Abstract
Background.
Increasing evidence suggests depot medroxyprogesterone acetate (DMPA) and intravaginal practices may be associated with human immunodeficiency virus (HIV-1) infection risk; however, the mechanisms are not fully understood. This study evaluated the effect of DMPA and intravaginal practices on the genital proteome and microbiome to gain mechanistic insights.
Methods.
Cervicovaginal secretions from 86 Kenyan women, including self-reported DMPA users (n = 23), nonhormonal contraceptive users (n = 63), and women who practice vaginal drying (n = 46), were analyzed using tandem-mass spectrometry.
Results.
We identified 473 human and 486 bacterial proteins from 18 different genera. Depot medroxyprogesterone acetate use associated with increased hemoglobin and immune activation (HBD, HBB, IL36G), and decreased epithelial repair proteins (TFF3, F11R). Vaginal drying associated with increased hemoglobin and decreased phagocytosis factors (AZU1, MYH9, PLAUR). Injury signatures were exacerbated in DMPA users who also practiced vaginal drying. More diverse (H index: 0.71 vs 0.45; P = .009) bacterial communities containing Gardnerella vaginalis associated with vaginal drying, whereas DMPA showed no significant association with community composition or diversity.
Conclusions.
These findings provide new insights into the impact of DMPA and vaginal drying on mucosal barriers. Future investigations are needed to confirm their relationship with HIV risk in women.
Keywords: Mucosa, proteomics, DMPA, Depot medroxyprogesterone acetate, microbiome, metaproteome, HIV, progestin, vaginal drying, genital injury
Women bear a disproportionate burden of new human immunodeficiency virus (HIV) infections in sub–Saharan Africa, accounting for 56% of new infections [1–3]. Given that a large proportion of these infections occur through heterosexual intercourse, investigations of the mucosal barrier of the female genital tract is of great interest because it is the first site of contact for HIV. Many epidemiological studies have identified behavioral, clinical, and biological factors linked to increased risk of HIV infection in women. One of these is the use of injectable hormonal contraceptives, such as depot medroxyprogesterone acetate (DMPA). Depot medroxyprogesterone acetate is the most popular injectable contraceptive used in sub–Saharan Africa and areas of high HIV risk and has been associated with a 1.2–1.7-fold increase in risk of HIV acquisition [4–7]. In addition, certain behaviors, such as vaginal washing and vaginal drying, are also popular in sub–Saharan Africa [8, 9]. Although vaginal washing with water has been associated with a 2.6-fold increased risk of acquiring HIV, vaginal washing with soap has been associated with a 3.8-fold increased risk, and vaginal drying is believed to negatively affect the mucosal barrier of the female genital tract, the impact of these intravaginal practices on the function of the mucosal barrier is not well understood [10–12].
Mucosal surfaces play a role in natural immunity and function as an ecological niche for various microbes. Any disruption in mucosal barrier function or its homeostasis at the host or microbial level can lead to increased HIV transmission risk [13–15]. Previous studies have shown that DMPA use is associated with increased levels of mucosal inflammatory mediators, such as cytokines and antimicrobial proteins [16–19], and an increased number of HIV target cells [20]. However, the reason for this increased inflammation is unknown, and little information has been generated on how it affects microbial communities or epithelial barriers in women in vivo. Furthermore, there has been a lack of studies evaluating the effect of intravaginal practices upon mucosal barriers, leaving a considerable gap in knowledge.
Mass spectrometry–based proteomics is an ideal technique that can be used to study the metaproteome of mucosal compartments, thus allowing us to study both host and bacterial components at mucosal surfaces. We recently used this approach to study how microbial diversity and function affect mucosal barrier function [21], mucosal processes underpinning target cell recruitment in the female genital tract [22], and immunological changes during the menstrual cycle [23]. The purpose of this study was to use a metaproteomics approach to evaluate the effect of DMPA and intravaginal practices upon the mucosal barrier and the microbiome of the female genital tract.
Methods
Study Population and Clinical Variables
All study participants were recruited through voluntary counseling and testing centers in Nairobi, Kenya, from September 2007 to December 2009, and represent a subset of a larger cohort study presented elsewhere [24]. All study participants were HIV-exposed, seronegative women in heterosexual relationships with HIV-positive partners. Eligible participants were aged >18 years, reported sexual intercourse with their partner ≥3 times in the 3 months before screening, and planned to remain together for the duration of the study (up to 24 months). Samples analyzed in this study included those that were collected 1 year after study enrollment. The women included in the subset for this study were grouped based on birth-control method used during this time—DMPA users (n = 23) or controls not using hormonal contraceptives (total: n = 63; condom only: n = 31; tubal ligation: n = 3; copper intrauterine device: n = 1; none: n = 27; natural/rhythm: n = 1)—and based on their self-reported vaginal drying practices: women who vaginal dry (n = 46) and those who do not (n = 40). Depot medroxyprogesterone acetate users self-reported using DMPA as their current birth control method and also reported lifetime use of the method (median = 12 months; interquartile range [IQR] = 1.5–22). All participants were tested for HIV-1, bacterial vaginosis, Trichomonas vaginalis, Treponema pallidum, and herpes simplex virus type 2 and reported any symptoms of vaginitis or cervicitis at the time of enrollment. Sociodemographic variables collected from these individuals also included age, sexual frequency in the previous month, vaginal cleansing practices, vaginal drying practices, partner viral load, and relationship length. Participants with incident pregnancies during the year of study follow-up were excluded from our analysis. Written informed consent was obtained from all study participants, and ethical approval was granted by institutional review boards at University of Washington (30243), Karolinska Institutet (2009/264-31/3), University of Manitoba (HS16416, H2013:200), and Kenyatta National Hospital (P151/07/2006).
Sample Collection
Samples were collected by 2 cotton-tipped swabs from the cervical os and posterior vaginal fornix. Both swabs were transferred into a vial containing 5 mL of phosphate-buffered saline, transferred on ice to the laboratory within 2 hours of collection, spun down to remove cellular debris, and cryopreserved at −80°C.
Proteomic Data Analysis
Fifty micrograms of protein from each sample were digested with trypsin and analyzed by tandem mass spectrometry using an Orbitrap Velos mass spectrometer as described previously [23]. Human peptide identity searching was performed using Mascot v2.4.0 against the SwissProt database restricting taxonomy to Human. Bacterial peptide identity searches were performed using a manually curated TrEMBL database containing the major identified genera identified from an initial search and taxa described previously by 16S rRNA studies (21 genera total) [25]. Search results were imported into Scaffold to validate the protein identifications, using the following criteria: ≤0.1% False Discovery Rate (FDR) for peptide identification, ≤1% FDR for protein identification, and at least 2 unique peptides identified per protein. Host proteome results were imported into Progenesis LC-Mass Spectrometry (MS) software to perform label-free differential protein expression analysis based on MS peak intensities. Feature detection, normalization, and quantification were all performed using default settings from the software. Microbial abundance was calculated by summing normalized total spectral counts from Scaffold for all proteins associated with each genus. Database construction and analysis details are described in the Supplementary Methods.
Statistical Analysis
Host Proteome Analysis
To adjust for underlying sources of variation within the data set, surrogate variable analysis was performed using the software R (version 3.2.2). Sociodemographic, clinical, and behavioral variables that were significantly different (P < .10) between DMPA users and controls were included in the adjustment model (Table 1). This included participant age, seminal exposure (semenogelin-2 levels detected by mass spectrometry), and self-reported vaginal washing and drying practices. Surrogate variable analysis selected an additional 6 surrogate variables to account for unexplained sources of variance. Proteomic data was fit to all clinical and surrogate variables using a linear model. To further eliminate any residual effects related to seminal exposure, we generated linear regression models between another seminal protein, semenogelin-1 (SEMG1), and all proteins in our data set and removed those that were significantly related to SEMG1 in our downstream DMPA-based analyses (Supplementary Table 1). Proteins that significantly differed between DMPA users and controls were determined by Student t test (P < .05). Hierarchical clustering was performed on proteins found to be differentially abundant between DMPA users and controls (P < .05) using complete linkage and the Kendall’s tau distance metric (Gene Cluster software, version 3.0). Gene ontology and pathway associations were determined using Ingenuity Pathway Analysis software. Right-tailed Fisher’s exact tests (Benjamini-Hochberg corrected) were used to calculate the probability that the association between each protein in the data set and the biological function or pathway was random. The top 5 activated and inhibited biological functions (based on the software’s z scores) are included in our analysis. Vaginal drying was assessed in the same manner, with the exception of DMPA use included in the adjustment model (Table 2).
Table 1.
Clinical Characteristics of Study Participants Stratified by Depot Medroxyprogesterone Acetate Use
| Clinical variable | Participants using DMPA | Participants using no or nonhormonal contraceptives | P value |
|---|---|---|---|
| Median age at enrollment | 27 (24–30) | 31 (25–37) | .01 |
| Sexual frequency in previous month, d | 4 (2–5.5) | 3 (2–7) | .76 |
| Vaginal washing | 9 (39) | 13 (21%) | .098 |
| Vaginal dryinga | 17 (74) | 28 (44%) | .03 |
| Relationship length, mo. | 4 (3–10) | 7 (2–15) | .34 |
| Partner log, viral load | 4.6 (3.4–4.9) | 4.6 (4–5.6) | .33 |
| STI results at enrolment, 1 y earlier | 7 (30) | 13 (21) | .39 |
| Bacterial vaginosis | 6 (26) | 8 (13) | .19 |
| Trichomonas vaginalis | 1 (4) | 2 (3) | 1.00 |
| Syphilis | 0 (0) | 1 (2) | 1.00 |
| Vaginitis | 0 (0) | 3 (5) | .56 |
| Cervicitis | 0 (0) | 1 (2) | 1.00 |
| HSV-2 | 16 (70) | 42 (67) | 1.00 |
Data are no. (%) or median (interquartile range).
Abbreviations: DMPA, depot medroxyprogesterone acetate; HSV-2, herpes simplex virus type 2; STI, sexually transmitted infection.
Vaginal drying was performed with a cloth/towel; 94% of individuals reported performing this practice always, 2% most of the time, 2% sometimes, and 2% did not answer about their frequency.
Table 2.
Clinical Characteristics of Study Participants Stratified by Women Who Practice Vaginal Drying and Controls
| Clinical variable | Participants who practice vaginal drying | Participants who do not practice vaginal drying | P value |
|---|---|---|---|
| Median age at enrollment | 29 (25–34) | 31 (26–36) | .26 |
| Last month sexual frequency (days) | 3 (2–6) | 5 (2–7) | .60 |
| Vaginal washing | 9 (23) | 13 (28) | .62 |
| DMPA use | 16 (35) | 6 (15) | .048 |
| Relationship length, mo | 5.5 (3–13) | 6 (2–11) | .77 |
| Partner log, viral load | 4.7 (4.3–5.4) | 4.4 (3.6–5.3) | .28 |
| STI results at enrolment, 1 y earlier | 11 (24) | 9 (23) | 1.00 |
| Bacterial vaginosis | 10 (22) | 4 (10) | .16 |
| Trichomonas vaginalis | 1 (2) | 2 (5) | .60 |
| Syphilis | 0 (0) | 1 (3) | .46 |
| Vaginitis | 1 (2) | 2 (5) | .60 |
| Cervicitis | 0 (0) | 1 (3) | .46 |
| HSV-2 | 27 (59) | 31 (78) | .07 |
Data are no (%) or median (interquartile range).
Abbreviations: DMPA, depot medroxyprogesterone acetate; HSV-2, herpes simplex virus type 2; STI, sexually transmitted infection.
Microbial Proteome Data Analysis
Unsupervised hierarchical clustering with average Euclidean linkage was performed in Gene Cluster (version 3.0) using genus-level microbial abundance proportions for each sample, and stacked bar charts were generated in GraphPad Prism (version 6.05). Shannon’s H index diversity scores were calculated in MatLab (version R2015b), and compared between groups using Mann-Whitney U tests in GraphPad Prism.
Discriminant Analysis
Least absolute shrinkage and selection operator (LASSO) and Partial least-squares discriminant analysis were applied to select a minimal protein signature that could best distinguish DMPA users from controls. For LASSO, K-fold cross-validation was used to determine the optimum value of the tuning parameter (“s”), such that the resulting model had the lowest possible mean squared error for prediction. Partial least-squares discriminant analysis was then used to assess how accurately the LASSO-based protein signature distinguished DMPA users from controls.
RESULTS
Clinical Characteristics of Study Participants
This cross-sectional study examined cervicovaginal secretions collected from 86 Kenyan women to independently assess the association between DMPA use, as well as demographic, clinical, and behavioral factors, including vaginal drying, and the host and microbial proteome (Table 1). This sample subset was representative of all other HIV-uninfected participants in terms of age, lifetime sexually transmitted infections, herpes simplex virus type 2 seropositivity, sex acts in the previous month, vaginal washing practices, vaginal drying practices, and partner viral load [24]. Stratified by DMPA use, women who used DMPA were younger (median age = 27 vs 31; P = .01), more likely to report vaginal washing (odds ratio [OR] 2.47; 95% confidence interval [CI] = .94–3.70; P = .10), and more likely to engage in vaginal drying practices (OR = 3.54; 95% CI = 1.13–5.92; P = .03).
Enhanced Signatures of Inflammation, Injury, and Reduced Epithelial Repair Observed among Depot Medroxyprogesterone Acetate Users and Women Who Practice Vaginal Drying
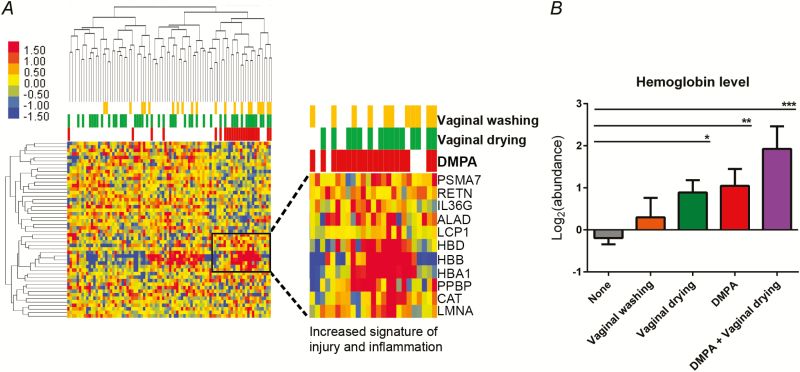
Mass spectrometry analysis of cervicovaginal secretions identified a total of 473 unique human proteins. Demographic, clinical, and behavioral variables were each analyzed to determine their impact on the genital proteome. Depot medroxyprogesterone acetate was shown to have the strongest impact on the genital proteome, affecting the largest number of proteins, followed by seminal exposure, vaginal washing, and vaginal drying (Supplementary Figure 1). Partner viral load and age had the smallest number of mucosal protein associations. Linear models were used to adjust for non-DMPA effects. Differential expression analysis of the adjusted data uncovered 62 proteins (13.1%) associated with the use of DMPA (Student’s t test, P < .05) (Supplementary Table 1). Factors significantly overabundant in DMPA users included components from blood (HBA1, HBB, HBD, ALAD, PPBP), inflammatory response proteins (IL36G, HMGB1, PPBP), and T-cell activation (GRB2, LCP1) (Figure 1A). Factors that were significantly underabundant in DMPA users included specific proteins involved in the maintenance and repair of the mucosal epithelial barrier (TFF3, GRN, F11R, KLK7, APOD, TMPRSS11E), phagocytosis (CAPG, CALR, CDC42), and protease inhibition (KNG1, SPINT1, TIMP2, SERPINF2). Gene ontology and pathway analysis showed that the major biological functions overrepresented in DMPA users were cell death, injury, and inflammation (z score > 1.3; P < .003), whereas underrepresented pathways included fibroblast proliferation and connective tissue adhesion (z score < −1.8; P < .002).
Figure 1.
Increased injury signals in the female genital tract mucosa are associated with the use of depot medroxyprogesterone acetate (DMPA) and vaginal drying. A, Hierarchical clustering of differentially abundant proteins among DMPA users and nonhormonal contraceptive user controls (Kendall’s tau distance metric, complete linkage). Inflammatory response proteins found to be overabundant in DMPA users are highlighted. B, Hemoglobin subunit delta levels were examined as a blood/injury biomarker. Individuals with levels 1.5 fold higher than the mean were considered to have an injury signature. Vaginal drying independently was significantly associated with an increased risk of injury (P = .01), as was DMPA (P = .004), and the combination of DMPA use and vaginal drying together was associated with an even greater injury signal (P = .0001). Statistical comparisons were performed using unpaired Student’s t tests. The comparisons shown are between each separate variable (vaginal wash, vaginal dry, DMPA, and DMPA with vaginal drying) and controls (women who do not vaginal wash or dry or use DMPA) where each variable was adjusted for in its own model.
Vaginal drying was associated with 30 differentially abundant proteins (Student’s t test; P < .05), as seen in comparison with women who did not practice vaginal drying (Supplementary Table 2). These included increased levels of blood proteins involved in oxygen transport (HBB, HBD) and cadherin binding (PLEC, SFN TRIM29). Proteins with known roles in focal adhesion and actin cytoskeleton organization (ACTR3, CFL1, EZR, MYH9, PLAUR, ZYX) were significantly lower. Pathway analysis identified phagocytosis as a major inhibited biological function (AZU1, EZR, MYH9, PADI4, PLAUR) (z score < −1.95; P < .002).
A comparative analysis showed that 5 factors were commonly affected by both DMPA and vaginal drying, including lower levels of antimicrobial defense proteins (AZU1, MYH9, TMPRSS11E) and higher levels of hemoglobin proteins (HBD, HBB). Independent adjusted models showed that hemoglobin protein levels were higher in women who practiced vaginal drying (fold change = 1.85; P = .01, Student’s t test) and those who used DMPA (fold change = 2.1; P = .004, Student’s t test) and that levels were greater still among DMPA users who also practiced vaginal drying (fold change = 3.8; P = .0001, Student`s t test Figure 1B) compared with controls who did not practice vaginal washing or vaginal drying and did not use DMPA. A multivariate model (Supplementary Figure 2) confirmed this barrier injury signature such that ALAD (delta-aminolevulinic acid dehydratase), a protein involved in porphyrin synthesis and a precursor for hemoglobin production, and barrier inflammation proteins (IL36G, HMGB1, HP) were positively associated with DMPA use. Meanwhile, proteins important for maintaining and repairing the mucosal barrier were concurrently decreased (TFF3, F11R) (Supplementary Table 3).
Vaginal Microbial Diversity Associates With Vaginal Drying Practices, But Not Depot Medroxyprogesterone Acetate Use
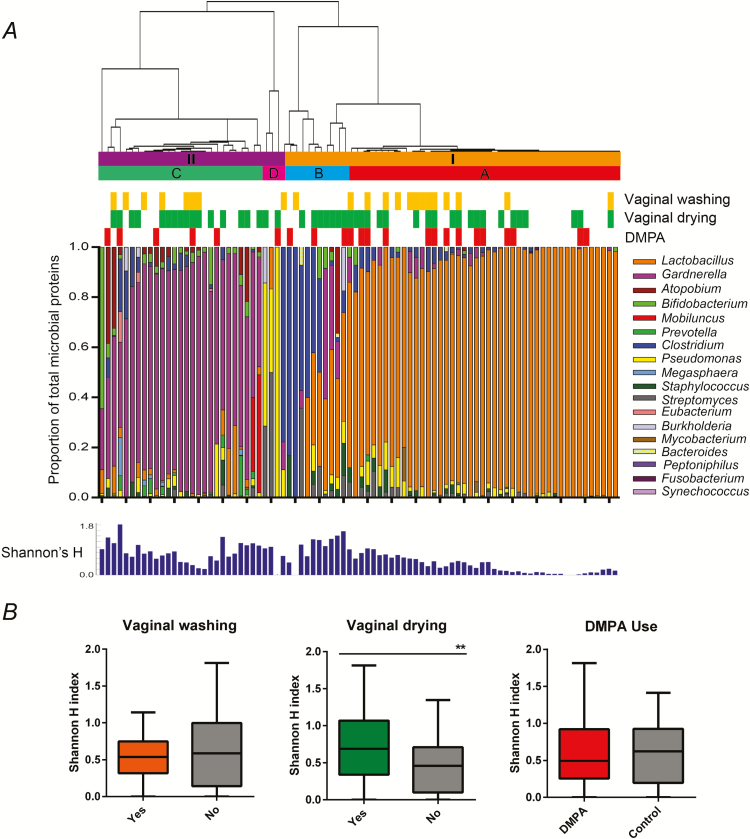
We identified 486 unique microbial proteins from 18 different genera. Clustering placed the major microbial communities into 2 groups: one dominated by predominantly Lactobacillus species (group I, n = 56, 65.1%) and the other dominated by non-Lactobacillus, including bacteria from the phyla Actinobacteria, Proteobacteria, and Bacteroidetes (group II, n = 30, 34.9%) (Figure 2A and Supplementary Figure 3). Group I included 2 subgroups, one mainly dominated by Lactobacillus (subgroup A, n = 45, 52.3%), and the other heterogeneous, containing Lactobacillus and Clostridium species (subgroup B, n = 11, 12.8%). Subgroup A species composition was 75% Lactobacillus iners, 14% Lactobacillus crispatus, and 11% other Lactobacillus species. Group II contained two subgroups, one predominantly made up of Gardnerella vaginalis with lower amounts of other anaerobes (subgroup C, n = 27, 31.4%), and a minor subgroup dominated by Pseudomonas and Streptomyces (subgroup D, n = 3, 3.5%).
Figure 2.
Vaginal bacterial profiles by mass spectrometry reveal 2 distinct microbial community structures. A, Bacterial abundances were calculated by summing normalized total spectral counts for all proteins associated with each genus. Hierarchical clustering of the samples was performed using unsupervised, average Euclidean linkage of the proportional bacterial abundance of each sample, resulting in 2 major clusters: one dominated by Lactobacillus (group I), and the other dominated by non-Lactobacillus bacteria (group II). Subgroups are as follows: A = Lactobacillus predominance; B = Lactobacillus/Clostridium heterogeneous; C = Gardnerella vaginalis predominance, D = Pseudomonas and Streptomyces predominance. B, Shannon’s diversity indices were compared between women who vaginally washed, vaginally dried, or used depot medroxyprogesterone acetate (DMPA) and their respective controls, showing increased ecological diversity associated with vaginal drying practices (Mann-Whitney U test, ** = P < .01).
There was no discernable relationship between DMPA use and vaginal washing with vaginal community structure based on bacterial community profiles or microbial diversity measurements. However, women who practiced vaginal drying had increased bacterial diversity (mean H index ± standard deviation: 0.71 ± 0.46) and lower Lactobacillus abundance compared with those who did not (H index: 0.45 ± 0.36; Mann-Whitney U test, P = .009) (Figure 2B).
DISCUSSION
It has been proposed that DMPA and intravaginal practices are associated with an increased risk of HIV acquisition [4, 5, 20, 26, 27], and the mechanisms behind this enhanced vulnerability have been partly explored but have not been fully elucidated [16, 18, 20]. Our study had several novel observations. First, DMPA use was associated with increased levels of skin inflammatory and hemoglobin proteins with concurrent decreased levels of factors responsible for maintaining the epithelial barrier [28–30], repairing damaged mucosal tissues [31, 32], and mediating inflammation [33–35]. Second, both DMPA use and vaginal drying independently associated with an increase in blood/injury biomarkers; however, both variables in combination (DMPA + vaginal drying) had the most severe injury signature, suggesting that these factors together may have compounding effects that lead to epithelial barrier weakness. Finally, vaginal microbiome alterations are evident in women who practice vaginal drying, leading to increased bacterial diversity and higher levels of anaerobic bacteria known to be associated with increased risk of HIV [36].
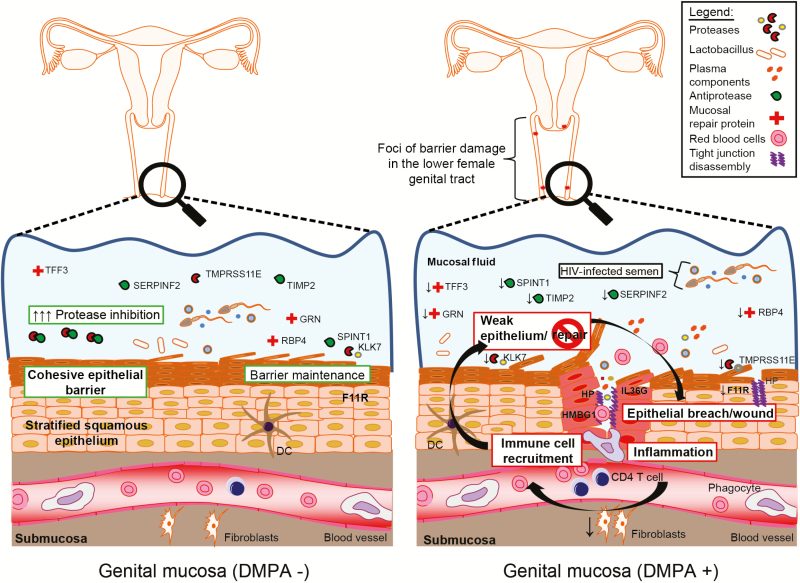
Depot medroxyprogesterone acetate has been previously associated with vaginal barrier thinning and weakness [37, 38], although this has not been the case in all studies [39, 40]. Maintaining a coherent epithelial barrier is critical for preventing migration of viruses into vaginal tissue [41]. Our findings suggest that these functions are impaired in women using DMPA, exemplified by signatures of activated bleeding and inflammation pathways, which could increase vulnerability to cervicovaginal barrier damage, particularly when faced with mechanical stress experienced during coitus. Indeed, our multivariate model showed an inverse relationship between increasing skin inflammatory proteins, including zonulin, with decreasing wound repair factors, such as trefoil factor 3, among DMPA users, which collectively could contribute to increased tissue permeability due to their known functions in tight junction disassembly [42] and tissue repair [31]. This agrees with observations of ectocervical thinning, decreased epithelial junction proteins, and an increase in barrier permeability with DMPA use by in vivo evaluation and from animal models [43, 44]. Signatures of inflammatory proteins involved in immune cell recruitment to sites of tissue injury–IL36G and HMBG1–were also upregulated [45, 46]. Although this study was not designed for the purpose of enumerating cervicovaginal immune cells, this agrees with previous observations of increased cervicovaginal HIV target cell numbers with DMPA use [20] and may represent pathways that are affected by DMPA underlying or driving their recruitment. Collectively, this suggests a model where epithelial damage at the molecular level of the genital mucosa affects barrier function, allowing HIV greater access to susceptible target cells, which are likely higher in frequency (Figure 3).
Figure 3.
Model of molecular effects of depot medroxyprogesterone acetate (DMPA) use on the vagina mucosa. The use of DMPA is associated with a molecular signature, which suggests that the genital epithelium is weak and likely more prone to injury when faced with any kind of mechanical stress. Breach signals are evident, leading to an increase in the release of inflammatory molecules, which serve to attract immune cells to the breach site. Proteins involved in this inflammatory signalling cascade are increased in women using DMPA, whereas mucosal protection and repair proteins are reduced. This lack of mucosal protection and reparation may further exacerbate risk of human immunodeficiency virus (HIV) infection in DMPA users as the portal of entry that is generated is likely slow to heal. Further to this, DMPA users who practice vaginal drying appear to exacerbate this phenomenon, which likely contributes to an even great risk of HIV infection due to this compounding effect.
Another critical component of vaginal health is the composition and function of the microbial community found within this compartment. Indeed, Lactobacillus-dominant communities are known to be important for vaginal health, as the secretion of lactic acid and antimicrobial peptides helps to maintain a low vaginal pH and deter opportunistic pathogens. A shift to other community types that contain facultative anaerobes or anaerobic bacteria such as Gardnerella vaginalis, Prevotella, Mobiluncus, Atopobium, as well as others, are associated with adverse reproductive health outcomes [15, 47, 48]. Here, we report that vaginal drying practices are associated with increased bacterial diversity and higher levels of heterogeneous communities containing G. vaginalis, with increased inflammatory signatures. This has particular relevance to HIV risk, given that women with higher microbial diversity and non-Lactobacillus communities have higher mucosal inflammation [49], increased target cells [25], and an impaired ability to repair wounds [21]. Therefore, vaginal drying practices may exacerbate HIV risk in women through modulation of vaginal microbiota, which could have consequences on mucosal barrier function and stimulation of cellular inflammatory processes.
This study was limited in that it was not designed to study DMPA usage. Menstrual cycle information was not available, and it is possible that inflammation signatures may have been affected by changes between menstrual cycle phases [23]. However, a comparison of our DMPA data set with these menstrual cycle data sets showed these signatures had little overlap (5%, 3 proteins), thus supporting the idea that the molecular signatures observed were likely restricted to DMPA use. Furthermore, samples were not collected from women who self-reported being on their period or were visibly menstruating.
In summary, DMPA use was significantly associated with signatures of epithelial wounding and reduced levels of proteins important for tissue repair. Intravaginal drying was associated with injury biomarkers and increased bacterial diversity that are related to increased mucosal inflammation [25], impaired wound healing [21], and increased HIV infection risk. This study provides new insight into the impact of DMPA use and vaginal drying on mucosal barriers, and future investigations are needed to confirm their relationship to HIV susceptibility risk in women.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. The authors would like to thank all of the study participants who donated their samples without which this study would not be possible. They would also like to thank the Mass Spectrometry Core Facility from the Public Health Agency of Canada, particularly Stuart McCorrister and Garrett Westmacott for technical support. Additional thanks to Souradet Shaw for reviewing the statistical models used in this study, and Drs Anna Häggmark-Månberg, Ulrika Qundos, and Frideborg Bradley and Ms Mariethe Ehnlund for technical support.
Disclaimer. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health, Fogarty International Center or Vanderbilt University.
Financial support. Research support for this study was provided by the Canadian Institutes for Health Research (TMI-138658 to A. B.); the National Institutes of Health (R01 AI0684316 and K24 AI087399 to C. F., 3D43TW000007-22S1 to B. L. G, 1K01AI098527 to B. L. G, P30 AI027757); the Fogarty International Center through Vanderbilt University (R24 TW007988); the Swedish Research Council; Science for Life Laboratory Stockholm; the ProNova VINN Excellence Centre for Protein Technology (VINNOVA, Swedish Governmental Agency for Innovation Systems); the Knut and Alice Wallenberg Foundation; the KTH Center for Applied Proteomics financed through the Erling-Persson Family Foundation; and the Manitoba Health Research Council (to K. D. B.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Karim QA, Kharsany AB, Frohlich JA, et al. Stabilizing HIV prevalence masks high HIV incidence rates amongst rural and urban women in KwaZulu-Natal, South Africa. Int J Epidemiol 2011; 40:922–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. UNAIDS. Global AIDS update—2016. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS (UNAIDS), 2016. [Google Scholar]
- 3. Glynn JR, Caraël M, Auvert B, et al. Why do young women have a much higher prevalence of HIV than young men? A study in Kisumu, Kenya and Ndola, Zambia. AIDS 2001; 15 Suppl 4:S51–60. [DOI] [PubMed] [Google Scholar]
- 4. Polis CB, Curtis KM, Hannaford PC, et al. An updated systematic review of epidemiological evidence on hormonal contraceptive methods and HIV acquisition in women. AIDS 2016; 30:2665–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morrison CS, Chen PL, Kwok C, et al. Hormonal contraception and the risk of HIV acquisition: an individual participant data meta-analysis. PLoS Med 2015; 12:e1001778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heffron R, Donnell D, Rees H, et al. Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. Lancet Infect Dis 2012; 12:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baeten JM, Benki S, Chohan V, et al. Hormonal contraceptive use, herpes simplex virus infection, and risk of HIV-1 acquisition among Kenyan women. AIDS 2007; 21:1771–7. [DOI] [PubMed] [Google Scholar]
- 8. Sandala L, Lurie P, Sunkutu MR, Chani EM, Hudes ES, Hearst N. ‘Dry sex’ and HIV infection among women attending a sexually transmitted diseases clinic in Lusaka, Zambia. AIDS 1995; 9 Suppl 1:S61–8. [PubMed] [Google Scholar]
- 9. Baleta A. Concern voiced over “dry sex” practices in South Africa. Lancet 1998; 352:1292. [DOI] [PubMed] [Google Scholar]
- 10. Ralph LJ, Gollub EL, Jones HE. Hormonal contraceptive use and women’s risk of HIV acquisition: priorities emerging from recent data. Curr Opin Obstet Gynecol 2015; 27:487–95. [DOI] [PubMed] [Google Scholar]
- 11. Myer L, Kuhn L, Stein ZA, Wright TC, Jr, Denny L. Intravaginal practices, bacterial vaginosis, and women’s susceptibility to HIV infection: epidemiological evidence and biological mechanisms. Lancet Infect Dis 2005; 5:786–94. [DOI] [PubMed] [Google Scholar]
- 12. McClelland RS, Lavreys L, Hassan WM, Mandaliya K, Ndinya-Achola JO, Baeten JM. Vaginal washing and increased risk of HIV-1 acquisition among African women: a 10-year prospective study. AIDS 2006; 20:269–73. [DOI] [PubMed] [Google Scholar]
- 13. Burgener A, McGowan I, Klatt NR. HIV and mucosal barrier interactions: consequences for transmission and pathogenesis. Curr Opin Immunol 2015; 36:22–30. [DOI] [PubMed] [Google Scholar]
- 14. Buve A, Jespers V, Crucitti T, Fichorova RN. The vaginal microbiota and susceptibility to HIV. AIDS 2014; 28:2333–44. [DOI] [PubMed] [Google Scholar]
- 15. Atashili J, Poole C, Ndumbe PM, Adimora AA, Smith JS. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS 2008; 22:1493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guthrie BL, Introini A, Roxby AC, et al. Depot medroxyprogesterone acetate use is associated with elevated innate immune effector molecules in cervicovaginal secretions of HIV-1-uninfected women. J Acquir Immune Defic Syndr 2015; 69:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Louw-du Toit R, Hapgood JP, Africander D. Medroxyprogesterone acetate differentially regulates interleukin (IL)-12 and IL-10 in a human ectocervical epithelial cell line in a glucocorticoid receptor (GR)-dependent manner. J Biol Chem 2014; 289:31136–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Irvin SC, Herold BC. Molecular mechanisms linking high dose medroxyprogesterone with HIV-1 risk. PLoS One 2015; 10:e0121135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Deese J, Masson L, Miller W, et al. Injectable progestin-only contraception is associated with increased levels of pro-inflammatory cytokines in the female genital tract. Am J Reprod Immunol 2015; 74:357–67. [DOI] [PubMed] [Google Scholar]
- 20. Byrne EH, Anahtar MN, Cohen KE, et al. Association between injectable progestin-only contraceptives and HIV acquisition and HIV target cell frequency in the female genital tract in South African women: a prospective cohort study. Lancet Infect Dis 2016; 16:441–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zevin AS, Xie IY, Birse K, et al. Microbiome composition and function drives wound-healing impairment in the female genital tract. PLoS Pathog 2016; 12:e1005889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Arnold KB, Burgener A, Birse K, et al. Increased levels of inflammatory cytokines in the female reproductive tract are associated with altered expression of proteases, mucosal barrier proteins, and an influx of HIV-susceptible target cells. Mucosal Immunol 2016; 9:194–205. [DOI] [PubMed] [Google Scholar]
- 23. Birse K, Arnold KB, Novak RM, et al. Molecular signatures of immune activation and epithelial barrier remodeling are enhanced during the luteal phase of the menstrual cycle: implications for HIV susceptibility. J Virol 2015; 89:8793–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guthrie BL, Lohman-Payne B, Liu AY, et al. HIV-1-specific enzyme-linked immunosorbent spot assay responses in HIV-1-exposed uninfected partners in discordant relationships compared to those in low-risk controls. Clin Vaccine Immunol 2012; 19:1798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Anahtar MN, Byrne EH, Doherty KE, et al. Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity 2015; 42:965–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brown JE, Ayowa OB, Brown RC. Dry and tight: sexual practices and potential AIDS risk in Zaire. Soc Sci Med 1993; 37:989–94. [DOI] [PubMed] [Google Scholar]
- 27. van de Wijgert JH, Chirenje ZM, Iliff V, et al. Effect of intravaginal practices on the vaginal and cervical mucosa of Zimbabwean women. J Acquir Immune Defic Syndr 2000; 24:62–7. [DOI] [PubMed] [Google Scholar]
- 28. Mandell KJ, McCall IC, Parkos CA. Involvement of the junctional adhesion molecule-1 (JAM1) homodimer interface in regulation of epithelial barrier function. J Biol Chem 2004; 279:16254–62. [DOI] [PubMed] [Google Scholar]
- 29. Igawa S, Kishibe M, Murakami M, et al. Tight junctions in the stratum corneum explain spatial differences in corneodesmosome degradation. Exp Dermatol 2011; 20:53–7. [DOI] [PubMed] [Google Scholar]
- 30. Sedghizadeh PP, Mallery SR, Thompson SJ, et al. Expression of the serine protease DESC1 correlates directly with normal keratinocyte differentiation and inversely with head and neck squamous cell carcinoma progression. Head Neck 2006; 28:432–40. [DOI] [PubMed] [Google Scholar]
- 31. Hoffmann W. Trefoil factors TFF (trefoil factor family) peptide-triggered signals promoting mucosal restitution. Cell Mol Life Sci 2005; 62:2932–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. He Z, Ong CH, Halper J, Bateman A. Progranulin is a mediator of the wound response. Nat Med 2003; 9:225–9. [DOI] [PubMed] [Google Scholar]
- 33. Mangan MS, Kaiserman D, Bird PI. The role of serpins in vertebrate immunity. Tissue Antigens 2008; 72:1–10. [DOI] [PubMed] [Google Scholar]
- 34. Schaefer BM, Maier K, Eickhoff U, Bechtel M, Kramer MD. Alpha 2-antiplasmin and plasminogen activator inhibitors in healing human skin wounds. Arch Dermatol Res 1996; 288:122–8. [DOI] [PubMed] [Google Scholar]
- 35. Bainbridge P. Wound healing and the role of fibroblasts. J Wound Care 2013; 22:8–407. [DOI] [PubMed] [Google Scholar]
- 36. Martin HL, Richardson BA, Nyange PM, et al. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis 1999; 180:1863–8. [DOI] [PubMed] [Google Scholar]
- 37. Miller L, Patton DL, Meier A, Thwin SS, Hooton TM, Eschenbach DA. Depomedroxyprogesterone-induced hypoestrogenism and changes in vaginal flora and epithelium. Obstet Gynecol 2000; 96:431–9. [DOI] [PubMed] [Google Scholar]
- 38. Butler K, Ritter J, Ellis S, et al. Analysis of putative mucosal SHIV susceptibility factors during repeated DMPA treatments in pigtail macaques. J Med Primatol 2015; 44:286–95. [DOI] [PubMed] [Google Scholar]
- 39. Bahamondes MV, Castro S, Marchi NM, et al. Human vaginal histology in long-term users of the injectable contraceptive depot-medroxyprogesterone acetate. Contraception 2014; 90:117–22. [DOI] [PubMed] [Google Scholar]
- 40. Bahamondes L, Trevisan M, Andrade L, et al. The effect upon the human vaginal histology of the long-term use of the injectable contraceptive Depo-Provera. Contraception 2000; 62:23–7. [DOI] [PubMed] [Google Scholar]
- 41. Carias AM, McCoombe S, McRaven M, et al. Defining the interaction of HIV-1 with the mucosal barriers of the female reproductive tract. J Virol 2013; 87:11388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang W, Uzzau S, Goldblum SE, Fasano A. Human zonulin, a potential modulator of intestinal tight junctions. J Cell Sci 2000; 113 Pt 24:4435–40. [DOI] [PubMed] [Google Scholar]
- 43. Tjernlund A, Carias AM, Andersson S, et al. Progesterone-based intrauterine device use is associated with a thinner apical layer of the human ectocervical epithelium and a lower ZO-1 mRNA expression. Biol Reprod 2015; 92:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Quispe Calla NE, Vicetti Miguel RD, Boyaka PN, et al. Medroxyprogesterone acetate and levonorgestrel increase genital mucosal permeability and enhance susceptibility to genital herpes simplex virus type 2 infection. Mucosal Immunol 2016; 9:1571–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schiraldi M, Raucci A, Muñoz LM, et al. HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J Exp Med 2012; 209:551–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gresnigt MS, van de Veerdonk FL. Biology of IL-36 cytokines and their role in disease. Semin Immunol 2013; 25:458–65. [DOI] [PubMed] [Google Scholar]
- 47. Haahr T, Jensen JS, Thomsen L, Duus L, Rygaard K, Humaidan P. Abnormal vaginal microbiota may be associated with poor reproductive outcomes: a prospective study in IVF patients. Hum Reprod 2016; 31:795–803. [DOI] [PubMed] [Google Scholar]
- 48. Onderdonk AB, Delaney ML, Fichorova RN. The human microbiome during bacterial vaginosis. Clin Microbiol Rev 2016; 29:223–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hong KH, Hong SK, Cho SI, et al. Analysis of the vaginal microbiome by next-generation sequencing and evaluation of its performance as a clinical diagnostic tool in vaginitis. Ann Lab Med 2016; 36:441–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.