Denosumab therapy is used to reduce skeletal‐related events in metastatic bone disease. Atypical femoral fracture in osteoporotic patients treated with denosumab have been reported but none in the context of higher dose and more frequent denosumab therapy for metastatic bone disease. This retrospective review assesses atypical femoral fracture incidence among patients with metastatic bone disease who had received oncologic dosing of denosumab for longer cumulative durations than previously reported in clinical trials.
Keywords: Denosumab, Atypical femoral fractures, Metastatic bone disease
Abstract
Background.
Denosumab therapy is used to reduce skeletal‐related events in metastatic bone disease (MBD). There have been reports of atypical femoral fracture (AFF) in osteoporotic patients treated with denosumab but none in the context of higher dose and more frequent denosumab therapy for MBD. The goal of this study was to assess the incidence of AFF in MBD.
Patients and Methods.
We conducted a retrospective review of 253 patients who received a minimum of 12 doses of denosumab at 120 mg each for MBD. To identify patients with asymptomatic atypical stress reactions in the lateral subtrochanteric femur (which precede fractures), we reviewed the skeletal images of 66 patients who had received at least 21 doses of denosumab for AFF features.
Results.
These patients received a median of 17 doses, with a median treatment duration of 23 months. There was 1 case of undiagnosed clinical AFF detected after chart review and 2 cases of subclinical atypical femoral stress reaction observed on imaging review after 23 doses of denosumab over 33 months, 28 doses over 27 months, and 21 doses over 21 months, respectively. Scout computed tomography films showed diffuse cortical thickening of diaphysis with localized periosteal reaction of lateral femoral cortex. Bone scan and magnetic resonance imaging scan of 2 patients with stress reactions confirmed the diagnosis.
Conclusion.
The incidence of clinical AFF in this context is 0.4% (1/253; 95% confidence interval [CI] 0.1%–2.2%), and the incidence of atypical femoral stress reaction based on imaging review is 4.5% (3/66; 95% CI 1.6%–12.5%). Clinicians should be aware of the clinical prodrome (which may or may not be present) and antecedent imaging changes associated with AFF.
Implications for Practice.
Among patients with metastatic bone disease treated with denosumab, cases of clinical and subclinical atypical femoral fracture (AFF) are rare. The one detected case of clinical fracture went unrecognized despite prodromic symptoms. Clinicians should be aware of (a) the potential prodrome of anterior thigh/groin pain and (b) subclinical imaging changes in the lateral femur, both of which may precede clinical AFF.
Introduction
Bisphosphonates are commonly used in patients with metastatic bone disease (MBD) to reduce the risk of skeletal‐related events (SRE) such as spinal cord compression, pathological fractures, the need for external beam radiation or surgery to bone, and hypercalcemia of malignancy. Bisphosphonate therapy, when given at frequent, often monthly intervals in patients with MBD, had been shown to delay the time to the first SRE and decrease the incidence of SRE [1], [2], [3], [4], [5]. However, bisphosphonate therapy for the treatment of osteoporosis has been associated with atypical femoral fracture (AFF), with an absolute risk of 3.2 to 50 cases per 100,000 person‐years for standard therapeutic regimens and 100 per 100,000 person‐years with long‐term use [6]. Our group [7] and Hayashi et al. [8] previously reported cases of AFF in patients with MBD who were treated with more frequent intravenous bisphosphonate therapy. More recently, in a review of 10,587 cancer patients who have received bisphosphonates for underlying osteopenia or osteoporosis, 23 AFF cases were identified, giving rise to an incidence of 0.05 cases per 100,000 person‐years. Out of these AFF cases, 7 had metastatic disease. The authors hypothesized that the lower incidence of AFF could be due to accumulation of bisphosphonates at active areas of bone resorption at bone metastases sites, leaving behind reduced amount of bisphosphonates to affect other skeletal sites [9].
Denosumab, a highly specific RANK (Receptor Activator of Nuclear Factor κ B) ligand inhibitor, is approved by the U.S. Food and Drug Administration for SRE prevention in MBD. Several randomized trials comparing denosumab with zoledronate in MBD demonstrated that denosumab delayed the time to first on‐study SRE and lowered the risk of first SRE by 16%–19% [10], [11], [12], [13], [14], [15], [16]. Although AFF has been reported in 8 patients whose osteoporosis was treated with denosumab (60 mg) administered at 6‐month intervals [17], [18], [19], [20], [21], [22], [23], there were no reports of AFF in the clinical trials of denosumab among patients with MBD despite the higher (120 mg) and more frequent doses (once monthly) [10], [11], [12], [13], [14], [15], [16]. Patients in the pivotal clinical trial leading to approval of denosumab for MBD received a median of 13 doses [11]; clinical data on more extended dosing, which could potentially increase the risk of AFF, is lacking. In this study, we retrospectively assessed AFF incidence among patients with MBD who have received oncologic dosing of denosumab for longer cumulative durations than previously reported in clinical trials.
Materials and Methods
Subjects
After obtaining institutional review board approval, we searched our institution's pharmacy database and identified 253 patients with secondary skeletal malignancies who received a minimum of 12 doses of subcutaneous denosumab (120 mg; Xgeva, Amgen, Thousand Oaks, CA) between February 2011 and June 2014. To identify patients with asymptomatic atypical stress reactions in the lateral subtrochanteric femur (which precede fractures), we reviewed the skeletal images of 66 patients who had received at least 21 doses of denosumab for AFF features. Medical records were reviewed for demographics, primary malignancy, prior bisphosphonate use, prior hormonal, steroid, and/or proton pump inhibitor therapy, skeletal imaging characteristics (when available), incidence of fracture, and clinical outcome.
Clinical Follow‐Up
Denosumab was administered subcutaneously at 120 mg every 4 weeks for the first year of therapy in most cases [10], [11], [12], [13], [14], [15], [16]. After the first year, denosumab was dosed once every 2 months but varied within a range of 1 to 6 months, depending on physician judgment. Skeletal imaging was performed based on the clinical course of disease, clinical suspicion for MBD progression, or suspicion for an adverse skeletal event. The follow‐up period was defined as the time of first denosumab dose to the time of latest clinical review or death.
Skeletal Evaluation
We reviewed available skeletal imaging modalities, including plain radiography of the femur, computed tomography (CT) scout scans, 18fluorodeoxyglucose (FDG)‐positron emission tomography (PET) scan, whole‐body radionuclide bone scan with 99mTc‐methylene diphosphonate, magnetic resonance imaging (MRI), and dual x‐ray absorptiometry bone mineral density (BMD) scans. Patients were assessed for evidence of AFF in accordance with the revised 2013 American Society for Bone and Mineral Research (ASBMR) task force criteria for diagnosis of AFF [6]. The ASBMR definition requires a location along the femoral diaphysis from just distal to the lesser trochanter to just proximal to the supracondylar flare and presence of four of the five Major Features: (a) fracture associated with minimal or no trauma; (b) fracture line originating at the lateral cortex with substantial transverse orientation; (c) complete fracture extending through both cortices that may be associated with a medial spike, or incomplete fracture involving only the lateral cortex; (d) non‐comminuted or minimally comminuted fracture; and (e) localized periosteal or endosteal thickening of the lateral cortex at the fracture site (“beaking” or “flaring”).
To identify asymptomatic atypical fractures or impending fractures using the ASBMR criteria, the imaging studies of patients who had received more than 21 doses (this amount of cumulative exposure was assumed to represent a higher risk group) of denosumab were independently reevaluated by P.B., an orthopedic surgeon, and A.F., an endocrinologist with expertise in metabolic bone disorders. The independent assessments of the reviewers were concordant.
Statistical Methods
Continuous data were presented as means and standard deviations or median and ranges, as appropriate, for each variable. Proportions were calculated for categorical data. The Wilson method was used for calculating confidence intervals for proportions. Analyses were performed using SPSS software (SPSS, Inc., Chicago, IL).
Results
Initial Clinico‐Pathological Characteristics
A total of 253 patients received at least 12 doses of 120 mg denosumab, with a median of 17 doses (range 12–35 doses). The median duration of denosumab treatment was 23 months (range 10–39 months), with a total of 491.2 years of cumulative denosumab exposure. Primary malignancies included breast cancer (69%), prostate cancer (16%), and thyroid cancer (4%). Half (53%) of the cohort had received prior bisphosphonate therapy, including zoledronate, palmidronate, alendronate, and risedronate. Because the majority of patients had breast or prostate cancer, 91% of the cohort had received prior hormone deprivation therapy. Twelve percent were receiving chronic steroid therapy, whereas 23% received proton pump inhibitors. There was a history of osteopenia in 39% and osteoporosis in 17%. See Table 1 for more details.
Table 1. Characteristics of the patients.
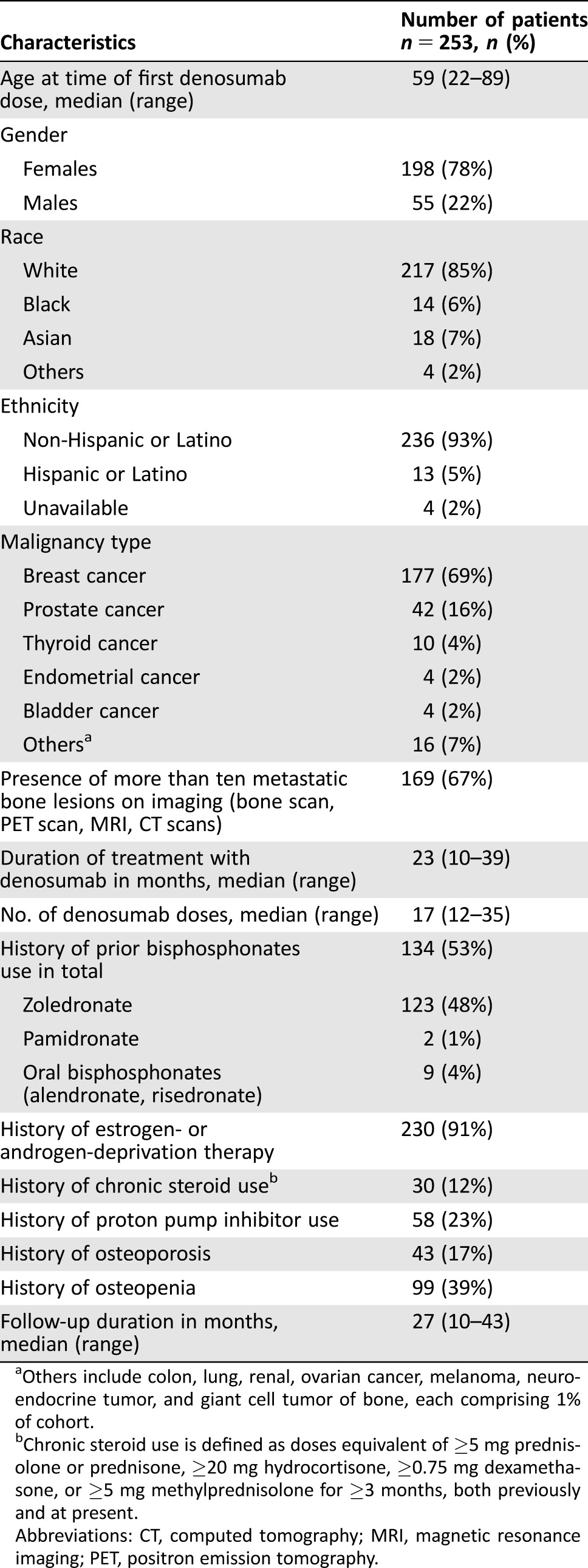
Others include colon, lung, renal, ovarian cancer, melanoma, neuroendocrine tumor, and giant cell tumor of bone, each comprising 1% of cohort.
Chronic steroid use is defined as doses equivalent of ≥5 mg prednisolone or prednisone, ≥20 mg hydrocortisone, ≥0.75 mg dexamethasone, or ≥5 mg methylprednisolone for ≥3 months, both previously and at present.
Abbreviations: CT, computed tomography; MRI, magnetic resonance imaging; PET, positron emission tomography.
Clinical Outcome During Follow‐Up
During a median follow‐up of 27 months (range 10–43 months; 585.2 patient‐years of follow‐up), 1 of 253 patients developed clinical AFF, detected by review of charts and confirmed on skeletal imaging. The incidence rate of clinical AFF was 170.9 per 100,000 patient‐years of follow‐up. Available imaging was reassessed by both P.B. and A.F. for signs of asymptomatic AFF (stress reaction) for 66 of the 70 patients who had received 21 denosumab doses or more. Skeletal images were unavailable for 4 of the 70 patients. Of the 66 evaluable patients, 63 had no imaging features characteristic of AFF on at least 1 imaging modality, while 2 other patients had evidence of undiagnosed subclinical atypical femoral stress reaction on scout CT scans, bone scans, and MRIs; another patient had right groin and upper thigh pain, which was attributed to degenerative etiology, but was later confirmed to have a clinical right AFF and also a stress reaction on the left femur.
Patient 1 was a 61‐year‐old woman with metastatic breast cancer treated with prior adjuvant therapy with tamoxifen, anastrozole and exemestane, and fulvestrant. Bone metastases were not widespread (<10 foci) on the most recent imaging. The patient received monthly pamidronate from 2001 until 2005 and monthly zoledronic acid from 2005 until discontinuation in 2007 due to tooth loosening. Total intravenous bisphosphonate exposure was 70 doses. She received 28 doses of denosumab (120 mg) over 27 months between December 2011 and March 2014. Denosumab at 120 mg was initially administered monthly and reduced in 2014 to once every 4 months. The patient had no fragility fracture or SRE. There was no reported trauma or any prodromal symptoms such as groin/thigh pain and no evidence of lateral femoral stress reaction on serial CT scans performed after completion of zoledronic acid therapy in 2007 (Fig. 1A) or on an October 2011 bone scan. The first sign of change in the lateral femur appeared in June 2014, after having received 28 doses of denosumab. A scout CT film showed radiographic features of diffuse cortical thickening of diaphysis with localized periosteal reaction (“beaking”) of the lateral cortex of her left femur (Fig. 1B). A subsequent bone scan showed increased uptake in the lateral cortex (Fig. 1C) at the site of “beaking,” confirming the diagnosis. An MRI of her left femur showed a focus of cortical thickening in the lateral cortex of the proximal third of the left femoral shaft with a small amount of overlying soft tissue edema. No intramedullary edema or suspicious osseous lesion was identified. These findings were consistent with a cortical stress reaction, and this lesion was believed to be at risk for progressing to insufficiency fracture (Fig. 1D). Denosumab was discontinued. Her BMD in August 2014 showed osteopenia with T scores of −1.7 and −1.5 at the femoral neck and the lumbar spine, respectively. Her serum calcium, alkaline phosphatase, thyroid stimulating hormone (TSH), renal and liver function, and 25‐hydroxy‐vitamin D level (42 ng/mL) were normal. Her bone turnover markers, assessed in April 2015, demonstrated low‐normal premenopausal values: fasting serum C‐telopeptide of 73 pg/mL and bone alkaline phosphatase of 6.5 mcg/L. Her comorbidities were type 2 diabetes mellitus (HbA1c 8.2%) and hypothyroidism post total thyroidectomy for benign goiter (on levothyroxine replacement).
Figure 1.
Imaging studies of a 61‐year‐old patient with metastatic breast cancer who developed an asymptomatic atypical femoral fracture. (A): Computed tomography (CT) scan scout image of the left femur in January 2014 showed no stress reaction. (B): CT scan scout image in June 2014 shows the asymptomatic atypical stress reaction with localized periosteal reaction (“beaking”) of the lateral cortex of the left femur and diffuse cortical thickening of the diaphysis. (C): A bone scan showed an increased uptake in the lateral cortex of left femoral shaft. (D): Magnetic resonance image of the femur (T1‐weighted) showing a focus of cortical thickening in the lateral cortex of the proximal third of the left femoral shaft with a small amount of overlying soft tissue edema.
The second patient with asymptomatic atypical femoral stress reaction was a 50‐year‐old woman with a history of hypothyroidism status post hemithyroidectomy for a Hürthle cell adenoma and metastatic breast cancer diagnosed in 2006, with diffuse bone metastases diagnosed in 2011. The patient had been treated with bilateral oophorectomy, chemotherapy, tamoxifen, letrozole, exemestane, investigational phosphoinositide 3‐kinase inhibitor, fulvestrant, everolimus, and a cyclin‐dependent kinase inhibitor. Monthly denosumab at 120 mg was begun in September 2011, with dosing frequency reduced to every 3 months in April 2013. Detection of asymptomatic femoral stress reaction prompted therapy discontinuation after a total of 21 doses of denosumab in 21 months. The patient had no prior bisphosphonate therapy. Despite the patient's cancer progression through multiple therapies, she had no weight loss or functional limitations. Her BMD scan from January 2013 showed osteopenic T scores of −1.8 and −1.3 at the lumbar spine and femoral neck, respectively. Metabolic workup showed no abnormalities, with normal calcium, TSH, and 25‐hydroxy‐vitamin D level (38 ng/mL). Bone turnover markers were low‐normal (urine N‐telopeptide of 10 nM bone collagen equivalents [BCE]/mM creatinine, fasting serum C‐telopeptide of 43 pg/mL, bone alkaline phosphatase of 12 U/L). The patient had no history of fragility fractures or SREs and no prodromal symptoms such as thigh/groin pain. Review of scout CT scans showed right lateral femoral thickening and likely stress reaction (Fig. 2A), confirmed by increased uptake on bone scan (Fig. 2B). MRI of the right femur (Fig. 2C) showed focal lateral cortical thickening proximally, with adjacent endosteal and periosteal edema, which was also consistent with stress reaction. In contrast, the right proximal femur did not demonstrate FDG‐avidity on a recent 18FDG‐PET/CT scan.
Figure 2.
A 50‐year‐old patient with metastatic breast cancer who developed asymptomatic stress reaction. (A): A computed tomography scan scout image of the right lateral femur shows thickening. (B): Bone scan showing an increased uptake in the lateral cortex of right femoral shaft. (C): Magnetic resonance image of the femur (T2‐weighted) showed focal lateral cortical thickening of the right proximal femur, with adjacent endosteal and periosteal edema, consistent with a stress reaction.
Patient 3 was a 70‐year‐old woman with a history of breast cancer diagnosed in 1998 and treated with adjuvant tamoxifen and letrozole until July 2011 when bone metastases were found. She had received 5 years of alendronate (2004–2009) for osteopenia while on aromatase inhibitor therapy until a drug holiday was initiated in 2009. At this time, urine NTx was low‐normal at 9 nM BCE/mM creatinine and 25‐hydroxy‐vitamin D level was replete at 43 ng/mL. T scores from a September 2009 BMD test were −2.0 and −1.4 at the lumbar spine and femoral neck, respectively, in the osteopenia range. Risedronate was started in September 2009 for 1 year (though with poor compliance). This was switched to denosumab in July 2011 when she developed bone metastases. Denosumab was dosed monthly (with a hiatus from July 2013 to January 2014) over 33 months until April 2014. At that point, after 23 doses of denosumab, the patient developed right groin and upper thigh pain, which was attributed to degenerative etiology. In retrospect, CT scan did show bilateral stress reactions at that time (Fig. 3A), and her bone scan also showed increased uptake in the right lateral femur, corroborating the site of stress reaction on CT scan (Fig. 3B). In October 2014, the patient suffered a subtrochanteric fracture of her right femur and underwent intramedullary rod placement; the treating orthopedist from an external institution noted that the fracture “was typical of bisphosphonate therapy” (Fig. 3C). On our imaging review, a contralateral (left‐sided) lateral femoral stress reaction was evident on the June 2015 scout CT film, and this was later demonstrated on radiograph (Fig. 3D). She eventually died of progressive disease (liver, lymph nodes, and innumerable bone metastases) in July 2015.
Figure 3.
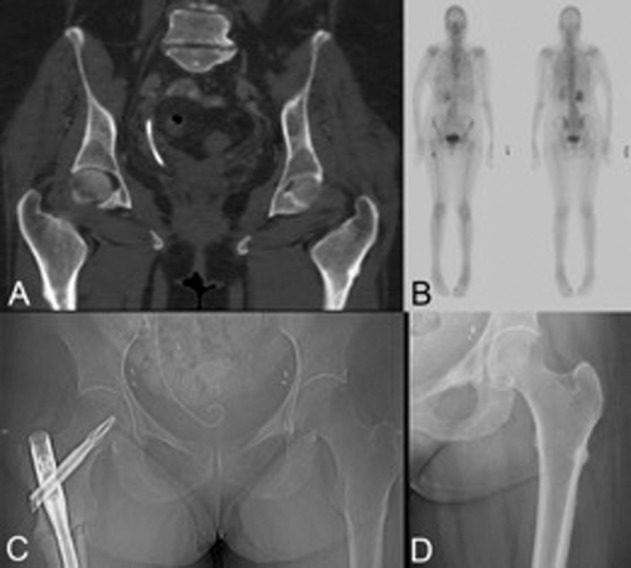
A 70‐year‐old patient with metastatic breast cancer who developed an asymptomatic atypical femoral fracture (AFF) following classic prodromal symptoms and who also developed an atypical stress reaction on the contralateral femur. (A): Coronal computed tomography (CT) image showing a bilateral stress reaction in the lateral femoral cortices; patient complained of right sided groin and thigh pain. (B): Bone scan corroborates CT findings of AFF. (C): Postoperative radiograph after intramedullary rod placement for AFF. (D): The left lateral cortex stress reaction (“beaking”) is evident on plain radiograph.
Discussion
We reviewed AFF incidence in 253 patients with MBD receiving denosumab therapy. There was one case of clinical AFF preceded by the typical prodromic symptoms, consistent with the revised ASBMR task force criteria for AFF [6], and two cases of subclinical atypical stress reaction of the femur, with localized periosteal reaction (“beaking”) of the lateral cortex of the left femur and diffuse cortical thickening of the diaphysis. These changes are thought to precede AFFs and warrant denosumab discontinuation. The interval between radiological changes typical of AFF and clinical fracture is unknown, so it is unknown whether the asymptomatic patients would have eventually experienced a clinical fracture. None of the three patients had FDG‐avid stress fractures on PET scan in contrast to some reports of FDG‐avid stress fractures [24].
The prolonged use of potent antiresorptives (bisphosphonates and denosumab) has been postulated to increase the risk of AFF by suppression of osteoblastic activity and accumulation of skeletal microdamage with reduced bone remodeling. This leads to reduced bone strength, especially at sites that are subjected to increased loading, resulting in stress fractures [25], [26]. Patient 1 received bisphosphonate therapy prior to denosumab, with her last dose of zoledronic acid given in 2007. She had no radiographic findings of atypical femoral stress reaction on CT scans and bone scans in 2007 and 2011. Denosumab was initiated in December 2011 and is thus temporally associated with the detection of the stress reaction first seen on imaging in June 2014. Thus, denosumab is likely to have contributed to the stress reaction in Patient 1, although there certainly could also be a contribution from prior bisphosphonates use. Similarly, Patient 3 also had prior oral bisphosphonate exposure; this could have increased the risk for AFF post denosumab. Patient 2 had no prior bisphosphonate exposure, and we therefore suspect that denosumab was the main etiologic factor for her atypical femoral stress reaction. Table 1 showed that only 4% of patients in our cohort used oral bisphosphonates (for osteopenia/osteoporosis); we did not capture data on use of intravenous zoledronic acid for osteopenia/osteoporosis and acknowledge this as a limitation. More than half (56%) of subjects had osteopenia or osteoporosis and may have been treated with bisphosphonates; we speculate that our review may have underreported the prevalence of oral bisphosphonate use (4%). Although 48% of the cohort was treated with zoledronic acid, in the overall cohort, we did not capture the dosing schedule (osteoporosis versus malignancy). Duration of bisphosphonate use was recorded solely for the three cases of atypical femoral stress reaction and AFF, which is a limitation to our study.
There have been no published reports of AFF in patients with solid tumors receiving denosumab to reduce the risk of SREs. In a meta‐analysis that included randomized controlled trials of denosumab and zoledronic acid in MBD involving 5,544 patients, of whom 2,776 had received denosumab, no AFF had been observed. However, the number of doses of denosumab received ranged from 3 to 20. In the few studies that reported median time on study follow‐up, the time of follow‐up was only 7 to 12 months per study subject [10], [11], [12], [13], [14], [15], [16], [27]. Furthermore, in our anecdotal experience, in the setting of complicated patients with advanced cancer, AFF may be confused for metastatic disease or simply not recognized. It should be noted that cortical bone metastases are very rare.
Clinical trial data regarding risk of AFF and “long term” therapy with denosumab at the osteoporosis dose (60 mg every 6 months) are largely limited to the FREEDOM extension trial [23]. There were no reports of AFF in the initial FREEDOM trial after 3 years of denosumab therapy [28]. In the FREEDOM extension trial [23], there was one positively adjudicated case of AFF in the long‐term denosumab treatment group after 7 years of continued denosumab therapy (4 years into the extension trial). In the group that initially received placebo for 3 years and then crossed over to denosumab treatment in the extension trial, there was one positively adjudicated case of AFF.
In our study, the 3 patients with atypical femoral stress reaction and/or fracture had received 28, 21, and 23 doses of denosumab over the course of 27, 21, and 33 months, respectively. All three patients had a history of aromatase inhibitor use, and one patient had a history of diabetes, now considered a risk factor for fragility fracture. The second ASBMR task force on AFF concluded that most cases of AFF occur after bisphosphonate therapy was administered for more than 3–5 years (in the setting of MBD) [6], [29], [30], [31], [32]. In advanced cancer involving the bone, retrospective data on AFF risk with monthly intravenous bisphosphonate demonstrated, in 4 AFF cases, a median of 43 doses over 66 months [7]. In the recent review of 10,587 cancer patients who have received bisphosphonates for underlying osteopenia or osteoporosis, the 23 AFF cases received zoledronate (n = 13) for a mean duration of 10.87 months, alendronate (n = 6) for 29.39 months, pamidronate (n = 3) for 2.17 months, and ibandronate (n = 2) for 3.13 months [9].
We note the following study limitations: (a) The denosumab treatment regimen was not standardized. (b) The follow‐up duration of our patients was relatively short (expected given the underlying nature of metastatic disease). (c) In addition, of 70 patients who received at least 21 doses of denosumab, imaging for 4 patients was unavailable for review. Our subgroup imaging analysis for subclinical skeletal changes is limited by virtue of the small number of patients included (n = 66); reviewing all 253 patients' imaging was not feasible. It is possible that some of the patients who received between 12 and 20 doses had minimally symptomatic or asymptomatic femoral lesions that were undetected. (d) Finally, definitive conclusions on the relative contribution of denosumab and bisphosphonate to AFF in Patient 1 and Patient 3 are not possible, although there is a strong temporal association with denosumab.
Conclusion
It is noteworthy that half of our cohort had prior treatment with intravenous bisphosphonates, which could theoretically increase the risk of subsequent AFF in the context of denosumab therapy. This retrospective study showed that the incidence rate of clinical AFF in the context of denosumab treatment for MBD, based on charts review, is 0.4% (1/253; 95% CI 0.1%–2.2%), and the incidence of atypical femoral stress reaction based on imaging review is 4.5% (3/66; 95% CI 1.6%–12.5%). Upon detection of the asymptomatic cases through our retrospective analysis, denosumab therapy was discontinued to minimize the risk of the lesions' progression to clinical AFF. In contrast, Patient 3, who died prior to our review, had clinical and radiographic evidence of a right atypical stress reaction that was undiagnosed and eventually progressed to right AFF. This patient also had a left femoral atypical stress reaction and was at risk for left AFF. This highlights the need for increased awareness of this clinically devastating complication. Physicians should educate patients treated with denosumab to report prodromic symptoms of AFF (thigh, hip, or groin pain), evaluate any such complaints for an incomplete femur fracture (atypical stress reaction), and assess AFF patients for signs of fracture in the contralateral limb. Furthermore, we emphasize the need for careful evaluation of the subtrochanteric and shaft region of the lateral femur for signs of atypical stress reaction on imaging studies done for other reasons in patients on long‐term denosumab therapy.
Footnotes
For Further Reading: Zhiyu Wang, Dan Qiao, Yaohong Lu et al. Systematic Literature Review and Network Meta‐Analysis Comparing Bone‐Targeted Agents for the Prevention of Skeletal‐Related Events in Cancer Patients With Bone Metastasis.
Implications for Practice: Bone metastasis (BM) can lead to skeletal‐related events (SREs), which can dramatically reduce patients' quality of life and even shorten survival. We used network meta‐analysis to evaluate the comparative effectiveness of bone‐targeted agents (BTAs) in reducing the morbidity of SREs in cancer patients with BM.We found that denosumab, zoledronate, and pamidronate were generally effective (compared with placebo), and denosumab was shown to be the most effective BTA. Reduction in the incidence of pathologic fractures and radiation was the main cause of reduced risk of SREs. The findings of this study highlight the importance of the use of BTAs in cancer patients with BM.
Author Contributions
Conception/Design: Samantha Peiling Yang, Patrick J. Boland, Azeez Farooki
Provision of study material or patients: Samantha Peiling Yang, Azeez Farooki
Collection and/or assembly of data: Samantha Peiling Yang, Tae Won B. Kim, Patrick J. Boland, Azeez Farooki
Data analysis and interpretation: Samantha Peiling Yang, Tae Won B. Kim, Patrick J. Boland, Azeez Farooki
Manuscript writing: Samantha Peiling Yang, Tae Won Kim, Azeez Farooki
Final approval of manuscript: Samantha Peiling Yang, Tae Won B. Kim, Patrick J. Boland, Azeez Farooki
Disclosures
Azeez Farooki: Oncomed, Celgene (C/A), Abbvie (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Lipton A, Theriault RL, Hortobagyi GN et al. Pamidronate prevents skeletal complications and is effective palliative treatment in women with breast carcinoma and osteolytic bone metastases: Long term follow‐up of two randomized, placebo‐controlled trials. Cancer 2000;88:1082–1090. [DOI] [PubMed] [Google Scholar]
- 2. Rosen LS, Gordon D, Kaminski M et al. Long‐term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: A randomized, double blind, multicenter, comparative trial. Cancer 2003;98:1735–1744. [DOI] [PubMed] [Google Scholar]
- 3. Kohno N, Aogi K, Minami H et al. Zoledronic acid significantly reduces skeletal complications compared with placebo in Japanese women with bone metastases from breast cancer: A randomized, placebo‐controlled trial. J Clin Oncol 2005;23:3314–3321. [DOI] [PubMed] [Google Scholar]
- 4. Saad F, Gleason DM, Murray R et al. Long‐term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone‐refractory prostate cancer. J Natl Cancer Inst 2004;96:879–882. [DOI] [PubMed] [Google Scholar]
- 5. Rosen LS, Gordon DH, Dugan W Jr et al. Zoledronic acid is superior to pamidronate for the treatment of bone metastases in breast carcinoma patients with at least one osteolytic lesion. Cancer 2004;100:36–43. [DOI] [PubMed] [Google Scholar]
- 6. Shane E, Burr D, Abrahamsen B et al. Atypical subtrochanteric and diaphyseal femoral fractures: Second report of a task force of the American Society for Bone and Mineral Research. J Bone and Miner Res 2014;29:1–23. [DOI] [PubMed] [Google Scholar]
- 7. Puhaindran ME, Farooki A, Steensma MR et al. Atypical subtrochanteric femoral fractures in patients with skeletal malignant involvement treated with intravenous bisphosphonates. J Bone Joint Surg Am 2011;93:1235–1242. [DOI] [PubMed] [Google Scholar]
- 8. Hayashi K, Aono M, Shintani K et al. Bisphosphonate‐related atypical femoral fracture with bone metastasis of breast cancer: Case report and review. Anticancer Res 2014;34:1245–1249. [PubMed] [Google Scholar]
- 9. Edwards BJ, Sun M, West DP et al. Incidence of atypical femur fractures in cancer patients: The MD Anderson Cancer Center Experience. J Bone Miner Res 2016;31:1569–1576. [DOI] [PubMed] [Google Scholar]
- 10. Stopeck AT, Lipton A, Body JJ et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: A randomized, double‐blind study. J Clin Oncol 2010;28:5132–5139 [DOI] [PubMed] [Google Scholar]
- 11. Lipton A, Fizazi K, Stopeck AT et al. Superiority of denosumab to zoledronic acid for prevention of skeletal‐related events: A combined analysis of 3 pivotal, randomised, phase 3 trials. Eur J Cancer 2012;48:3082–3092. [DOI] [PubMed] [Google Scholar]
- 12. Henry DH, Costa L, Goldwasser F et al. Randomized, double‐blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol 2011;29:1125–1132. [DOI] [PubMed] [Google Scholar]
- 13. Fizazi K., Carducci M, Smith M et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration‐resistant prostate cancer: A randomised, double‐blind study. Lancet 2011;377:813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Scagliotti GV, Hirsh V, Siena S et al. Overall survival improvement in patients with lung cancer and bone metastases treated with denosumab versus zoledronic acid: Subgroup analysis from a randomized phase 3 study. J Thorac Oncol 2012;7:1823–1829. [DOI] [PubMed] [Google Scholar]
- 15. Martin M, Bell R, Bourgeois H et al. Bone‐related complications and quality of life in advanced breast cancer: Results from a randomized phase III trial of denosumab versus zoledronic acid. Clin Cancer Res 2012;18:4841–4849. [DOI] [PubMed] [Google Scholar]
- 16. Henry D, Vadhan‐Raj S, Hirsh V et al. Delaying skeletal‐related events in a randomized phase 3 study of denosumab versus zoledronic acid in patients with advanced cancer: An analysis of data from patients with solid tumors. Support Care Cancer 2014;22:679–687. [DOI] [PubMed] [Google Scholar]
- 17. Paparodis R, Buehring B, Pelley EM et al. A case of an unusual subtrochanteric fracture in a patient receiving denosumab. Endocr Pract 2013;19:e64–e68. [DOI] [PubMed] [Google Scholar]
- 18. Thompson RN, Armstrong CL, Heyburn G. Bilateral atypical femoral fractures in a patient prescribed denosumab ‐ A case report. Bone 2014;61:44–47. [DOI] [PubMed] [Google Scholar]
- 19. Villiers J, Clark DW, Jeswani T et al. An atraumatic femoral fracture in a patient with rheumatoid arthritis and osteoporosis treated with denosumab. Case Rep Rheumatol 2013;2013: 249872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Drampalos E, Skarpas G, Barbounakis N et al. Atypical femoral fractures bilaterally in a patient receiving denosumab. Acta Orthop 2014;85:3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schilcher J, Aspenberg P. Atypical fracture of the femur in a patient using denosumab – A case report. Acta Orthop 2014;85:6–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Khow KS, Yong TY. Atypical femoral fracture in a patient treated with denosumab. J Bone Miner Metab 2015;33:355–358. [DOI] [PubMed] [Google Scholar]
- 23. Bone HG, Chapurlat R, Brandi ML et al. The effect of three or six years of denosumab exposure in women with postmenopausal osteoporosis: Results from the FREEDOM extension. J Clin Endocrinol Metab 2013;98:4483–4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fayad LM, Kamel IR, Kawamoto S et al. Distinguishing stress fractures from pathologic fractures: A multimodality approach. Skeletal Radiol 2005;34:245–259 [DOI] [PubMed] [Google Scholar]
- 25. Mashiba T, Hirano T, Turner CH et al. Suppressed bone turnover by bisphosphonates increases microdamage accumulation and reduces some biomechanical properties in dog rib. J Bone Miner Res 2000;15:613–620. [DOI] [PubMed] [Google Scholar]
- 26. Açil Y, Möller B, Niehoff P et al. The cytotoxic effects of three different bisphosphonates in‐vitro on human gingival fibroblasts, osteoblasts and osteogenic sarcoma cells. J Craniomaxillfac Surg 2012;40:e229–e235. [DOI] [PubMed] [Google Scholar]
- 27. Zheng GZ, Chang G, Lin FX et al. Meta‐analysis comparing denosumab and zoledronic acid for treatment of bone metastases in patients with advanced solid tumours. Eur J Cancer Care 2016. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 28. McClung MR, Lewiecki EM, Cohen SB et al. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med 2006;354:821–831. [DOI] [PubMed] [Google Scholar]
- 29. Shane E, Burr D, Ebeling PR et al. Atypical subtrochanteric and diaphyseal femoral fractures: Report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2010;25:2267–2294. [DOI] [PubMed] [Google Scholar]
- 30. Giusti A, Hamdy NA, Papapoulos SE. Atypical fractures of the femur and bisphosphonate therapy: A systematic review of case/case series studies. Bone 2010;47:169–180. [DOI] [PubMed] [Google Scholar]
- 31. Donnelly E, Saleh A, Unnanuntana A et al. Atypical femoral fractures: Epidemiology, etiology, and patient management. Curr Opin Support Palliat Care 2012;6:348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rizzoli R, Akesson K, Bouxsein M et al. Subtrochanteric fractures after long‐term treatment with bisphosphonates: A European Society on Clinical and Economic Aspects of Osteoporosis and Osteoarthritis, and International Osteoporosis Foundation Working Group Report. Osteoporos Int 2011;22:373–390. [DOI] [PMC free article] [PubMed] [Google Scholar]




