Abstract
Objective
Knee osteoarthritis (OA) is a broadly applied diagnosis that may encompass multiple subtypes of pain. The purpose of this study was to identify phenotypes of knee OA, using measures from the following pain-related domains: 1) knee OA pathology, 2) psychological distress, and 3) altered pain neurophysiology.
Methods
Data were selected from a total of 3494 participants at Visit #6 of the Osteoarthritis Initiative (OAI) study. Latent Class Analysis was applied to the following variables: radiographic OA severity, quadriceps strength, Body Mass Index (BMI), Charlson Comorbidity Index (CCI), Center for Epidemiologic Studies Depression subscale (CES-D), Coping Strategies Questionnaire-Catastrophizing subscale (CSQ-Cat), number of bodily pain sites, and knee joint tenderness at 4 sites. Resulting classes were compared on the following demographic and clinical factors: age, sex, pain severity, disability, walking speed, and use of arthritis-related healthcare.
Results
A four-class model was identified. Class 1 (4% of the study population) had higher CCI scores. Class 2 (24%) had higher knee joint sensitivity. Class 3 (10%) had greater psychological distress. Class 4 (62%) had lesser radiographic OA, little psychological involvement, greater strength, and less pain sensitivity. Additionally, Class 1 was the oldest, on average. Class 4 was the youngest, had the lowest disability, and least pain. Class 3 had the worst disability and most pain.
Conclusions
Four distinct pain phenotypes of knee OA were identified. Psychological factors, comorbidity status, and joint sensitivity appear to be important in defining phenotypes of knee OA-related pain.
Introduction
For centuries, knee osteoarthritis (OA) has been recognized by clinicians as an important health condition that often occurs in older adults, and the clinical episode is typically spurred by patient reports of knee pain and associated limitations in function and participation (1). However, within the last century, the diagnosis “knee OA” has come to be defined primarily by joint or tissue characteristics—most notably radiographic indicators of cartilage degeneration and osteophyte formation—which have relatively poor specificity to the experience of pain. Epidemiological studies suggest that only half of all older adults with knee pain demonstrate radiographic signs of OA, and approximately half of all older adults with radiographic OA do not report pain (2). In recognition of this discrepancy, a definition of OA has been proposed to encompass—not only radiographic abnormalities—but a wide array of mechanical, biochemical, hereditary, and structural processes (3). This definition has been criticized as so broad as to be largely unhelpful in guiding intervention strategies or clarifying OA disease pathogenesis (4).
We have suggested that conceptual underpinnings of the construct, “knee OA” should prioritize clinically relevant aspects of the condition, not necessarily markers of joint pathology or other imprecise surrogates of the clinical condition (5). Knee pain is complex and may be influenced by factors that are not unique to the knee joint, including psychological factors (e.g. depression, pain-related fear and anxiety) (6–8) and changes in neural sensitization (9, 10). Some of these factors may help to explain the observed discrepancy between symptom severity and radiographic structural findings (11).
Considering the myriad factors that have been implicated in 1) knee OA pathology and 2) knee pain, and considering that knee OA has been broadly applied as a diagnosis (having been used to describe the experience of knee pain for approximately 4.3 million people in the US) (12), it seems possible that subtypes of pain may now exist under this one diagnostic umbrella. For example, while some patients may experience pain due to OA-related damage of the knee joint, others may have pain attributable to changes in the neurological processing of nociceptive information. Still others may experience pain that is exacerbated by maladaptive cognitions or comorbid psychological distress. Such heterogeneity in knee pain could explain why 10–34% of patients continue to have pain following total knee replacement surgery (13), or why promising pain therapies have been shown to be ineffective when studied across the entire population of people diagnosed with knee OA (14).
The purpose of this study was to determine pain phenotypes in knee OA by applying Latent Class Analysis (LCA) to a variety of factors that have been implicated in the experience of pain. In LCA, unobserved latent variables are inferred from the observed measures, and participants can then be classified into subgroups or “phenotypes” according to the resulting latent variable structure. LCA is generally regarded as superior to traditional cluster analyses (15), in that it allows for greater variation in the types of discriminating variables used (e.g., continuous, categorical, or counts), greater flexibility in the types of models that can be estimated, and greater accuracy of classification. For this cross sectional analysis, variables were selected from visit #6 of the publicly available Osteoarthritis Initiative study. Visit #6 was chosen because of the availability of multiple measures related to the following pain domains: 1) knee OA pathology, 2) psychological distress, and 3) altered pain neurophysiology. We hypothesized that LCA would yield a 3-class model describing these domains (i.e. LCA would yield a group of participants with high levels of psychological distress and relatively low levels of joint pathology, a group of participants with high systemic pain sensitivity, and a group of participants with high levels of joint pathology and relatively low levels of systemic pain sensitivity and psychological distress). A secondary aim of this study was to examine any resulting classes for differences in demographic variables (age and sex distribution) and clinical characteristics (knee pain severity, functional limitation, and tendency to seek arthritis-related healthcare).
Patients and Methods
Data from Visit #6 (4-year follow-up) of the Osteoarthritis Initiative (OAI) study were selected for this cross sectional analysis. The OAI study is a longitudinal observational study intended to provide information on the incidence and progression of knee OA in older adults. At the time of this analysis, data were available up to 72 months following a baseline assessment. Specific inclusion and exclusion criteria have been described elsewhere and are also publically available (https://oai.epi-ucsf.org/datarelease/docs/StudyDesignProtocol.pdf) (16). Briefly, participants were eligible for inclusion if they possessed both symptomatic and radiographic knee OA at the baseline assessment or if they possessed risk factors for the near-term development of both symptomatic and radiographic knee OA. Exclusion criteria included: rheumatoid arthritis, bilateral knee arthroplasty or plans to undergo bilateral knee arthroplasty within three years, comorbid conditions that might interfere with participation over 4 years, bilateral end-stage osteoarthritis, positive pregnancy test, use of ambulatory aids other than a straight cane, or inability/unwillingness to participate in testing (including several MRI contraindications). Publicly available datasets for all assessments are made accessible online (https://oai.epi-ucsf.org/datarelease/default.asp).
Visit #6 was chosen for this analysis because of the array of potentially important pain-related variables available at this assessment. In order to prioritize our analysis around the experience of pain, participants were included if they reported knee symptoms (answered “yes” to the question, “During the past 12 months, have you had any pain, aching, or stiffness in or around your knee?”). For knee-specific (unilaterally assessed) variables, the measurement from the more painful limb was chosen for analysis. In total, the LCA was applied to data from 3494 participants. Characteristics of the study population are provided in Table 1.
Table 1.
Characteristics of Study Population (n= 3494). Values are reported as Mean ± Standard Deviation unless otherwise stated.
| Women, n (%) | 2069 (59.2) | |
| Age, years | 64.9 ± 9.0 | |
| WOMAC score | 15.6 ± 16.2 | |
| Knee pain, NPRS | 3.6 ± 2.8 | |
| Currently seeking healthcare for Arthritis, n (%) | 305 (7.2) | |
| Walking speed, m/s | 1.30 ± 0.22 | |
| Charlson Comorbity Score | 0.55 ± 1.05 | |
| Body Mass Index; kg/m2 | 28.9 ± 5.0 | |
| Knee extensor strength, N | 317 ± 122 | |
| Radiographic Severity, n (%) | (n=2915) | |
| K/L grade 0 | 873 (30.0) | |
| K/L grade 1 | 421 (14.5) | |
| K/L grade 2 | 802 (27.5) | |
| K/L grade 3 | 528 (18.1) | |
| K/L grade 4 | 288 (9.9) | |
| CES-D score | 7.24 ± 7.54 | |
| CSQ-Cat score | 0.67 ± 1.16 | |
| Number of Bodily Pain Sites | 1.59 ± 2.25 | |
| Knee Joint Tenderness Present on Physical Exam, n (%) | (n=3312) | |
| Medial Joint Tenderness, n (%) | 1060 (35.5) | |
| Lateral Joint Tenderness, n (%) | 756 (25.3) | |
| Positive Patellar Grind Test, n (%) | 504 (17.0) | |
| Pes Anserine Tenderness, n (%) | 992 (33.3) | |
CCI: Charlson Comorbidity Index
BMI: Body Mass Index
K/L: Kellgren-Lawrence
CES-D: Center for Epidemiologic Studies—Depression Subscale
CSQ-Cat: Coping Strategies Questionnaire—Catastrophizing Subscale (2-question modified version)
WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index
NPRS: Numeric Pain Rating Scale
Measures used to determine class structure (phenotyping variables)
Radiographic severity of knee OA
Although the specificity of radiography in capturing the experience of knee pain appears to be limited, a relationship exists between severity of knee OA and pain; people with more severe radiographic OA appear more likely to report pain, and people with pain-discrepant knees (i.e. experiencing more frequent knee pain in one knee compared to the other) appear particularly likely to report more pain in the knee that displays greater evidence of radiographic degeneration (17). Weight-bearing fixed-flexion knee radiographs were taken and graded according to the Kellgren-Lawrence (K/L) scale, a 4-point scale describing cartilage degeneration and the formation of osteophytes (18). K/L grades of the more painful knee were chosen for analysis.
Body Mass Index (BMI), defined as the ratio of weight (in kilograms) to height (in meters, squared), has been associated with pain and mobility deficits in knee OA and may contribute to progression of the disease, especially in cases of joint malalignment (19, 20). Associations have also been observed between pain severity and kinematic and kinetic measures of lower extremity loading (21). Thus, this variable was chosen as a potential marker of pain related to excessive joint loading.
Knee extensor strength
Quadriceps femoris weakness is strongly associated with the presence of pain in knee OA and may predispose patients to worsening pain over time via an inability to attenuate forces through the knee joint (22, 23). Knee extensor strength, measured in Newtons (N), was assessed with the Good Strength Chair (Metitur Oy, Jyvaskyla, Finland) as previously described (24, 25). The maximal voluntary isometric strength over three trials was selected for analysis. The reliability and validity of this test has been previously reported (26).
Tenderness of the knee joint—an item on the clinical diagnostic criteria for knee OA—(27, 28) could be considered an indicator of joint-specific osteoarthritic damage and may also result from sensitization of peripheral nociceptors. For the purposes of this analysis, four dichotomous variables were selected to capture the presence of knee joint tenderness, palpation tenderness (yes/no) on physical exam at 3 locations: 1) medial joint line, 2) lateral joint line, 3) pes anserine bursa, and 4) data from the patellar grind test (pain with test: yes/no) were also used as an indicator of patellofemoral joint sensitivity. To standardize the examination, examiners routinely calibrated palpation pressure using a Chatillon dolorimeter (16).
Comorbidity status
The modified Charlson Comorbidity Index (CCI), assesses the number and severity of 13 health conditions (e.g. heart attack, stroke, diabetes, cancer) by self-report. This questionnaire has demonstrated excellent test-retest reliability and has been validated in terms of excellent agreement with an extensive, chart-review based version of the index (29). Scores ranged between 0 and 12 in our dataset, with higher scores indicating a greater impact of comorbidities on patients’ health. Originally validated as a predictor of mortality in hospitalized patients (30), we chose to use this measure as a surrogate of systemic factors (other than knee OA) that could influence the experience of pain. For example, the CCI has been associated with measures of systemic inflammation (31), which could play a role in neurological pain sensitization (32).
Number of pain sites
To capture the presence of widespread pain, and as a surrogate of central pain sensitization, the number of pain sites on the body was summed for each participant. The presence of pain—by self-report—at each site over the previous 30 days was used in the calculation. Available sites included the neck, as well as right and left measurements for the elbow, wrist, hand, foot, ankle, and shoulder. A score of 13 represented the highest possible score, and scores ranged between 0 and 13 in our dataset. Joint pain summing in areas other than the affected joint is considered a valid indicator of pain comorbidity in knee OA (33).
Depression
The Center for Epidemiologic Studies-Depression (CES-D) scale is a 10-item scale that has been used extensively to measure depressive symptoms in knee osteoarthritis (6, 34–36). A total score of 60 points is possible, with scores > 16 thought to indicate the presence of mild depression.
Pain Catastrophizing
A modified version of the coping strategies questionnaire-catastrophizing subscale (CSQ-CAT) was used for this analysis. This two-item questionnaire (possible score: 0–6 points) asks participants to rate the frequency with which they demonstrate the following pain coping responses: “It is terrible and I think it is never going to get any better,” and “I feel I can’t stand it anymore.” Higher scores indicate more frequent catastrophizing responses. This scale has demonstrated construct validity with the larger coping strategies questionnaire and criterion validity to measures of pain and physical function (37). Feelings of helplessness surrounding a chronic pain experience are thought to be maladaptive, potentially contributing to a cycle of activity avoidance that ultimately results in the exacerbation of pain (38). Pain catastrophizing has been implicated in poor pain prognosis for people with knee OA and in individuals undergoing total knee replacement surgery (7, 39, 40).
Comparison of variables across phenotypes
Participant demographics and clinical characteristics were selected for comparison across any phenotypes that resulted from the LCA. Data were again drawn from Visit #6 of the OAI study. Demographic measures consisted of participant age and sex. Clinical characteristics included assessments of pain intensity, functional limitation, and need for arthritis-related healthcare. Self-reported knee pain intensity (during the previous week) was assessed by an 11-point Numeric Pain Rating Scale (NPRS). The Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index was used to assess self-reported functional limitations, and a 20-meter timed walking test was used to assess self-selected walking speed (SSWS). Participants’ need for arthritis-related healthcare was captured by self-report, in response to the question, “Are you currently seeing a doctor or other healthcare professional for arthritis?”
Data Analysis
Each of the selected phenotyping variables was examined individually in bivariate regression analyses against pain severity (NPRS over the previous week) to confirm a significant association with pain. LCA was then performed using Mplus, Version 7.0 (Muthen & Muthen). For the purposes of this study, models with an increasing number of classes were estimated until the best-fitting model was observed. Model fit was evaluated by observing differences in fit statistics—Bayesian Information Criterion (BIC) and Akaike Information Criterion (AIC)—with models of differing numbers of classes. A Lo-Mendell-Rubin likelihood ratio test (LRT) was also used to assess the goodness of fit of a model of n classes, relative to a model of n-1 classes (41). This information, in combination with model interpretability, was used to select the model that best represented the selected data.
Results
Determination of class structure
Latent Class Analyses were conducted for models comprising 2–5 classes. Although fit statistics—BIC and AIC—improved marginally with models of increasing class, the 5-class model did not outperform the 4-class model according to Lo-Mendell-Rubin LRT (p = 0.111). The 4-class model appeared to be a better fit than a 3-class model, according to LRT (p = 0.0324). Furthermore, the smallest resulting class in the 4-class model represented approximately 4% of the study population, whereas the smallest class in the 5-class model represented only 1.9% of the study population (66 participants of the 3494 available). Thus, based on significant likelihood ratio tests and overall model interpretability, we concluded that a 4-class model provided the best fit to the data. This model demonstrated an entropy value of 0.80, which indicates acceptable precision for assigning individuals to their appropriate class.
After determining the class structure, individual participants were assigned to their most likely class based on model posterior probabilities. Phenotyping variables were then compared across classes using one-way ANOVAs and post-hoc least significant difference tests, as well as chi-square tests for categorical variables. Analyses were performed using Statistical Analysis Software (SAS, SAS Institute Inc., Cary, NC). Differences in pain phenotypes are detailed in Table 2. Briefly, Class 1 (representing 4% of the study population) had higher CCI scores than the other classes. Within this class, the most common comorbidities were: diabetes (43.5% of this phenotype), cancer (39.3%), renal disease (34.8%), heart attack (26.9%), stroke (24.7%) asthma (21.4%), and Chronic Obstructive Pulmonary Disease (COPD) (15.75%). Class 2 (24% of the study population) had higher frequencies of knee joint tenderness. Class 3 (10%) demonstrated higher levels of psychological distress (CES-D and CSQ-Cat scores), and greater numbers of pain sites. Finally, Class 4 (62%) had lesser radiographic OA, relatively little psychological distress, greater strength, and low frequencies of knee joint tenderness on physical exam.
Table 2.
Characteristics of “phenotyping” variables across the 4 pain phenotypes. Data are presented as mean ± SD unless otherwise indicated.
| Variable | Class 1 (n=148) |
Class 2 (n=854) |
Class 3 (n=337) |
Class 4 (n=2155) |
F (p-value) |
χ2(3) (p-value) |
|---|---|---|---|---|---|---|
| CCI Score | 4.1 ± 1.5a | 0.4 ± 0.7b | 0.7 ± 0.9c | 0.3 ± 0.6d | 1261.68 (<0.0001) | |
| BMI, kg/m2 | 30.8 ± 5.3a | 29.7 ± 5.3a | 30.3 ± 5.2a | 28.2 ± 4.7b | 34.17 (<0.0001) | |
| Knee extensor strength, N | 277 ± 85a | 259 ± 96a | 281 ± 119a | 349 ± 124b | 116.42 (<0.0001) | |
| Radiographic Severity | 1.83 ± 1.33a | 1.81 ± 1.32a | 1.96 ± 1.37a | 1.49 ± 1.32b | 18.53 (<0.0001) | |
| K/L grade 0, % | 24.8 | 24.8 | 23.2 | 33.8 | ||
| K/L grade 1, % | 11.9 | 12.7 | 11.1 | 61.0 | ||
| K/L grade 2, % | 30.3 | 30.4 | 26.3 | 26.3 | ||
| K/L grade 3, % | 22.0 | 20.9 | 24.9 | 15.5 | ||
| K/L grade 4, % | 11.0 | 11.2 | 14.5 | 8.4 | ||
| CES-D score | 10.8 ± 8.5a | 7.5 ± 7.5b | 14.7 ± 10.6c | 5.7 ± 5.9d | 170.74 (<0.0001) | |
| CSQ-Cat score | 1.0 ± 1.2a | 0.5 ± 0.6b | 3.5 ± 1.0c | 0.3 ± 0.5d | 2167.35 (<0.0001) | |
| Number of Pain Sites | 2.3 ± 2.9a | 2.4 ± 2.6a | 3.1 ± 3.2b | 1.0 ± 1.5c | 155.67 (<0.0001) | |
| Medial Joint Tenderness, % | 49.1a | 83.7b | 55.2a | 8.4c | 1459.94 (<0.0001) | |
| Lateral Joint Tenderness, % | 26.8a | 59.8b | 42.1c | 6.1d | 905.92 (<0.0001) | |
| Positive Patellar Grind Test, % | 21.6a | 29.6a | 28.9a | 8.7b | 208.66 (<0.0001) | |
| Pes Anserine Tenderness, % | 43.8a | 74.1b | 49.8a | 10.4c | 1076.01 (<0.0001) |
CCI: Charlson Comorbidity Index
BMI: Body Mass Index
K/L: Kellgren-Lawrence
CES-D: Center for Epidemiologic Studies—Depression Subscale
CSQ-Cat: Coping Strategies Questionnaire—Catastrophizing Subscale (modified version)
Note: Values sharing a common subscript letter (i.e., a, b, c, d) failed to differ significantly at alpha = 0.05 in post hoc testing.
Comparison of Classes
After determining the class structure using pain-related phenotyping variables, individual participants were assigned to their most likely class based on model posterior probabilities and compared on a number of demographic and clinical characteristics. Group comparisons were again performed using one-way ANOVAs and chi-square tests. Demographic measures consisted of participant age and sex. Clinical characteristics included assessments of knee pain intensity (NPRS), functional limitation (WOMAC and SSWS), and the need for arthritis-related healthcare. A significant group effect was observed for all variables examined (Table 3). For age, post-hoc testing revealed that participants in Class 1 were significantly older, on average, than participants in other classes (p<0.0001 for all post-hoc comparisons). Participants in Class 2 were also significantly older compared to participants in Classes 3 (p=0.001) and Class 4 (p<0.0001). Classes 3 and 4 did not differ significantly in mean age (p=0.383). Class 2 represented proportionally more females than the other classes (p<0.0001 for all post-hoc comparisons), and Classes 1 and 4 did not differ significantly in sex distribution (p=0.696). Participants in Class 3 reported significantly higher levels of knee pain, on average, than participants in any of the other classes (p=0.002 in comparison with Class 1; p<0.0001 in post-hoc comparison with Class 2), whereas participants in Class 4 reported the lowest overall pain levels (p<0.0001 for comparisons to all other classes). The same pattern of differences was also observed with WOMAC scores. Participants in Class 1 demonstrated slower walking speed than participants in Class 2 (p<0.0001), Class 3 (p=0.049) and Class 4 (p<0.0001). Participants in Class 3 sought healthcare for arthritis at higher rates than participants in Class 1 (p=0.013) and Class 4 (p<0.0001) but not Class 2 (p=0.083). Participants in Class 1 did not differ significantly from participants in Class 4 (p=0.138) in rates of arthritis-related healthcare seeking.
Table 3.
Class differences in demographic characteristics and pain/health outcomes.
| Variable | Class 1 (n=148) “Higher CCI Scores” | Class 2 (n=854) “Knee Joint Tenderness” | Class 3 (n=337) “Psychological Distress” | Class 4 (n=2155) “Mild OA” | F (p-value) | χ2(3) (p-value) |
|---|---|---|---|---|---|---|
| Women, % | 52.7 a | 78.8 b | 64.7 c | 51.0 a | 202.07 (p<0.0001) | |
| Age, years | 69.5 ± 9.0 a | 65.8 ± 9.0 b | 63.9 ± 9.4 c | 64.4 ± 8.9 c | 19.71 (p<0.0001) | |
| WOMAC score | 27.5 ± 19.9 a | 20.2 ± 15.6 b | 32.5 ± 20.5 c | 10.4 ± 12.2 d | 307.57) (p<0.0001) | |
| Knee pain, NPRS | 5.1 ± 2.9 a | 4.3 ± 2.6 b | 6.0 ± 2.8 c | 2.9 ± 2.5 d | 185.81 (p<0.0001) | |
| Walking speed (m/s) | 1.13 ± 0.22 a | 1.26 ± 0.21 b | 1.18 ± 0.23 c | 1.35 ± 0.20 d | 110.68 (p<0.0001) | |
| Currently seeking healthcare for arthritis, % | 8.8 a | 13.4 b | 17.5 b | 5.4 a | 87.32 (p<0.0001) |
WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index
NPRS: Numeric Pain Rating Scale (knee pain)
Note: Values sharing a common subscript letter (i.e., a, b, c, d) failed to differ significantly at alpha = 0.05 in post hoc testing.
Stability of class structure
Although it was not possible to perform the LCA at multiple time-points due to a lack of availability of several of the measures in the OAI dataset (e.g. pain catastrophizing was first assessed at Visit #6), a preliminarily assessment of model stability was undertaken via the examination of four phenotyping variables at multiple time-points predating Visit #6. Knee extensor strength, CES-D scores, K/L grades, and CCI scores were examined at all available time-points between Baseline and Visit #6 (4 year follow-up). Using the class membership elucidated in the LCA (based on data from visit #6), means were calculated for each variable and plotted over time (Figure 1). The ordering of variables across classes (lowest to highest mean values) demonstrated consistency over time.
Figure 1.
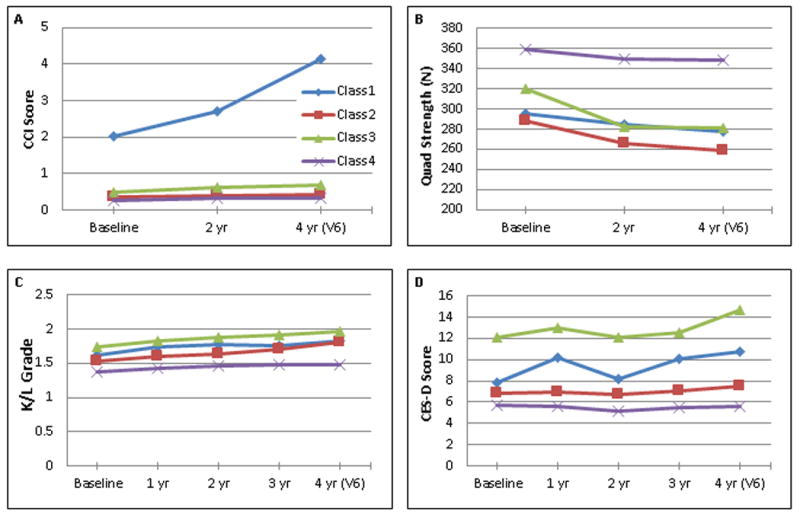
Examination of phenotyping variables by class over time. Latent Class Analysis was applied to data from Visit 6 (V6). A) Charlson Comorbidity Index (CCI) score, B) isometric quadriceps force production, C) radiographic severity, as measured by Kellgren-Lawrence (K/L) grade, D) Center for Epidemiologic Studies—Depression subscale (CES-D) Score. Data were plotted based on availability. Not all measures were available at every yearly assessment.
Discussion
This analysis supports and expands upon the results of previous studies, by 1) providing additional evidence that subgroups exist within the heterogeneous population of people with knee OA, and 2) elucidating potential pain-specific subgroups to help explain the observed heterogeneity. There were 4 classes—or pain phenotypes—that resulted from the LCA performed in this study; Class 1: a group of people with higher levels of comorbidity, Class 2: a group of people with higher levels of knee joint tenderness and relatively weak quadriceps, Class 3: a group of people with higher levels of psychological distress and more severe radiographic OA, and Class 4: a group of people with relatively mild radiographic OA, relatively low levels of psychological distress and comorbidity, and the highest average quadriceps strength.
We hypothesized a 3-class structure to the LCA, with phenotypes characterized by 1) high levels of psychological distress and low levels of joint pathology, 2) high levels of systemic pain sensitivity and low levels of joint pathology, and 3) high levels of joint pathology, low levels of pain sensitivity and low levels of psychological distress. Although a 4-class structure emerged from our analysis, the results appear partially consistent with our hypothesis, in that a phenotype of knee OA-related pain was largely defined by psychological distress. Approximately 10% of the study population (Class 3) was classified under this phenotype, which compares favorably with the prevalance of a “depressed” phenotype in a recent subgrouping analysis (42). Depression and pain catastrophizing have been implicated in the etiology of chronic pain, via either diathesis-stress (43), or fear-avoidance type mechanisms, and patients who exhibit signs of depression or catastrophizing have poorer pain prognosis following many interventions, including knee replacement surgery (44–46). However, contrary to our hypothesis, participants in Class 3 did not exhibit lower levels of radiographic OA. This suggests against the idea that psychological distress simply exacerbates pain for people with mild radiographic disease.
It is perhaps notable that the two phenotypes that exhibited the most severe radiographic OA (Class 1 and Class 3) reported the highest levels of pain, on average, while also possessing signs of psychological distress (Class 3) or other health comorbidities (as indicated by high CCI scores, in the case of Class 1). Thus, for people reporting high levels of knee pain, our results appear to reinforce the idea of knee OA as more than just a knee problem, even in the presence of demonstrable radiographic disease. Future work should focus on understanding the influence of comorbid illness or psychological distress on the experience of knee pain, so that comprehensive management strategies can be developed to target joint-specific issues in addition to the comorbid conditions that make up the clinical phenotype.
In our analysis, approximately 4% of the study population appeared to be defined predominantly by higher CCI scores (Class 1). We chose to use CCI scores to assist in determining class structure, as a marker of systemic factors that could influence the experience of pain. Elevated systemic inflammation has been associated with CCI score (47), and various pro-inflammatory factors have been implicated in pain sensitization for people with OA (48, 49). Quantitative sensory testing, which could aid in the identification of pain sensitization in study participants (and could perhaps help further discern possible mechanisms of sensitization within the nervous system), was not performed in the OAI study. Future work may benefit from the inclusion of such measures, especially considering that pain sensitization might arise via multiple peripherally or centrally mediated mechanisms (50). Sensitization that co-travels with the presence of inflammatory comorbidities might warrant a different management strategy than sensitization that co-travels with psychological distress or severe radiographic disease.
Classes 1, 2 and 3 demonstrated significantly reduced quadriceps strength relative to class 4. Approximately 24% of the participants in our sample were classified under a phenotype (Class 2) that demonstrated the weakest average quadriceps strength as well as significantly higher rates of knee joint sensitivity. This group was also predominantly female, which may help to explain some of the observed sex differences in knee OA prevalence. Quadriceps weakness has been associated with knee-OA-related pain in a number of studies, and the presence of quadriceps weakness was found to predict the development of symptomatic OA (but not necessarily radiographic OA) in an analysis of the Multicenter Osteoarthritis Study (MOST) data (51). The quadriceps muscle acts to attenuate forces during knee joint loading and may help protect the joint from damage due to repetitive or jarring physical stress (52, 53). Thus, the elucidation of a group of people exhibiting both quadriceps weakness and knee joint tenderness may identify a phenotype for which strength training of the quadriceps is particularly beneficial. Studies investigating the efficacy of strength training interventions for people with knee OA might consider incorporating assessments of joint tenderness to explore whether patients with this phenotype have a differential response to strengthening.
This is not the first attempt to empirically subgroup the diagnosis, “knee OA.” In 2011, Knoop et al used cluster analysis to describe a 5-group structure, where group differences were defined according radiographic severity, depression, BMI, and muscle strength (42). Other studies have attempted to examine pain trajectories over time or to characterize subgroups based only on radiographic features (54). Largely by design (by selection of alternative phenotyping variables), the phenotypes elucidated in our analysis differ from what has previously been described. However, some common themes have also emerged. First of all, pain intensity appears to vary by phenotype, and phenotypes with high levels of pain appear to possess other distinguishing characteristics related to factors such as psychological distress, joint sensitivity, or health status. Therefore, assessments that capture these characteristics of knee OA may warrant consideration in future work, as possible markers of knee OA diagnostic categories or modifiers of treatment effect. Secondly, the idea of knee OA as a progressive clinical condition is challenged. Although pain severity varies substantially across the population of people with knee OA, within-phenotype pain levels remain relatively stable over time (54, 55). Our results also provide preliminary evidence for the stability of other phenotypic characteristics over multiple years of assessment. Therefore, the potential—not only to prevent progression—but also to reduce pain and improve function through specific interventions targeting pain phenotypes appears worthy of further study.
The LCA performed in this study is exploratory by nature. The phenotypes resulting from LCA are determined by the variables chosen for analysis. We attempted to choose variables based on our own conceptual model and previous studies that have identified multiple important pain-related variables. To our knowledge, this if the first analysis to examine variables across pain-relevant domains, with the intention of developing our diagnostic understanding of knee OA more specifically around the experience of knee pain. Therefore, we also chose to define our study population based on the presence of symptoms, rather than to exclude people lacking radiographic evidence of knee OA. Perhaps for this reason, a large percentage of study participants appeared to demonstrate relatively mild knee OA. For example, participants in Class 4 made up a majority of the study population (62%) and were the highest functioning, with a SSWS of 1.35 m/s. This compares favorably with normative values for healthy adults in that age range (56). Furthermore, participants in our analysis appeared unlikely to seek healthcare for arthritis—only 7.2% of the study population sought healthcare for arthritis within the previous year—which may limit the generalizability of our findings to clinical settings. However, we felt it was important to include anyone with symptoms in our analysis—regardless of their healthcare seeking behavior—because of the potential to identify people outside the healthcare system who might stand to benefit from arthritis-related care. For example, while only 8.8% of participants in Class 1 reported seeking healthcare for arthritis (compared to 13.4% in Class 2 and 17.5% in Class 3), these participants demonstrated relatively high pain levels and the lowest average walking speed of any phenotype. Thus, comprehensive interventions aimed specifically at the characteristics of this phenotype appear to have the potential to produce meaningful improvements while meeting an otherwise-unmet healthcare need.
Although the LCA performed in this study suggests a 4-class structure, our characterization of classes as “pain phenotypes,” warrants further work. First of all, phenotypic structure should be replicated in other datasets, including study populations that are representative of the clinical population. Secondly, the inclusion of other pain-relevant variables (such as measures of nociceptor sensitization) should be considered. Thirdly, the stability of phenotypes over time should be formally assessed in cohort studies. Our work provides preliminary evidence suggesting that change occurs slowly and distinctions between classes are largely retained over time, but we could not assess class structure via LCA at multiple time points due to the lack of availability of many measures at other assessment times. For example, catastrophizing was first assessed at visit #6 in the OAI study. Finally, the response of phenotypes to targeted interventions should be assessed. If pain phenotypes provide a meaningful conceptual approach to understanding knee OA, phenotype-specific interventions should ultimately prove to be more effective than traditional therapies or therapies applied across the heterogeneous knee OA population.
In conclusion, the LCA performed in this study yielded 4 classes, which may define pain phenotypes of knee OA that warrant targeted intervention strategies. Characteristics of the elucidated phenotypes could also be assessed in future studies to determine phenotype-specific treatment response or prognosis.
Significance and Innovations.
The diagnosis, “knee OA” should ideally prioritize factors that are relevant to the clinical experience of knee pain. By applying Latent Class Analysis (LCA) to multiple pain-related variables, we have elucidated 4 phenotypes: 1) higher comorbidity burden, 2) higher knee joint sensitivity, 3) psychological distress and more widespread pain, and 4) greater strength, less severe radiographic OA, low levels of psychological distress, and low frequencies of knee joint tenderness on physical exam.
The two phenotypes that exhibited the highest levels of pain also demonstrated the most severe radiographic OA, while possessing signs of increased psychological distress (Class 3) or higher numbers of other health comorbidities (Class 1). Thus, for people reporting high levels of knee pain, our results appear to reinforce the idea of knee OA as more than just a knee problem, even in the presence of demonstrable radiographic disease.
High rates of knee joint tenderness helped to identify a phenotype (Class 2) that may also demonstrate quadriceps weakness. Studies investigating the efficacy of strength training for people with knee OA should consider incorporating assessments of joint tenderness to explore whether patients with this phenotype have a differential response to strengthening.
Attempts should be made to replicate this class structure in other study populations, and formal examinations of class stability over time should be examined in future cohort studies.
Acknowledgments
Funding source: This research was funded in part by the National Institutes of Health (T32-AG000279, R21-AG044710) and Foundation for Physical Therapy (Promotion of Doctoral Studies I and II).
References
- 1.Dequeker J, Luyten FP. The history of osteoarthritis-osteoarthrosis. Ann Rheum Dis. 2008;67(1):5–10. doi: 10.1136/ard.2007.079764. [DOI] [PubMed] [Google Scholar]
- 2.Bedson J, Croft PR. The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskelet Disord. 2008;9:116. doi: 10.1186/1471-2474-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandt KD, Dieppe P, Radin EL. Etiopathogenesis of osteoarthritis. Rheum Dis Clin North Am. 2008;34(3):531–59. doi: 10.1016/j.rdc.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Brandt KD, Dieppe P, Radin E. Etiopathogenesis of osteoarthritis. Med Clin North Am. 2009;93(1):1–24, xv. doi: 10.1016/j.mcna.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Kittelson AJ, George SZ, Maluf KS, Stevens-Lapsley JE. Future directions in painful knee osteoarthritis: harnessing complexity in a heterogeneous population. Phys Ther. 2014;94(3):422–32. doi: 10.2522/ptj.20130256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scopaz KA, Piva SR, Wisniewski S, Fitzgerald GK. Relationships of fear, anxiety, and depression with physical function in patients with knee osteoarthritis. Arch Phys Med Rehabil. 2009;90(11):1866–73. doi: 10.1016/j.apmr.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Somers TJ, Keefe FJ, Pells JJ, Dixon KE, Waters SJ, Riordan PA, et al. Pain catastrophizing and pain-related fear in osteoarthritis patients: relationships to pain and disability. J Pain Symptom Manage. 2009;37(5):863–72. doi: 10.1016/j.jpainsymman.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heuts PH, Vlaeyen JW, Roelofs J, de Bie RA, Aretz K, van Weel C, et al. Pain-related fear and daily functioning in patients with osteoarthritis. Pain. 2004;110(1–2):228–35. doi: 10.1016/j.pain.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 9.Arendt-Nielsen L, Nie H, Laursen MB, Laursen BS, Madeleine P, Simonsen OH, et al. Sensitization in patients with painful knee osteoarthritis. Pain. 2010;149(3):573–81. doi: 10.1016/j.pain.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Arendt-Nielsen L, Egsgaard LL, Petersen KK, Eskehave TN, Graven-Nielsen T, Hoeck HC, et al. A mechanism-based pain sensitivity index to characterize knee osteoarthritis patients with different disease stages and pain levels. Eur J Pain. 2014 doi: 10.1002/ejp.651. [DOI] [PubMed] [Google Scholar]
- 11.Finan PH, Buenaver LF, Bounds SC, Hussain S, Park RJ, Haque UJ, et al. Discordance Between Pain and Radiographic Severity in Knee Osteoarthritis Findings From Quantitative Sensory Testing of Central Sensitization. Arthritis Rheum-Us. 2013;65(2):363–72. doi: 10.1002/art.34646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dillon CF, Rasch EK, Gu Q, Hirsch R. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991–94. J Rheumatol. 2006;33(11):2271–9. [PubMed] [Google Scholar]
- 13.Beswick AD, Wylde V, Gooberman-Hill R, Blom A, Dieppe P. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open. 2012;2(1):e000435. doi: 10.1136/bmjopen-2011-000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lapane KL, Yang S, Driban JB, Liu SH, Dube CE, McAlindon TE, et al. Effects of prescription non-steroidal anti-inflammatory agents on symptoms and disease progression among patients with knee osteoarthritis. Arthritis & rheumatology. 2014 doi: 10.1002/art.38933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madgison J, Vermunt JK. Latent class models for clustering: A comparison with K-means. Canadian Journal of Marketing Research. 2002;(20):37–44. [Google Scholar]
- 16.Nevitt M, Felson D, Lester G. The Osteoarthritis Initiative: Protocol for the Cohort Study 2006 [7/24/15] Available from: https://oai.epi-ucsf.org/datarelease/docs/StudyDesignProtocol.pdf.
- 17.Neogi T, Felson D, Niu J, Nevitt M, Lewis CE, Aliabadi P, et al. Association between radiographic features of knee osteoarthritis and pain: results from two cohort studies. BMJ. 2009;339:b2844. doi: 10.1136/bmj.b2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Creamer P, Lethbridge-Cejku M, Hochberg MC. Factors associated with functional impairment in symptomatic knee osteoarthritis. Rheumatology (Oxford) 2000;39(5):490–6. doi: 10.1093/rheumatology/39.5.490. [DOI] [PubMed] [Google Scholar]
- 20.Felson DT, Goggins J, Niu J, Zhang Y, Hunter DJ. The effect of body weight on progression of knee osteoarthritis is dependent on alignment. Arthritis Rheum. 2004;50(12):3904–9. doi: 10.1002/art.20726. [DOI] [PubMed] [Google Scholar]
- 21.Thorp LE, Sumner DR, Wimmer MA, Block JA. Relationship between pain and medial knee joint loading in mild radiographic knee osteoarthritis. Arthritis Rheum. 2007;57(7):1254–60. doi: 10.1002/art.22991. [DOI] [PubMed] [Google Scholar]
- 22.Hall MC, Mockett SP, Doherty M. Relative impact of radiographic osteoarthritis and pain on quadriceps strength, proprioception, static postural sway and lower limb function. Ann Rheum Dis. 2006;65(7):865–70. doi: 10.1136/ard.2005.043653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Reilly SC, Jones A, Muir KR, Doherty M. Quadriceps weakness in knee osteoarthritis: the effect on pain and disability. Ann Rheum Dis. 1998;57(10):588–94. doi: 10.1136/ard.57.10.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sattler M, Dannhauer T, Hudelmaier M, Wirth W, Sanger AM, Kwoh CK, et al. Side differences of thigh muscle cross-sectional areas and maximal isometric muscle force in bilateral knees with the same radiographic disease stage, but unilateral frequent pain - data from the osteoarthritis initiative. Osteoarthritis Cartilage. 2012;20(6):532–40. doi: 10.1016/j.joca.2012.02.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riddle DL, Stratford PW. Impact of pain reported during isometric quadriceps muscle strength testing in people with knee pain: data from the osteoarthritis initiative. Phys Ther. 2011;91(10):1478–89. doi: 10.2522/ptj.20110034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Curb JD, Ceria-Ulep CD, Rodriguez BL, Grove J, Guralnik J, Willcox BJ, et al. Performance-based measures of physical function for high-function populations. J Am Geriatr Soc. 2006;54(5):737–42. doi: 10.1111/j.1532-5415.2006.00700.x. [DOI] [PubMed] [Google Scholar]
- 27.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29(8):1039–49. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 28.Peat G, Thomas E, Duncan R, Wood L, Hay E, Croft P. Clinical classification criteria for knee osteoarthritis: performance in the general population and primary care. Ann Rheum Dis. 2006;65(10):1363–7. doi: 10.1136/ard.2006.051482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34(1):73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 31.Vanfleteren LE, Spruit MA, Groenen M, Gaffron S, van Empel VP, Bruijnzeel PL, et al. Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine. 2013;187(7):728–35. doi: 10.1164/rccm.201209-1665OC. [DOI] [PubMed] [Google Scholar]
- 32.Bliddal H, Danneskiold-Samsoe B. Chronic widespread pain in the spectrum of rheumatological diseases. Best practice & research Clinical rheumatology. 2007;21(3):391–402. doi: 10.1016/j.berh.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Siemons L, ten Klooster PM, van de Laar MA, van den Ende CH, Hoogeboom TJ. Validity of summing painful joint sites to assess joint-pain comorbidity in hip or knee osteoarthritis. BMC Musculoskelet Disord. 2013;14:234. doi: 10.1186/1471-2474-14-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sale JE, Gignac M, Hawker G. The relationship between disease symptoms, life events, coping and treatment, and depression among older adults with osteoarthritis. J Rheumatol. 2008;35(2):335–42. [PubMed] [Google Scholar]
- 35.Maly MR, Costigan PA, Olney SJ. Determinants of self-report outcome measures in people with knee osteoarthritis. Arch Phys Med Rehabil. 2006;87(1):96–104. doi: 10.1016/j.apmr.2005.08.110. [DOI] [PubMed] [Google Scholar]
- 36.Riddle DL, Kong X, Fitzgerald GK. Psychological health impact on 2-year changes in pain and function in persons with knee pain: data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2011;19(9):1095–101. doi: 10.1016/j.joca.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riddle DL, Jensen MP. Construct and criterion-based validity of brief pain coping scales in persons with chronic knee osteoarthritis pain. Pain Med. 2013;14(2):265–75. doi: 10.1111/pme.12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vlaeyen JW, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain. 2000;85(3):317–32. doi: 10.1016/S0304-3959(99)00242-0. [DOI] [PubMed] [Google Scholar]
- 39.Riddle DL, Wade JB, Jiranek WA, Kong X. Preoperative pain catastrophizing predicts pain outcome after knee arthroplasty. Clin Orthop Relat Res. 2010;468(3):798–806. doi: 10.1007/s11999-009-0963-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Somers TJ, Keefe FJ, Godiwala N, Hoyler GH. Psychosocial factors and the pain experience of osteoarthritis patients: new findings and new directions. Curr Opin Rheumatol. 2009;21(5):501–6. doi: 10.1097/BOR.0b013e32832ed704. [DOI] [PubMed] [Google Scholar]
- 41.Nylund KL, Asparoutiov T, Muthen BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Structural Equation Modeling-a Multidisciplinary Journal. 2007;14(4):535–69. [Google Scholar]
- 42.Knoop J, van der Leeden M, Thorstensson CA, Roorda LD, Lems WF, Knol DL, et al. Identification of phenotypes with different clinical outcomes in knee osteoarthritis: data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken) 2011;63(11):1535–42. doi: 10.1002/acr.20571. [DOI] [PubMed] [Google Scholar]
- 43.Turk DC. A diathesis-stress model of chronic pain and disability following traumatic injury. Pain Res Manag. 2002;7(1):9–19. doi: 10.1155/2002/252904. [DOI] [PubMed] [Google Scholar]
- 44.Forsythe ME, Dunbar MJ, Hennigar AW, Sullivan MJ, Gross M. Prospective relation between catastrophizing and residual pain following knee arthroplasty: two-year follow-up. Pain Res Manag. 2008;13(4):335–41. doi: 10.1155/2008/730951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brander V, Gondek S, Martin E, Stulberg SD. Pain and depression influence outcome 5 years after knee replacement surgery. Clin Orthop Relat Res. 2007;464:21–6. doi: 10.1097/BLO.0b013e318126c032. [DOI] [PubMed] [Google Scholar]
- 46.Brander VA, Stulberg SD, Adams AD, Harden RN, Bruehl S, Stanos SP, et al. Predicting total knee replacement pain: a prospective, observational study. Clin Orthop Relat Res. 2003;(416):27–36. doi: 10.1097/01.blo.0000092983.12414.e9. [DOI] [PubMed] [Google Scholar]
- 47.Haupt TH, Petersen J, Ellekilde G, Klausen HH, Thorball CW, Eugen-Olsen J, et al. Plasma suPAR levels are associated with mortality, admission time, and Charlson Comorbidity Index in the acutely admitted medical patient: a prospective observational study. Critical care. 2012;16(4):R130. doi: 10.1186/cc11434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lundborg C, Hahn-Zoric M, Biber B, Hansson E. Glial cell line-derived neurotrophic factor is increased in cerebrospinal fluid but decreased in blood during long-term pain. Journal of neuroimmunology. 2010;220(1–2):108–13. doi: 10.1016/j.jneuroim.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 49.Lee YC, Lu B, Bathon JM, Haythornthwaite JA, Smith MT, Page GG, et al. Pain sensitivity and pain reactivity in osteoarthritis. Arthritis Care Res (Hoboken) 2011;63(3):320–7. doi: 10.1002/acr.20373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10(9):895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Segal NA, Torner JC, Felson D, Niu J, Sharma L, Lewis CE, et al. Effect of thigh strength on incident radiographic and symptomatic knee osteoarthritis in a longitudinal cohort. Arthritis Rheum. 2009;61(9):1210–7. doi: 10.1002/art.24541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stefanik JJ, Guermazi A, Zhu Y, Zumwalt AC, Gross KD, Clancy M, et al. Quadriceps weakness, patella alta, and structural features of patellofemoral osteoarthritis. Arthritis Care Res (Hoboken) 2011;63(10):1391–7. doi: 10.1002/acr.20528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jefferson RJ, Collins JJ, Whittle MW, Radin EL, O’Connor JJ. The role of the quadriceps in controlling impulsive forces around heel strike. Proceedings of the Institution of Mechanical Engineers Part H, Journal of engineering in medicine. 1990;204(1):21–8. doi: 10.1243/PIME_PROC_1990_204_224_02. [DOI] [PubMed] [Google Scholar]
- 54.Nicholls E, Thomas E, van der Windt DA, Croft PR, Peat G. Pain trajectory groups in persons with, or at high risk of, knee osteoarthritis: findings from the Knee Clinical Assessment Study and the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2014;22(12):2041–50. doi: 10.1016/j.joca.2014.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Collins JE, Katz JN, Dervan EE, Losina E. Trajectories and risk profiles of pain in persons with radiographic, symptomatic knee osteoarthritis: data from the osteoarthritis initiative. Osteoarthritis Cartilage. 2014;22(5):622–30. doi: 10.1016/j.joca.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steffen TM, Hacker TA, Mollinger L. Age- and gender-related test performance in community-dwelling elderly people: Six-Minute Walk Test, Berg Balance Scale, Timed Up & Go Test, and gait speeds. Phys Ther. 2002;82(2):128–37. doi: 10.1093/ptj/82.2.128. [DOI] [PubMed] [Google Scholar]


