The development of immune checkpoint inhibitors represents a major breakthrough in cancer therapy. This systematic review and meta‐analysis of randomized controlled trials was conducted to compare summary toxicity endpoints and clinically relevant adverse events between PD‐1/PD‐L1 inhibitors and chemotherapy.
Keywords: Chemotherapy, PD‐1/PD‐L1 inhibitor, Meta‐analysis, Systematic review, Toxicity
Abstract
Background.
Compared with chemotherapy, significant improvement in survival outcomes with the programmed death receptor‐1 (PD‐1) inhibitors nivolumab and pembrolizumab and the programmed death‐ligand 1 (PD‐L1) inhibitor atezolizumab has been shown in several types of advanced solid tumors. We conducted a systematic review and meta‐analysis to compare safety and tolerability between PD‐1/PD‐L1 inhibitors and chemotherapy.
Methods.
PubMed and American Society of Clinical Oncology (ASCO) databases were searched 1966 to September 2016. Eligible studies included randomized controlled trials (RCTs) comparing single‐agent U.S. Food and Drug Administration–approved PD‐1/PD‐L1 inhibitors (nivolumab, pembrolizumab, or atezolizumab) with chemotherapy in cancer patients reporting any all‐grade (1–4) or high‐grade (3–4) adverse events (AEs), all‐ or high‐grade treatment‐related symptoms, hematologic toxicities and immune‐related AEs, treatment discontinuation due to toxicities, or treatment‐related deaths. The summary incidence, relative risk, and 95% confidence intervals were calculated.
Results.
A total of 3,450 patients from 7 RCTs were included in the meta‐analysis: 4 nivolumab, 2 pembrolizumab, and 1 atezolizumab trials. The underlying malignancies included were non‐small cell lung cancer (4 trials) and melanoma (3 trials). Compared with chemotherapy, the PD‐1/PD‐L1 inhibitors had a significantly lower risk of all‐ and high‐grade fatigue, sensory neuropathy, diarrhea and hematologic toxicities, all‐grade anorexia, nausea, and constipation, any all‐ and high‐grade AEs, and treatment discontinuation. There was an increased risk of all‐grade rash, pruritus, colitis, aminotransferase elevations, hypothyroidism, and hyperthyroidism, and all‐ and high‐grade pneumonitis with PD1/PD‐L1 inhibitors.
Conclusion.
PD‐1/PD‐L1 inhibitors are overall better tolerated than chemotherapy. Our results provide further evidence supporting the favorable risk/benefit ratio for PD‐1/PD‐L1 inhibitors.
Implications for Practice.
We conducted a systematic review and meta‐analysis to compare summary toxicity endpoints and clinically relevant adverse events between programmed death receptor‐1 (PD‐1)/programmed death‐ligand 1 (PD‐L1) inhibitors and chemotherapy. PD1/PD‐L1 inhibitors were associated with a lower risk of treatment‐related symptoms (fatigue, anorexia, nausea, diarrhea, constipation, and sensory neuropathy) but a higher risk of immune‐related adverse events (AEs). Summary toxicity endpoints favor PD1/PD‐L1 inhibitors (any all‐ and high‐grade AEs and treatment discontinuation). PD1/PD‐L1 inhibitors are overall better tolerated than chemotherapy. In addition to efficacy data from trials, our findings provide useful information for clinicians for well‐balanced discussions with their patients on the risks and benefits of treatment options for advanced cancer.
Introduction
The development of immune checkpoint inhibitors (ICIs) represents a major breakthrough in cancer therapy. ICIs enhance antitumor immune responses by releasing the “brakes” on the immune system [1]. They are designed to block immunosuppressive receptors expressed on the surface of T lymphocytes such as cytotoxic T‐lymphocyte–associated antigen 4, programmed death receptor‐1 (PD‐1), and the programmed death‐ligand 1 (PD‐L1) expressed on tumor cells and tumor‐infiltrating immune cells [2]. ICIs targeting the PD‐1/PD‐L1 pathway have shown especially significant improvement in progression‐free survival and overall survival (OS) compared with standard care in different advanced solid tumors [3], [4], [5], [6], [7], [8], [9]. Their superior efficacy led to U.S. Food and Drug Administration (FDA) approval of PD‐1 inhibitors, nivolumab, and pembrolizumab for the treatment of unresectable or metastatic melanoma and advanced non‐small cell lung cancer (NSCLC) in the second‐line setting [10], [11]. Nivolumab is also approved for patients with metastatic renal cell carcinoma following prior treatment with an anti‐angiogenic therapy [10]. Recently, the FDA approved the PD‐L1 inhibitor atezolizumab for the treatment of locally advanced or metastatic urothelial carcinoma after prior platinum‐based chemotherapy [12]. Based on the remarkable durable responses in single‐arm trials, the FDA approved nivolumab for relapsed or refractory Hodgkin's lymphoma and pembrolizumab for recurrent or metastatic head and neck squamous cell carcinoma [10], [11]. The indications for these agents are expected to continue expanding as they are studied in different treatment settings, including first‐line therapy for advanced disease and a wide variety of other malignancies [13].
Treatment decision‐making for patients with advanced cancer is a major challenge for oncologists. The goals of therapy are often palliative: prolongation of survival, control of symptoms, and maintenance or improvement of quality of life. In order to attain these goals, it is essential to have a balanced discussion of treatment options that focuses on the benefits and risks of each treatment, taking into account patient preferences and values. Currently, both novel immunotherapy agents and traditional cytotoxic chemotherapy are approved treatment options for advanced cancer. In addition to efficacy data derived from trials of impact on survival outcomes, a comprehensive understanding of the toxicity profile of immunotherapy compared chemotherapy is needed for informed treatment decisions. Inhibition of the immune checkpoints can lead to immune dysregulation that clinically manifests with symptoms similar to autoimmune disease. These side effects are termed immune‐related adverse events (AEs) and include dermatologic, gastrointestinal, hepatic, endocrine, and pulmonary events [14]. In addition to these unique AEs, classical chemotherapy toxicities, such as fatigue, anorexia, nausea, and diarrhea, have also been seen in patients treated with the PD‐1/PD‐L1 inhibitors [3], [4], [5], [6], [7], [8], [9]. These treatment‐related symptoms are important, as they affect patients' quality of life [15], [16]. However, to date, there has been no systematic comparison of tolerability between PD‐1/PD‐L1 inhibitors and chemotherapy. We conducted a systematic review and meta‐analysis of randomized controlled trials (RCTs) to compare summary toxicity endpoints and clinically relevant AEs between PD‐1/PD‐L1 inhibitors and chemotherapy.
Materials and Methods
Data Source
This analysis was performed in accordance with the preferred reporting items for systematic reviews and meta‐analyses statement [17]. Two authors (TFN and SSS) conducted independent reviews of PubMed from January 1966 to September 30, 2016. Search terms included “nivolumab,” “pembrolizumab,” and “atezolizumab.” The search was limited to clinical trials. We also searched abstracts and virtual meeting presentations utilizing the same search terms from the American Society of Clinical Oncology (ASCO) conferences held through September 2016 to identify relevant studies. Independent searches of the Web of Science, Embase, and Cochrane electronic databases were also performed. In instances of duplicate publications, only the most complete, recent, and up‐to‐date report of the study was included.
Study Selection
Clinical trials that met the following criteria were included: (a) phase II and III trials in patients with cancer; (b) random assignment of participants to treatment with single‐agent PD‐1/PD‐L1 inhibitor or chemotherapy; and (c) reporting of events or event rate and sample size for any all‐grade (1–4) or high‐grade (3–4) AEs, individual all‐ or high‐grade AEs, treatment discontinuation for AEs, or treatment‐related deaths. For the individual AEs, we included immune‐related AEs (rash, pruritus, colitis, hypothyroidism, hyperthyroidism, hypophysitis, hepatitis, and pneumonitis), hematologic toxicities (neutropenia, anemia, and thrombocytopenia), and the core set of 12 clinically relevant symptoms recommended for assessment in clinical trials by the National Cancer Institute's Symptom Management and Health‐Related Quality of Life Steering Committee [18]. These 12 symptoms are fatigue, insomnia, pain, anorexia, dyspnea, cognitive problems, anxiety, nausea, depression, sensory neuropathy, constipation, and diarrhea. Reviewers (TFN and SSS) independently screened reports that included the key terms by their titles and abstracts for relevance. Then, full texts of relevant articles were retrieved to assess eligibility. The references of relevant reports were also reviewed manually to identify additional studies.
Data Extraction
Two investigators (TFN and SSS) independently performed data extraction. Any discrepancies between reviewers were resolved by consensus. The following information was recorded for each study: first author's name, year of publication, trial phase, masking, underlying malignancy, treatment arms, number of patients available for analysis, age, follow‐up duration, Common Terminology Criteria for AEs (CTCAE), any all‐ or high‐grade AEs, individual all‐ or high‐grade AEs (fatigue, insomnia, pain, anorexia, dyspnea, cognitive problems, anxiety, nausea, depression, sensory neuropathy, constipation, diarrhea, neutropenia, thrombocytopenia, anemia, rash, pruritus, colitis, hypothyroidism, hyperthyroidism, hypophysitis, alanine aminotransferase (ALT)/ aspartate aminotransferase (AST) elevations, and pneumonitis), treatment discontinuation for AEs, and treatment‐related deaths. AEs were recorded according to the CTCAE. The quality of trials was rated using the five‐point Jadad scale, which is based on the reporting of randomization method, blinding method, withdrawals, and dropouts [19].
Statistical Analysis
The primary objective of this study was to compare toxicity between PD‐1/PD‐L1 inhibitors and chemotherapy. The relative risk (RR), corresponding 95% confidence intervals (CIs), and incidence of toxicity outcomes were calculated. We calculated the RRs and CIs with data extracted from RCTs and assessed the incidence of toxicity events in patients assigned to PD‐1/PD‐L1 inhibitors compared with chemotherapy in the same trial. To calculate the 95% CIs, the variance of the log‐transformed study‐specific RR was derived using the delta method. For studies reporting zero AEs in any arm, we applied a classic half‐integer continuity correction to calculate the RR and variance. For the calculation of RRs, we used random‐ or fixed‐effects models depending on the heterogeneity of included studies. Statistical heterogeneity in results between studies included in the meta‐analysis was examined using Cochrane's Q statistic, and inconsistency was quantified with I2 statistic (100% × (Q − df)/Q) [20]. The assumption of homogeneity was considered invalid for p values less than .10. Summary RRs were calculated using random‐ or fixed‐effects models depending on the heterogeneity of included studies. When substantial heterogeneity was not observed, the pooled estimate calculated based on the fixed‐effects model was reported by using the inverse variance method. When substantial heterogeneity was observed, the pooled estimate calculated based on the random‐effects model was reported by using the DerSimonian and Laird method, which considers both within‐study and between‐study variations [21]. For the calculation of incidence, the proportion of patients with adverse outcomes and 95% CIs was derived from each trial. We used a random‐effects model to produce a pooled overall estimate for incidence of adverse outcomes. We evaluated publication bias using funnel plots and the Begg and Egger tests [22], [23]. A two‐tailed p value of less than .05 was considered statistically significant. Statistical analyses were performed using the comprehensive meta‐analysis program (Version 2, Biostat, Englewood, NJ, USA).
Results
Search Results and Patient Characteristics
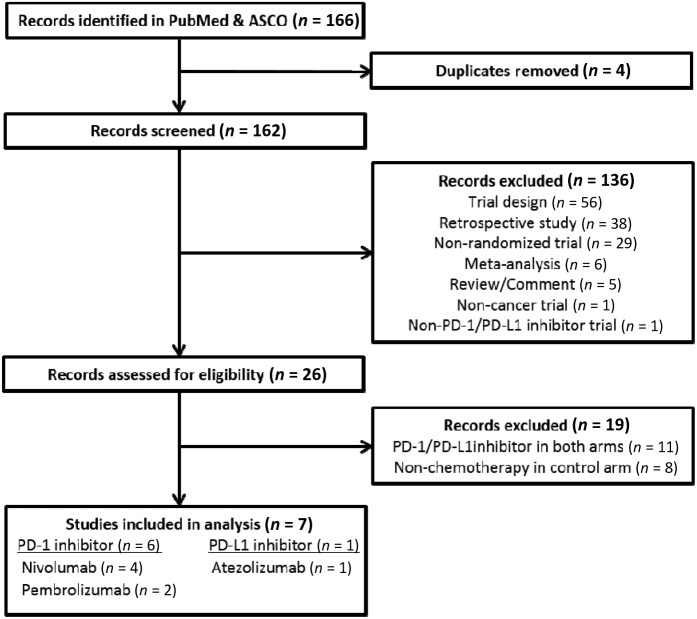
Our search strategy yielded 166 potentially relevant records in the PubMed and ASCO databases, of which 159 publications were excluded. Our selection process and reasons for study exclusion are shown in Figure 1. A total of four phase III, one phase II/III, and two phase II randomized clinical trials were considered eligible for the meta‐analysis. A total of 3,450 patients (PD‐1/PD‐L1 inhibitors: 2,090; chemotherapy: 1,360) were included in the analysis from four nivolumab trials, two pembrolizumab trials, and one atezolizumab trial. The underlying malignancies were NSCLC (4 trials) and melanoma (3 trials). The baseline characteristics in each trial are presented in Table 1.
Figure 1.
Flow diagram: selection process for the studies.
Abbreviations: ASCO, American Society of Clinical Oncology; PD‐1, programmed death receptor‐1; PD‐L1, programmed death‐ligand 1.
Table 1. Characteristics of the studies included in the meta‐analysis.
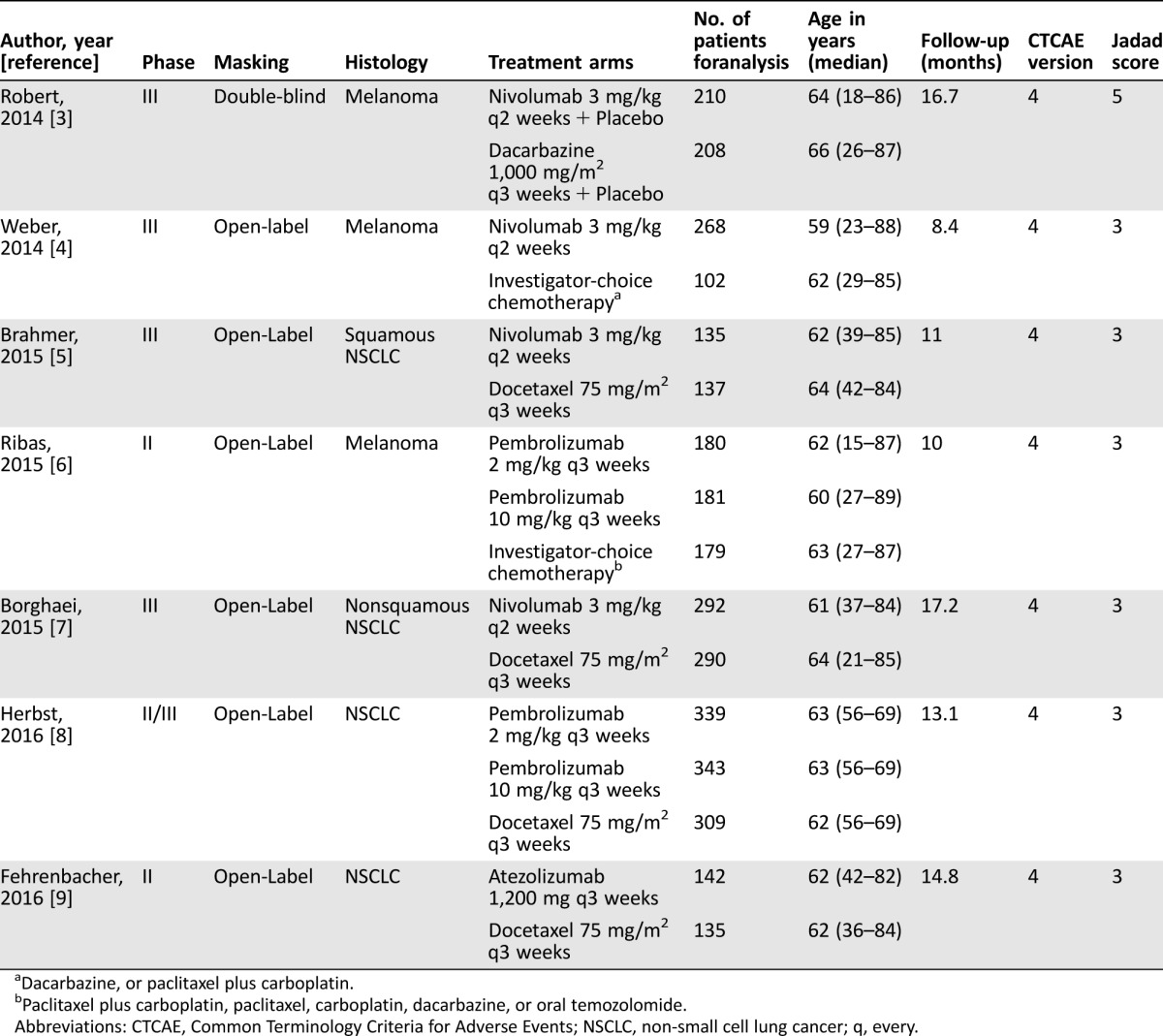
Dacarbazine, or paclitaxel plus carboplatin.
Paclitaxel plus carboplatin, paclitaxel, carboplatin, dacarbazine, or oral temozolomide.
Abbreviations: CTCAE, Common Terminology Criteria for Adverse Events; NSCLC, non‐small cell lung cancer; q, every.
Comparison of Toxicity Profiles
Summary Toxicity Endpoints.
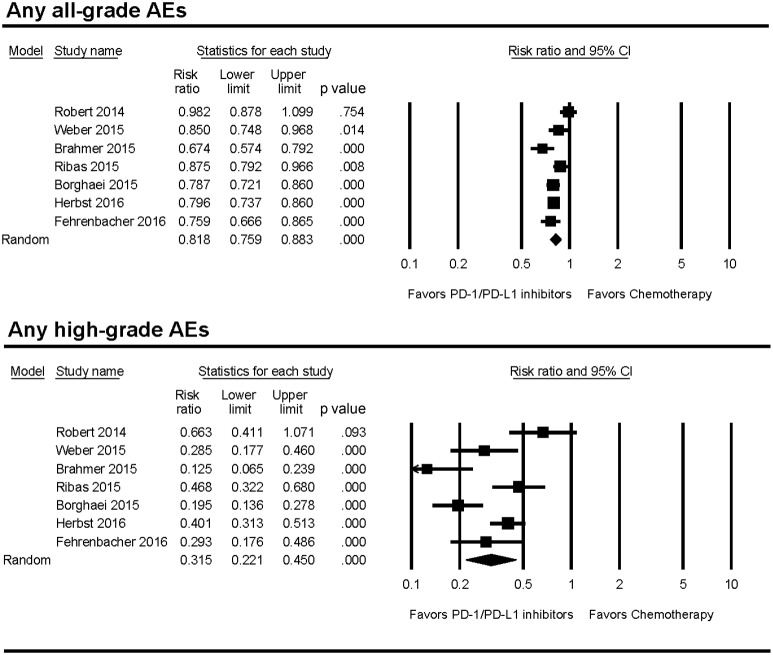
The incidence of any all‐grade (67.6% versus 82.9%) or high‐grade (11.4% versus 35.7%) AEs was lower in PD‐1/PD‐L1 inhibitors compared with chemotherapy (Table 2). PD‐1/PD‐L1 inhibitors also had significantly lower risk of any all‐grade (RR 0.82; p < .001) and high‐grade AEs (RR 0.32; p < .001; Fig. 2). Patients treated with PD‐1/PD‐L1 inhibitors stopped therapy for toxicity less frequently than those treated with chemotherapy (4.5% versus 11.1%); the RR of treatment discontinuation due to AEs was 0.44 (p < .001). Deaths attributed to study treatment occurred in 7 patients in the PD‐1/PD‐L1 inhibitor group and 11 patients in the chemotherapy group. There was no significant difference in the incidence of treatment‐related deaths between the two groups. The random‐effects model was used for the RR analysis of any all‐ and high‐grade AEs because there was significant heterogeneity among the studies. The test for heterogeneity was not significant for treatment discontinuation and treatment‐related deaths. Therefore, the fixed‐effects model was used for these RR analyses. The RR and incidence for each AE are summarized in Table 2.
Figure 2.
Forest plots of relative risk of any all‐ and high‐grade AEs associated with PD‐1/PD‐L1 inhibitors versus chemotherapy.
Abbreviations: AE, adverse event; CI, confidence interval; PD‐1, programmed death receptor‐1; PD‐L1, programmed death‐ligand 1.
Table 2. Incidence and RR of summary toxicity endpoints, including 95% CI and number of trials in each analysis.
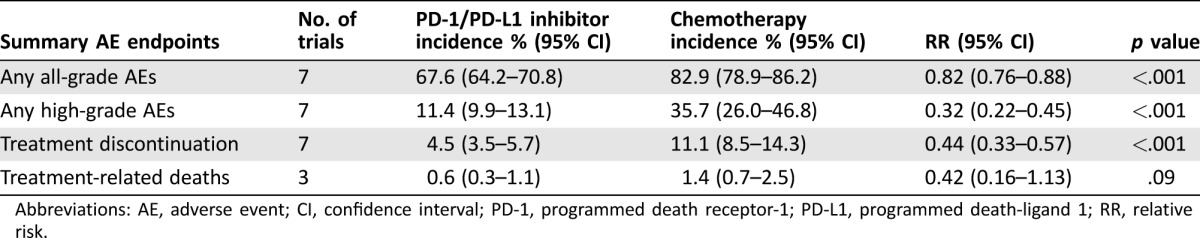
Abbreviations: AE, adverse event; CI, confidence interval; PD‐1, programmed death receptor‐1; PD‐L1, programmed death‐ligand 1; RR, relative risk.
Clinically Relevant Treatment‐Related Symptoms.
Of the core set of 12 clinically relevant symptoms, fatigue, anorexia, nausea, constipation, diarrhea, and pain were reported in all 7 trials. The overall incidence of all‐grade or high‐grade pain in the trials could not be obtained because only incidences of pain in specific sites were reported. Data for sensory neuropathy were available from six trials, and data for insomnia and dyspnea were available from three trials. Only one trial reported anxiety, and no trial reported cognitive problems or depression. Based on the availability of data, summary incidence and RR were calculated for the following eight symptoms: fatigue, anorexia, nausea, constipation, diarrhea, sensory neuropathy, insomnia, and dyspnea. PD‐1/PD‐L1 inhibitors were associated with a significantly lower risk for six of the eight evaluated all‐grade AEs when compared with chemotherapy (Table 3): fatigue (19.1% versus 27.7%; RR 0.69), anorexia (10.2% versus 15.8%; RR 0.65), nausea (11.5% versus 28.4%; RR 0.41), constipation (5.2% versus 9.9%; RR 0.57), diarrhea (10.2% versus 17.3%; RR 0.61), and sensory neuropathy (1.2% versus 8.6%; RR 0.16). The risk of three high‐grade AEs was significantly lower in the PD‐1/PD‐L1 inhibitor group compared with the chemotherapy group: fatigue (0.7% versus 4.0%; RR 0.19), sensory neuropathy (0.3% versus 1.1%; RR 0.20), and diarrhea (0.6% versus 1.9%; RR 0.30). We found no statistically significant differences between the groups for all‐grade insomnia, dyspnea, high‐grade anorexia, nausea, constipation, insomnia, or dyspnea.
Table 3. Incidence and RR of individual AEs, including 95% CI and number of trials in each analysis.
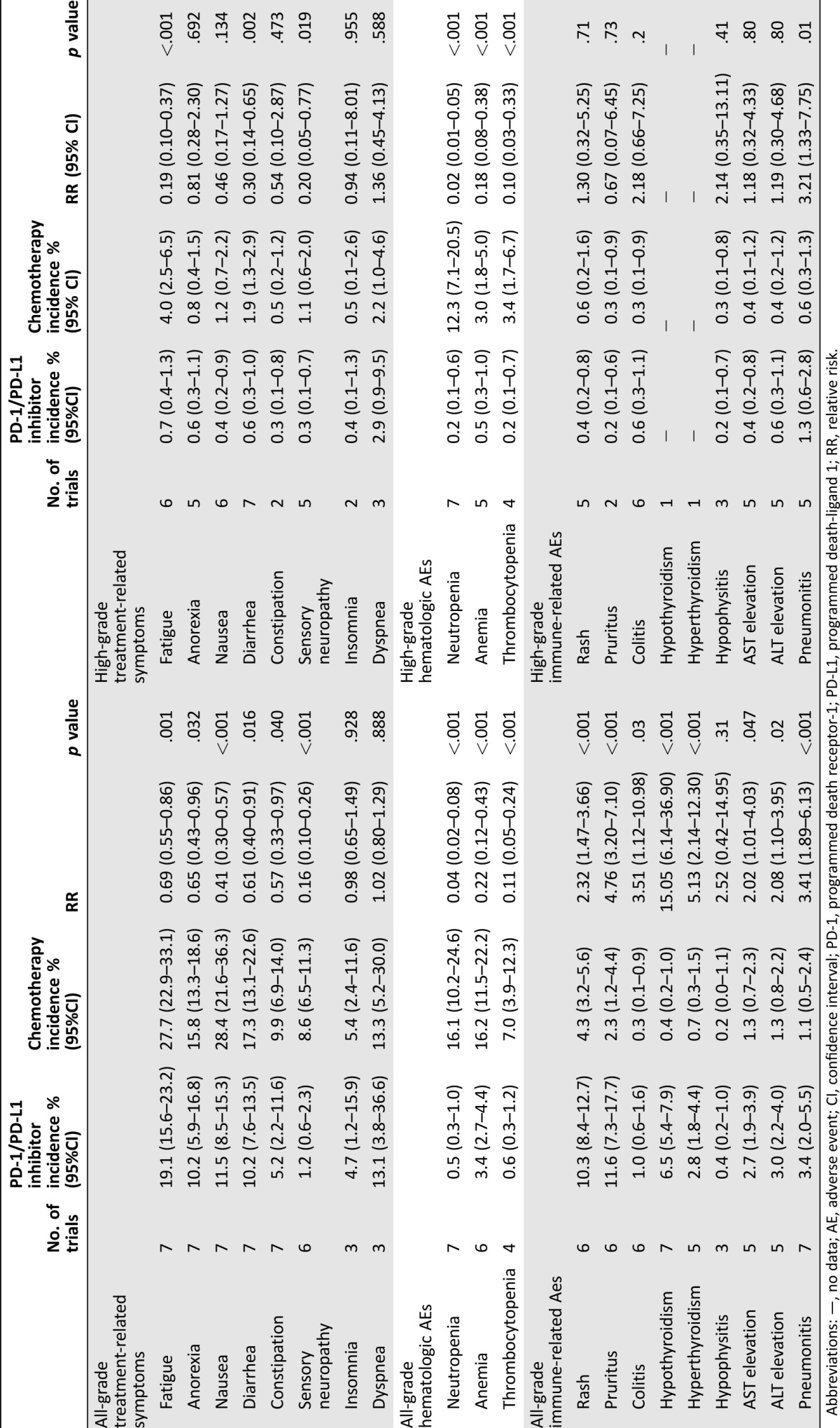
Abbreviations: —, no data; AE, adverse event; CI, confidence interval; PD‐1, programmed death receptor‐1; PD‐L1, programmed death‐ligand 1; RR, relative risk.
Hematologic Toxicities.
Patients treated with PD‐1/PD‐L1 inhibitors had a significantly lower risk of all‐grade neutropenia (0.5% versus 16.1%; RR 0.04), anemia (3.4% versus 16.2%; RR 0.22), and thrombocytopenia (0.6% versus 7.0%; RR 0.11). A risk of high‐grade neutropenia, anemia, and thrombocytopenia was also statistically significant lower in the PD‐1/PD‐L1 inhibitor group compared with the chemotherapy group (Table 3).
Immune‐Related AEs.
PD‐1/PD‐L1 inhibitors were associated with a significantly higher risk for all‐grade immune‐related AEs, including dermatologic (rash and pruritus), gastrointestinal (colitis), hepatic (AST/ALT elevations), endocrine (hypothyroidism and hyperthyroidism), and pulmonary (pneumonitis) events. There was also a small but statistically significant increase in the risk of high‐grade pneumonitis with PD‐1/PD‐L1 inhibitors compared with chemotherapy (1.3% versus 0.6%; RR 3.21).
Exploratory Subgroup Analysis
To investigate possible reasons for heterogeneity, we did subgroup analyses with regard to the RRs by type of chemotherapy regimen (docetaxel versus others) and type of tumor (NSCLC versus melanoma). As all NSCLC trials used docetaxel and all melanoma trials used non‐docetaxel regimens in the chemotherapy arms, the same results were obtained from the subgroup analyses according to chemotherapy type and tumor type. Using Q statistics, there were significant differences in the risk of all‐grade fatigue, anorexia, and diarrhea, all‐ and high‐grade nausea, and any all‐grade AEs between these subgroups. Otherwise, a similar toxicity profile in comparison with PD‐1/PD‐L1 inhibitors was observed in both chemotherapy subgroups. The exploratory subgroup analyses are summarized in Table 4.
Table 4. RR of toxicities according to chemotherapy type in control arm.
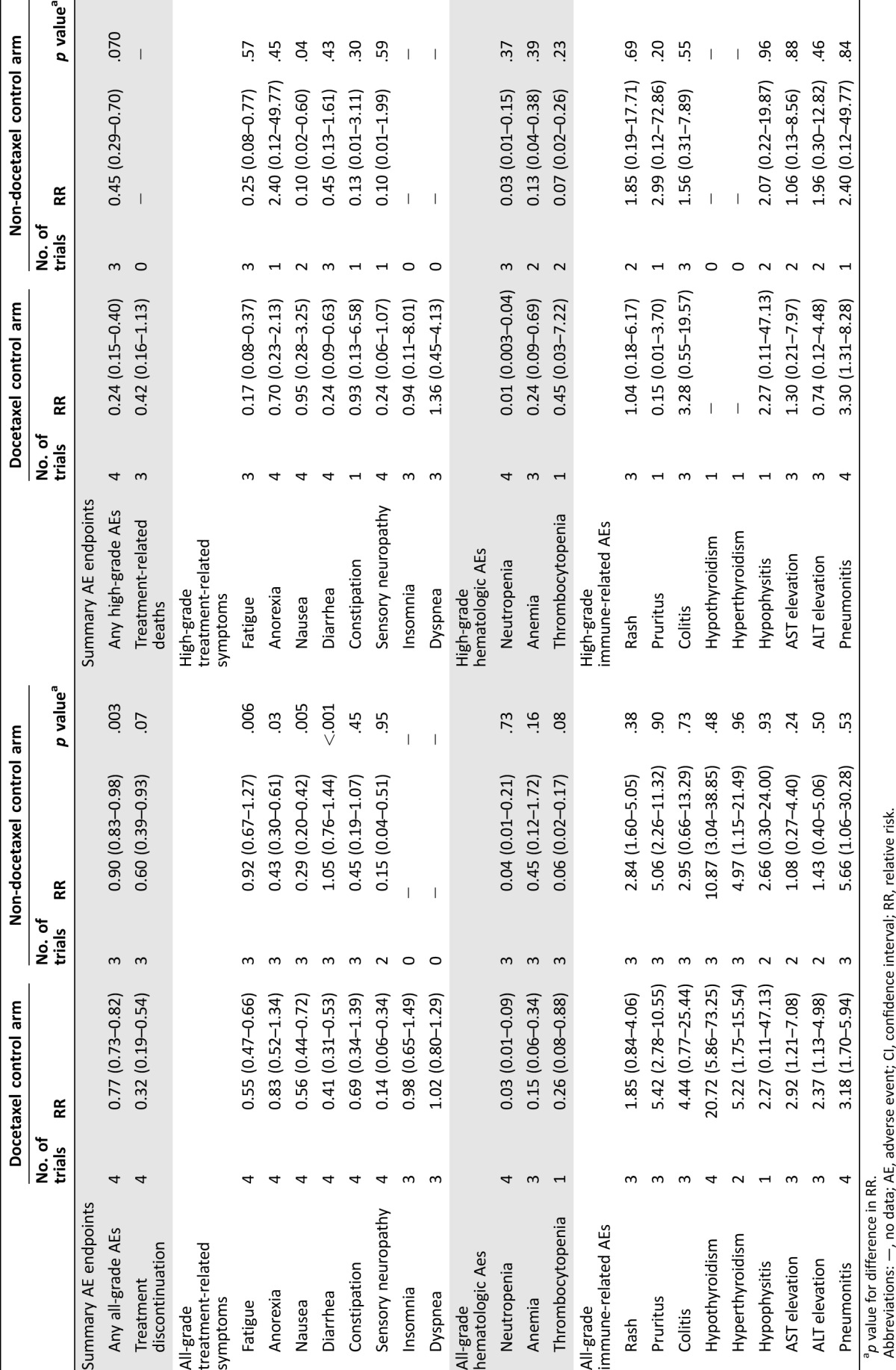
p value for difference in RR.
Abbreviations: —, no data; AE, adverse event; CI, confidence interval; RR, relative risk.
Study Quality and Publication Bias
Six trials were open label, whereas one trial was double blind placebo controlled. The Jadad score ranged from 3 to 5 with a mean was 3.3, indicating that overall study quality was fair (Table 1). For RR of all‐grade constipation and pneumonitis and high‐grade colitis, the Egger test suggested some evidence of publication bias. However, the Begg tests showed no evidence of bias (p > .05). This difference in the results obtained from the two methods may be due to a greater statistical power of the Egger test [24].
Discussion
We compared the tolerability of ICIs targeting PD1/PD‐L1 pathway and standard‐of‐care chemotherapy in patients with advanced cancer by performing a meta‐analysis of RCTs. PD1/PD‐L1 inhibitors were associated with a lower risk of treatment‐related symptoms (fatigue, anorexia, nausea, diarrhea, constipation, and sensory neuropathy) and hematologic toxicities. However, there was an increased risk of immune‐related AEs, including dermatologic, gastrointestinal, hepatic, endocrine, and pulmonary events in patients treated with PD1/PD‐L1 inhibitors. Most of these events were low‐grade, but high‐grade events were described, especially pneumonitis [25]. Clinicians need to be aware of the risk of these unique toxicities and manage them appropriately according to the algorithm for diagnosis and treatment adapted from guidelines used across anti‐PD‐1/PD‐L1 trials [26].
PD1/PD‐L1 inhibitors were associated with a lower risk of treatment‐related symptoms (fatigue, anorexia, nausea, diarrhea, constipation, and sensory neuropathy) and hematologic toxicities. However, there was an increased risk of immune‐related AEs, including dermatologic, gastrointestinal, hepatic, endocrine, and pulmonary events in patients treated with PD1/PD‐L1 inhibitors.
Our analysis of summary toxicity endpoints revealed a lower risk of any all‐ and high‐grade AEs and treatment discontinuation in the PD1/PD‐L1 inhibitor group. Importantly, absolute difference in risk and RR was more substantial for any high‐grade AEs (11.4% versus 35.7%, RR 0.32) than for any all‐grade AEs (67.6% versus 82.9%, RR 0.82), and this is related to the lower incidence of treatment discontinuation due to toxicities (4.5% versus 11.1%, RR 0.44) in the PD1/PD‐L1 inhibitor group compared with the chemotherapy group. Overall, PD1/PD‐L1 inhibitors in these clinical trials were better tolerated than chemotherapy.
CTCAE has been the standard method to evaluate toxicities and has been widely used in cancer clinical trials for more than 2 decades. AEs are graded based on the clinician's assessment of toxicity [27]. However, studies have shown that clinician‐measured CTCAEs underestimated the incidence and/or severity of symptoms actually experienced by cancer patients [28]. Basch et al. longitudinally collected clinician, as compared with patient, adverse symptom reports from 163 patients with lung cancer receiving chemotherapy [29]. This study found that clinician CTCAE assessments are better in predicting unfavorable clinical events, such as death and emergency room admissions, but that patients generally reported symptoms earlier and more frequently that better reflected their daily health status. The authors concluded that clinician and patient‐reported measures are complementary, each providing clinically meaningful information. Based on these findings, patient‐reported outcomes (PRO) have been added to recent cancer clinical trials. The recent clinical trials of PD1/PD‐L1 inhibitors have incorporated the European Organization for the Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire C30 (QLQ‐C30) and other PRO measures [3], [4], [5], [6], [7], [8], [9]. Of the clinical trials included in our meta‐analysis, two trials reported the results of PROs. In the clinical trial of pembrolizumab versus chemotherapy in advanced melanoma, EORTC QLQ‐C30 data were collected at baseline and at week 12 [6]. A significant deterioration in the global health status quality‐of‐life score (10 points or more) was experienced by 7% to 12% fewer patients in the pembrolizumab group compared with the chemotherapy group. Patients treated with pembrolizumab had consistently smaller decrements in the symptoms scales for fatigue, nausea, anorexia, diarrhea, and constipation. Recently, Long et al. reported PRO outcomes in the trial of nivolumab versus dacarbazine in patients with advanced melanoma [30]. The EORTC QLQ‐C30 was evaluated at baseline and every 6 weeks while on treatment. Compared with dacarbazine, patients treated with nivolumab maintained better global health longer (hazard ratio [HR] 0.65; 95% CI 0.46–0.92) and better physical function (HR 0.60; 95% CI 0.42–0.87). These results suggested that PD1 inhibitors were better tolerated than chemotherapy based on the patients' perspective and were consistent with the findings in our meta‐analysis.
Based on the efficacy and favorable toxicity profile of ICIs, their utility in the treatment of older patients and patients with impaired functional status is of great interest. Our group performed a meta‐analysis of nine RCTs comprising 5,265 patients to compare the efficacy of ICIs between younger and older patients. We showed that ICIs significantly improved OS compared with controls in both younger (HR 0.75; 95% CI 0.68–0.82) and older (HR 0.73; 95% CI 0.62–0.87) patients, using an older age cut‐point of 65–70 years [31].
Recently, the FDA performed a pooled analysis of 1,030 patients from four registration trials of nivolumab for advanced cancer [32]. Toxicity was reported separately for three age groups (<65 years, 616 patients; 65 to <70 years, 414 patients; and ≥70 years, 212 patients). The incidence of any grade 1–2 and grade 3–4 toxicities were, respectively, <65 years: 39% and 44%; 65 to <70 years: 35% and 45%; ≥70 years: 37% and 46%. The frequency and severity of AEs were similar across the age groups in this retrospective analysis. To assess the efficacy and safety in more general patient population, a phase IV study of nivolumab was conducted in patients with advanced NSCLC [33]. This trial included 65 patients with performance status (PS) 2. Notably, there was no obvious difference in efficacy and toxicity outcomes between PS 2 patients and PS 0–1 patients. As PS is a crude measure of patients' functional status, further studies of PD1/PD‐L1 inhibitors in older and/or frail patients using geriatric assessments are warranted to evaluate efficacy and safety as well as health‐related quality‐of‐life outcomes.
Our study has some limitations. First, the results described here are affected by the limitations of individual clinical trials that were selected for this meta‐analysis. As five of six included trials used an open‐label design, these trials were liable to ascertainment bias. Second, this is a meta‐analysis at the study level; therefore, variables at the patient level were not included in the analysis. Thus, we could not establish risk factors associated with the development of toxicities. Third, the patients in studies selected for our meta‐analysis were a select group of patients with good PS who were recruited into clinical trials conducted at academic centers. The actual incidence of toxicities in patients with organ dysfunction and/or an impaired functional status is likely to be higher in clinical practice. However, the patient selection into the trails in our study is unlikely to introduce bias into the RR analysis of the toxicities. Finally, significant heterogeneity was observed in the included studies for some of the planned RR analyses. We minimized heterogeneity influence by using the random‐effects model and also performed exploratory subgroup analyses based on type of chemotherapy regimen (docetaxel versus others) and type of tumor (NSCLC versus melanoma). As there were differences in the risk of some individual AEs between the subgroups, the observed heterogeneity may be partially explained by the differences in these factors.
Conclusion
Our analysis suggests that PD1/PD‐L1 inhibitors are better tolerated than standard‐of‐care chemotherapy in patients with advanced cancer. In addition to the efficacy results from trials, our findings provide useful information for clinicians for well‐balanced discussions with their patients on the risks and benefits of treatment options for advanced cancer. Further research on this promising immunotherapeutic approach is needed to assess efficacy, toxicity, and PROs in older patients and patients with poorer health status who are generally not included in the clinical trials.
Acknowledgment
This is an updated analysis of the meta‐analysis we presented at the American Society of Clinical Oncology Annual Meeting, June 3–7, 2016.
Footnotes
For Further Reading: Grainne M. O'Kane, Catherine Labbé, Mark K. Doherty et al. Monitoring and Management of Immune‐Related Adverse Events Associated With Programmed Cell Death Protein‐1 Axis Inhibitors in Lung Cancer. The Oncologist 2017;22:70‐80.
Implications for Practice: The potential adverse events of immune checkpoint inhibitors differ from conventional chemotherapy and can require a multidisciplinary approach. Continued education is important for all physicians to ensure optimal care for patients.
Author Contributions
Conception/Design: Tomohiro F. Nishijima, Hyman B. Muss
Provision of study material or patients: Tomohiro F. Nishijima, Shlomit S. Shachar
Collection and/or assembly of data: Tomohiro F. Nishijima, Shlomit S. Shachar
Data analysis and interpretation: Tomohiro F. Nishijima, Kirsten A. Nyrop, Hyman B. Muss
Manuscript writing: Tomohiro F. Nishijima, Shlomit S. Shachar, Kirsten A. Nyrop, Hyman B. Muss
Final approval of manuscript: Tomohiro F. Nishijima, Shlomit S. Shachar, Kirsten A. Nyrop, Hyman B. Muss
Disclosures
The authors indicated no financial relationships.
References
- 1. Ribas A. Releasing the brakes on cancer immunotherapy. N Engl J Med 2015;373:1490–1492. [DOI] [PubMed] [Google Scholar]
- 2. Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol 2015;33:1974–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Robert C, Long GV, Brady B et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320–330. [DOI] [PubMed] [Google Scholar]
- 4. Weber JS, D'Angelo SP, Minor D et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti‐CTLA‐4 treatment (CheckMate 037): A randomised, controlled, open‐label, phase 3 trial. Lancet Oncol 2015;16:375–384. [DOI] [PubMed] [Google Scholar]
- 5. Brahmer J, Reckamp KL, Baas P et al. Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med 2015;373:123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ribas A, Puzanov I, Dummer R et al. Pembrolizumab versus investigator‐choice chemotherapy for ipilimumab‐refractory melanoma (KEYNOTE‐002): A randomised, controlled, phase 2 trial. Lancet Oncol 2015;16:908–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Borghaei H, Paz‐Ares L, Horn L et al. Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015;373:1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Herbst RS, Baas P, Kim DW et al. Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): A randomised controlled trial. Lancet 2016;387:1540–1550. [DOI] [PubMed] [Google Scholar]
- 9. Fehrenbacher L, Spira A, Ballinger M et al. Atezolizumab versus docetaxel for patients with previously treated non‐small‐cell lung cancer (POPLAR): A multicentre, open‐label, phase 2 randomised controlled trial. Lancet 2016;387:1837–1846. [DOI] [PubMed] [Google Scholar]
- 10.Nivolumab (Opdivo) prescribing information. Available at http://packageinserts.bms.com/pi/pi_opdivo.pdf. Accessed September 30, 2016.
- 11.Pembrolizumab (Keytruda) prescribing information. Available at http://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf. Accessed September 30, 2016.
- 12.Atezolizumab (Tecentriq) prescribing information. Available at http://www.gene.com/download/pdf/tecentriq_prescribing.pdf. Accessed September 30, 2016.
- 13.ClinicalTrials.gov. Available at https://clinicaltrials.gov. Accessed September 30, 2016.
- 14. Michot JM, Bigenwald C, Champiat S et al. Immune‐related adverse events with immune checkpoint blockade: A comprehensive review. Eur J Cancer 2016;54:139–148. [DOI] [PubMed] [Google Scholar]
- 15. Bacon CG, Giovannucci E, Testa M et al. The association of treatment‐related symptoms with quality‐of‐life outcomes for localized prostate carcinoma patients. Cancer 2002;94:862–871. [DOI] [PubMed] [Google Scholar]
- 16. Butler L, Bacon M, Carey M et al. Determining the relationship between toxicity and quality of life in an ovarian cancer chemotherapy clinical trial. J Clin Oncol 2004;22:2461–2468. [DOI] [PubMed] [Google Scholar]
- 17. Moher D, Liberati A, Tetzlaff J et al. Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. J Clin Epidemiol 2009;62:1006–1012. [DOI] [PubMed] [Google Scholar]
- 18. Reeve BB, Mitchell SA, Dueck AC et al. Recommended patient‐reported core set of symptoms to measure in adult cancer treatment trials. J Natl Cancer Inst 2014;106:dju129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jadad AR, Moore RA, Carroll D et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials 1996;17:1–12. [DOI] [PubMed] [Google Scholar]
- 20. Higgins JP, Thompson SG, Deeks JJ et al. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 22. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–1101. [PubMed] [Google Scholar]
- 23. Egger M, Davey Smith G, Schneider M et al. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta‐analysis: Power of statistical tests and prevalence in the literature. J Clin Epidemiol 2000;53:1119–1129. [DOI] [PubMed] [Google Scholar]
- 25. Nishino M, Sholl LM, Hodi FS et al. Anti‐PD‐1‐related pneumonitis during cancer immunotherapy. N Engl J Med 2015;373:288–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Naidoo J, Page DB, Li BT et al. Toxicities of the anti‐PD‐1 and anti‐PD‐L1 immune checkpoint antibodies. Ann Oncol 2015;26:2375–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v.4 data files. Available at http://evs.nci.nih.gov/ftp1/CTCAE/About.html. Accessed October 20, 2016.
- 28. Xiao C, Polomano R, Bruner DW. Comparison between patient‐reported and clinician‐observed symptoms in oncology. Cancer Nurs 2013;36:E1–E16. [DOI] [PubMed] [Google Scholar]
- 29. Basch E, Jia X, Heller G et al. Adverse symptom event reporting by patients vs clinicians: Relationships with clinical outcomes. J Natl Cancer Inst 2009;101:1624–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Long GV, Atkinson V, Ascierto PA et al. Effect of nivolumab on health‐related quality of life in patients with treatment‐naïve advanced melanoma: Results from the phase III CheckMate 066 study. Ann Oncol 2016;27:1940–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nishijima TF, Muss HB, Shachar SS et al. Comparison of efficacy of immune checkpoint inhibitors (ICIs) between younger and older patients: A systematic review and meta‐analysis. Cancer Treat Rev 2016;45:30–37. [DOI] [PubMed] [Google Scholar]
- 32. Singh H, Kim G, Maher VE et al. FDA subset analysis of the safety of nivolumab in elderly patients with advanced cancers J Clin Oncol 2016;34:(suppl; abstr; 10010). [Google Scholar]
- 33. Hussein M, McCleod M, Chandler J et al. Safety and efficacy of nivolumab in an ongoing trial of a PD‐L1+/‐ patient population with metastatic non‐small cell lung cancer. Abstract ORAL02.02. Abstract presented at: 16th World Conference on Lung Cancer; September 6–9, 2015; Denver, Colorado.