Abstract
Febrile illness-related epilepsy syndrome is a catastrophic epileptic encephalopathy that is highly refractory to most antiepileptic drugs leading to high morbidity and mortality. The authors report the use of a pediatric infusion protocol of continuous intravenous magnesium sulfate for the control of seizures in 2 children with febrile illness-related epilepsy syndrome refractory to multiple antiepileptic drugs in a pediatric intensive care unit of a tertiary care children’s hospital. Both patients, 2 and 16 years of age, respectively, were treated with continuous magnesium sulfate infusion. Serum magnesium concentrations ranging from 2.1 to 5 mmol/L were achieved. Seizure reduction and cessation were noted in 1 patient with magnesium more than 3.0 mmol/L. No significant adverse effects were observed. Magnesium sulfate infusions can be safely used in pediatric refractory status epilepticus. Magnesium sulfate can be considered in the management of children with febrile illness-related epilepsy syndrome.
Keywords: febrile illness-related epilepsy syndrome, refractory status epilepticus, magnesium sulfate infusion
Febrile illness-related epilepsy syndrome is a catastrophic epileptic encephalopathy characterized by focal or multifocal seizures arising from the neocortical regions with a yet undefined etiology. This rare entity comprises a small minority of all patients with status epilepticus.1 Previously described by several terms, including “Devastating epileptic encephalopathy in school-age children” by Mikaeloff et al,2 it is now called “febrile illness-related epilepsy syndrome.”1 The characteristic clinical picture is one of a previously well child with an initial transient fever phase beginning 2 to 10 days prior to presentation, followed by recurrent focal seizures that evolve rapidly into refractory status epilepticus without specific neurological features. Treatment with different antiepileptic drugs, immunosuppressive agents, plasmapheresis, and vagus nerve stimulation is often disappointing.1,3 There are some reports of partial therapeutic success with ketogenic diet4 and immunoglobulins.5 Mortality rate is about 11.7% with 93% of the survivors having refractory epilepsy and significant neuropsychological sequelae.1,4,6,7 Most survivors had moderate to severe learning disabilities and only 18% retained normal cognitive level.
Intravenous magnesium has been used in status epilepticus in patients with juvenile-onset Alpers’ syndrome with both clinical and neurophysiological improvement.8 We present 2 children with febrile illness-related epilepsy syndrome who were treated with continuous magnesium sulfate infusion.
Case Report 1
A previously well 2-year-old boy with a 5-day history of fever and upper respiratory tract symptoms was admitted after a brief self-aborted seizure. There were no prior infective contacts. He had six 10-minute seizures over 10 hours. Seizures continued despite intravenous lorazepam, phenytoin, and phenobarbital loading. Convulsive seizures stopped in the pediatric intensive care unit, but subtle eye blinking and lip twitching were seen along with left facial and limb twitching. He was intubated and intravenous midazolam infusion commenced. Computed tomography scan of the brain was normal. Magnetic resonance imaging (MRI) of the brain showed subtle leptomeningeal enhancement. Electroencephalography demonstrated frontal–central bisynchronous discharges consistent with status epilepticus. Barbiturate coma was induced with intravenous thiopental to achieve burst suppression.
He was investigated extensively for possible causes of meningoencephalitis, including a thorough infective and autoimmune screen, all of which were negative. He was treated with intravenous acyclovir, ceftriaxone, and methylprednisolone. N-methyl-d-aspartate receptor (NMDA), glutamic acid decarboxylase, and voltage-gated potassium channel receptor antibodies tested negative.
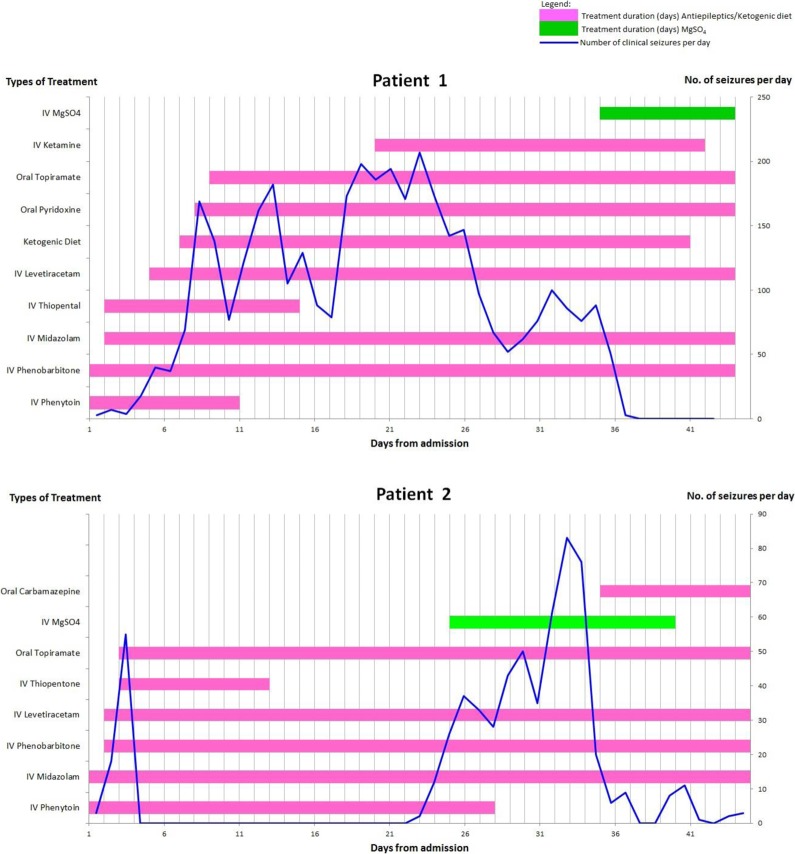
The patient was briefly seizure-free for just over a day on intravenous thiopental, phenytoin, and high-dose midazolam infusion. Frequent electrographic and clinical seizures (100-150 per day) persisted, despite phenobarbital, immunoglobulins, levetiracetam, ketogenic diet, oral pyridoxine, and topiramate (Figure 1 - Patient 1). Repeat electroencephalography on day 12 showed frequent electrographic seizures with increased frequency during the nighttime. The interictal background alternated between burst suppression pattern and irregular mixed theta, delta, and multifocal spikes with trains of bilateral asynchronous nonrhythmic posterior sharp activity seen at the left temporal and right posterior parietal–occipital region.
Figure 1.
Time line of clinically observed seizures and treatment with medication, ketogenic diet, and magnesium sulfate (MgSO4) for the 2 patients. Patient 1 had clinical and electrographic seizure cessation on achieving MgSO4 levels of 3 mmol/L (upper panel). Patient 2 had persistent electrographic seizures despite maximal dosing of MgSO4 (lower panel).
Thiopental was weaned off on day 13 at a phenobarbital dose of 22 mg/kg/d. Seizure frequency increased to 10 per hour, despite intravenous phenobarbital loading. Intravenous ketamine was added, but seizure frequency increased to 25 per hour beyond 30 μg/kg/min and ketamine infusion was reduced. Repeat MRI of the brain on day 33 showed bilateral microinfarcts (0.3 cm) in the frontoparietal region and generalized atrophy with increased ventricular size.
Seizure frequency fluctuated (Figure 1 - Patient 1) and rescue treatments were considered—inhaled isoflurane, intravenous lidocaine, and magnesium sulfate.9 Magnesium sulfate was chosen for its comparatively milder side effect profile. A literature search revealed no existing pediatric dosing regimen for intravenous magnesium sulfate infusion in the management of febrile illness-related epilepsy syndrome. A dosing protocol (Table 1) was drawn up based on magnesium sulfate eclampsia protocol.9 Magnesium sulfate infusion was initiated on day 35 with a loading dose of 50 mg/kg followed by a maintenance infusion of 20 mg/kg/h. Baseline seizure frequency was about 8 to 10 per day, and baseline magnesium level was 0.6 mmol/L. As magnesium sulfate infusion rate increased from 20 to 31 mg/kg/h, patient’s seizure frequency decreased. Clinical and electrographic seizures ceased on day 37 at magnesium levels of 3 mmol/L.
Table 1.
KKH Magnesium Sulfate Dosing Protocol for Refractory Status Epilepticus.
|
Magnesium Sulphate for Refractory Status Epilepticus
(For patients who are not responsive to standard Status Epilepticus management) | |
|---|---|
| Preparation available in KKH Formulary: 49.3% w/v MgSO4 (10mmol/5mL) 1ml = 2 mmol Mg2+ = 493 mg MgSO4 | |
| Target serum level: 2-4 mmol/l | Endpoint: Electroencephalographic seizure control |
|
Dosing regimen: Loading: 50mg/kg over 30 minutes (Max = 4g/dose) Maintenance: start at 20mg/kg/hr (range 20 mg/kg/hr – 40mg/kg/hr) Maximum infusion dose: 40mg/kg/hr (Recommended maximum daily dose = 40g/day) | |
|
Dilution: Loading: Load 50mg/kg MgSO4 (≈ 0.1ml/kg of 49.3% w/v MgSO4) over 30 minutes (Max rate : 150mg/min) Dilute every 1 ml of 49.3% MgSO4 with 2.5 ml 0.9% NaCl | |
|
Maintenance (for CENTRAL LINE): Dilute 500mg Mg (=1ml 49.3% w/v MgSO4) × Body Weight with 0.9% NaCl to a total volume of 50 ml (1ml/hr = 10 mg/kg/hr) Run between 1ml/hr to 4ml/kg/hr (= 10mg/kg/hr to 40 mg/kg/hr) | |
Occasional premature ventricular contractions were noted on cardiac monitoring on day 2 of magnesium sulfate infusion, when magnesium level was 2.76 mmol/L and total calcium (Ca) level was 1.54 mmol/L (corrected Ca 1.9 = mmol/L; ionized Ca = 1.07). The premature ventricular contractions resolved with calcium chloride infusion, and repeat total calcium was 1.74 mmol/L. No other adverse effects with magnesium sulfate were noted. Magnesium sulfate infusion rate was titrated to maintain a magnesium level around 3 mmol/L. Repeat electroencephalography showed no electrographic seizures. The ketogenic diet and ketamine infusion were weaned off over the next 2 days. Magnetic resonance imaging was not repeated as the patient was critically ill. Unfortunately, the patient developed pneumonia with septic shock on day 38, which led to multiorgan failure and he passed away on day 43.
Case Report 2
A 16-year-old male with febrile illness-related epilepsy syndrome presented with 5 days of fever and malaise and three 10-minute seizures over 4 hours without interictal recovery. He presented initially at another hospital and had persistent seizures, despite intravenous lorazepam, valproic acid, and phenytoin. Following transfer, he had a generalized tonic–clonic seizure and 2 episodes of eye and lip twitching. He was intubated and started on intravenous midazolam infusion, intravenous levetiracetam, phenobarbital, and phenytoin. Seizures increased from 3 to 55 per day. Thiopental coma was induced, phenobarbital and midazolam doses increased, and oral topiramate added. No further clinical seizure was observed after initiation of barbiturate coma, which was stopped on day 12 over concerns of pancreatitis possibly secondary to thiopental.
Magnetic resonance imaging of the brain on day 1 showed no significant abnormalities. Repeat MRI on day 14 showed symmetrical signal abnormalities in both thalami. He was empirically treated with intravenous acyclovir and ceftriaxone. Infective and autoimmune etiologies were investigated and were negative. Pulsed methylprednisolone and immunoglobulins were given. N-methyl-d-aspartate receptor, glutamic acid decarboxylase, and voltage-gated potassium channel antibodies later returned negative.
No clinical seizures were seen from day 3 to day 23 on high-dose midazolam infusion, intravenous phenobarbital, intravenous levetiracetam, intravenous phenytoin, and oral topiramate. Brief bursts of bizarre waveforms lasting 10 to 30 seconds were observed on the electroencephalography on day 10 to day 13 of pediatric intensive care unit admission but were not consistent with electrographic seizures. On day 24, he developed facial twitching of the cheek and lips. Repeat phenobarbital and phenytoin levels were stable. He continued to have a significant number of both electrographic and clinical seizures (about 26 per day), despite loading and increased maintenance dose with levetiracetam.
Magnesium sulfate treatment was started for refractory status epilepticus, with 50 mg/kg magnesium sulfate loading followed by a continuous infusion at 10 mg/kg/h for a target magnesium level of 3 to 5 mmol/L. As the patient weighed 80 kg, the loading dose and total magnesium dose were capped at 4 g and 40 g/d, respectively, per adult eclampsia protocol recommendations. The baseline clinical seizure frequency was about 14 per day when baseline magnesium level was 0.77 mmol/L. Magnesium level reached 2.13 mmol/L on day 26 when infusion rate was increased to 30 mg/kg/h. There was no corresponding improvement in the patient’s seizure control in the subsequent days (about 32-83 per day). To avoid exceeding the maximum daily magnesium dose, magnesium infusion was not escalated. Patient developed urticarial rash around the time of initiation of magnesium sulfate and intravenous piperacillin–tazobactam which was started for Pseudomonas aeruginosa urinary tract infection. However, the rash improved and resolved spontaneously. Clinically observed seizures decreased from day 35 but electrographic seizures persisted on the electroencephalography recording. Magnesium sulfate infusion was weaned off after 14 days (Figure 1 - Patient 2).
Discussion
Magnesium sulfate was safely administered in our 2 patients with febrile illness-related epilepsy syndrome, with seizure cessation in one. Magnesium sulfate treatment in eclamptic seizures9 is established. Target magnesium levels for eclamptic convulsions treatment are between 3.5 and 5 mmol/L, achieved with a loading dose followed by continuous magnesium sulfate infusion.9 Common side effects are mild: flushing (19.7%), nausea and vomiting (3.3%), and muscle weakness (1.4%). Serious adverse effects are rare and include respiratory depression (1.0%) and cardiovascular side effects such as hypotension and tachycardia (0.72%).10,11 Adverse effects are often dose related.
The use of magnesium sulfate in seizures due to other etiologies is not well studied. There were 2 case reports of magnesium sulfate in noneclamptic status epilepticus. Fisher et al12 reported an unsuccessful attempt to terminate myoclonic status epilepticus with magnesium sulfate infusion. Magnesium was infused to achieve levels as high as 14.2 mEq/L, which resulted in magnesium-related neuromuscular blockade and accompanying cessation of visible myoclonus; however, the electroencephalography still revealed ongoing blunted sharp-wave activity. On the other hand, Visser et al8 reported some success in juvenile-onset Alpers’ syndrome with polymerase gamma 1 deficiency (POLG1 deficiency). Magnesium sulfate infusion resulted in clinical improvement and rapid extubation of 2 patients without significant side effects.
The exact anticonvulsant mechanism of magnesium sulfate is not known. Magnesium is hypothesized to potentially modulate seizure activity by reducing excitation through blocking NMDA receptors13 and by acting as a voltage-dependent calcium channel antagonist to prevent membrane depolarization. Single oral dose of magnesium can inhibit NMDA-induced convulsions in mice in a dose-dependent manner.14 Zou et al15 found an improved response to treatment of infantile spasms with adrenocorticotropic hormone and intravenous magnesium sulfate compared to adrenocorticotropic hormone treatment alone (79% vs 53%).
The temporal relationship between magnesium sulfate initiation and seizure cessation is compelling in patient 1, although causality is not proven. As treatment was initiated on day 35, it is possible the acute phase of febrile illness-related epilepsy syndrome was naturally waning. Magnesium sulfate treatment failed in the second patient, with cessation of clinical seizures but persistence of electrographic seizures at levels of 2.50 mmol/L. This lack of efficacy could be due to a decoupling effect on clinical and electrographic seizures or inability to reach a target magnesium level of 3 mmol/L due to the maximum total daily dose of 40 g being reached. Higher total daily doses to achieve the desired magnesium level might have improved response but were not given due to safety considerations. As many of the adverse events in magnesium sulfate treatment are dose related, for safety our protocol follows a 4-hourly step-up dosing where magnesium levels are drawn at 4-hourly intervals along with continuous blood pressure, respiratory rate, and urine output monitoring. To our knowledge, this is the first case report describing the use of continuous intravenous magnesium sulfate for the treatment of febrile illness-related epilepsy syndrome in children.
Conclusion
Magnesium sulfate infusion can be safely used in children with refractory status epilepticus due to febrile illness-related epilepsy syndrome. Magnesium levels of 3.5 to 5 mmol/L can be useful. Further safety data are required for cases with children having high body weight.
Footnotes
Author Contributions: WWT – Literature search, development of protocol, study data collection, analysis, interpretation, manuscript drafting, review and revisions; DWSC – Literature search, development of protocol, study data collection, analysis, interpretation, manuscript drafting, review and revisions; JHL – Manuscript review and revisions; TT – Manuscript review and revisions; APM – Literature search, development of protocol, manuscript review and revisions; YHC – Literature search, development of protocol, study data analysis & interpretation, manuscript review and revisions.
Ethical Approval: We confirm that we have read the journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. van Baalen A, Hausler M, Boor R, et al. Febrile infection-related epilepsy syndrome (FIRES): a nonencephalitic encephalopathy in childhood. Epilepsia. 2010;51 (7):1323–1328. [DOI] [PubMed] [Google Scholar]
- 2. Mikaeloff Y, Jambaque I, Hertz-Pannier L, et al. Devastating epileptic encephalopathy in school-aged children (DESC): a pseudo encephalitis. Epilepsy Res. 2006;69 (1):67–79. [DOI] [PubMed] [Google Scholar]
- 3. Sahin M, Menache CC, Holmes GL, Riviello JJ. Outcome of severe refractory status epilepticus in children. Epilepsia. 2001;42 (11):1461–1467. [DOI] [PubMed] [Google Scholar]
- 4. Nabbout R, Mazzuca M, Hubert P, et al. Efficacy of ketogenic diet in severe refractory status epilepticus initiating fever induced refractory epileptic encephalopathy in school age children (FIRES). Epilepsia. 2010;51 (10):2033–2037. [DOI] [PubMed] [Google Scholar]
- 5. Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12 (2):157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Caraballo RH, Reyes G, Avaria MF, et al. Febrile infection-related epilepsy syndrome: a study of 12 patients. Seizure. 2013;22 (7):553–559. [DOI] [PubMed] [Google Scholar]
- 7. Howell KB, Katanyuwong K, Mackay MT, et al. Long-term follow-up of febrile infection-related epilepsy syndrome. Epilepsia. 2012;53 (1):101–110. [DOI] [PubMed] [Google Scholar]
- 8. Visser NA, Braun KP, Leijten FS, van Nieuwenhuizen O, Wokke JH, van den Bergh WM. Magnesium treatment for patients with refractory status epilepticus due to POLG1-mutations. J Neurol. 2011;258 (2):218–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Euser AG, Cipolla MJ. Magnesium sulfate for the treatment of eclampsia: a brief review. Stroke. 2009;40 (4):1169–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Which anticonvulsant for women with eclampsia? Evidence from the collaborative eclampsia trial. Lancet. 1995;345(8963):1455–1463. [PubMed] [Google Scholar]
- 11. Altman D, Carroli G, Duley L, et al. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359 (9321):1877–1890. [DOI] [PubMed] [Google Scholar]
- 12. Fisher RS, Kaplan PW, Krumholz A, Lesser RP, Rosen SA, Wolff MR. Failure of high-dose intravenous magnesium sulfate to control myoclonic status epilepticus. Clin Neuropharmacol. 1988;11 (6):537–544. [DOI] [PubMed] [Google Scholar]
- 13. Cotton DB, Hallak M, Janusz C, Irtenkauf SM, Berman RF. Central anticonvulsant effects of magnesium sulfate on N-methyl-d-aspartate-induced seizures. Am J Obstet Gynecol. 1993;168 (3 pt 1):974–978. [DOI] [PubMed] [Google Scholar]
- 14. Decollogne S, Tomas A, Lecerf C, Adamowicz E, Seman M. NMDA receptor complex blockade by oral administration of magnesium: comparison with MK-801. Pharmacol Biochem Behav. 1997;58 (1):261–268. [DOI] [PubMed] [Google Scholar]
- 15. Zou LP, Wang X, Dong CH, Chen CH, Zhao W, Zhao RY. Three-week combination treatment with ACTH + magnesium sulfate versus ACTH monotherapy for infantile spasms: a 24-week, randomized, open-label, follow-up study in China. Clin Ther. 2010;32 (4):692–700. [DOI] [PubMed] [Google Scholar]