Humans live within a surprisingly narrow window of body temperature (Tb) with core Tb typically regulated at 36.5–37.5°C. Symptoms of mild hypothermia occur below 35°C and severe hypothermia (<28°C) is often lethal. We generally think that precise temperature regulation is a common characteristic of mammals but it is not. The core Tb of many small mammals can vary by several degrees in species that use daily torpor or can drop to 0–5°C for days or weeks during hibernation.1 Entry into torpor or hibernation involves suppressing ATP-expensive metabolic processes, stabilizing macromolecules with chaperones and antioxidant defenses, switching to a lipid-based fuel economy, and regulating Tb over torpor/arousal cycles.1 Medically-induced mild hypothermia is a current treatment for some human conditions (e.g. open heart surgery, traumatic brain injury) but more uses of managed hypothermia and/or inducible torpor (e.g., organ preservation for transplant, long term space flight) could be achieved with enhanced understanding of the mechanisms that allow many mammalian species to transition to/from torpor and endure wide variations in core Tb without injury.
To do this, multiple issues that cause metabolic injury need resolution; one being apoptosis (programmed cell death). Both extrinsic (hormones, cytokines, growth factors) and intrinsic signals (changes in calcium, ATP, oxygen, nutrients, other stressors) can initiate apoptosis.2 A common negative effect of low temperature on cells is disruption of plasma membrane potential difference due to differential temperature effects on ion channels versus ATP-driven ion pumps resulting in dissipation of ion gradients, cell swelling, and inhibition of ATP generation.1 Such events, along with hypoxia, oxidative stress, and nutrient limitation accompany hibernation but hibernators have developed adaptive responses including anti-apoptosis mechanisms, altered signal transduction mechanisms, reversible posttranslational modifications of proteins, and differential microRNA expression to limit apoptosis (Fig. 1).2–4 Our recent studies of apoptosis in hibernators focused on the intrinsic (mitochondrial) pathway which involves mitochondrial outer membrane permeabilization (MOMP), release of pro-apoptotic proteins into the cytoplasm and activation of apoptotic protease activating factor (APAF).2,3
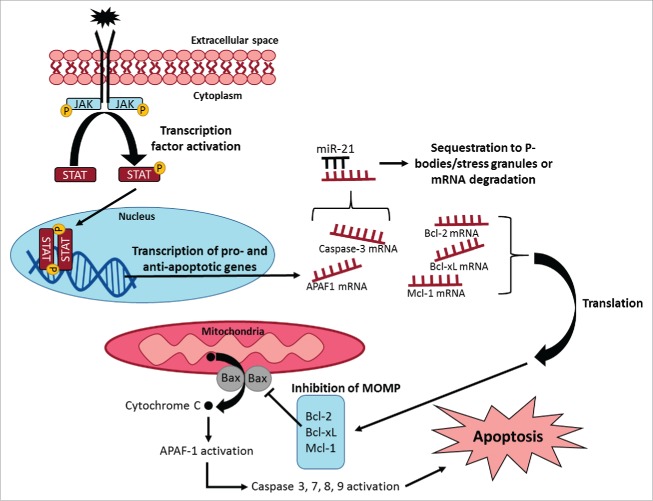
Figure 1.
Summary showing regulation of apoptotic signaling pathways in different subcellular organelles. Intrinsic or extrinsic signals may promote reversible protein phosphorylation of signaling molecules (e.g. Janus kinases and STATs) and activation of gene expression of pro- and anti-apoptotic proteins. Exported mRNA transcripts may be bound by microRNAs (e.g., miR-21) to direct transcripts for sequestration and degradation, or transcripts can be translated into pro-apoptotic (e.g. Bax, Bad) or anti-apoptotic (e.g., Bcl-2, Bcl-xL, Mcl-1) proteins. Pro-apoptotic proteins may be responsible for cytochrome C release and cysteine protease activation leading to apoptosis. Anti-apoptotic proteins may inhibit cytochrome C release during mitochondrial outer membrane permeabilization (MOMP). The relative levels of pro- and anti-apoptotic proteins control the fate of the cell.
Apoptosis is regulated by pro- and anti-apoptotic Bcl protein family members and by caspase inhibitor proteins. Using thirteen-lined ground squirrels (Ictidomys tridecemlineatus), we studied the responses to hibernation by pro-survival Bcl proteins in white fat.2 The caspase inhibitor x-IAP appeared to be the main inhibitor of apoptosis during prolonged torpor, when metabolic rate and Tb are low. However, levels of Mcl-1 (a MOMP inhibitor) increased as animals aroused and rewarmed, a process that increases both oxygen consumption and reactive oxygen species generation, the latter being a common trigger for apoptosis. No other Bcl-2 family members were upregulated, suggesting that apoptosis prevention in white fat is linked with specific targets. Comparable analysis of ground squirrel heart, brown adipose, skeletal muscle, liver and kidney showed upregulation of no more than 2 anti-apoptotic Bcl-2 family members during hibernation but brain elevated 4 protective proteins.3 Hence, tissue functionality and/or susceptibility to damage may dictate the anti-apoptosis defenses required by each tissue to endure torpor/arousal.
STAT transcription factors, phosphorylated by Janus kinases, are known to control expression of proteins that regulate apoptosis and so we explored hibernation responses by the Janus kinase-STAT pathway in white fat, skeletal muscle and heart of squirrels.2,3 Phosphorylation of pro-apoptotic STAT1 and anti-apoptotic STAT3 and STAT5 increased during torpor in each tissue, but without changes in Janus kinase phosphorylation. This indicated a role for STATs in controlling apoptotic protein expression in hibernators but the question of whether Janus kinase signaling is involved remains unanswered.2,3
Reversible protein phosphorylation by kinases of the Akt pathway helps to modify or shut down non-essential processes during torpor/hibernation.5 Low Tb can lead to enzyme inactivation and membrane depolarization, which can initiate apoptosis in animals without the proper defenses.1 However, hibernating species can reversibly phosphorylate ATP-dependent ion transporters to block ion loss from cells, helping to avoid triggering cell death.1 Protein kinases including Akt and glycogen synthase kinase 3β are known to be anti-apoptotic and are elevated in ground squirrels entering and exiting hibernation. Akt was also activated in the brain of bats during arousal from hibernation.5 This suggests that there is an important link between the Akt pathway (involved in regulating energy homeostasis and membrane potential) and avoidance of cell death in hibernators, especially when Tb changes dramatically during entrance and exit from torpor.
Apoptosis can also be regulated at the mRNA transcript level by microRNAs. MicroRNAs bind to complementary mRNA sequences to tag them for degradation or sequestration into subcellular particles for storage. MicroRNA control over specific mRNAs can regulate entire metabolic processes, including apoptosis.4 For instance, miR-21 binds to mRNA transcripts of pro-apoptotic proteins APAF-1, caspase-3 and PCNA4, leading to their degradation/sequestration to prevent cell death. MiR-21 levels rose in squirrel muscle during hibernation but decreased in brown fat and also in muscle of little brown bats, suggesting that they regulate apoptosis in a tissue- and species-specific manner.4 New research also shows that low temperature strengthens binding interactions of microRNAs and target mRNAs.4 Hence, temperature-sensitive microRNA target recognition could be an unrecognized but crucial mechanism that facilitates torpor by suppressing the translation of pro-apoptotic mRNA at low Tb.
Many small mammals may decrease their Tb by 10–15°C during episodes of daily torpor and hibernating mammals can endure core Tb values approaching 0°C.1 The recent research reported here shows that hibernators can use selective gene/protein expression to achieve MOMP inhibition and/or prevent caspase activation, reversible protein phosphorylation to suppress metabolic rate and maintain ion homeostasis, and differential microRNA expression to inhibit pro-apoptotic signaling. Although this research serves as a great starting point, more will need to be done to replicate hibernator cellular conditions in humans in order to treat muscle atrophy, ischemia-reperfusion injury, and extend organ viability for transplants.
References
- [1].Van Breukelen F, Krumschnabel G, Podrabsky JE . Vertebrate cell death in energy-limited conditions and how to avoid it: what we might learn from mammalian hibernators and other stress-tolerant vertebrates.. Apoptosis 2010; 15:386-99; PMID:20127173; http://dx.doi.org/ 10.1007/s10495-010-0467-y [DOI] [PubMed] [Google Scholar]
- [2].Logan SM, Luu BE, Storey KB. Turn down genes for WAT? Activation of anti-apoptosis pathways protects white adipose tissue in metabolically depressed thirteen-lined ground squirrels.. Mol Cell Biochem 2016; 416:47-62; PMID:27032768; http://dx.doi.org/ 10.1007/s11010-016-2695-0 [DOI] [PubMed] [Google Scholar]
- [3].Logan SM, Tessier SN, Tye J, Storey KB. Response of the JAK-STAT pathway to mammalian hibernation in 13-lined ground squirrel striated muscle.. Mol Cell Biochem 2016; 414:115-27; PMID:26885984; http://dx.doi.org/ 10.1007/s11010-016-2665-6 [DOI] [PubMed] [Google Scholar]
- [4].Biggar KK, Storey KB. Insight into post-transcriptional gene regulation: stress-responsive microRNAs and their role in the environmental stress survival of tolerant animals.. J Exp Biol 2015; 218:1281-9; PMID:25954040; http://dx.doi.org/ 10.1242/jeb.104828 [DOI] [PubMed] [Google Scholar]
- [5].McMullen DC, Hallenbeck JM. Regulation of Akt during torpor in the hibernating ground squirrel, Ictidomys tridecemlineatus.. J Comp Physiol B Biochem Syst Environ Physiol 2010; 180:927-934; http://dx.doi.org/ 10.1007/s00360-010-0468-8 [DOI] [PMC free article] [PubMed] [Google Scholar]