Abstract
Background
Differentiation of malignant from benign liver tumors remains a challenging problem. In recent years, mass spectrometry (MS) technique has emerged as a promising strategy to diagnose a wide range of malignant tumors. The purpose of this study was to establish classification models to distinguish benign and malignant liver tumors and identify the liver cancer-specific peptides by mass spectrometry.
Material/Methods
In our study, serum samples from 43 patients with malignant liver tumors and 52 patients with benign liver tumors were treated with weak cation-exchange chromatography Magnetic Beads (MB-WCX) kits and analyzed by the Matrix-Assisted Laser Desorption Time of Flight Mass Spectrometry (MALDI-TOF-MS). Then we established genetic algorithm (GA), supervised neural networks (SNN), and quick classifier (QC) models to distinguish malignant from benign liver tumors. To confirm the clinical applicability of the established models, the blinded validation test was performed in 50 clinical serum samples. Discriminatory peaks associated with malignant liver tumors were subsequently identified by a qTOF Synapt G2-S system.
Results
A total of 27 discriminant peaks (p<0.05) in mass spectra of serum samples were found by ClinPro Tools software. Recognition capabilities of the established models were 100% (GA), 89.38% (SNN), and 80.84% (QC); cross-validation rates were 81.67% (GA), 81.11% (SNN), and 86.11% (QC). The accuracy rates of the blinded validation test were 78% (GA), 84% (SNN), and 84% (QC). From the 27 discriminatory peptide peaks analyzed, 3 peaks of m/z 2860.34, 2881.54, and 3155.67 were identified as a fragment of fibrinogen alpha chain, fibrinogen beta chain, and inter-alpha-trypsin inhibitor heavy chain H4 (ITIH4), respectively.
Conclusions
Our results demonstrated that MS technique can be helpful in differentiation of benign and malignant liver tumors. Fibrinogen and ITIH4 might be used as biomarkers for the diagnosis of malignant liver tumors.
MeSH Keywords: Biological Markers; Carcinoma, Hepatocellular; Mass Spectrometry
Background
Malignant liver tumors are considered to be one of the major public health problems in the world because of late diagnosis and failure of treatments. There are mainly three kinds of malignant liver tumors in adults: hepatocellular carcinoma (HCC), cholangiocarcinoma (CCA), and metastatic liver tumors. HCC is the most common and highly lethal liver tumor. Approximately 500,000 people die of HCC each year, and more than 50% of the cases occur in China. It is the fifth leading cause of cancer death worldwide, and the Asia-Pacific region is a high-prevalence area [1]. CCA, the second most common primary hepatic malignancy, originates from the bile duct epithelial cells and accounts for 10% to 20% of the deaths from hepatobiliary malignancies [2]. Metastatic liver tumors are malignant tumors in the liver that have spread from other areas of the body. Clinically, these malignant liver tumors are generally asymptomatic in early stages and easily lead to missed diagnosis. In addition, they also require differentiation from benign liver lesions to avoid unnecessary interventions. Therefore, it is important to develop accurate diagnostic methods to distinguishing between benign and malignant liver tumors [3].
At present, serologic markers, such as α-fetoprotein (AFP) and imaging technology, are the main methods to detect malignant liver tumors. However, serologic markers are limited by their low sensitivity, and imaging methods, such as computed tomography (CT) and magnetic resonance imaging (MRI), are limited by their high cost. Ultrasound and elastography have been used as screening methods for liver tumors, but their accuracy was only about 70% [4–6]. In addition, these imaging methods have the risk of radiation exposure. In recent years, mass spectrometry (MS) has been used in clinical diagnosis and medical research fields, including discovery of cancer biomarkers, diagnosis of bacterial infection, and identification of mutations and genotypes of virus [7–11]. Mass spectrometry technique is more precise, rapid, and cost-effective than the traditional methods. In this study, we applied Matrix-Assisted Laser Desorption Time of Flight Mass Spectrometry (MALDI-TOF-MS) combined with weak cation-exchange chromatography Magnetic Beads (MB-WCX) to establish classification models to distinguish benign and malignant liver tumors and identify the liver cancer-specific peptides by liquid chromatography-tandem mass spectrometry (LC-MS/MS).
Material and Methods
Subjects
All serum samples were obtained from 302 Military Hospital, Beijing, China. Subjects included 66 patients with malignant liver tumors (34 HCC, 22 CCA, and 10 metastatic liver tumors) and 79 patients with benign liver tumors (34 liver cirrhosis lesions, 30 liver hemangiomas, 11 hepatic cysts, 3 hepatic adenomas, and 1 focal nodular hyperplasia). The study characteristics are shown in Table 1. All study subjects were first-visit outpatients who were newly diagnosed with focal liver lesions by ultrasound. The final diagnoses were made by liver histopathology or MRI based on guidelines from the Ministry of Health of the People’s Republic of China [12] and the guideline from the Chinese Society of Hepatology and the Chinese Society of Infectious Diseases [13,14]. Some patients with the following conditions were excluded: other systemic disease such as diabetes and hypertension; prior surgery, interventional therapy, radiotherapy, chemotherapy, and other invasive treatment; and severe complications such as upper gastrointestinal bleeding and hepatic encephalopathy. The study procedures were approved by the Ethics Committee of the 302 Military Hospital of China, and written informed consent obtained from each subject.
Table 1.
Clinical information of the liver tumor patients enrolled in this study.
| Variable | Malignant liver tumors (n=66) | Benign liver tumors (n=79) |
|---|---|---|
| Age, yr | 53 (29–83) | 48 (23–70) |
| Sex | ||
| Male | 52 | 60 |
| Female | 14 | 19 |
| Set | ||
| Training set | 43 | 52 |
| Validation set | 23 | 27 |
| Diagnostic methods | ||
| MRI | 66 | 79 |
| Histopathology | 36 | 9 |
| Etiology | ||
| HBV | 48 | 52 |
| HCV | 13 | 17 |
| Others | 5 | 10 |
| Laboratory data | ||
| Bilirubin (μmol/L) | 18.5 (3.6–293.9) | 13.3 (2.5–193.4) |
| ALT (U/L) | 35 (9–1194) | 27 (9–118) |
| AST (U/L) | 44 (10–930) | 27 (11–99) |
| Albumin (g/L) | 36.4±5.5 | 39.3±5.9 |
ALT – alanine aminotransferase; AST – aspartate aminotransferase. Data was shown as median (range) or mean ±SD if normally distributed.
Sample preparation
The serum samples were collected in a 5 mL vacuum blood collection tube without anticoagulant, then centrifuged for 5 min at 12,000 g at room temperature. The serum samples were distributed into 1.5 mL aliquots and stored at −80°C. The frozen samples should be thawed at room temperature for 15 min before use. MB-WCX (Bruker Daltonik, Germany) kits were used according to the manufacturer’s protocol. We added 10 μL of MB-WCX beads and 10 μL of binding solution (BB) in 200 μL PCR tubes. Then we added 5 μL of serum samples and mixed thoroughly by pipetting up and down. Subsequently, samples were incubated at room temperature for 5 min, and then we used a magnetic separator to collect the beads. The supernatant was removed, and the magnetic beads were washed three times using washing solution (WB). Finally, we added 5 μL of elution solution (EB) and 4 μL of stabilization buffer (SB) to elute the peptide fraction from the magnetic beads.
MALDI-TOF-MS analysis
We transferred 1 μL of supernatant from the PCR tubes onto a 384 ground steel target plate, and then the samples were air-dried. Every dried sample was mixed with 1 μL of MALDI matrix (60 mg of α-cyano-4-hydroxycinnamic acid [HCCA] dissolved in 1 mL of acetonitrile and 1 mL of 2% trifluoroacetic acid [TFA]; Bruker Daltonik, Germany). Measurements were performed using an Autoflex MALDI-TOF-MS (Bruker Daltonik, Germany) instrument and FlexControl software (version 3.0; Bruker). The parameter settings were as follows: positive linear mode (LP); mass range: 1000 Da to 20,000 Da; laser power: 60%. For each spot, 500×6 shots were acquired. The mass calibration was performed with the Peptide Calibration Standard (700–3500 Da; Bruker Daltonik) and Peptide Calibration Standard I (5000–20,000 Da; Bruker Daltonik). To evaluate the reproducibility of the MB-WCX bead kits and instrument, sera from eight normal subjects were treated by MB-WCX bead, and each of them was detected for 8 repeats. The mean value of the coefficient of variance (CV) for all peaks was calculated to evaluate intra- and inter-reproducibility.
Data analysis and model generation
Data analysis was performed with ClinPro Tools Software and Flex analysis software (version 3.0; Bruker). All the spectra were normalized, and baseline subtraction and smoothing were performed with Flex analysis software. Peaks with signal-to-noise ratio (S/N) >5 were picked out, and statistical analysis was performed. Data that were normally distributed were analyzed with Student’s t tests, and non-normally distributed data were analyzed by the Wilcoxon test. In order to construct the diagnostic models to distinguish benign and malignant liver tumors, all samples were randomly divided into 2 subsets: training set and validation set. A total of 95 serum samples (43 malignant liver tumor patients and 52 benign liver lesion patients) were used to construct classification models as training samples. Three different machine-learning algorithms were used as follows: genetic algorithm (GA), supervised neural network (SNN), and quick classifier (QC). To confirm the clinical applicability of the models constructed, the blinded validation test was conducted with 50 other serum samples. Liver histopathology or MRI results served as the gold standard. Accuracy, sensitivity, specificity, positive and negative predictive values (PPV and NPV), and Youden’s index were calculated to assess the performances of the models.
Identification of peptide markers
Samples purified by MB-WCX beads were desalted using ZipTipC18 pipette (Millipore, Bedford, Massachusetts, USA) tips according to the procedure described below. ZipTips were activated and equilibrated with 50% (vol/vol) acetonitrile. Then, a TFA-acidified sample was applied to the ZipTip, followed by two washes with 10 μL of 0.1% (vol/vol) TFA solution. Bound proteins were eluted using a 50% (vol/vol) acetonitrile solution containing 0.1% (vol/vol) TFA. Analysis of the eluted peptides was performed using a qTOF Synapt G2-S system (Waters, Pittsburgh, Pennsylvania, USA) equipped with an ESI source operated in the positive ion mode. The source temperature was set at 100°C with a flow rate of 200 nL/min. The capillary and cone voltages were set to 3000 and 40 V. MS data were collected for m/z values in the range of 100 Da and 2000 Da with a scan time of 0.5 s, and MS/MS data were collected between 50 Da and 1600 Da with a scan time of 0.2 s. The MS/MS data were searched on Mascot (http://www.matrixscience.com). Peptide mass tolerance was 10 ppm, fragment ion mass tolerance was 0.01, and the mass type of the parent peptide and peptide fragment was at monoisotopic.
Results
Discrepancy analysis of mass spectra
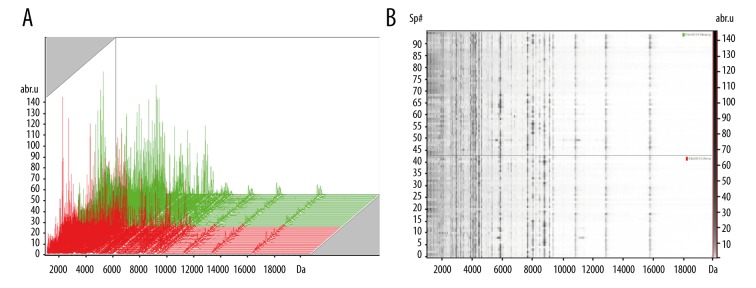
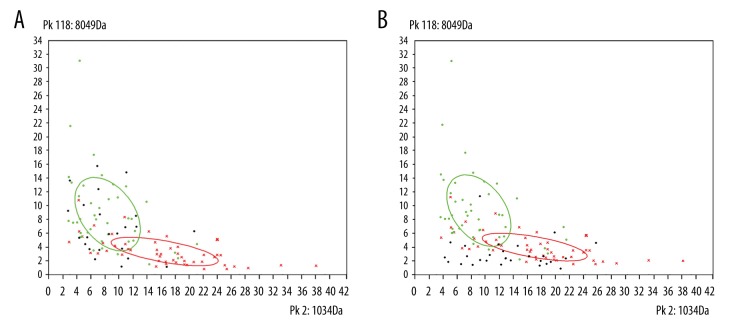
We performed a reproducible study for the MB-WCX bead and instrument. The CV of inter-reproducibility was 18.02% (4.46~32.16%), and that of intra-reproducibility was 14.97% (4.03~55.4%). After discrepancy analysis of serum peptidome fingerprints by using ClinPro Tools Software, a total of 129 peaks were found and 27 of them were significantly different (p<0.05) between the benign and malignant liver tumor groups. The statistical results for the 27 peaks are shown in Table 2. We also found that 16 peaks increased significantly in the malignant liver tumor group; in contrast, the other 11 peaks were clearly reduced in the malignant liver tumor group. To demonstrate the differences visually, the simulated two-dimensional (2D) gel electrophoresis map and mass spectra map are shown in Figure 1. The 2D peak distribution map of first 2 peaks showed that the 2 groups of peaks were separated completely, and we could discriminate the benign and malignant liver tumors easily (Figure 2).
Table 2.
ClinProTools peak statistics for the differential peaks between malignant and benign liver tumors.
| Index | Mass | DAve | PTTA | PWKW | PAD | Ave (benign) | Ave (malignant) | SD (benign) | SD (malignant) | CV (benign) | CV (malignant) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Down-regulated peptides in malignant group | |||||||||||
| 2 | 1034 | 9.77 | <0.000001 | 0.00000213 | 0.000172 | 19.91 | 10.13 | 9.16 | 5.6 | 46.02 | 55.26 |
| 29 | 2860.34** | 2.32 | <0.000001 | <0.000001 | <0.000001 | 4.69 | 2.38 | 2.27 | 0.89 | 48.31 | 37.54 |
| 60 | 4105.74 | 1.69 | <0.000001 | <0.000001 | 0.0028 | 4.44 | 2.74 | 1.57 | 1.04 | 35.3 | 37.91 |
| 5 | 1072.04 | 8.03 | 0.00000125 | 0.00000272 | 0.00000723 | 16.47 | 8.44 | 8.25 | 4.41 | 50.11 | 52.28 |
| 117 | 8035.96 | 9.04 | 0.00000399 | <0.000001 | <0.000001 | 11.77 | 2.73 | 10.13 | 2.46 | 86.09 | 90.05 |
| 3 | 1052.51* | 11.31 | 0.00000309 | 0.00000116 | <0.000001 | 22.13 | 10.82 | 12.52 | 5.7 | 56.55 | 52.65 |
| 30 | 2881.54** | 3.33 | 0.00000314 | <0.000001 | <0.000001 | 5.79 | 2.46 | 3.67 | 0.8 | 63.44 | 32.35 |
| 11 | 1281.81* | 4.37 | 0.00000629 | 0.0000296 | 0.00000404 | 10.88 | 6.52 | 4.93 | 2.82 | 45.25 | 43.29 |
| 23 | 2660.13 | 5.62 | 0.0000227 | 0.00000641 | 0.0000552 | 12.93 | 7.31 | 5.84 | 5.06 | 45.18 | 69.24 |
| 10 | 1277.26 | 4.83 | 0.0000294 | 0.000017 | <0.000001 | 10.01 | 5.18 | 6.19 | 2.86 | 61.83 | 55.18 |
| 7 | 1081.8 | 4.21 | 0.00013 | 0.000206 | 0.00000118 | 10.22 | 6.01 | 5.89 | 2.83 | 57.58 | 47.07 |
| Up-regulated peptides in malignant group | |||||||||||
| 118 | 8049.18 | 6.27 | <0.000001 | <0.000001 | <0.000001 | 3.74 | 10.02 | 2.38 | 6.15 | 63.69 | 61.42 |
| 8 | 1222.97 | 4.65 | <0.000001 | 0.00000322 | 0.00000323 | 4.88 | 9.53 | 2.62 | 4.51 | 53.7 | 47.34 |
| 9 | 1238.43 | 4.88 | 0.00000106 | 0.00000136 | 0.00000244 | 5.52 | 10.41 | 2.86 | 4.86 | 51.84 | 46.73 |
| 58 | 4068.99* | 10.41 | 0.00000134 | <0.000001 | <0.000001 | 11.56 | 21.96 | 4.63 | 10.51 | 40.03 | 47.84 |
| 57 | 4062.03* | 13.51 | 0.00000309 | <0.000001 | <0.000001 | 8.08 | 21.59 | 4.88 | 14.66 | 60.41 | 67.89 |
| 55 | 3968.97* | 5.29 | 0.00000309 | 0.00000116 | <0.000001 | 2.03 | 7.33 | 0.85 | 5.83 | 41.65 | 79.58 |
| 54 | 3952.94* | 6.43 | 0.00000314 | <0.000001 | <0.000001 | 3.19 | 9.62 | 1.15 | 7.15 | 36.06 | 74.28 |
| 6 | 1077.69 | 7.04 | 0.00000309 | 0.00000641 | 0.0000754 | 8.63 | 15.67 | 4.48 | 7.48 | 51.9 | 47.7 |
| 56 | 4050.90* | 7 | 0.00000499 | 0.0000096 | 0.288 | 13.02 | 20.02 | 6.56 | 6.13 | 50.41 | 30.64 |
| 59 | 4086.99 | 5.06 | 0.0000077 | 0.00000694 | 0.0466 | 9.95 | 15.01 | 4.54 | 4.82 | 45.63 | 32.13 |
| 4 | 1066.07 | 10.49 | 0.0000251 | 0.000011 | <0.000001 | 9.99 | 20.48 | 5.84 | 13.37 | 58.44 | 65.27 |
| 26 | 2709.6 | 2.36 | 0.0000997 | 0.000025 | <0.000001 | 2.71 | 5.07 | 1.36 | 3.13 | 50.07 | 61.72 |
| 41 | 3155.66** | 8.07 | 0.00013 | 0.00000653 | <0.000001 | 2.66 | 10.73 | 1.36 | 11.43 | 51.24 | 106.54 |
| 69 | 4295.6 | 5.67 | 0.000166 | 0.0000417 | <0.000001 | 5.18 | 10.85 | 4.6 | 7.39 | 88.85 | 68.15 |
| 42 | 3240.26 | 4.97 | 0.000179 | 0.0000296 | <0.000001 | 6.34 | 11.31 | 4.21 | 6.45 | 66.45 | 57 |
| 68 | 4277.79 | 5.24 | 0.000179 | <0.000001 | <0.000001 | 2.33 | 7.58 | 1.17 | 7.63 | 49.94 | 100.67 |
The peaks are sorted according to the P-value in descending order. PTTA – p-value of t-test or ANOVA test; PWKW – p-value of Wilcoxon test or Kruskal-Wallis test; PAD – p-value of Anderson-Darling test.
The identified peptide peaks with Mascot score lower than threshold value.
The identified peptide peaks with Mascot score higher than threshold value.
Figure 1.
(A) The whole mass spectra for all samples (green, benign group; red, malignant group). (B) The simulated two-dimensional gel electrophoresis views of all samples (the upload, benign group; the download, malignant group).
Figure 2.
(A) Two-dimensional (2D) peak distribution view of peptides with m/z 1034 (x-axis) and 8049 (y-axis) for malignant validation set samples. (B) 2D peak distribution view of peptides with m/z 1034 (x-axis) and 8049 (y-axis) for benign validation set samples. Red cross, malignant group of training set; green hollow circle, benign group of training set; black solid circle, validation set.
Establishment of classification models for liver tumors
We used the ClinPro Tools software to analyze the data and establish three identified models (GA, SNN, and QC models). Results of identification of malignant liver tumors by the 3 models were as follows: the GA model had a cross-validation of 81.67% and a recognition capability of 100%, the QC model had a cross-validation of 86.11% and a recognition capability of 80.84%, and the SNN model and a cross-validation of 81.11% and a recognition capability of 89.38%. From the results shown in Table 3 we could infer that by using these algorithm models, we could achieve above an 80% accuracy rate for detecting malignant liver tumors. Therefore, blinded validation study with more clinical samples should be done with the follow-up research.
Table 3.
Validation results of three liver tumor classified models.
| Recognition rate (%) | Cross-validation rate (%) | |||||
|---|---|---|---|---|---|---|
| Malignant | Benign | Total | Malignant | Benign | Total | |
| GA | 100.00 | 100.00 | 100.00 | 83.33 | 80.00 | 81.67 |
| SNN | 88.37 | 90.38 | 89.38 | 72.22 | 90.00 | 81.11 |
| QC | 67.44 | 94.23 | 80.84 | 72.22 | 100.00 | 86.11 |
Blinded validation of clinical samples
A total of 50 serum samples, including 23 malignant liver tumors and 27 benign liver tumors, were successfully analyzed as validation samples by MALDI-TOF-MS models. Among these 50 samples, 39 (78%) were correctly identified by the GA models with 4 false negatives and 7 false positives, giving a sensitivity of 82.61% and a specificity of 74.07%. A total of 42 (84%) samples were correctly identified by both the SNN and QC models with 5 false negatives and 3 false positives, giving a sensitivity of 78.26% and a specificity of 88.89%. The diagnostic performances of 3 algorithm models are listed in Table 4.
Table 4.
Diagnostic performances of 3 algorithms models with blinded validation samples.
| GA | SNN | QC | |
|---|---|---|---|
| Accuracy (%) | 78.00 | 84.00 | 84.00 |
| Sensitivity (%) | 82.61 | 78.26 | 78.26 |
| Specificity (%) | 74.07 | 88.89 | 88.89 |
| Positive predictive values (%) | 73.08 | 85.71 | 85.71 |
| Negative predictive values (%) | 83.33 | 82.76 | 82.76 |
| Positive likelihood ratio | 3.19 | 7.04 | 7.04 |
| Negative likelihood ratio | 0.23 | 0.24 | 0.24 |
| Youden’s index | 0.57 | 0.67 | 0.67 |
Peptide identification
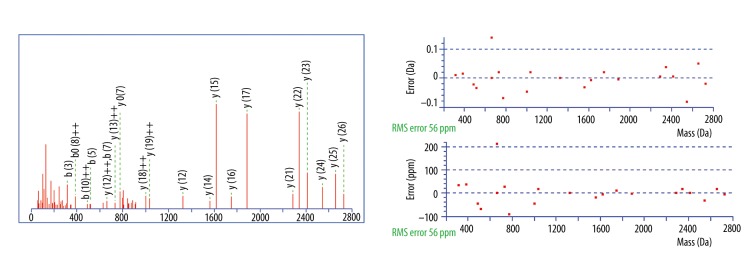
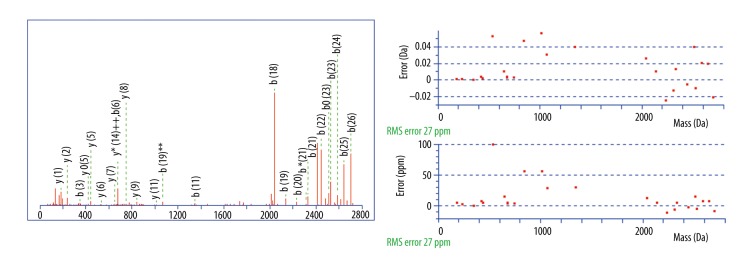
A total of 27 differential peaks were found by using the peptidomic profiling analysis, but only 10 were identified (Table 5). Among them, peaks at m/z 2860.34, 2881.54, and 3155.67 were positively identified by a Mascot database search. The peptide 2860.34 Da was identified with high probability as a fragment of fibrinogen alpha chain with the amino acid sequence MADEAGSEADHEGTHSTKRGHAKSRPV; the MASCOT score was 67. The peptide 2881.54 Da was a fragment of fibrinogen beta chain with the amino acid sequence GHRPLDKKREEAPSLRPAPPPISGGGY; the MASCOT score was 65. The peptide 3155.67 Da was a fragment of inter-alpha-trypsin inhibitor heavy chain H4 with the amino acid sequence NVHSGSTFFKYYLQGAKIPKPEASFSPR; the MASCOT score was 72. Mascot Search Results of 3 identified peptides are shown in Figure 3–5.
Table 5.
Summary of proteins identified by LC-MS/MS.
| Mr | ppm | Score | Expect | Protein source | Peptide |
|---|---|---|---|---|---|
| 2860.34 | 5.12 | 67 | 0.0023 | Fibrinogen alpha chain | K.MADEAGSEADHEGTHSTKRGHAKSRPV.R |
| 4062.03 | −19.05 | 6 | 4.4e+003 | Histone acetyltransferase | RQQQLQHRLQQAQMLRRRMASMQRTGVVGQQQGL |
| 4068.99 | −3.24 | 1 | 1.6e+004 | Angiomotin | ATSGVKAHPPVTSAPLSPPQPNDLYKNPTSSSEFYKAQG |
| 1052.50 | −27.57 | 7 | 7e+002 | Constitutive coactivator of PPAR-gamma-like protein 2 | REKNHLQE |
| 3968.96 | 15.6 | 16 | 5.3e+002 | Dedicator of cytokinesis protein 1 | FDWVIMNMVQNKVFLRAINQYADMLNKKFLDQ |
| 3952.94 | 13.3 | 18 | 3e+002 | Neurexin-3 | RVKLMVNLDCIRINCNSSKGPETLYAGQKLNDNE |
| 2881.54 | 3.13 | 65 | 0.0022 | Fibrinogen beta chain | R.GHRPLDKKREEAPSLRPAPPPISGGGY.R |
| 4050.90 | −4.70 | 9 | 2.3e+003 | Receptor-type tyrosine-protein phosphatase O | VDILGLVSEMRSYRMSMVQTEEQYIFIHQCVQL |
| 3155.67 | 15.5 | 72 | 0.00084 | Inter-alpha-trypsin inhibitor heavy chain H4 | R.NVHSGSTFFKYYLQGAKIPKPEASFSPR.R |
| 1281.81 | 29.0 | 8 | 43 | Uncharacterized protein C17orf59 homolog | VANNLQLKIRL |
Figure 3.
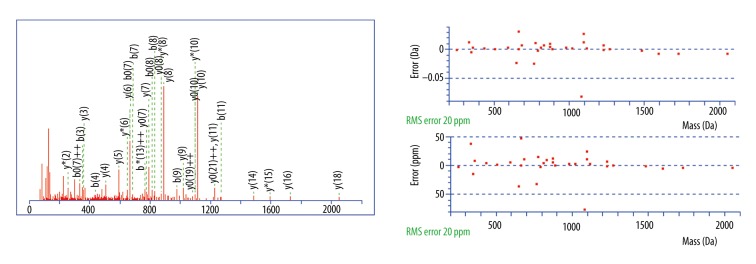
MS/MS fragmentation and sequence data for identified peptides of Mascot Search Results: The m/z 2860.34 peak (MADEAGSEADHEGTHSTKRGHAKSRPV) was identified as a fragment of fibrinogen alpha chain with an ion score of 67.
Figure 4.
MS/MS fragmentation and sequence data for identified peptides of Mascot Search Results: The m/z 2881.54 peak (GHRPLDKKREEAPSLRPAPPPISGGGY) was identified as a fragment of fibrinogen beta chain with an ion score of 65.
Figure 5.
MS/MS fragmentation and sequence data for identified peptides of Mascot Search Results: The m/z 3155.67 peak (NVHSGSTFFKYYLQGAKIPKPEASFSPR) was identified as a fragment of inter-alpha-trypsin inhibitor heavy chain H4 with an ion score of 72.
Discussion
Malignant liver tumors always present as focal lesions in liver, but not all focal lesions are malignant. Most benign tumors also present similar clinical and imaging features, and they do not require aggressive treatment. Therefore, it is very important for making a treatment plan to differentiate benign from malignant tumors. Despite widespread current availability of advanced imaging techniques, the distinction between benign and malignant liver tumors remains very difficult to determine. In addition, advanced imaging techniques require complex procedures or high costs that many patients can’t afford. Liver biopsy was considered the reference method for the diagnosis of malignant liver tumors, but it has many limitations such as invasiveness, sampling error, and inter-observer variability [15]. However, compared with imaging and histological methods, serum tests are more convenient and rapid with a relatively low cost, and they also can be used for dynamic monitoring.
MALDI-TOF-MS is a powerful tool for the detection and identification of proteins, peptides, polysaccharides, nucleic acids, and other biological molecules, and it has been used in clinical diagnosis and medical research successfully. In the past few years, MALDI-TOF-MS has been used for the expression analysis of low-molecular-weight serum proteins and peptides. As we know, human serum contains many different peptides that are thought to be fragments of large proteins. Some of these peptides may have the potential to be biomarkers for prognosis or diagnosis of diseases, because their presence/absence or relative abundances are correlated with health status of patients. MB-WCX were developed for enrichment and purification of low-mass peptides and proteins (1–20 kDa) directly from the biological samples prior to MALDI-TOF-MS analysis. Many studies have shown that it is an effective strategy to increase the sensitivity and reproducibility of the mass spectra [16–18].
There are many published reports that focused on the mass spectrometry technique in evaluation of liver diseases [19–22]. Mass spectrometry proteomic analysis for differentiating malignant liver tumors is also feasible now. He et al. established a neural network model to diagnose whether HCC patients had accompanying bone metastasis, and the sensitivity and specificity of this model were 85.29% and 85.71%, respectively [23]. Sandanayake et al. developed a predictive model to detect biliary tract cancer (BTC), and it had a sensitivity of 79.5% and a specificity of 83.9% in discriminating BTC from benign biliary disease [24]. Scarlett et al. developed a classification model to distinguish cholangiocarcinoma (CC) serum samples from benign serum samples, and it had 65.0% sensitivity and 70.0% specificity [25]. However, these studies were for diagnosis of a particular cancer and not for distinguishing malignant from benign liver tumors. Our study indicated that MALDI-TOF-MS can be helpful in differentiation of malignant liver tumors from benign liver tumors.
Although the MALDI-TOF-MS method has greatly improved the possibility of finding new potential biomarkers for diseases, interference of protein produced due to a stress reaction or chronic disease is a well-known problem in biomarker research. For example, some patients with terminal malignant tumors always have accompanying severe complications or have undergone invasive treatment; thus, their serum components have changed greatly. In order to rule out the influence of the patient’s physical condition, some patients who had invasive treatment, other chronic disease, and severe complications were excluded in our study. In addition, we handled all the serum samples using MB-WCX beads and detected them on the same day to avoid day-to-day variation. In this study, we applied MALDI-TOF-MS technique combined with MB-WCX kits to analyze 95 serum samples from 43 patients with malignant liver tumors and 52 patients with benign liver lesions. A total of 27 differentially expressed peaks (p<0.05) were found: 16 of them were over-expressed in patients with malignant liver tumors, whereas the others were under-expressed. Three peptide peaks of m/z 2860.34, 2881.54, and 3155.67 were identified with high probability as a fragment of fibrinogen alpha chain, fibrinogen beta chain, and inter-alpha-trypsin inhibitor heavy chain H4 (ITIH4), respectively. Fibrinogen, which participates in the process of blood clotting, is synthesized in the liver. Many previous studies had shown that fragments of fibrinogen alpha or beta chains were associated with liver tumors [21,24,26,27]. ITIH4, an acute-phase glycoprotein produced primarily in the liver, is involved in liver development and stabilization of the extracellular matrix (ECM), and its expression is altered in liver disease [28]. Noh et al. [29] confirmed that ITIH4 could be used as a diagnostic and prognostic indicator in patients with hepatitis B virus-associated hepatocellular carcinoma. In the present study, we have demonstrated that fragments of fibrinogen and ITIH4 have been obviously altered in the serum of malignant liver tumors patients, and they have the potential to be developed as ideal biomarkers for the identification of malignant liver tumors, which will be studied in our further work.
The classification models based on these differential peaks showed different performances. The GA model obtained an 81.67% cross-validation rate and a 100% recognition rate. The QC model obtained an 86.11% cross-validation rate and a 80.84% recognition rate. The SNN model obtained an 81.11% cross-validation rate and a 89.38% recognition rate. They can be used for distinguishing between benign and malignant liver tumors and also can meet clinical-application needs after further confirmation. The results of blinded validation are robust, since the accuracy rate of these models is around 80%. The GA model obtained 82.61% sensitivity and 74.07% specificity, which was better sensitivity and lower specificity compared with the QC and SNN models. Both the QC and SNN models obtained 78.26% sensitivity and 88.89% specificity. We believe that a combination of three models might be a more reliable approach for the screening of liver tumors.
There are still some things to be improved in our study. The numbers of samples tested in our study limited the reliability of the models, and the results of the blinded validation study are not yet reliable enough for routine clinical diagnosis. Despite this, we found some specific peaks to distinguish benign and malignant liver tumors, and then we established three classification models. The results of validation were very robust.
Conclusions
MALDI-TOF-MS can be helpful in differentiation of benign and malignant liver tumors. MALDI-TOF-MS combined with MB-WCX should be used as an additive method with conventional imaging methods. From the results of identification using the LC-MS/MS method, we found that fibrinogen and ITIH4 might be used as biomarkers for the diagnosis of malignant liver tumors.
Footnotes
Source of support: This work was supported by the Capital Medical Development Project of China (2014-2-5031)
References
- 1.El Serag HB, Rudolph KL. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(7):2557–76. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 2.Blechacz B, Gores GJ. Cholangiocarcinoma: Advances in pathogenesis, diagnosis, and treatment. Hepatology. 2008;48(1):308–21. doi: 10.1002/hep.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamphues C, Engel S, Denecke T, et al. Safety of liver resection and effect on quality of life in patients with benign hepatic disease: Single center experience. BMC Surg. 2011;11:16. doi: 10.1186/1471-2482-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tian L, Wang Y, Xu D, et al. Serological AFP/Golgi protein 73 could be a new diagnostic parameter of hepatic diseases. Int J Cancer. 2011;129(8):1923–31. doi: 10.1002/ijc.25838. [DOI] [PubMed] [Google Scholar]
- 5.Özmen E, Adaletli I, Kayadibi Y, et al. The impact of share wave elastography in differentiation of hepatic hemangioma from malignant liver tumors in pediatric population. Eur J Radiol. 2014;83(9):1691–97. doi: 10.1016/j.ejrad.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Ma X, Zhan W, Zhang B, et al. Elastography for the differentiation of benign and malignant liver lesions: A meta-analysis. Tumour Biol. 2014;35(5):4489–97. doi: 10.1007/s13277-013-1591-4. [DOI] [PubMed] [Google Scholar]
- 7.Taneja S, Ahmad I, Sen S, et al. Plasma peptidome profiling of acute hepatitis E patients by MALDI-TOF/TOF. Proteome Sci. 2011;9:5. doi: 10.1186/1477-5956-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J, Wang X, Lin S, et al. Identification of kininogen-1 as a serum biomarker for the early detection of advanced colorectal adenoma and colorectal cancer. PLoS One. 2013;8(7):e70519. doi: 10.1371/journal.pone.0070519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huijbers A, Velstra B, Dekker TJ, et al. Proteomic serum biomarkers and their potential application in cancer screening programs. Int J Mol Sci. 2010;11:4175–93. doi: 10.3390/ijms11114175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cobo F. Application of maldi-tof mass spectrometry in clinical virology: A review. Open Virol J. 2013;27:84–90. doi: 10.2174/1874357920130927003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiu F, Gao YH, Jiang CG, et al. Serum proteomic profile analysis for endometrial carcinoma detection with MALDI-TOF MS. Arch Med Sci. 2010;6:245–52. doi: 10.5114/aoms.2010.13903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. [Guidelines for diagnosis and treatment of primary hepatocellular carcinoma]. Chin Clin Oncol. 2011;16(10):929–46. [in Chinese] [Google Scholar]
- 13.Chinese Society of Hepatology and Chinese Society of Infectious Diseases, Chinese Medical Association. [The guidelines of prevention and treatment for chronic hepatitis B (2010 version)]. Chin J Exp Clin Infect Dis (Electronic Version) 2011;5(1):79–100. [in Chinese] [Google Scholar]
- 14.Hepatotogy Branch, Infectious and Parasitology branch, Chinese Medical Association. [Guideline of prevention and treatment of hepatitis C]. Zhonghua Yu Fang Yi Xue Za Zhi. 2004;38:210–15. [in Chinese] [PubMed] [Google Scholar]
- 15.Waghray A, Murali AR, Menon KN. Hepatocellular carcinoma: From diagnosis to treatment. World J Hepatol. 2015;7(8):1020–29. doi: 10.4254/wjh.v7.i8.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang J, Yang SY, Hu XY, et al. Serum peptidome profiling in patients with lung cancer. Anat Rec (Hoboken) 2010;293(12):2027–33. doi: 10.1002/ar.21267. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Jin H, Li L, et al. Detection of murine toxoplasmosis using magnetic bead-based serum peptide profiling by MALDI-TOF MS. Vector Borne Zoonotic Dis. 2012;12(6):462–66. doi: 10.1089/vbz.2011.0742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang J, Yang J, Gao Y, et al. Identification of potential serum proteomic biomarkers for clear cell renal cell carcinoma. PLoS One. 2014;9(11):e111364. doi: 10.1371/journal.pone.0111364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orvisky E, Drake SK, Martin BM, et al. Enrichment of low molecular weight fraction of serum for MS analysis of peptides associated with hepatocellular carcinoma. Proteomics. 2006;6(9):2895–902. doi: 10.1002/pmic.200500443. [DOI] [PubMed] [Google Scholar]
- 20.Schwegler EE, Cazares L, Steel LF, et al. SELDI-TOF MS profiling of serum for detection of the progression of chronic hepatitis C to hepatocellular carcinoma. Hepatology. 2005;41(3):634–42. doi: 10.1002/hep.20577. [DOI] [PubMed] [Google Scholar]
- 21.Tian L, Wang Y, Xu D, et al. The differential diagnostic model for serous peptidomics in HBV carriers established by MALDI-TOF-MS analysis. Clin Biochem. 2014;47(1–2):56–62. doi: 10.1016/j.clinbiochem.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 22.Noguchi Y, Kurokawa MS, Okuse C, et al. Serum peptides, represented by complement 3f des-arginine, are useful for prediction of the response to pegylated interferon-α plus ribavirin in patients with chronic hepatitis C. Hepatol Res. 2013;43(7):743–56. doi: 10.1111/hepr.12018. [DOI] [PubMed] [Google Scholar]
- 23.He J, Zeng ZC, Xiang ZL, Yang P. Mass spectrometry-based serum peptide profiling in hepatocellular carcinoma with bone metastasis. World J Gastroenterol. 2014;20(11):3025–32. doi: 10.3748/wjg.v20.i11.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sandanayake NS, Camuzeaux S, Sinclair J, et al. Identification of potential serum peptide biomarkers of biliary tract cancer using MALDI MS profiling. BMC Clin Pathol. 2014;14(1):7. doi: 10.1186/1472-6890-14-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scarlett CJ, Saxby AJ, Nielsen A, et al. Proteomic profiling of cholangiocarcinoma: Diagnostic potential of SELDI-TOF MS in malignant bile duct stricture. Hepatology. 2006;44(3):658–66. doi: 10.1002/hep.21294. [DOI] [PubMed] [Google Scholar]
- 26.An Y, Bekesova S, Edwards N, Goldman R. Peptides in low molecular weight fraction of serum associated with hepatocellular carcinoma. Dis Markers. 2010;29(1):11–20. doi: 10.3233/DMA-2010-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tessitore A, Gaggiano A, Cicciarelli G, et al. Serum biomarkers identification by mass spectrometry in high-mortality tumors. Int J Proteomics. 2013;2013:125858. doi: 10.1155/2013/125858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chandler KB, Brnakova Z, Sanda M, et al. Site-specific glycan microheterogeneity of inter-alpha-trypsin inhibitor heavy chain H4. J Proteome Res. 2014;13(7):3314–29. doi: 10.1021/pr500394z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noh CK, Kim SS, Kim DK, et al. Inter-alpha-trypsin inhibitor heavy chain H4 as a diagnostic and prognostic indicator in patients with hepatitis B virus-associated hepatocellular carcinoma. Clin Biochem. 2014;47(13–14):1257–61. doi: 10.1016/j.clinbiochem.2014.05.002. [DOI] [PubMed] [Google Scholar]