (2004) and Hinam and Clair (2008) reported higher heterophil to lymphocyte ratios (H/L; the avian equivalent to N/L-ratio) in Eurasian treecreeper nestlings and Northern sawwhet owls (Aegolius acadius) in smaller compared to larger forest patches. On the other hand, in male ovenbirds (Seiurus aurocapilla) fragmentation was associated with lower H/L-ratios compared to males from the continuous forest, even though only during the breeding season (Mazerolle and Hobson, 2002).
In contrast to our hypothesis, we found an association between chronic stress and habitat disturbance in a small, highly social, cave-roosting species, but not in the larger, foliage-roosting species (R. trifoliatus and R. sedulus). Foliage-roosting species may be less resilient to forest modification and individuals suffering from chronic stress may have emigrated or deceased of starvation or disease already at the early beginning of habitat modification. In the consequence, we would not have been able to sample them.
Indeed, the total number of captured individuals was generally lower in actively logged and fragmented compared with recovering forest for most species including R. sedulus, which was completely absent in fragmented forest (although sampling effort was similar in all habitat types). Thus, this species may be highly vulnerable to local extinction in human-modified habitats. Due to the fact that a small home range size increases extinction risk in bats (Jones et al., 2003) one should attribute particular attention on species with small home ranges for conservation management.
Effect of habitat alteration on total white blood cell counts
In three rhinolophid bat species, we used TWBC counts as a measure of the cellular immunity. White blood cells (leukocytes) are energetically costly to produce and maintain and commonly used in comparative field research (Nunn, 2002; Young et al., 2013; Schneeberger et al., 2013a; Tian et al., 2015). Compared to similar-sized insectivorous bat species from the Neotropics, we found low mean values of leukocytes per field. Schneeberger et al. (2013a) reported between 1.8 (Micronycteris microtis) and 3.9 (M. hirsuta) leukocytes per visual field while the range in our study was only 0.27–0.32 leukocytes per field. Stockmaier et al. (2015) reported similar numbers of leukocytes (0.85) in the similar-sized, insectivorous Pallas’ mastiff bat (Molossus molossus).
In one species, R. sedulus, we found a significant effect of habitat disturbance, with significantly lower numbers of leukocytes in individuals from actively logged than individuals from recovering forests, which is consistent with findings in diademed sifaks (Propithecus diadema; Irwin et al., 2010) and agile antechinus (Johnstone et al., 2012b). In these other studies, authors suggested that animals suffered from an impaired immune system function, yet no experimental tests were performed on functional aspects of the immune system in support of this notion. High numbers of leukocytes can either indicate an individual in good condition that is well prepared to fight an infection or an individual currently fighting an infection (Salvante, 2006). Because all animals appeared healthy on visual examination, we support the former explanation and suggest that individuals in actively logged forest suffered from a weakened immune system. In support with this, a previous study on Brazilian free-tailed bats showed that individuals roosting in certain disturbed locations (human-made bridges) can have lower cellular immune function compared to individuals roosting in natural caves (Allen et al., 2009).
In R. sedulus a large proportion of leukocytes consisted of lymphocytes (36.73 ± 24.28%), a cell type that is used to counter viral infections by killing infected cells and the activation of the cytokine and antibody response (Baker and Zhou, 2015). Therefore, R. sedulus with low leukocyte numbers might be particularly prone to shed pathogens, potentially causing zoonotic spillovers when humans come into contact with this species. In Southeast Asia, besides flying foxes also larger insectivorous bats are occasionally hunted for their meat (Mildenstein et al., 2016), as it may be the case for R. sedulus.
Rhinolopohus sedulus seems to be less resilient to habitat disturbance compared with R. borneensis and R. trifoliatus that might develop reduced numbers of leukocytes during later stages of habitat conversion. Like R. trifoliatus, but contrasting to R. borneensis, R. sedulus is smaller compared to the other congeneric species. Although N/L-ratios of individuals in R. sedulus were not elevated in actively logged compared to recovering forest, a non-stress related mechanism affecting all immune cells in a similar way might have led to the reduction in TWBC counts.
Conclusions
Overall, we found evidence that in some, but not all studied paleotropical bat species human habitat disturbances may be associated with a decrease in body mass, elevated chronic stress and reduced investment in cellular components of the immune system. We identified foliage-roosting species as particularly sensitive to habitat deterioration, which may predispose them as potential sources for viral spillovers events when humans encroach into natural habitats. Presumably, a prolonged period of habitat disturbances will result in chronic stress and an impaired immune system in more species, especially because forest modification during our study only lasted for a short period of time. A follow-up study at a later stage of fragmentation may clarify whether and which bat species are resilient to human disturbances over a longer period.
Acknowledgments
We thank the scientific committee of the SAFE project for the opportunity to be part in an extraordinary study of land-use change. We are thankful to all research assistants, Ryan Gray and fellow researchers at SAFE, especially Lonnie Baking, Victoria Kemp and David Bennett, for vital support and great company in the field. We are grateful to Katja Pohle for help in the laboratory analyses, Karin Schneeberger and three anonymous reviewers for helpful comments on an earlier draft of the manuscript. We thank the Sabah Biodiversity Center, Yayasan Sabah and Sabah Forestry Department for permission to conduct research in Sabah, and Yayasan Sime Darby for supporting the SAFE project.
Funding
This work was supported by the German Research Foundation (DFG Priority Programme 1596) and the UK Natural Environment Research Council (Human Modified Tropical Forests programme under the Land-use Options for Maintaining Biodiversity and Ecosystem Function consortium). The publication of this article was funded by the Open Access Fund of the Leibniz Association.
Author contributions
C.V., M.S., H.B., G.C. and A.S. designed the study. H.B., A.S., M.S. and C.V. contributed to research permit acquisition. A.S. and M.S. undertook field work. H.B. provided facilities to store the samples in Malaysia. G.C. and A.S. analyzed the samples. A.C., A.S., C.V., G.C. and M.S. conducted statistical analyses of the data. All authors interpreted the results and wrote the paper.
References
- Allen LC, Turmelle AS, Mendonca MT, Navara KJ, Kunz TH, McCracken GF (2009) Roosting ecology and variation in adaptive and innate immune system function in the Brazilian free-tailed bat (Tadarida brasiliensis). J Comp Physiol B 179: 315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M, Schountz T, Wang LF (2013) Antiviral immune responses of bats: a review. Zoonoses Public Health 60: 104–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker ML, Zhou P (2015) Bat immunology In: Wang L-F, Cowled C, eds, Bats and Viruses, Vol 1 John Wiley & Sons, Inc, Hoboken, pp 327–348. [Google Scholar]
- Barnosky AD, Matzke N, Tomiya S, Wogan GOU, Swartz B, Quental TB, Marshall C, McGuire JL, Lindsey EL, Maguire KC et al. (2011) Has the Earth's sixth mass extinction already arrived. Nature 471: 51–57. [DOI] [PubMed] [Google Scholar]
- Boonstra R. (2013) Reality as the leading cause of stress: rethinking the impact of chronic stress in nature. Funct Ecol 27: 11–23. [Google Scholar]
- Brook CE, Dobson AP (2015) Bats as ‘special’ reservoirs for emerging zoonotic pathogens. Trends Microbiol 23: 172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan KL. (2000) Stress and the evolution of condition-dependent signals. Trends Ecol Evolut 15: 156–160. [DOI] [PubMed] [Google Scholar]
- Chua KB, Bellini WJ, Rota PA, Harcourt BH, Tamin A, Lam SK, Ksiazek TG, Rollin PE, Zaki SR, Shieh WJ et al. (2000) Nipah virus: a recently emergent deadly paramyxovirus. Science 288: 1432–1435. [DOI] [PubMed] [Google Scholar]
- Davis AK. (2005) Effect of handling time and repeated sampling on avian white blood cell counts. J Field Ornithol 76: 334–338. [Google Scholar]
- Davis AK, Maney DL, Maerz JC (2008) The use of leukocyte profiles to measure stress in vertebrates: a review for ecologists. Funct Ecol 22: 760–772. [Google Scholar]
- Demas GE, Zysling DA, Beechler BR, Muehlenbein MP, French SS (2011) Beyond phytohaemagglutinin: assessing vertebrate immune function across ecological contexts. J Anim Ecol 80: 710–730. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, Miller AH, McEwen BS, Spencer RL (1996) Stress-induced changes in blood leukocyte distribution. Role of adrenal steroid hormones. J Immunol 157: 1638–1644. [PubMed] [Google Scholar]
- Diaz M, Santos T, Telleria JL (1999) Effects of forest fragmentation on the winter body condition and population parameters of an habitat generalist, the wood mouse Apodemus sylvaticus: a test of hypotheses. Acta Oecol 20: 39–49. [Google Scholar]
- Dickens MJ, Romero LM (2013) A consensus endocrine profile for chronically stressed wild animals does not exist. Gen Comp Endocrinol 191: 177–189. [DOI] [PubMed] [Google Scholar]
- Dinno A. (2015) Nonparametric pairwise multiple comparisons in independent groups using dunn's test. Stata J 15: 292–300. [Google Scholar]
- Ellis RD, McWhorter TJ, Maron M (2012) Integrating landscape ecology and conservation physiology. Landsc Ecol 27: 1–12. [Google Scholar]
- Epstein J, Field H (2016) Anthropogenic epedemics: the ecology of bat-borne viruses and our role in their emergence In: Bats and Viruses: A New Frontier of Emerging Infectious Diseases, Wang LF and Cowled C, eds, John Wiley & Sons, Inc, Hoboken, New Jersey, pp 249–280. [Google Scholar]
- Estrada-Villegas S, Meyer CFJ, Kalko EKV (2010) Effects of tropical forest fragmentation on aerial insectivorous bats in a land-bridge island system. Biol Conserv 143: 597–608. [Google Scholar]
- Ewers RM, Boyle MJW, Gleave RA, Plowman NS, Benedick S, Bernard H, Bishop TR, Bakhtiar EY, Chey VK, Chung AYC (2015) Logging cuts the functional importance of invertebrates in tropical rainforest. Nat Commun 6: doi:10.1038/ncomms7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. (1985) Phylogenies and the comparative method. Am Nat 125: 1–15. [Google Scholar]
- Field A. (2005) Discovering Statistics Using SPSS. Ed 2 Sage, London. [Google Scholar]
- Fitzherbert EB, Struebig MJ, Morel A, Danielsen F, Brühl CA, Donald PF, Phalan B (2008) How will oil palm expansion affect biodiversity. Trends Ecol Evol 23: 538–545. [DOI] [PubMed] [Google Scholar]
- Fox J, Monette G (1992) Generalized collinearity diagnostics. J Am Stat Assoc 87(417): 178–183. [Google Scholar]
- Fox J, Weisberg S (2011) An {R} Companion to Applied Regression, Ed 2 Sage, Thousands Oaks, USA. [Google Scholar]
- Gaveau DLA, Sloan S, Molidena E, Yaen H, Sheil D, Abram NK, Ancrenaz M, Nasi R, Quinones M, Wielaard N (2014) Four decades of forest persistence, clearance and logging on Borneo. PLoS ONE 9: e101654: doi:10.1371/journal.pone.0101654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson L, Lee TM, Koh LP, Brook BW, Gardner TA, Barlow J, Peres CA, Bradshaw CJ, Laurance WF, Lovejoy TE (2011) Primary forests are irreplaceable for sustaining tropical biodiversity. Nature 478: 378–381. [DOI] [PubMed] [Google Scholar]
- Grafen A. (1989) The phylogenetic regression. Philos Trans R Soc Lond B Biol Sci 326: 119–157. [DOI] [PubMed] [Google Scholar]
- Habel JC, Mulwa RK, Gassert F, Roedder D, Ulrich W, Borghesio L, Husemann M, Lens L (2014) Population signatures of large-scale, long-term disjunction and small-scale, short-term habitat fragmentation in an afromontane forest bird. Heredity 113: 205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad NM, Brudvig LA, Clobert J, Davies KF, Gonzalez A, Holt RD, Lovejoy TE, Sexton JO, Austin MP, Collins CD (2015) Habitat fragmentation and its lasting impact on Earth's ecosystems. Sci Adv 1(2): e1500052: doi:10.1126/sciadv.1500052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon LJ, Weir JT, Brock CD, Glor RE, Challenger W (2008) Geiger: investigating evolutionary radiations. Bioinformatics 24: 129–131. [DOI] [PubMed] [Google Scholar]
- Harrel FE. (2001) Regression Modeling Strategies. Springer, New York. [Google Scholar]
- Hinam HL, Clair CCS (2008) High levels of habitat loss and fragmentation limit reproductive success by reducing home range size and provisioning rates of Northern saw-whet owls. Biol Conserv 141: 524–535. [Google Scholar]
- Hinsley SA. (2000) The costs of multiple patch use by birds. Landscape Ecol 15: 765–775. [Google Scholar]
- Hothorn T, Bretz F, Westfall P (2008). Simultaneous inference in general parametric models. Biom J 50(3): 346–363. [DOI] [PubMed] [Google Scholar]
- Irwin MT, Junge RE, Raharison J-L, Samonds KE (2010) Variation in physiological health of diademed sifakas across intact and fragmented forest at Tsinjoarivo, Eastern Madagascar. Am J Primatol 72: 1013–1025. [DOI] [PubMed] [Google Scholar]
- Johnstone CP, Lill A, Reina RD (2011) Response of the agile antechinus to habitat edge, configuration and condition in fragmented forest. PLoS ONE 6(11): e27158: doi:10.1371/journal.pone.0027158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone CP, Lill A, Reina RD (2012. a) Does habitat fragmentation cause stress in the agile antechinus? A haematological approach. J Comp Physiol B 182: 139–155. [DOI] [PubMed] [Google Scholar]
- Johnstone CP, Lill A, Reina RD (2014) Habitat loss, fragmentation and degradation effects on small mammals: analysis with conditional inference tree statistical modelling. Biol Conserv 176: 80–98. [Google Scholar]
- Johnstone CP, Reina RD, Lill A (2010) Impact of anthropogenic habitat fragmentation on population health in a small, carnivorous marsupial. J Mammal 91: 1332–1341. [Google Scholar]
- Johnstone CP, Reina RD, Lill A (2012. b) Interpreting indices of physiological stress in free-living vertebrates. J Comp Physiol B 182: 861–879. [DOI] [PubMed] [Google Scholar]
- Jones G, Jacobs DS, Kunz TH, Willig MR, Racey PA (2009) Carpe noctem: the importance of bats as bioindicators. Endanger Species Res 8: 93–115. [Google Scholar]
- Jones KE, Purvis A, Gittleman JL (2003) Biological correlates of extinction risk in bats. Am Nat 161: 601–614. [DOI] [PubMed] [Google Scholar]
- Khan FAA, Solari S, Swier VJ, Larsen PA, Abdullah MT, Baker RJ (2010) Systematics of Malaysian woolly bats (vespertilionidae: Kerivoula) inferred from mitochondrial, nuclear, karyotypic, and morphological data. J Mammal 91: 1058–1072. [Google Scholar]
- Kingston T, Francis CM, Akbar Z, Kunz TH (2003) Species richness in an insectivorous bat assemblage from Malaysia. J Trop Ecol 19: 67–79. [Google Scholar]
- Kingston T, Lim BL, Akbar Z (2006) Bats of Krau Wildlife Reserve. University Kebangsaan Malaysia, Penerbit UKM, Bangi. [Google Scholar]
- Kunz TH, Anthony THP (1982) Age estimation and post-natal growth in the bat Myotis lucifugus. J Mammal 63(1): 23–32. [Google Scholar]
- Kunz TH, Hodgkinson R, Weise CD (2009) Methods of capturing and handling bats In: Kunz TH, Parsons S, eds, Ecological and Behavioral Methods for the Study of Bats, Ed 2, The Johns Hopkins University Press, Baltimore, pp 36–56. [Google Scholar]
- Kunz TH, Weise CD (2009) Methods and devices for marking bats In: Kunz TH, Parsons S, eds, Ecological and Behavioral Methods for the Study of Bats, Ed 2, The Johns Hopkins Uníversity Press, Baltimore, pp 36–56. [Google Scholar]
- Lau SKP, Li KSM, Huang Y, Shek C-T, Tse H, Wang M, Choi GKY, Xu H, Lam CSF, Guo R, et al. (2010) Ecoepidemiology and complete genome comparison of different strains of severe acute respiratory syndrome-related rhinolophus bat coronavirus in China reveal bats as a reservoir for acute, self-limiting infection that allows recombination events. J Virol 84: 2808–2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurance WF, Sayer J, Cassman KG (2014) Agricultural expansion and its impacts on tropical nature. Trends Ecol Evol 29: 107–116. [DOI] [PubMed] [Google Scholar]
- Lens L, Van Dongen S, Matthysen E (2002) Fluctuating asymmetry as an early warning system in the critically endangered taita thrush. Conserv Biol 16: 479–487. [Google Scholar]
- Leroy EM, Kumulungui B, Pourrut X, Rouquet P, Hassanin A, Yaba P, Délicat A, Paweska JT, Gonzalez J-P, Swanepoel R (2005) Fruit bats as reservoirs of ebola virus. Nature 438: 575–576. [DOI] [PubMed] [Google Scholar]
- Li W, Shi Z, Yu M, Ren W, Smith C, Epstein JH, Wang H, Crameri G, Hu Z, Zhang H et al. (2005) Bats are natural reservoirs of sars-like coronaviruses. Science 310: 676–679. [DOI] [PubMed] [Google Scholar]
- Luis AD, Hayman DTS, O'Shea TJ, Cryan PM, Gilbert AT, Pulliam JRC, Mills JN, Timonin ME, Willis CKR, Cunningham AA et al. (2013) A comparison of bats and rodents as reservoirs of zoonotic viruses: are bats special?. Proc R Soc B 280: 20122753; doi:10.1098/rspb.2012.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlier ME, DeFries RS, Kim PS, Koplitz SN, Jacob DJ, Mickley LJ, Myers SS (2015) Fire emissions and regional air quality impacts from fires in oil palm, timber, and logging concessions in indonesia. Environ Res Lett 10: 085005. [Google Scholar]
- Martins EP, Hansen TF (1997) Phylogenies and the comparative method: a general approach to incorporating phylogenetic information into the analysis of interspecific data. Am Nat 149: 646–667. [Google Scholar]
- Mazerolle DF, Hobson KA (2002) Physiological ramifications of habitat selection in territorial male ovenbirds: Consequences of landscape fragmentation. Oecologia 130: 356–363. [DOI] [PubMed] [Google Scholar]
- Meyer FJ, Fruend J, Lizano WP, Kalko EKV (2008) Ecological correlates of vulnerability to fragmentation in neotropical bats. J App Ecol 45: 381–391. [Google Scholar]
- Meyer CF, Struebig MJ, Willig MR (2016) Responses of tropical bats to habitat fragmentation, logging, and deforestation In: Voigt CC, Kingston T, eds, Bats in the Anthropocene: Conservation of Bats in a Changing World, Ed 1, Springer, Cham, Germany, pp 63–103. [Google Scholar]
- Mildenstein T, Tanshi I, Racey P (2016) Exploitation of bats for bushmeat and medicine In: Voigt CC, Kingston T, eds, Bats in the Anthropocene: Conservation of Bats in a Changing World, Ed 1, Springer, Cham, Germany, pp 325–375. [Google Scholar]
- Newbold T, Hudson LN, Phillips HRP, Hill SLL, Contu S, Lysenko I, Blandon A, Butchart SHM, Booth HL, Day J et al. (2014) A global model of the response of tropical and sub-tropical forest biodiversity to anthropogenic pressures. Proc Biol Sci 281 (1792): 20141371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norberg UM. (1981) Allometry of bat wings and legs and comparison with bird wings. Philos Trans R Soc Lond B Biol Sci 292: 359–398: doi:10.1098/rstb.1981.0034. [Google Scholar]
- Nunn CL. (2002). A comparative study of leukocyte counts and disease risk in primates. Evolution, 56(1): 177–190. [DOI] [PubMed] [Google Scholar]
- O'Brien RM. (2007) A caution regarding rules of thumb for variance inflation factors. Qual Quant, 41(5): 673–690. [Google Scholar]
- Paradis E, Claude J, Strimmer K (2004) Ape: analyses of phylogenetics and evolution in r language. Bioinformatics 20: 289–290. [DOI] [PubMed] [Google Scholar]
- Payne J, Francis C, Phillipps K (1985) Field Guide to the Mammals of Borneo. The Sabah Society and WWF Malaysia, Kota Kinabalu. [Google Scholar]
- Plowright RK, Field HE, Smith C, Divljan A, Palmer C, Tabor G, Daszak P & Foley JE (2008) Reproduction and nutritional stress are risk factors for Hendra virus infection in little red flying foxes (Pteropus scapulatus). Proc Biol Sci 275: 861–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2016). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria: http://www.R-project.org. (last accessed 4 August 2016). [Google Scholar]
- Revell LJ. (2012) Phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecol Evol 3: 217–223. [Google Scholar]
- Romero LM. (2002) Seasonal changes in plasma glucocorticoid concentrations in free-living vertebrates. Gen Comp Endocrinol 128: 1–24. [DOI] [PubMed] [Google Scholar]
- Romero LM. (2004) Physiological stress in ecology: lessons from biomedical research. Trends Ecol Evol 19: 249–255. [DOI] [PubMed] [Google Scholar]
- Romero LM, Wikelski M (2010) Stress physiology as a predictor of survival in Galapagos marine iguanas. Proc Biol Sci 277: 3157–3162: doi:10.1098/rspb.2010.0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvante KG. (2006) Techniques for studying integrated immune function in birds. Auk 123: 575–586. [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU (2000) How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21: 55–89. [DOI] [PubMed] [Google Scholar]
- Sazali SN, Besar K, Abdullah MT (2011) Phylogenetic analysis of the Malaysian Rhinolopus and Hipposideros inferred from partial mitochondrial DNA cytochrome b gene sequences. Pertanika J Trop Agric Sci 34: 281–294. [Google Scholar]
- Schneeberger K, Czirják GÁ, Voigt CC (2013. a) Measures of the constitutive immune system are linked to diet and roosting habits of neotropical bats. PLoS ONE 8: e54023: doi:10.1371/journal.pone.0054023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeberger K, Czirják GÁ, Voigt CC (2013. b) Inflammatory challenge increases measures of oxidative stress in a free-ranging, long-lived mammal. J Exp Biol 216: 4514–4519. [DOI] [PubMed] [Google Scholar]
- Stockmaier S, Dechmann DKN, Page RA, O'Mara MT (2015) No fever and leucocytosis in response to a lipopolysaccharide challenge in an insectivorous bat. Biol Lett 11: 20150576: doi:10.1098/rsbl.2015.0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struebig MJ, Turner A, Giles E, Lasmana F, Tollington S, Bernard H, Bell D (2013) Quantifying the biodiversity value of repeatedly logged rainforests: gradient and comparative approaches from Borneo. Adv Ecol Res 48: 183–224. [Google Scholar]
- Struebig MJ, Kingston T, Petit EJ, Le Comber SC, Zubaid A, Mohd-Adnan A, Rossiter SJ (2011) Parallel declines in species and genetic diversity in tropical forest fragments. Ecol Lett 14: 582–590. [DOI] [PubMed] [Google Scholar]
- Struebig MJ, Sujarno R (2006) Forest bat surveys using harp traps—a practical manual and identification key for the bats of Kalimantan, Indonesia. The Kalimantan Bat Conservation Project and Bat Conservation International, Balikpapan.
- Suorsa P, Helle H, Koivunen V, Huhta E, Nikula A, Hakkarainen H (2004) Effects of forest patch size on physiological stress and immunocompetence in an area-sensitive passerine, the Eurasian treecreeper (Certhia familiaris): an experiment. Proc Biol Sci 271: 435–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suorsa P, Huhta E, Nikula A, Nikinmaa M, Jantti A, Helle H, Hakkarainen H (2003) Forest management is associated with physiological stress in an old-growth forest passerine. Proc Biol Sci 270: 963–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J, Courtiol A, Schneeberger K, Greenwood AD, Czirják GÁ (2015) Circulating white blood cell counts in captive and wild rodents are influenced by body mass rather than testes mass, a correlate of mating promiscuity. Fun Ecol 29: 823–829: doi:10.1111/1365–2435.12394. [Google Scholar]
- Towner JS, Amman BR, Sealy TK, Carroll SAR, Comer JA, Kemp A, Swanepoel R, Paddock CD, Balinandi S, Khristova ML et al. (2009) Isolation of genetically diverse Marburg viruses from Egyptian fruit bats. PLoS Pathog 5 (7): e1000536: doi:10.1371/journal.ppat.100050356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turmelle AS, Allen LC, Jackson FR, Kunz TH, Rupprecht CE & McCracken GF (2010) Ecology of rabies virus exposure in colonies of Brazilian free-tailed bats (Tadarida brasiliensis) at natural and man-made roosts in Texas. Vector Borne Zoonotic Dis 10: 165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villafuerte R, Litvaitis JA, Smith DF (1997) Physiological responses by lagomorphs to resource limitations imposed by habitat fragmentation: implications for condition-sensitive predation. Can J Zool 75: 148–151. [Google Scholar]
- Voigt CC, Cruz-Neto A (2009) Energetic analyses of bats In: Kunz TH, Parsons S, eds, Ecological and Behavioral Methods for the Study of Bats, Ed 2 The John Hopkins University Press, Baltimore, pp 623–645. [Google Scholar]
- Wang LF, Walker PJ, Poon LLM (2011) Mass extinctions, biodiversity and mitochondrial function: are bats ‘special’ as reservoirs for emerging viruses. Curr Opin Virol 1: 649–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibbelt G, Moore MS, Schountz T, Voigt CC (2010) Emerging diseases in chiroptera: why bats. Biol Lett, rsbl20100267: doi:10.1098/rsbl.2010.0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikelski M, Cooke SJ (2006) Conservation physiology. Trends Ecol Evol 21: 38–46. [DOI] [PubMed] [Google Scholar]
- Wingfield JC. (2005) The concept of allostasis: coping with a capricious environment. J Mammal 86: 248–254. [Google Scholar]
- Wingfield JC, Jacobs J, Hillgarth N (1997) Ecological constraints and the evolution of hormone-behavior interrelationships In: Carter CS, Lederhendler II, Kirkpatrick B, eds, Integrative Neurobiology of Affiliation, Vol 807, New Yor Academy of Sciences, New York, pp 22–41. [DOI] [PubMed] [Google Scholar]
- Young H, Griffin RH, Wood CL, Nunn CL (2013). Does habitat disturbance increase infectious disease risk for primates. Ecol Lett 16: 656–663. [DOI] [PubMed] [Google Scholar]