Abstract
Growth of Rhodobacter capsulatus with molecular dinitrogen as the sole N source via the alternative Fe-only nitrogenase requires all seven gene products of the anfHDGK-1-2-3 operon. In contrast to mutant strains carrying lesions in the structural genes of nitrogenase (anfH, anfD, anfG, and anfK), strains defective for either anf1, anf2, or anf3 are still able to reduce the artificial substrate acetylene, although with diminished activity. To obtain further information on the role of Anf1, we screened an R. capsulatus genomic library designed for use in yeast two-hybrid studies with Anf1 as bait. Two genes, which we propose to call ranR and ranT (for genes related to alternative nitrogenase), coding for products that interact with Anf1 were identified. A ranR mutant exhibited a phenotype similar to that of an anf1 mutant strain (no growth with N2 in the absence of molybdenum, but significant reduction of acetylene via the Fe-only nitrogenase), whereas a ranT mutant retained the ability to grow diazotrophically, but growth was clearly delayed compared to the parental strain. In contrast to the situation for anf1, expression of neither ranR nor ranT was regulated by ammonium or molybdenum. A putative role for Anf1, RanR, and RanT in the acquisition and/or processing of iron in connection with the Fe-only nitrogenase system is discussed.
The phototrophic purple bacterium Rhodobacter capsulatus synthesizes two distinct nitrogenases, a conventional molybdenum nitrogenase (nif encoded) and an alternative, heterometal-free, “iron-only” nitrogenase (anf encoded) (30, 31). Several characteristic features distinguish the Fe nitrogenase from the Mo-containing system. (i) While the dinitrogenase component of the conventional nitrogenase (MoFe protein) consists of four subunits forming an α2β2 tetramer, the analogous component of the Fe nitrogenase (FeFe protein) contains an additional small subunit (AnfG) to form an α2β2δ2 hexameric structure (28). (ii) The FeMo cofactor (FeMoco) and the FeFe cofactor (Fe-only cofactor) (FeFeco) probably have homologous structures (17). The only fundamental structural difference between these two cofactors apparently is that the molybdenum atom of FeMoco is replaced by iron in the FeFe cofactor. (iii) The catalytic activity (N2 and C2H2 reduction) of the Fe nitrogenase is comparatively low (10 to 20% of the rates determined for the Mo enzyme). A typical feature of the alternative enzyme system is its ability to partly reduce acetylene to ethane (2 to 4%). (iv) The most significant catalytic property is the relatively high H2-producing activity of the Fe-only nitrogenase. Under optimal conditions for N2 reduction, the activity ratio (moles of N2 reduced/moles of H2 produced) is almost 1:8 compared to 1:1 in the case of the Mo nitrogenase (28).
The synthesis and activities of both nitrogenases are tightly controlled at different regulatory levels by ammonium and other environmental factors (for a review, see reference 21). Under nitrogen-limiting conditions (in the absence of ammonium), transcription of nifA or anfA, which encode the transcriptional activators of all the other nif or anf genes, respectively, is activated by NtrC. In addition to ammonium control, transcription of anfA is inhibited by traces of molybdenum via the molybdate-dependent repressor proteins MopA and MopB (18). Therefore, only in the absence of both ammonium and molybdenum is anfA expressed, and in turn, AnfA activates transcription of the anfHDGK-1-2-3 operon, which is located downstream of anfA (Fig. 1) (15, 31). All seven gene products of the anfHDGK-1-2-3 operon are essential for diazotrophic growth via the Fe nitrogenase. In contrast to mutants carrying lesions in anfH, anfD, anfG, or anfK, strains defective for either anf1, anf2, or anf3 are able to reduce the artificial substrate acetylene (references 15 and 22 and this study).
FIG. 1.
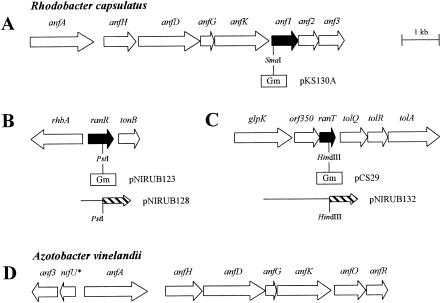
Organization of anf and ran genes involved in nitrogen fixation via the iron-only nitrogenases in R. capsulatus (A to C) and A. vinelandii (D). The locations and sizes of genes are given by arrows carrying their respective gene designations. The A. vinelandii nifU* gene is predicted to code for a truncated NifU protein in addition to the full-length nifU gene forming part of the nifUSV operon. Black arrows emphasize the R. capsulatus anf1 gene and two genes coding for Anf1 interactors. Below these genes, the locations of interposon cassettes ([Gm], gentamicin resistance) are shown. Hybrid plasmids pKS130A, pNIRUB128, and pNIRUB132, used to create anf1, ranR, and ranT mutant strains, respectively, are based on mobilizable narrow-host-range plasmids (Table 1). Hybrid plasmids pNIRUB128 (ranR-lacZ) and pNIRUB132 (ranT-lacZ) are based on the mobilizable broad-host-range plasmid pML5. The [Gm] interposon and the lacZ gene (hatched arrow) are not drawn to scale.
A similar organization of anf genes, forming the monocistronic anfA transcriptional unit and the anfHDGKOR operon, has been described for Azotobacter vinelandii (13, 14, 27), with anfO and anfR being equivalent to R. capsulatus anf1 and anf2, respectively (15, 22). In contrast to the situation in R. capsulatus, the A. vinelandii anf3 homologue does not belong to the anfHDGKOR operon but is located upstream of and in the opposite orientation to anfA (Fig. 1). Like R. capsulatus anf1, anf2, and anf3 mutants, A. vinelandii anfO and anfR mutants cannot reduce N2 to ammonia but are still able to reduce acetylene via the iron-only nitrogenase (25). To date, no A. vinelandii mutant defective in the anf3-like gene has been constructed.
A. vinelandii AnfO exhibits some similarity to the N-terminal part of dinitrogenase reductases (including a cysteine residue involved in coordination of the [4Fe-4S] cluster) and to the heme-binding domain of P-450 cytochromes, suggesting that AnfO might be an iron-containing protein (14, 25). In contrast to AnfO, no putative conserved domain has been detected for AnfR. Anf3 might be a flavin-nucleotide-binding protein, suggesting a possible role of Anf3 in electron transport.
The objective of this study was to further characterize the role of R. capsulatus Anf1 in the nitrogen fixation process by protein interaction analyses. For this purpose, we used the yeast two-hybrid system to search for proteins interacting with R. capsulatus Anf1. Screening of an R. capsulatus genomic library (26) identified two interactors of Anf1. Mutants defective for the corresponding genes (designated ranR and ranT) exhibited phenotypes strongly suggesting that the respective products play pivotal roles in the activity of the alternative nitrogenase in R. capsulatus.
MATERIALS AND METHODS
Strains, plasmids, and growth conditions.
The bacterial strains, yeast strains, and plasmids used in this study are shown in Table 1. Methods for conjugational plasmid transfer between Escherichia coli S17-1 and R. capsulatus and the selection of mutants, rich medium (PY), minimal medium (RCV), molybdenum-free minimal medium, growth conditions, and antibiotic concentrations were as previously described (16, 23, 29, 31).
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristicsa | Source or reference |
|---|---|---|
| E. coli | ||
| DH5α | Host for plasmid amplification | 8 |
| S17-1 | RP4-2 (Tc::Mu) (Km::Tn7) integrated in the chromosome | 34 |
| R. capsulatus | ||
| B10S | Spontaneous Smr mutant of R. capsulatus B10 | 16 |
| KS36 | nifHDK deletion derivative of B10S; Spcr | 35 |
| TD6 | nifHDK deletion derivative of B10S; KmranfA::[Spcr] | This study |
| S. cerevisiae CG1945 | Host for R. capsulatus genomic library | 7, 26 |
| Plasmids | ||
| pCS4 | pGBT9.C derivative containing DBD-anf1 | This study |
| pCS28 | pK18 derivative containing ranT::[Gmr] | This study |
| pCS29 | pCS28 derivative containing Tcr-mob from pJQ18 | This study |
| pGAD424.C | GAL4-AD; LEU2 Ampr | 1 |
| pGAD-5 | pGAD424.C derivative containing AD-ranT in-frame fusion | This study |
| pGAD-8 | pGAD424.C derivative containing AD-ranT in-frame fusion | This study |
| pGAD-K16 | pGAD424.C derivative containing AD-ranR in-frame fusion | This study |
| pGAD-P1 | pGAD424.C derivative containing AD-ranR in-frame fusion | This study |
| pGBT9.C | GAL4-DBD; TRP1 Ampr | 1 |
| pKS130A | Mobilizable pBR325 derivative containing anf1::[Gmr]; Ampr Cmr Tcr | This study |
| pKS131A | pPHU236 derivative containing anfA-lacZ; Tcr | 18 |
| pML5 | Broad-host-range lacZ transcriptional fusion vector; Tcr | 19 |
| pNIRUB96-II | pSUP202 derivative containing [Kmr] from pBSL14 | This study |
| pNIRUB120 | pBluescript KS derivative containing ranR | This study |
| pNIRUB121 | pNIRUB120 derivative containing ranR::[Gmr] | This study |
| pNIRUB123 | pNIRUB121 derivative containing Kmr-mob from pNIRUB96-II | This study |
| pNIRUB125 | pSUP202 derivative containing ranT | This study |
| pNIRUB128 | pML5 derivative containing ranR-lacZ | This study |
| pNIRUB132 | pML5 derivative containing ranT-lacZ fusion | This study |
| pSA16 | pSUP401 derivative containing anf1-lacZ; Tcr | This study |
| pTD7-2I | pSUP401 derivative containing anfH-lacZ; Tcr | 4 |
| pWKR202 | Cmr Kmr Gmr | 3 |
Amp, ampicillin; Cm, chloramphenicol; Gm, gentamicin; Km, kanamycin; Sm, streptomycin; Spc, spectinomycin; Tc, tetracycline.
Identification of gene products interacting with Anf1 by yeast two-hybrid studies.
The R. capsulatus anf1 gene was PCR amplified with chromosomal DNA of the wild-type strain B10S. Appropriate oligonucleotides were designed for amplification of the full-length anf1 gene flanked by restriction sites, allowing the generation of an in-frame fusion with the DNA-binding domain (DBD) encoded by the E. coli-yeast shuttle vector pGBT9.C (GAL4-DBD). The resulting hybrid plasmid, pCS4 (DBD-Anf1), was used as bait to screen an R. capsulatus genomic library based on the E. coli-yeast shuttle vector pGAD424.C carrying the activation domain (GAL4-AD). The construction and screening of the R. capsulatus genomic library created for use in yeast two-hybrid studies was described previously (26).
Construction of R. capsulatus ranR and ranT interposon mutants.
A 1.3-kb DNA fragment containing the ranR gene was PCR amplified using synthetic primers (5′-GCGGGCGCCTCCTCCTGTGG-3′ and 5′-ACATCCGGGGCGCTTCTTGA-3′) prior to ligation into the SmaI site of pK18. To get rid of the vector-encoded PstI site, a 1.3-kb BamHI-KpnI fragment was subsequently cloned into pBluescript KS (resulting in the hybrid plasmid pNIRUB120) before a 2.6-kb PstI fragment carrying the gentamicin resistance cassette from plasmid pWKR202 was cloned into the remaining PstI site within the ranR coding region (pNIRUB121). In the last step, a 4.5-kb BamHI fragment from pNIRUB96-II (encompassing a kanamycin resistance gene and the mobilization (mob) site of RP4) was cloned into pNIRUB121, leading to the mobilizable hybrid plasmid pNIRUB123, which was used to create an R. capsulatus ranR mutant. Methods for the conjugational transfer of pNIRUB123 into R. capsulatus and selection of interposon mutants (marker rescue) were as previously described (16, 23, 31).
In a parallel approach, a 1.5-kb DNA fragment carrying the ranT gene and adjacent regions was PCR amplified using synthetic primers (5′-CGATGCCGAGATCGGCGC-3′ and 5′-CGCGATTTCTTCGAAGGC-3′). The PCR fragment was blunt-end cloned into the SmaI site of a pK18 derivative (pK18 ΔHindIII) before a 2.6-kb HindIII fragment carrying the gentamicin resistance cassette from plasmid pWKR202 was cloned into the single HindIII site within the ranT coding region, resulting in the hybrid plasmid pCS28. Subsequently, a 9.0-kb PstI fragment from pJQ18 (encompassing a tetracycline resistance gene and the mob site of RP4) was cloned into pCS28, leading to the mobilizable hybrid plasmid pCS29, which was used to create an R. capsulatus ranT mutant.
Construction of lacZ reporter gene fusions and β-galactosidase assays.
To create a fusion between the R. capsulatus ranR gene and the promoterless lacZ gene from E. coli, a 0.6-kb BamHI-PstI fragment (carrying the ranR promoter region) from plasmid pNIRUB120 was cloned into the mobilizable broad-host-range vector pML5, resulting in the hybrid plasmid pNIRUB128 (Fig. 1). Similarly, a fusion between R. capsulatus ranT and lacZ was generated by cloning a 1.8-kb BamHI-HindIII fragment (carrying the orf350-ranT promoter) from plasmid pNIRUB125 into pML5, resulting in the hybrid plasmid pNIRUB132 (Fig. 1).
To determine the β-galactosidase activities of R. capsulatus strains carrying either pNIRUB128 (ranR-lacZ) or pNIRUB132 (ranT-lacZ), cultures were grown in molybdenum-free minimal medium under either nitrogenase-derepressing or nitrogenase-repressing conditions. For growth under nitrogenase-derepressing conditions, serine was added to final concentrations of 9.5 mM. Nitrogenase-repressing conditions were achieved by addition of 15 mM NH4Cl to the medium. When required, Na2MoO4 was added at a final concentration of 10 μM. Growth was followed up to the late exponential phase, and the β-galactosidase activities of R. capsulatus strains were determined by the sodium dodecyl sulfate-chloroform method (12, 24).
Determination of in vivo and in vitro nitrogenase activities.
R. capsulatus strains used for activity studies were grown phototrophically in minimal medium in vacuum bottles according to the method of Schneider et al. (30). Maximal nitrogenase activity was obtained when cultures were grown overnight at 33°C in media containing 0.15 to 0.35 mM ferric citrate, 2.5 mM serine, and 0.02% yeast extract. Extract preparations and determination of the nitrogenase activities of whole cells (by acetylene reduction) and cell extracts (by H2 production) were performed as described previously (28).
Western immunoblot analysis.
Rabbit antisera containing monospecific polyclonal antibodies against the purified dinitrogenase component of the iron-only nitrogenase (FeFe protein) were employed in Western immunoblot experiments, which were conducted according to the procedure described by Siemann et al. (33).
RESULTS AND DISCUSSION
Identification of two proteins interacting with R. capsulatus Anf1 by yeast two-hybrid studies.
R. capsulatus Anf1, Anf2, and Anf3 (like A. vinelandii AnfO and AnfR) are essential for growth with N2 as the sole N source via the alternative iron-only nitrogenase, but corresponding mutants are still able to reduce the artificial substrate acetylene (15, 22, 25) (Fig. 2; see Table 3). A similar alteration in substrate specificity has been observed for the Mo-dependent nitrogenase in nifV mutants that are unable to incorporate homocitrate into the FeMo cofactor of the molybdenum nitrogenase (10, 11). Involvement of Anf1, Anf2, and Anf3 in maturation of the cofactor of the alternative nitrogenase (FeFeco) was discussed previously (22). Alternatively, one might also consider other roles for these proteins, e.g., functions in the assembly of the alternative nitrogenase or in electron supply. In any case, knowledge about proteins interacting (at least transiently) with these Anf proteins should give new insights into the mechanism(s) underlying the observed alterations in substrate specificity in the respective mutant strains.
FIG. 2.
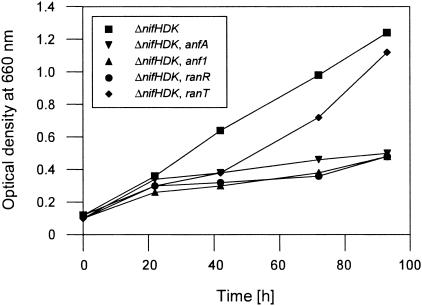
Diazotrophic growth of the R. capsulatus parental strain, KS36, and selected mutant derivatives. To analyze diazotrophic growth via the alternative nitrogenase, R. capsulatus strains were grown in molybdenum-free minimal medium. KS36 (ΔnifHDK), TD6 (ΔnifHDK anfA), KS36-KS130A (ΔnifHDK anf1), KS36-NIRUB123 (ΔnifHDK ranR), and KS36-CS29 (ΔnifHDK ranT) are represented.
TABLE 3.
Nitrogenase activities of selected R. capsulatus mutant strains
| Straina | Relevant characteristics | Nitrogenase activityb
|
Diazotrophic growthc | |||
|---|---|---|---|---|---|---|
| C2H2 reduction
|
H2 production
|
|||||
| Sp Act | % | Sp Act | % | |||
| KS36 | ΔnifHDK | 25.1 | 100 | 156 | 100 | + |
| KS36-KS130A | ΔnifHDK anf1 | 17.2 | 68 | 69 | 44 | − |
| KS36-NIRUB123 | ΔnifHDK ranR | 6.5 | 26 | 24 | 15 | − |
| KS36-CS29 | ΔnifHDK ranT | 18.9 | 75 | 8 | 5 | +d |
R. capsulatus strains were grown under phototrophic conditions optimal for derepression of Fe nitrogenase as described in Materials and Methods.
Nitrogenase activity was determined in vivo by the acetylene reduction assay and in vitro by hydrogen production. Specific activities are given in units per milligram of protein with standard deviations in the range of 15 to 20%.
See Fig. 2.
Delayed growth.
To identify proteins interacting with Anf1, we made use of the yeast two-hybrid system as described previously (26). The hybrid plasmid pCS4 (a pGBT9.C derivative encoding an in-frame fusion between the DNA-binding domain of the GAL4 transcription factor and Anf1: DBD-Anf1) served as bait for screening an R. capsulatus genomic library based on a low-copy-number GAL4 system (26). After ruling out self-activation mediated by DBD-Anf1, the hybrid plasmid pCS4 was transformed into the Saccharomyces cerevisiae reporter strain CG1945, which carried the R. capsulatus library based on the E. coli-yeast shuttle vector pGAD424.C, coding for the AD of the GAL4 transcription factor. Transformants were selected for expression of both reporter genes, HIS3 and lacZ, and positive clones were rechecked as described earlier to exclude false positives (26). Plasmid DNA was isolated from four true-positive clones (pGAD-K16, pGAD-P1, pGAD-5, and pGAD-8), and the inserts were characterized by DNA sequence analyses. Genes coding for putative Anf1 interactors were identified by BLAST searches in the R. capsulatus genome database (9; http://www.ergo-light.com). Two plasmids, pGAD-K16 and pGAD-P1, contained an in-frame fusion between the AD and the full-length open reading frame 1065 (orf1065). Since the insert sizes of both plasmids differ remarkably (Table 2), it seems unlikely that the two plasmids descended from a single ancestor. Similarly, plasmids pGAD-5 and pGAD-8 carried an in-frame fusion between the AD and the full-length coding region of open reading frame 349 (orf349). Again, different insert sizes (Table 2) suggest that the two plasmids were not related and therefore descended from two independent clones.
TABLE 2.
Proteins that interact with Anf1 identified by screening of an R. capsulatus DNA library for use in yeast two-hybrid studies
| Plasmida | Insert size (bp) | In-frame fusion to AD |
|---|---|---|
| pGAD-K16 | 875 | Orf1065 (RanR) |
| pGAD-P1 | 1,268 | Orf1065 (RanR) |
| pGAD-5 | 2,057 | Orf349 (RanT) |
| pGAD-8 | 1,102 | Orf349 (RanT) |
Protein-protein interactions were assayed in yeast strain CG1945 carrying plasmid pCS4 (DBD-Anf1) and the indicated library plasmid (AD fusion).
As described below, gene inactivation revealed that the orf1065 product is essential for growth with N2 as the sole N source, and Orf349 is required for maximal growth rates with N2 under Mo-deficient conditions via the alternative nitrogenase. Therefore, we propose to call these open reading frames ranR and ranT, respectively (for genes related to alternative nitrogenase, with ranR coding for a putative regulator and ranT encoding a putative thioesterase).
Since R. capsulatus anf1, anf2, and anf3 mutants show the same phenotype (inability to grow with N2 via the alternative nitrogenase but retaining the ability to reduce acetylene) (15, 22), one might speculate that the respective gene products form a complex in R. capsulatus. To test this assumption, we carried out yeast two-hybrid studies of interactions between defined protein partners as described previously (26), but we could not demonstrate interactions for either Anf1-Anf1, Anf1-Anf2, or Anf1-Anf3 (data not shown). However, if all three gene products are essential to form a stable complex, the failure to detect such interactions in yeast does not necessarily exclude complex formation in R. capsulatus.
The ranR and ranT gene products play important roles during diazotrophic growth via the iron-only nitrogenase.
The genetic maps of the ranR and ranT gene regions are shown in Fig. 1. Since the gene upstream of ranR, rhbA, reads in the opposite direction and ranR is separated from tonB by 114 bp, it seems likely that the ranR gene is monocistronic. Database searches revealed that the ranR gene product (predicted to consist of 216 amino acid residues; molecular weight, 24,237) exhibits strong similarity to the TetR family of bacterial regulators.
The coding regions of ranT and orf350 overlap by 1 bp, and therefore, it seems likely that both genes are transcriptionally and translationally coupled. The deduced ranT gene product (133 amino acids; molecular weight, 15,101), which exhibits distinct similarity to the thioesterase superfamily, is highly conserved among bacteria. The thioesterase family contains a wide variety of enzymes involved in lipid metabolism. In contrast to RanT, database searches suggest that the orf350 gene product (215 amino acids) might be limited to phototrophic purple bacteria.
Since yeast two-hybrid studies suggested that RanR and RanT interact with the Anf1 protein, which is essential for diazotrophic growth via the alternative nitrogenase, we carried out gene inactivation experiments to examine the roles of the respective genes in the nitrogen fixation process. For this purpose, we constructed the hybrid plasmids pNIRUB123 and pCS29 (Fig. 1) (see Materials and Methods). In these plasmids, a gentamicin resistance cassette (Gmr) was used to disrupt the coding regions of ranR (pNIRUB123) and ranT (pCS29). The two plasmids were introduced into the R. capsulatus wild-type strain B10S and the nifHDK deletion strain KS36, respectively, and marker rescue (exchange of the wild-type genes for the mutated genes carrying Gmr) resulting in corresponding ranR and ranT mutant strains was selected as described earlier (23). The resulting strains were called NIRUB123 (ranR), KS36-NIRUB123 (nifHDK ranR), CS29 (ranT), and KS36-CS29 (nifHDK ranT). Analyses of strains KS36-NIRUB123 and KS36-CS29 allowed us to examine the influence of ranR and ranT mutations on the Fe-only nitrogenase system without interference from the nifHDK-encoded Mo nitrogenase.
The above-mentioned null mutant strains were easily obtained, ruling out the possibility that mutations in either ranR or ranT are lethal, at least under the standard growth conditions based on PY rich-medium plates under either aerobic or anaerobic conditions (see Materials and Methods). In addition, we analyzed the growth of wild-type R. capsulatus and the respective mutant strains in RCV minimal medium with ammonium as an N source under photoheterotrophic (anaerobic) conditions. All mutant strains exhibited growth properties comparable to those of the parental strains B10S (wild type) and KS36 (nifHDK). Furthermore, the mutant strains NIRUB123 (ranR) and CS29 (ranT) were able to grow diazotrophically, like the wild-type strain B10S, in the presence of molybdenum (data not shown), strongly suggesting that RanR and RanT are dispensable for growth with N2 via the Mo nitrogenase.
To analyze the roles of RanR and RanT in N2 fixation via the alternative nitrogenase, the parental strain, KS36 (nifHDK), and corresponding mutant strains were grown under diazotrophic conditions in molybdenum-free medium (Fig. 2). Mutant strain TD6 (nifHDK anfA), which is defective for both nitrogenase systems, served as a negative control. The parental strain, KS36, showed normal diazotrophic growth, whereas the mutant strains KS36-KS130A (nifHDK anf1) and KS26-NIRUB123 (nifHDK ranR) were no longer able to grow with N2 as the sole N source. Therefore, like Anf1, RanR is essential for diazotrophic growth via the iron-only nitrogenase. In contrast to anf1 and ranR mutant strains, strain KS36-CS29 (nifHDK ranT) was able to grow diazotrophically, although this growth was delayed (a prolonged lag phase) compared to the parental strain, KS36 (nifHDK). In summary, the observed phenotypes clearly corroborate the notion that both Anf1 interactors originally identified by yeast two-hybrid studies play important roles during diazotrophic growth via the alternative nitrogenase.
Analysis of in vivo and in vitro activities of the alternative nitrogenase from ranR and ranT mutant strains.
Both R. capsulatus anf1 and A. vinelandii anfO mutants cannot reduce the natural substrate N2 via the alternative nitrogenase but are still able to reduce the artificial substrate acetylene (15, 25) (Table 3). Therefore, we asked whether mutant strains devoid of the Anf1 interactors RanR and RanT also exhibit this altered substrate specificity. For this purpose, the R. capsulatus strain KS36 (nifHDK) and selected mutant strains were grown under Fe nitrogenase-derepressing conditions (in Mo-depleted medium) until the mid-exponential growth phase prior to determination of in vivo nitrogenase activity by the acetylene reduction assay (Table 3). Like the anf1 mutant KS36-KS130A, which showed ∼2/3 of the activity of the nifHDK parental strain, the strains defective for ranR (KS36-NIRUB123) and ranT (KS36-CS29) reduced acetylene to ethylene as well, although the ranR strain did so at a distinctly lower rate than the anf1 mutant.
As mentioned above, a typical feature of the Fe nitrogenase is its ability to partly reduce acetylene to ethane. Mutations concerning the structure of the cofactor or the close cofactor environment within the FeFe protein component may influence the degree of ethane formation and the affinity of acetylene to the Fe nitrogenase. However, the proportion of ethane produced from acetylene (∼3%), as well as the Km values determined for C2H2 (∼18 kPa), in the parental strain, KS36, and the respective ranR and ranT mutant strains were identical.
To rule out the possibility that the observed differences in nitrogenase activities correlated with different levels of nitrogenase, crude extracts of the same cultures used before for the estimation of acetylene reduction were examined by Western blot analysis using an antiserum raised against the FeFe protein component of the alternative nitrogenase of R. capsulatus (28). Indeed, comparable amounts of the FeFe protein were detected in extracts of the parental strain, KS36, and the mutant strains defective for anf1 (KS36-KS130A), ranR (KS36-NIRUB123), and ranT (KS36-CS29), suggesting that mutations in the corresponding genes did not influence the accumulation of the alternative nitrogenase (data not shown). A somewhat exceptional position is taken by the ranT mutant strain. In accordance with the delayed N2-dependent growth (Fig. 2), a significant time lag in Fe nitrogenase formation and activity development was observed when cells were grown with serine as an N source (data not shown). While the other strains reached their maximum enzyme contents and catalytic activities after 18 to 20 h of incubation at 33°C, the ranT mutant needed 6 to 8 h longer. Thus, although expression and/or maturation of the functionally intact Fe nitrogenase was delayed in the ranT mutant, the final degrees of accumulation of Fe nitrogenase were identical in all strains. In conclusion, it seems likely that the observed differences in substrate reduction rates (Table 3) have to be explained exclusively by actual differences in the catalytic behavior and not by variations in enzyme concentrations in the respective mutants.
The high rate of proton reduction (∼7 times the acetylene reduction rate) is the most characteristic catalytic feature of Fe nitrogenases (28). We therefore also examined proton reduction (measured as H2 production) catalyzed by the Fe-only nitrogenases from the anf1, ranR, and ranT mutant strains. To avoid interference between nitrogenase-dependent H2 production and hydrogenase-mediated H2 consumption, H2 production was analyzed in an in vitro system based on soluble cell extracts (separated from particulate hydrogenase) (32). Data obtained for in vitro activity of the alternative nitrogenase from the parental strain, KS36, and its derivatives met expectations in that (i) extracts from the parental strain and from mutants defective for either anf1 (KS36-KS130A) or ranR (KS36-NIRUB123) mediated significant levels of H2 production and (ii) the relative activities of these extracts roughly paralleled those determined for the in vivo activities (measured as acetylene reduction) of the respective strains (Table 3). On the other hand, extracts from the ranT mutant (KS36-CS29) displayed only very low H2-producing activity (Table 3). Since the C2H2-reducing activities of ranT mutant extracts were correspondingly low (only ∼10% of the whole-cell activity), it can be concluded that low-H2 evolving activity is not a specific catalytic feature of that mutant. It rather appears likely that the Fe nitrogenase system of the ranT strain is distinctly more labile than the analogous enzymes from the other strains, becoming largely inactivated during cell disruption and subsequent steps of extract preparation.
Expression of ranR and ranT is not regulated by ammonium or molybdenum.
The anf1 gene belongs to the anfHDGK-1-2-3 operon, and consequently, its transcription is repressed by both ammonium and molybdenum (15, 18) (Table 4). Since Anf1 and its interactors, RanR and RanT, were shown to play important roles during diazotrophic growth via the alternative nitrogenase, we asked whether expression of ranR and/or ranT might also be regulated by ammonium and/or molybdenum. As a prerequisite for transcriptional analysis of ranR and ranT, we constructed the hybrid plasmids pNIRUB128 (ranR-lacZ) and pNIRUB132 (ranT-lacZ). Both lacZ reporter fusions (Fig. 1) (see Materials and Methods) are based on the mobilizable broad-host-range plasmid pML5. After conjugational transfer of the hybrid plasmids pNIRUB128 and pNIRUB132 into the R. capsulatus wild-type strain B10S, the resulting reporter strains were grown under phototrophic conditions in minimal medium supplemented with ammonium, serine, and/or molybdenum. The results shown in Table 4 may be summarized as follows. (i) Control strains containing anfA-lacZ, anfH-lacZ, or anf1-lacZ reporter fusions exhibited the well-known expression pattern of anf genes. Maximal expression of these genes occurred under nitrogenase-derepressing conditions (with serine as the sole N source and in the absence of molybdenum), whereas only background levels of expression were found after cultivation of cells in the presence of either ammonium or molybdenum. (ii) In contrast, expression of neither ranR nor ranT was regulated by the nitrogen source or by the availability of molybdenum. Therefore, despite the functional connections among Anf1, RanR, and RanT, the expression patterns of both ranR and ranT clearly differed from those of anf1 and other anf genes.
TABLE 4.
Expression of selected lacZ reporter fusions in R. capsulatus
| Plasmid | Relevant characteristics | β-Galactosidase activitya
|
||
|---|---|---|---|---|
| + N/−Mo | −N/−Mo | −N/+Mo | ||
| pKS131A | anfA-lacZ | 95 ± 36 | 2,016 ± 138 | 0 ± 0 |
| pTD7-2I | anfH-lacZ | 52 ± 26 | 1,913 ± 317 | 38 ± 14 |
| pSA16 | anf1-lacZ | 51 ± 47 | 445 ± 97 | 0 ± 0 |
| pNIRUB128 | ranR-lacZ | 675 ± 136 | 538 ± 76 | 644 ± 76 |
| pNIRUB132 | ranT-lacZ | 1,395 ± 30 | 1,333 ± 105 | 1,847 ± 70 |
R. capsulatus strains were grown under phototrophic conditions with either 15 mM ammonium (+N) or 9.5 mM serine (−N) as the sole source of nitrogen. Na2MoO4 (+Mo) was added at a final concentration of 10 μM.
Conclusions.
This paper is based on yeast two-hybrid studies which led to the identification of two new genes, ranR and ranT, coding for proteins interacting with Anf1. The respective gene products were shown to play important roles during growth with N2 via the alternative iron-only nitrogenase, thereby confirming that the protein interactions observed in the yeast system indeed reflect a functional connection in R. capsulatus. In contrast to known anf genes, the expression of ranR and ranT was not repressed by ammonium or molybdenum. Therefore, these genes would not have been detected in a general search for ammonium- or molybdenum-regulated genes, e.g., by microarray experiments.
Despite the similarity of RanR and RanT to the TetR family of bacterial regulators and the thioesterase superfamily, respectively, no clear function can be assigned to RanR or RanT that would obviously explain their roles (or the role of Anf1) in the activity of the alternative nitrogenase. However, there are several hints leading us to propose the involvement of these proteins and the products of adjacent genes in the acquisition and/or processing of iron. (i) Yersinia pestis requires a thioesterase domain for the production of a siderophore, yersiniabactin, which is involved in iron uptake under Fe deficiency (2). Based on the similarity of RanT to thioesterases, one might assume a similar function for RanT in siderophore biosynthesis in R. capsulatus. (ii) The genes located downstream of R. capsulatus ranT (Fig. 1) exhibit strong similarity to tolQRA from Pseudomonas aeruginosa and other gram-negative bacteria (5). These genes are known to code for systems involved in macromolecule transport across the outer membrane, and therefore, one might speculate that the respective R. capsulatus gene products are involved in siderophore uptake. Remarkably, like R. capsulatus, A. vinelandii harbors a ranT-like gene directly followed by a tolQ-like gene (http://www.azotobacter.org). (iii) The deduced product of the gene located immediately upstream of ranR (Fig. 1) exhibits significant similarity to RhbA, which is known to catalyze the first step in the biosynthesis of a siderophore from Sinorhizobium meliloti, rhizobactin 1021 (20). (iv) The deduced product of the gene located downstream of ranR (Fig. 1) shows some similarity to TonB from Erwinia chrysanthemi, which is involved in transport of all the ferrisiderophores used by this bacterium as iron sources (6). (v) Last but not least, the Anf1 homologue AnfO itself has been suggested to be an iron-containing protein (14, 25).
The hints enumerated above suggest the involvement of Anf1 and the products of the ranR and ranT gene regions in acquisition and/or processing of iron. It seems unlikely, however, that RanR and RanT have general functions in Fe metabolism, since the growth rates of corresponding mutants via the molybdenum nitrogenase system or in the presence of ammonium were indistinguishable from those of the wild type, whereas diazotrophic growth via the iron-only nitrogenase, as well as the catalytic activity of the enzyme system, were clearly affected. It is worth mentioning that the latter effects could not be compensated for by increasing the Fe concentration in the culture medium up to 10-fold (data not shown). Therefore, assuming that the ran gene products may somehow be involved in synthesis of a siderophore-like component, this putative siderophore cannot be essential per se but may play a distinctive role in the iron-only nitrogenase system. Taking into consideration that the only fundamental structural difference between the cofactors of the two nitrogenases apparently is that in the FeFeco the molybdenum atom of FeMoco is replaced by iron (17), one might speculate that Anf1, RanR, and RanT play roles in acquisition and/or insertion of this special Fe atom into the FeFeco.
Acknowledgments
We thank A. Pawlowski, F. Rosenau, S. Weidner, and S. Wilhelm for helpful discussions and F. Narberhaus for critically reading the manuscript.
This work was supported by financial grants from Fonds der Chemischen Industrie and Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Bartel, P. L., J. Roecklein, D. J. Gupta, and S. Fields. 1996. A protein linkage map of Escherichia coli bacteriophage T7. Nat. Genet. Res. 12:72-77. [DOI] [PubMed] [Google Scholar]
- 2.Bobrov, A. G., V. A. Geoffroy, and R. D. Perry. 2002. Yersiniabactin production requires the thioesterase domain of HMWP2 and YbtD, a putative phosphopantetheinylate transferase. Infect. Immun. 70:4204-4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drepper, T., S. Groβ, A. F. Yakunin, P. C. Hallenbeck, B. Masepohl, and W. Klipp. 2003. Role of GlnB and GlnK in ammonium control of both nitrogenase systems in the phototrophic bacterium Rhodobacter capsulatus. Microbiology 149:2203-2212. [DOI] [PubMed] [Google Scholar]
- 4.Drepper, T., K. Raabe, D. Giaourakis, M. Gendrullis, B. Masepohl, and W. Klipp. 2002. The Hfq-like protein NrfA of the phototrophic purple bacterium Rhodobacter capsulatus controls nitrogen fixation via regulation of nifA and anfA expression. FEMS Microbiol. Lett. 215:221-227. [DOI] [PubMed] [Google Scholar]
- 5.Duan, K., E. R. Lafontaine, S. Majumdar, and P. A. Sokol. 2000. RegA, iron, and growth phase regulate expression of the Pseudomonas aeruginosa tol-oprL gene cluster. J. Bacteriol. 182:2077-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Enard, C., and D. Expert. 2000. Characterization of a tonB mutation in Erwinia chrysanthemi 3937: TonBEch is a member of the enterobacterial TonB family. Microbiology 146:2051-2058. [DOI] [PubMed] [Google Scholar]
- 7.Feilotter, H. E., G. J. Hannon, C. J. Ruddel, and D. Beach. 1994. Construction of an improved host strain for two-hybrid screening. Nucleic Acids Res. 22:1502-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 9.Haselkorn, R., A. Lapidus, Y. Kogan, C. Vlcek, J. Paces, V. Paces, P. Ulbrich, T. Pecenkova, D. Rebrekov, A. Milgram, M. Mazur, R. Cox, N. Kyrpides, N. Ivanova, V. Kapatral, T. Los, A. Lykidis, N. Mikhailova, G. Reznik, O. Vasieva, and M. Fonstein. 2001. The Rhodobacter capsulatus genome. Photosyn. Res. 70:43-52. [DOI] [PubMed] [Google Scholar]
- 10.Hoover, T. R., J. Imperial, J. Liang, P. W. Ludden, and V. K. Shah. 1988. Dinitrogenase with altered substrate specificity results from the use of homocitrate analogues for in vitro synthesis of the iron-molybdenum cofactor. Biochemistry 27:3647-3652. [DOI] [PubMed] [Google Scholar]
- 11.Hoover, T. R., J. Imperial, P. W. Ludden, and V. K. Shah. 1988. Homocitrate cures the NifV− phenotype in Klebsiella pneumoniae. J. Bacteriol. 170:1978-1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hübner, P., J. C. Willison, P. M. Vignais, and T. A. Bickle. 1991. Expression of regulatory nif genes in Rhodobacter capsulatus. J. Bacteriol. 173:2993-2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joerger, R. D., M. R. Jacobson, and P. E. Bishop. 1989. Two nifA-like genes required for expression of alternative nitrogenases by Azotobacter vinelandii. J. Bacteriol. 171:3258-3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joerger, R. D., M. R. Jacobson, R. Premakumar, E. D. Wolfinger, and P. E. Bishop. 1989. Nucleotide sequence and mutational analysis of the structural genes (anfHDGK) for the second alternative nitrogenase from Azotobacter vinelandii. J. Bacteriol. 171:1075-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klipp, W., S. Angermüller, S. Astroth, P.-B. Kamp, M. Kern, M. Kutsche, S. Leimkühler, and A. Paschen. 1995. Regulation of molybdenum and alternative nitrogenase in the photosynthetic purple bacterium Rhodobacter capsulatus, p. 201-206. In I. A. Tikhonovich, N. A. Provorov, V. I. Romanov, and W. E. Newton (ed.), Nitrogen fixation: fundamentals and applications. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 16.Klipp, W., B. Masepohl, and A. Pühler. 1988. Identification and mapping of nitrogen fixation genes of Rhodobacter capsulatus: duplication of a nifA-nifB region. J. Bacteriol. 170:693-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krahn, E., R. Weiss, M. Krockel, J. Groppe, G. Henkel, P. Cramer, X. Trautwein, K. Schneider, and A. Müller. 2002. The Fe-only nitrogenase from Rhodobacter capsulatus: identification of the cofactor, an unusual, high-nuclearity iron-sulfur cluster, by Fe K-edge EXAFS and 57Fe Mössbauer spectroscopy. J. Biol. Inorg. Chem. 7:37-45. [DOI] [PubMed] [Google Scholar]
- 18.Kutsche, M., S. Leimkühler, S. Angermüller, and W. Klipp. 1996. Promoters controlling expression of the alternative nitrogenase and the molybdenum uptake system in Rhodobacter capsulatus are activated by NtrC, independent of σ54, and repressed by molybdenum. J. Bacteriol. 178:2010-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Labes, M., A. Pühler, and R. Simon. 1990. A new family of RSF1010-derived expression and lac-fusion broad-host-range vectors for Gram-negative bacteria. Gene 89:37-46. [DOI] [PubMed] [Google Scholar]
- 20.Lynch, D., J. O'Brien, T. Welch, P. Clarke, P. O. Cuiv, J. H. Crosa, and M. O'Connell. 2001. Genetic organization of the region encoding regulation, biosynthesis, and transport of rhizobactin 1021, a siderophore produced by Sinorhizobium meliloti. J. Bacteriol. 183:2576-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masepohl, B., T. Drepper, A. Paschen, S. Groβ, A. Pawlowski, K. Raabe, K.-U. Riedel, and W. Klipp. 2002. Regulation of nitrogen fixation in the phototrophic purple bacterium Rhodobacter capsulatus. J. Mol. Microbiol. Biotechnol. 4:243-248. [PubMed] [Google Scholar]
- 22.Masepohl, B., and W. Klipp. 1996. Organization and regulation of genes encoding the molybdenum nitrogenase and the alternative nitrogenase in Rhodobacter capsulatus. Arch. Microbiol. 165:80-90. [Google Scholar]
- 23.Masepohl, B., W. Klipp, and A. Pühler. 1988. Genetic characterization and sequence analysis of the duplicated nifA/nifB gene region of Rhodobacter capsulatus. Mol. Gen. Genet. 212:27-37. [DOI] [PubMed] [Google Scholar]
- 24.Miller, J. H. 1972. Experiments in molecular genetics, p. 352-355. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 25.Mylona, P. V., R. Premakumar, R. N. Pau, and P. E. Bishop. 1996. Characteristics of orf1 and orf2 in the anfHDGK genomic region encoding nitrogenase 3 of Azotobacter vinelandii. J. Bacteriol. 178:204-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pawlowski, A., K.-U. Riedel, W. Klipp, P. Dreiskemper, S. Groβ, H. Bierhoff, T. Drepper, and B. Masepohl. 2003. Yeast two-hybrid studies on interaction of proteins involved in regulation of nitrogen fixation in the phototrophic bacterium Rhodobacter capsulatus. J. Bacteriol. 185:5240-5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Premakumar, R., M. R. Jacobson, T. M. Loveless, and P. E. Bishop. 1992. Characterization of transcripts expressed from nitrogenase-3 structural genes of Azotobacter vinelandii. Can. J. Microbiol. 38:929-936. [DOI] [PubMed] [Google Scholar]
- 28.Schneider, K., U. Gollan, M. Dröttboom, S. Selsemeier-Voigt, and A. Müller. 1997. Comparative biochemical characterization of the iron-only nitrogenase and the molybdenum nitrogenase from Rhodobacter capsulatus. Eur. J. Biochem. 244:789-800. [DOI] [PubMed] [Google Scholar]
- 29.Schneider, K., A. Müller, K.-U. Johannes, E. Diemann, and J. Kottmann. 1991. Selective removal of molybdenum traces from growth media of N2-fixing bacteria. Anal. Biochem. 193:292-298. [DOI] [PubMed] [Google Scholar]
- 30.Schneider, K., A. Müller, U. Schramm, and W. Klipp. 1991. Demonstration of a molybdenum- and vanadium-independent nitrogenase in a nifHDK-deletion mutant of Rhodobacter capsulatus. Eur. J. Biochem. 195:653-661. [DOI] [PubMed] [Google Scholar]
- 31.Schüddekopf, K., S. Hennecke, U. Liese, M. Kutsche, and W. Klipp. 1993. Characterization of anf genes specific for the alternative nitrogenase and identification of nif genes required for both nitrogenases in Rhodobacter capsulatus. Mol. Microbiol. 8:673-684. [DOI] [PubMed] [Google Scholar]
- 32.Seefeldt, L. C., L. C. McCollum, C. M. Doyle, and D. J. Arp. 1987. Immunological and molecular evidence for a membrane-bound, dimeric hydrogenase in Rhodopseudomonas capsulata. Biochim. Biophys. Acta 914:299-303. [Google Scholar]
- 33.Siemann, S., K. Schneider, K. Behrens, A. Knöchel, W. Klipp, and A. Müller. 2001. FeMo cofactor biosynthesis in a nifE− mutant of Rhodobacter capsulatus. Eur. J. Biochem. 268:1940-1952. [DOI] [PubMed] [Google Scholar]
- 34.Simon, R., U. Priefer, and A. Pühler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Bio/Technology 1:784-791. [Google Scholar]
- 35.Wang, G., S. Angermüller, and W. Klipp. 1993. Characterization of Rhodobacter capsulatus genes encoding a molybdenum transport system and putative molybdenum-pterin-binding proteins. J. Bacteriol. 175:3031-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]