This study examines the extent and resource encumbrance of myelodysplastic syndromes in patients who have failed hypomethylating agent treatment. Considering the cost of illness and research to date, this study aims to improve understanding of the clinical importance of this unmet need.
Keywords: Myelodysplastic syndromes, Cost of illness, Incidence, Prevalence
Abstract
Background.
Although hypomethylating agents (HMAs) are effective and approved therapies for patients with myelodysplastic syndromes (MDS), many patients do not benefit from treatment, and nearly all ultimately stop responding to HMAs. The incidence and cost burden of HMA failure are unknown yet needed to appreciate the magnitude and significance of such failure.
Methods.
We analyzed a de‐identified dataset of over 5 million individuals with private health insurance in the U.S. to estimate MDS incidence, prevalence, and treatments. Based on MDS provider interviews, a conceptual model of MDS patient management was constructed to create a new, claims‐relevant and drug development‐relevant definition of HMA treatment failure. This algorithm was used to define resource encumbrance of MDS patients in whom HMA treatment failed.
Results.
We estimated an MDS incidence rate of ∼70 cases per 100,000 enrollees per year and a prevalence of 155 cases per 100,000 enrollees. The proportion of MDS patients receiving HMA treatment was low (∼3%), and treatment was typically initiated within 1 year of the first MDS claim. Notably, HMA‐treated individuals were older and had more comorbidities than the overall MDS cohort. Total health care costs of managing MDS patients after HMA failure were high (∼$77,000 during the first 6 months) and were driven primarily by non‐pharmacy costs.
Conclusion.
This study quantifies for the first time the burden of significant unmet need in caring for MDS patients following HMA treatment failure.
Implications for Practice.
U.S.‐based treatment patterns among MDS patients demonstrate the significant clinical, financial, and health care burden associated with HMA failure and call for active therapies for this patient population.
Introduction
The myelodysplastic syndromes (MDS) are most commonly diagnosed in older individuals, and hypomethylating agent (HMA) treatments improve clinical outcomes in 40%–60% of patients [1], [2]. However, approximately 40% of MDS patients fail to achieve clinical improvement after HMA treatment, and nearly all patients eventually suffer from progressive disease. Unfortunately, there is no approved salvage treatment after HMA failure, and the prognosis is grim [3]. The actual incidence of HMA treatment failure is unknown. Furthermore, the cost burden after such failure remains unexamined. Studies by our group and others have generally been limited to the period following MDS diagnosis [1], and no data exist on incidence and burden of MDS after first‐line treatment failure. As a result, the significance of this clinical unmet need is unknown. We examined the extent and resource encumbrance of MDS patients who have failed HMA to improve understanding of the clinical importance associated with this unmet need.
Materials and Methods
This analysis comprised a series of linked studies using a commercial claims database and informed by a conceptual model developed in collaboration with disease area experts. The database used was the Optum Clinformatic Data Mart, a Health Insurance Portability and Accountability Act‐compliant administrative claims database of 13 million annual lives, with adjudicated pharmacy and medical claims submitted by providers, health care facilities, and pharmacies. Claims include information on physician visits, medical procedures, hospitalizations, drugs dispensed, and tests performed. Also available are member enrollment and benefit information as well as limited patient, provider, and hospital demographic information. All major U.S. regions are represented. The data used for this study were from calendar years 2008–2012. The study was exempt from institutional review board approval.
To study the epidemiology and initial treatment of the disease, we first estimated the prevalence and incidence of MDS patients. Prevalent patients were those with a medical claim with a diagnosis of MDS (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD‐9‐CM] code of 238.7x) in any diagnosis field in the 2009 calendar year (the identification [ID] period) and who were continuously enrolled for all of 2008 (pre‐ID period) and 2009. Incident patients were the subset with an ICD‐9‐CM code for MDS in 2009 and no codes for MDS or unspecified anemia in 2008. A subgroup of prevalent patients with new HMA treatment was defined by identifying patients with no HMA treatment in 2008 followed by HMA treatment in 2009.
Demographic data were reported for all groups. Incidence and prevalence were reported stratified by age and sex by dividing the number of incident or prevalent patients by the appropriate denominator (e.g., the total population in the database in the relevant age and sex stratum). The proportions of patients receiving chemotherapy, supportive care, and “watch and wait” were reported. All data transformations and statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
To better understand the conceptual model of MDS management, we conducted in‐depth, semi‐structured interviews with nine oncology and hematology specialists (eight physicians, one nurse practitioner) who care for substantial numbers of MDS patients. The interviews were organized around five topic areas: (a) diagnosis, including information gathered in the clinical setting to support the diagnostic process, tests performed to rule out alternative diagnoses, and risk assessment; (b) initial management decision based on risk assessment; (c) management choice and the role of risk assessment; (d) disease progression assessment, such as timing and information used; and (e) management choice(s) during disease progression. Based on these interviews, we developed and validated a conceptual model describing current MDS management decision‐making options and pathways.
A definition of second‐line treatment was developed to incorporate the findings from the conceptual model. HMA failure was defined as those MDS patients who had disease progression while on first‐line HMA or stopped responding to first‐line HMA. Specifically, we identified patients with “HMA failure” in this study as those who (a) stopped initial HMA for ≥2 months, (b) switched to a second HMA, or (c) experienced “HMA prolongation,” continuing the initial HMA for >7 months. The third criterion was added to reflect the experts' observation that continuing HMA treatment beyond 6 months reflected a heterogeneous mix of patients, including complete responders, partial responders, those with stable disease, and some who were continued on HMA due to lack of alternative therapy options.
A patient cohort was developed using the database described above with the index period extended to encompass calendar years 2009–2011 to maximize sample size. All patients who met one of the three criteria listed above and were continuously enrolled for ≥1 year before and 6 months after the index date were included. The index date was 2 months after the last HMA for those who stopped, the date of the new HMA for those who switched, and the first day the initial HMA was used beyond 7 months for those who continued. For these patients, we reported demographics and patient‐related information as well as overall and MDS‐specific health care costs and utilization. MDS‐specific costs included medication costs (HMAs, lenalidomide, erythropoietin‐stimulating agents [ESAs], growth factors, and blood transfusions) and costs for claims with a primary diagnosis of MDS, acute myeloid leukemia (AML), anemia, thrombocytopenia, neutropenia, or pancytopenia. Utilization included office and emergency department (ED) visits and hospital stays, with MDS‐specific utilization defined in an analogous way to MDS‐specific cost.
Results
Epidemiology and Treatment
We identified 9,209 patients with MDS in 2009 in the Optum Clinformatic Data Mart who were enrolled in 2008–2009. Of these, 4,151 (45%) patients had no prior diagnosis of MDS or unspecified anemia in 2008. During this same period, there were 5,942,153 continuously enrolled members for an overall MDS incidence in this database of 69.9/100,000 enrollees per year. Women had higher MDS incidence than men: 75.7/100,000 versus 63.1/100,000, respectively. Men appeared to be diagnosed at older ages. The highest observed incidence in women was among those aged 50–64 years (111.5/100,000) and next among those aged 65–74 years (101.2/100,000), whereas in men the highest incidence was among those aged 65–74 years (106.1/100,000) and next among those aged 75+ years (97.1/100,000). Overall MDS prevalence was 155.0/100,000, and the pattern was similar to that for incidence: higher among women (87.1 versus 42.9/100,000), peaking at a lower age for women (50–64 years) than men (65–74 years).
Next, we studied HMA initiation among incident patients. Beginning at the date of MDS diagnosis, we followed the 4,151 incident patients until disenrollment, the end of available data, or observed HMA treatment initiation. By 1 year after diagnosis, 2.3% had initiated HMA treatment, with 2.7% and 2.9% initiating treatment by 2 years and 3 years, respectively.
Taking a broader look at MDS treatments for the 9,209 MDS patients, 3.9% were observed to have received some chemotherapy, 18.6% received supportive care (ESAs, growth factors, or transfusions) with or without chemotherapy, and 80.1% received neither chemotherapy nor supportive care (Table 1). Similar to findings in the newly incident MDS subset, 257 of 9,209 (2.8%) were treated with HMAs at some time during 2009, with 166 initiating in that year (Table 1). The mean age of patients with new HMA treatment was 72.8 years (standard deviation [SD] 9.1) versus 63.9 years (SD 17.1) in all MDS patients. Among HMA initiators, 39.8% were female versus 57.8% of MDS patients overall. Charlson Comorbidity Index was 4.2 (SD 3.2) and the number of chronic conditions was 7.0 (SD 2.4) in patients initiating HMA compared with 3.0 (SD 3.1) and 5.3 (SD 2.6), respectively, in all patients. Nearly three quarters (74.1%) of newly HMA‐treated patients also received supportive care. Among HMA initiators, treatment involved azacitidine (AZA) in 76.5% of patients and decitabine (DEC) in 30.1%, with 6.6% of patients receiving both. In patients treated with HMA at any time (n = 257), 76.7% were treated with AZA, 32.3% with DEC, and 8.9% with both. Newly treated patients remained on HMA for a median 154 (AZA) or 117 (DEC) days before stopping. There was no difference in duration of treatment between the two HMAs (p = .532).
Table 1. Treatment of MDS patientsa.
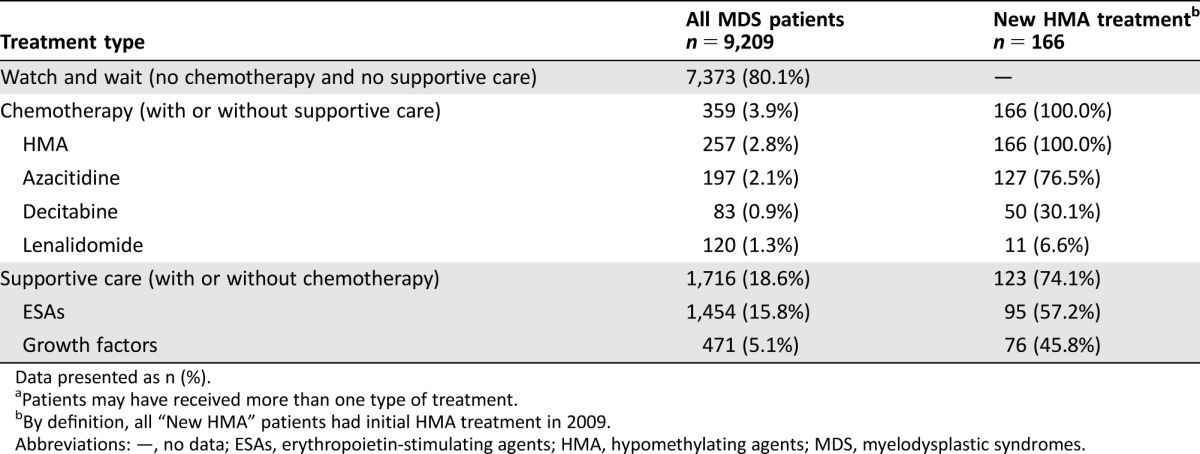
Data presented as n (%).
Patients may have received more than one type of treatment.
By definition, all “New HMA” patients had initial HMA treatment in 2009.
Abbreviations: —, no data; ESAs, erythropoietin‐stimulating agents; HMA, hypomethylating agents; MDS, myelodysplastic syndromes.
Conceptual Model of MDS
MDS patient clinical providers gave consistent responses regarding the major elements of the diagnostic process, including history and physical, routine lab tests (e.g., complete blood counts, metabolic panels), and bone marrow biopsy. Providers calculated International Prognostic Scoring System or Revised International Prognostic Scoring System on almost all treatment‐naïve patients [4]. Several risk score assessments were often needed for the same patient in order to fulfill different purposes (e.g., clinical trial entry criteria, billing, treatment decision‐making). Respondents agreed that most low‐risk MDS patients undergo a period of watchful waiting. Methods used to assess disease progression varied between respondents, but complete blood counts and/or bone marrow aspirations and biopsies were cited most often. High‐risk MDS was cited as a trigger for HMA treatment, but “high‐risk” definitions varied substantially. Approximately two thirds of respondents kept patients on HMAs indefinitely in the absence of treatment‐related adverse effects. Definitions of treatment failure and treatment choices after first‐line failure varied greatly. In general, the most frequently used therapies included clinical trials, induction chemotherapy, and best supportive care, and respondents' definitions of transplant eligibility varied.
Treatment Choice in Refractory MDS After First‐Line HMA Failure
Based on the conceptual model, we identified 402 refractory MDS patients in the Optum Database: 335 who had stopped their initial HMA, 32 who switched HMA, and 35 who continued on treatment >7 months (i.e., experienced “HMA prolongation”). Age (mean 72.9 years, SD 9.1 years) and sex (39.8% female) were similar for these groups.
Just under half (198/402, 49.3%) of MDS patients in whom first‐line HMA failed subsequently initiated chemotherapy in the 6 months following (Table 2). A second HMA was the most common chemotherapy (30.1% AZA and 18.4% DEC), while 4.5% of patient initiated lenalidomide. During the same period, 61.4% of patients initiated supportive care (ESAs, growth factors, or transfusions). Rates of treatment with chemotherapy fell over successive periods following initial eligibility for second‐line treatment, with chemotherapy used in 49.3% of the 402 patients initially (months 1–6) but in only 32.6% of the 95 patients remaining in the sample by months 19–24 (Table 2). Supportive care followed a similar pattern.
Table 2. Second‐line MDS treatment received over successive 6‐month periods.
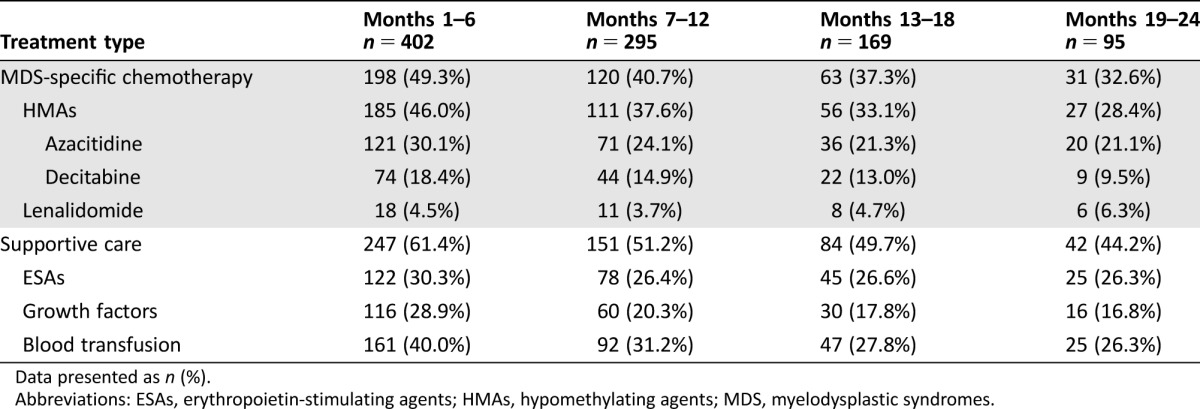
Data presented as n (%).
Abbreviations: ESAs, erythropoietin‐stimulating agents; HMAs, hypomethylating agents; MDS, myelodysplastic syndromes.
Resource Burden After Failing First‐Line HMA
Next, we examined health care resource use in MDS patients in whom initial treatment failed (Table 3). The 402 MDS patients who failed first‐line HMA had a mean of 26 (SD 21.1, median 23) office visits during the first 6 months, declining to 20.5 (SD 18.2, median 15) in the 95 remaining patients by months 19–24. At least 1 visit to the ED was recorded in 19.7% of patients during the first 6 months after the index HMA failure date. At least 1 hospitalization was observed in 32.6% of patients during the first 6 months after the index date, and 9.5% had 3 or more hospitalizations. The mean length of stay for MDS‐specific hospitalizations was 13 days (SD 16.5, median 5; Table 3).
Table 3. Health care utilization over successive 6‐month periods following second‐line treatment initiation.
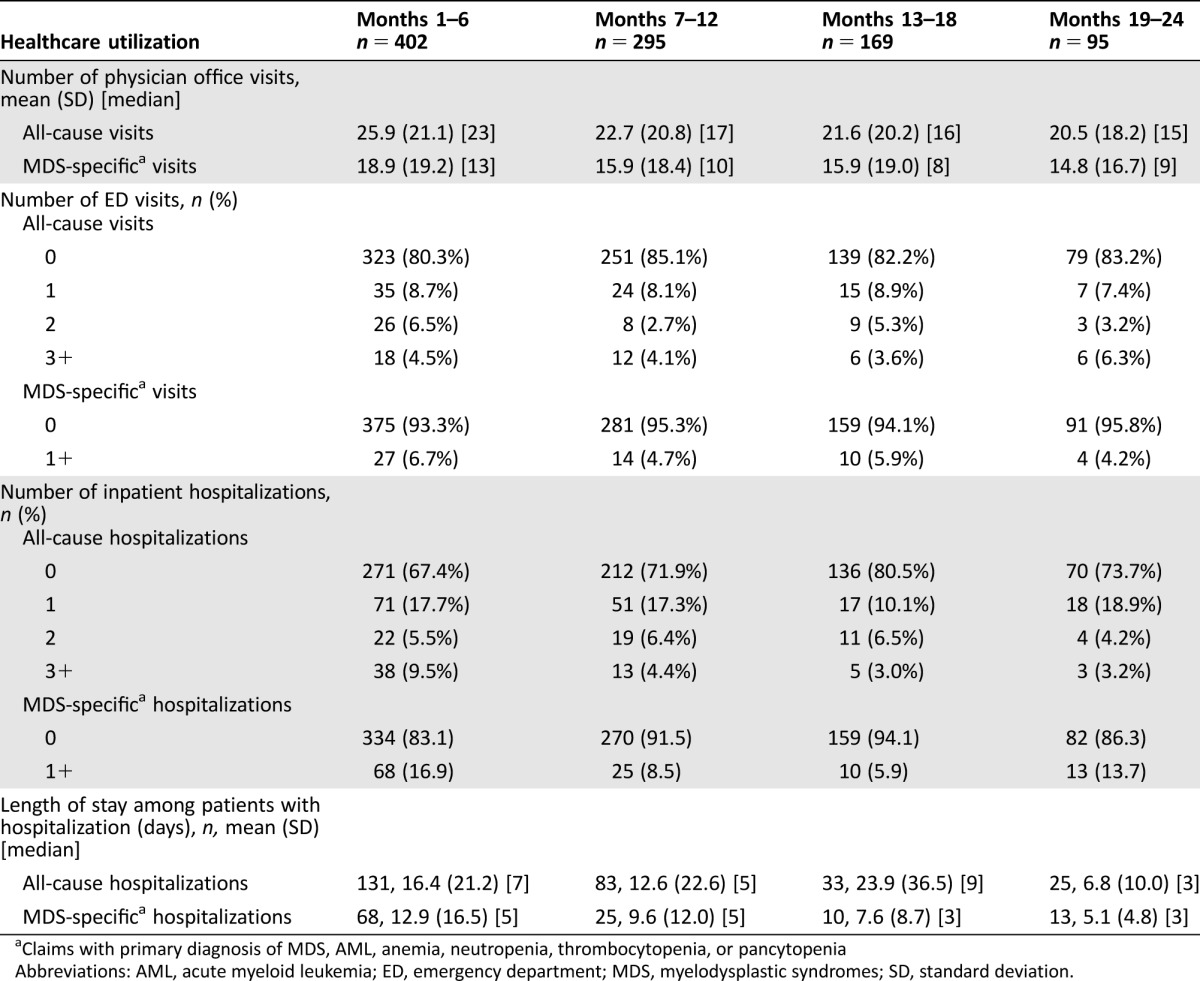
Claims with primary diagnosis of MDS, AML, anemia, neutropenia, thrombocytopenia, or pancytopenia
Abbreviations: AML, acute myeloid leukemia; ED, emergency department; MDS, myelodysplastic syndromes; SD, standard deviation.
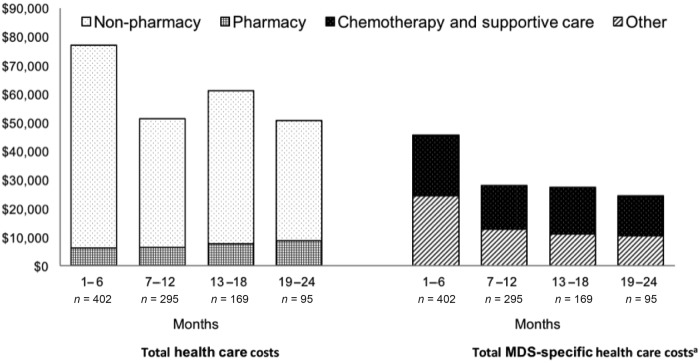
Among patients in whom initial treatment failed, the mean total cost of care was $76,945 (SD $92,764) during the first 6 months and declined to $50,732 (SD $77,885) in the 95 patients remaining at months 19–24 (Fig.). These costs comprise those of chemotherapy (HMAs and lenalidomide), supportive care (ESAs, growth factors, and blood transfusions), and claims with a primary diagnosis of MDS, AML, anemia, thrombocytopenia, neutropenia, or pancytopenia. The mean MDS‐specific health care cost was $45,564—or 59% of total costs—during the first 6 months after the index HMA failure date, with a similar decline over time as that of total costs. Chemotherapy and supportive care comprised 43%–46% of MDS‐specific costs.
Discussion
In this report, we reveal several novel results regarding the incidence, prevalence, and treatment patterns of MDS, with specific focus on the unfortunate yet inevitable scenario of failing HMA treatment. Using a U.S. commercial health insurance claims database, we found an MDS incidence of 69.9/100,000, which is close to what we found in our previous Surveillance, Epidemiology, and End Results (SEER)‐Medicare study (75/100,000) [5]. MDS prevalence in the current study was estimated to be 155/100,000, which is within range of prior reports [1], [6]. Based on these results, it is estimated that almost 500,000 people in the U.S. are living with MDS.
Using a commercial insurance database, we found that a very small proportion (less than 3%) of MDS patients were treated with HMAs. If HMA treatment was initiated, it typically began within a year of the first MDS claim. Our estimates are lower than others made when using SEER‐Medicare data, where approximately 10% of MDS patients used HMAs [7]. These differences may be due to misclassification of disease status when using ICD‐9‐based algorithms to identify patients in claims data and that older patients identified in claims may be healthier than and not representative of their non‐employed counterparts in Medicare. Our results are, however, consistent with other claims‐based estimates [8]. Although prior studies documented that older MDS patients are more likely to receive supportive care rather than disease‐modifying treatment, we show that this reluctance to treat MDS occurs at all ages [7], [9]. In these prior studies, age alone was linked to abstention from active treatment, which led the investigators to surmise that ageism may explain treating physicians' unwillingness to administer HMA treatment to older MDS patients. Whereas age discrimination is certainly possible and has been detected throughout medical practice [10], [11], we found that HMA‐treated individuals were older rather than younger MDS patients. Moreover, the HMA‐treated individuals had an increased number of comorbidities compared with the entire MDS cohort. One explanation for the seemingly contrasting results is that the prior studies limited their examinations to individuals aged 65 years or older; on the other hand, this study had no age restrictions and therefore was able to detect HMA use in patients at younger ages. There could be many reasons for lower usage of HMA in younger MDS patients, including but not limited to the use of higher‐intensity chemotherapy regimens and/or direct application of allogeneic stem cell transplant for an attempt at curative treatment. Regardless of the reason, the case for age discrimination in MDS is not settled and deserves a closer evaluation using larger datasets.
From interviews with MDS providers, there was congruence in almost all aspects of care for the MDS patient except when defining the occurrence of first‐line treatment failure, choice of treatment after failing first‐line treatment, and determining transplant eligibility. The clinical management of MDS still depends upon determining the numbers of circulating peripheral blood cells and examining bone marrow cell morphology, which are included in the MDS International Working Group 2006 categories of Failure and Disease Progression [12]. However, these assessments, and especially bone marrow examination, are notoriously difficult because of the challenges in distinguishing a panoply of dysplastic phenotypes from normal hematopoiesis [13], [14]. As such, the respondent providers reported the use of a variety of clinical observations such as number and depth of cytopenias, presence of dysplastic hematopoiesis, enumeration of myeloblasts, and chromosome analysis (i.e., cytogenetics and fluorescence in situ hybridization) to guide care. More recently, molecular mutation‐based models have enabled a greater degree of discrimination in predicting response to HMA [15]; however, these have not been validated and there exists no consensus on such predictions. Consequently, defining treatment success or failure will rely upon bone marrow and peripheral blood count assessments and marrow function as determined by transfusion needs.
Nevertheless, we created a new definition of HMA failure for the use in conducting claims‐based analyses. Two of the criteria for HMA failure (i.e., stopped HMA for more than 2 months, switched from one HMA to another) are obvious from a clinical perspective. However, the third criterion (i.e., continuing on HMA for more than 7 months, or “HMA prolongation”) could be misconstrued if only applied to day‐to‐day clinical practice. The two intents of the third criterion were to provide index dates for calculating resource utilization and to create a placeholder for a discussion of therapy strategy. Whereas the clinical respondents reported prescribing HMA treatments indefinitely until MDS progression or unacceptable toxicities, for the purposes of this study, a specific time point had to be chosen. When longer time points of 1 year or longer were chosen, there were too few patients in the HMA cohort to conduct resource utilization calculations. Thus, continuing HMA for more than 7 months was chosen for practical reasons and to represent a time point at which a hypothetical second active therapy could be prescribed. When applying our new billing claims definition of HMA failure, only a small minority of patients (9%) fit in the third criterion, while the vast majority of patients (83%) had HMA failure as defined by stopping HMA for more than 2 months. We conducted a sensitivity analysis excluding patients in the third group. The results were very similar to those from the base case approach. In this report, we concentrate on the entire group to follow the conceptual model developed based on expert guidance.
Certainly claims‐based reporting has its vagaries, including incorrect coding, lack of pathology verification, lack of coding for non‐reimbursable or low‐reimbursable events, and underreporting of comorbidities. Despite these challenges, claims‐based procedures represent the only comprehensive method for quantifying the financial burden of illness and for that reason were used in this study.
In MDS patients in whom HMA therapy failed, supportive care was the most commonly used subsequent therapy, followed closely by treatment with a second HMA. Of note, the medical literature and National Comprehensive Cancer Network guidelines do not provide high levels of evidence to the clinical practice of administering ESAs or myeloid growth factors after HMA failure, despite its apparent use in conventional oncology practice. This phenomenon may point to an educational opportunity on the appropriateness of growth factor support, but it also exposes a desperate need for more active therapies in MDS. Interestingly, lenalidomide was administered in a small number of patients after HMA failure. Unfortunately, the claims data didn't permit interrogation of disease characteristics' linking with second‐line treatment choice. Nonetheless, these constrained practice patterns reflect the paucity of choices for treating MDS patients in whom HMA agents have failed and beg for novel therapies to fill this void. It would appear that with more clinical trial awareness on the part of the providers and patients, another option that offers hope for improving the outcome of these patients could be clinical trials.
The cost of caring for MDS patients after first‐line HMA failure was high, at nearly $80,000 per patient during the first 6 months and approximately $130,000 for the subsequent 12 months (Figure 1). Among those living beyond 1.5 years, costs were approximately $50,000 for a 6‐month period. For all time periods, these costs mostly comprised chemotherapy and supportive care expenses and were higher than the costs of caring for a newly diagnosed MDS patient, as we have previously published [16]. Of note, the MDS‐specific costs likely underestimated the true disease‐related costs, as they excluded diagnoses such as pneumonia, sepsis, and bleeding that may in fact be related to MDS. As all‐cause costs may contrarily be overestimates, the true costs of caring for MDS patients after first‐line HMA failure are likely to be somewhere in between the MDS‐specific and all‐cause estimates presented here.
Figure 1.
Mean health care costs over successive 6‐month periods following second‐line treatment initiation. aIncludes chemotherapy (HMAs and lenalidomide) and supportive care (ESAs, growth factors, and blood transfusions) costs, as well as costs for claims with a primary diagnosis of MDS, AML, anemia, thrombocytopenia, neutropenia, and pancytopenia.
Abbreviations: AML, acute myeloid leukemia; ESA, erythropoietin‐stimulating agents; HMA, hypomethylating agents; MDS, myelodysplastic syndromes.
Conclusion
In clinical practice terms, results from this study define an important inflection point in the current‐day care of the MDS patient. Given our findings of high incidence and costly outcomes after first‐line HMA failure, this event in the lifetime of an MDS patient should be recognized as a critical happenstance worthy of a planned visit inclusive of reconsidering long‐term goals and changing treatment bearing based on these revisited goals. In particular, if HMA therapy was initially prescribed as palliation and the patient continues to request palliative efforts, then this visit would instigate discussions about clinical trials and end‐of‐life care. However, if the patient expresses curative intent, then this visit would initiate discussions about allogeneic hematopoietic cell transplant (for eligible patients) and clinical trials [17]. Results from this study support the recognition of this planned and documented visit after HMA failure as an important measure for defining high‐quality care among patients with MDS. Meeting and documenting this time point ensures that the costs of care are commensurate with the au fait patient's wishes and simultaneously aligns health care to patient dignity and checks cost of care.
Acknowledgments
This research was presented at the 13th International Symposium on Myelodysplastic Syndromes in April 2015. The Leukemia & Lymphoma Society supported C.R.C. with a Scholar in Clinical Research award (2400‐13). Select results described in this paper were presented at the American Society of Hematology 56th Annual Meeting in December 2014 and the MDS Foundation 13th International Symposium of MDS in May 2015. This research was funded by Onconova Therapeutics, Inc.
Footnotes
For Further Reading: Christopher R. Cogle, Bart L. Scott, Thomas Boyd et al. Oral Azacitidine (CC‐486) for the Treatment of Myelodysplastic Syndromes and AcuteMyeloid Leukemia.
Implications for Practice: Injectable azacitidine can prolong survival, reduce transfusions, and improve quality of life compared with conventional care regimens in patients with higher‐risk myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML). An oral formulation improves convenience and eliminates injection‐site reactions but also enables testing of novel, longer term, low‐dose schedules that may enhance therapeutic activity of azacitidine by increasing exposure to cycling malignant cells. In early phase trials, oral azacitidine (CC‐486) in extended dosing regimens was biologically and clinically active in patients with MDS and AML. Oral azacitidine is being further evaluated in an ongoing phase III program.
Author Contributions
Conception/Design: Christopher R. Cogle, Sandra E. Kurtin, Tanya G. K. Bentley, Michael S. Broder, Eunice Chang, Scott Megaffin, Sudipto Mukherjee, Michael E. Petrone
Provision of study material or patients: Sandra E. Kurtin, Tanya G. K. Bentley, Michael S. Broder, Scott Megaffin
Collection and/or assembly of data: Tanya G. K. Bentley, Michael S. Broder, Eunice Chang
Data analysis and interpretation: Christopher R. Cogle, Sandra E. Kurtin, Tanya G. K. Bentley, Michael S. Broder, Eunice Chang, Scott Megaffin, Steven Fruchtman, Sudipto Mukherjee, Michael E. Petrone
Manuscript writing: Christopher R. Cogle, Tanya G. K. Bentley, Michael S. Broder, Sudipto Mukherjee, Michael E. Petrone
Final approval of manuscript: Christopher R. Cogle, Sandra E. Kurtin, Tanya G. K. Bentley, Michael S. Broder, Eunice Chang, Scott Megaffin, Steven Fruchtman, Sudipto Mukherjee, Michael E. Petrone
Disclosures
Christopher R. Cogle: IMS Expert Services (ET); Sandra E. Kurtin: Celgene, Takeda, Genentech, Pharmacyclis (C/A); Tanya G. K. Bentley: PHAR, LLC (E); Michael S. Broder: PHAR, LLC (E); Eunice Chang: PHAR, LLC (E); Scott Megaffin: Onconova Therapeutics, Inc. (E); Steven Fruchtman: CMO, Onconova Therapeutics (E, OI); Sudipto Mukherjee: Ariad Pharmaceuticals, Novartis, PHAR, LLC (C/A), Novartis, IIT (RF). The other author indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Cogle CR. Incidence and burden of the myelodysplastic syndromes. Curr Hematol Malig Rep 2015;10:272–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fenaux P, Mufti GJ, Hellstrom‐Lindberg E et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher‐risk myelodysplastic syndromes: A randomised, open‐label, phase III study. Lancet Oncol 2009;10:223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Prébet T, Gore SD, Esterni B et al. Outcome of high‐risk myelodysplastic syndrome after azacitidine treatment failure. J Clin Oncol 2011;29:3322–3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Greenberg PL, Tuechler H, Schanz J et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood 2012;120:2454–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cogle CR, Craig BM, Rollison DE et al. Incidence of the myelodysplastic syndromes using a novel claims‐based algorithm: High number of uncaptured cases by cancer registries. Blood 2011;117:7121–7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sekeres MA. Epidemiology, natural history, and practice patterns of patients with myelodysplastic syndromes in 2010. J Natl Compr Canc Netw 2011;9:57–63. [DOI] [PubMed] [Google Scholar]
- 7. Wang R, Gross CP, Maggiore RJ et al. Pattern of hypomethylating agents use among elderly patients with myelodysplastic syndromes. Leuk Res 2011;35:904–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hatoum HT, Lin SJ, Buchner D et al. Use of hypomethylating agents and associated care in patients with myelodysplastic syndromes: A claims database study. Curr Med Res Opin 2011;27:1255–1262. [DOI] [PubMed] [Google Scholar]
- 9. Sekeres MA, Schoonen WM, Kantarjian H et al. Characteristics of US patients with myelodysplastic syndromes: Results of six cross‐sectional physician surveys. J Natl Cancer Inst 2008;100:1542–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bowling A. Ageism in cardiology. BMJ 1999;319:1353–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bowling A. Honour your father and mother: Ageism in medicine. Br J Gen Pract 2007;57:347–348. [PMC free article] [PubMed] [Google Scholar]
- 12. Cheson BD, Greenberg PL, Bennett JM et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood 2006;108:419–425. [DOI] [PubMed] [Google Scholar]
- 13. Font P, Loscertales J, Benavente C et al. Inter‐observer variance with the diagnosis of myelodysplastic syndromes (MDS) following the 2008 WHO classification. Ann Hematol 2013;92:19–24. [DOI] [PubMed] [Google Scholar]
- 14. Font P, Loscertales J, Soto C et al. Interobserver variance in myelodysplastic syndromes with less than 5% bone marrow blasts: Unilineage vs. multilineage dysplasia and reproducibility of the threshold of 2% blasts. Ann Hematol 2015;94:565–573. [DOI] [PubMed] [Google Scholar]
- 15. Bejar R, Lord A, Stevenson K et al. TET2 mutations predict response to hypomethylating agents in myelodysplastic syndrome patients. Blood 2014;124:2705–2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cogle CR, Ortendahl JD, Bentley TG et al. Cost‐effectiveness of treatments for high‐risk myelodysplastic syndromes after failure of first‐line hypomethylating agent therapy. Expert Rev Pharmacoecon Outcomes Res 2016;16:275–284. [DOI] [PubMed] [Google Scholar]
- 17. Zeidan AM, Kharfan‐Dabaja MA, Komrokji RS. Beyond hypomethylating agents failure in patients with myelodysplastic syndromes. Curr Opin Hematol 2014;21:123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]