Abstract
A growing number of studies have found a link between outdoor air pollution and cognitive function among older adults. Psychosocial stress is considered an important factor determining differential susceptibility to environmental hazards and older adults living in stressful neighborhoods may be particularly vulnerable to the adverse health effects of exposure to hazards such as air pollution. The objective of this study is to determine if neighborhood social stress amplifies the association between fine particulate matter air pollution (PM2.5) and poor cognitive function in older, community-dwelling adults. We use data on 779 U.S. adults ages 55 and older from the 2001/2002 wave of the Americans’ Changing Lives study. We determined annual average PM2.5 concentration in 2001 in the area of residence by linking respondents with EPA air monitoring data using census tract identifiers. Cognitive function was measured using the number of errors on the Short Portable Mental Status Questionnaire (SPMSQ). Exposure to neighborhood social stressors was measured using perceptions of disorder and decay and included subjective evaluations of neighborhood upkeep and the presence of deteriorating/abandoned buildings, trash, and empty lots. We used negative binomial regression to examine the interaction of neighborhood perceived stress and PM2.5 on the count of errors on the cognitive function assessment. We found that the association between PM2.5 and cognitive errors was stronger among older adults living in high stress neighborhoods. These findings support recent theoretical developments in environmental health and health disparities research emphasizing the synergistic effects of neighborhood social stressors and environmental hazards on residents’ health. Those living in socioeconomically disadvantaged neighborhoods, where social stressors and environmental hazards are more common, may be particularly susceptible to adverse health effects of social and physical environmental exposures.
Keywords: cognitive function, neighborhoods, air pollution, psychosocial stress, USA
INTRODUCTION
There are significant social, health, and economic costs of cognitive impairment to older adults, their families, and society. Older adults with poor cognitive functioning are at greater risk of poor physical and mental health outcomes (Frisoni et al. 2000; Yaffe et al. 1999) and increased risk of dementia, a disabling condition associated with high caregiving burden and cost (Langa et al. 2001; Hurd et al. 2013). Efforts to identify modifiable risk factors associated with cognitive impairment and decline have largely focused on the role of individual-level risk factors, such as education, smoking, physical activity, and diet (Beydoun et al. 2014). An increasing number of studies have also identified neighborhood-level physical and social stressors that may have an adverse impact on cognitive health in older adults. There is growing evidence, for instance, that older adults living in areas with higher concentrations of outdoor air pollution have worse cognitive function and are at greater risk of cognitive decline (Power et al. 2016). Several studies have also linked neighborhood social stressors, such as poverty and disorder, to poor cognitive function (Wu, Prina, and Brayne 2014).
Neighborhood social and environmental stressors, such as socioeconomic disadvantage and air pollution, tend to cluster together geographically (Hajat et al. 2013) and may interact with one another. Acknowledging the potential interrelationships between social and physical environmental hazards, several researchers have called for increased attention to identifying the synergistic effects of neighborhood social and physical environmental factors on health (Clougherty and Kubzansky 2009; Gee and Payne-Sturges 2004; McEwen and Tucker 2011; Morello-Frosch and Shenassa 2006; Wright and Steinbach 2001). Gee and Payne-Sturgis (2004) have proposed a conceptual framework for integrating social and environmental exposures that acknowledges psychosocial stress as a “vulnerability factor” linking social conditions and environmental hazards. Community-based social stressors, such as perceptions of disorder and decay, may be considered susceptibility factors because exposure to these stressors often translates into individual psychosocial stress (Hill, Ross, and Angel 2005). Exposure to psychosocial stressors can lower the brain’s threshold for neurotoxicity (Lupien et al. 2009), which may increase risk for neurodegeneration and compromised cognitive function from environmental toxicants such as air pollution. Yet, there is little empirical research examining neighborhood social stressors as a vulnerability factor in the relationship between environmental hazards and cognitive function. One prior study found that living in stressful neighborhoods amplified the adverse influence of environmental lead exposure on cognitive function (Glass et al., 2009). To our knowledge, however, no study to date has examined whether the relationship between air pollution and cognitive function is stronger among those living in more stressful neighborhoods.
We used data on adults ages 55 and older from the Americans’ Changing Lives Study, a national, population-based sample of community-dwelling adults, to investigate the role of neighborhood stress in the association between outdoor residential air pollution and cognitive function among older adults. Prior research using ACL data demonstrated a link between fine particulate matter air pollution (PM2.5) and cognitive function in older adults (Ailshire and Clarke 2015). The current study builds on this work by determining if the adverse association of PM2.5 with cognitive function is stronger among older adults living in more stressful neighborhoods. Understanding how community-based social stressors and environmental hazards interact to influence health may help to identify individuals and communities that are at increased risk for poor cognitive functioning.
BACKGROUND
The accumulating evidence suggests ambient air pollution, especially particulate matter, can have adverse consequences for cognitive function among older adults. Fine particulate matter (PM2.5) is an air pollutant consisting of small, inhalable particles with aerodynamic diameters less than 2.5 microns (μm) that are produced primarily from combustion and industrial sources. PM2.5 is of particular interest for understanding air pollution effects on the aging brain because it is ubiquitous in the air we breath and, once inhaled, fine particles can pass into systemic circulation, leading to increased inflammation, and may ultimately translocate from the lungs into other organ systems such as the brain (Heusinkveld et al. 2016; Peters et al. 2015) where they can cause damage and pathophysiological changes consistent with cognitive decline and impairment. A link between PM2.5 and cognitive function has been reported in several recent studies of older adults. For instance, prior research using cross-sectional data has found worse cognitive function among older adults living in areas with higher concentrations of PM2.5 (Ailshire and Crimmins 2014; Ailshire and Clarke 2015; Gatto et al. 2014; Ranft et al. 2009; Schikowski et al. 2015). The pollution-cognition relationship has also been demonstrated in longitudinal data, with two studies finding an association between PM2.5 and greater cognitive decline in older adults (Weuve et al. 2012; Tonne et al. 2014).
Research in animals suggests there are neurodegenerative effects of exposure to particulate matter air pollution. Prior studies have found associations between exposure to high levels of ambient air pollution and increased brain inflammation and accumulation of beta-amyloid, which is implicated in the pathogenesis of Alzheimer’s Disease (Calderón-Garcidueñas et al. 2012; Levesque et al. 2011). Researchers have also linked air pollutant-induced inflammation and neurodegeneration to cognitive deficits in humans (Calderón-Garcidueñas et al. 2008). These studies examined the effects of a high dosage of particulate matter exposure, much higher than the concentrations of ambient PM2.5 typically found in the United States. However, chronic exposure to even small doses of a pollutant may increase risk for neurodegeneration as individuals age (Bandyopadhyay 2016). Two recent U.S. studies with neuroimaging data on older adults found an association between living in areas with higher PM2.5 concentrations and changes in brain structure, including white matter loss (Chen et al. 2015) and reduced cerebral volume (Wilker et al. 2015).
Neighborhood social stressors have also been linked to differential risk for poor cognitive functioning among community-dwelling older adults. Prior research has found that older U.S. adults living in socioeconomically disadvantaged neighborhoods, where social stressors may be more common, have poorer cognitive function (Aneshensel et al. 2011; Wight et al. 2006) and experience faster rates of cognitive decline (Sheffield and Peek 2009). Exposure to neighborhood deprivation, which includes social and economic stressors such as housing affordability and crime, has also been linked to worse cognitive function among older adults in England (Lang et al. 2008). Although two studies of older adults living in Chicago, IL found no association between poor neighborhood social conditions and cognitive function, net of individual-level characteristics, (Clarke et al. 2011; Clarke et al. 2015) those exposed to worse social environments did experience a faster rate of cognitive decline over time. Exposure to neighborhood social stressors may induce a psychosocial stress response that puts individuals at increased risk of experiencing levels of neurodegeneration commonly observed among those with cognitive impairment.
Perceptions of neighborhood problems, such as disorder and decay, can be a source of psychological distress for residents (Steptoe and Feldman 2001), and chronic exposure to neighborhood stressors may cause a physiological response that results in dysregulation of the systems responsible for the production of stress hormones (Taylor, Repetti, and Seeman 1997). As the “key target organ” for stress, the brain is of particular interest in the study of the impacts of psychosocial stressors on health (McEwen and Tucker 2011). The body responds to stress through a series of neural and endocrine reactions that protect the body and promote adaptation. Physiological responses to stress include chronic activation of the hypothalamic-pituitary-adrenal (HPA) axis and immune system, which can culminate in overproduction of glucocorticoid hormones (e.g., cortisol) and cytokines (McEwen and Tucker 2011). Overproduction of glucocorticoids and cytokines has been linked to damage to the structure and function of the brain consistent with memory impairment and decreased cognitive function (Lupien et al. 2009). Chronic activation of the stress response can also increase risk for hardening of arteries and chronic hypertension, cardiovascular disease risk factors that increase risk for cognitive impairment (Nash and Fillit 2006). In addition, it has long been hypothesized that prolonged exposure to stress hormones can increase neuronal susceptibility to insults, which can accelerate the rate of neuronal damage from exposure to toxicants (McEwen et al. 1992). Thus, the extent of damage to the brain from environmental toxicants such as air pollution may be amplified by exposure to neighborhood social stressors.
Although Gee and Payne-Sturgis (2004) and others have called for an integration of social and environmental exposures in health research, there are few existing studies examining the potential compounding effects of neighborhood social stress and environmental hazards on health. Gee and Takeuchi (2004) examined the interaction between perceived traffic stress in the neighborhood and a measure of vehicular burden and found worse physical and mental health among those living in neighborhoods with more cars on the road and who reported the most traffic-related stress. Only one study, to our knowledge, has examined the effects of neighborhood social stressors and environmental hazards on cognitive function in older adults. Using data on older adults from the Baltimore Memory Study, Glass and colleagues (2009) found that the presence of neighborhood stressors exacerbated the adverse association of environmental lead exposure with cognitive function. The aim of the current study is to determine if neighborhood stressors amplify the association between outdoor air pollution and cognitive function in older adults.
MATERIALS AND METHODS
Data
Individual-level data come from the Americans’ Changing Lives (ACL) survey. The ACL began in 1986 as a nationally representative study of non-institutionalized U.S. adults ages 25 and older. The study sample was obtained using a stratified, multistage area probability sample with oversampling of black adults and adults ages 60 and older. Follow-up interviews were conducted with respondents in 1989, 1994, 2001/2002, and 2011/2012. We use the 2001/2002 survey wave because it was the first wave of data collection to occur after wide spread national monitoring of PM2.5 began in the late 1990’s. The 2001/2002 follow-up interview was conducted via telephone, or in face-to-face interviews when necessary, with 1,787 respondents or their proxies (n=95), representing 74 percent of the surviving baseline sample.
We limit the sample to 1,077 respondents who were ages 55 years or older at the time of the survey, the age group with the highest risk of having poor cognitive function (Rönnlund et al. 2005). We omitted 87 respondents whose proxy completed the survey and therefore did not take the cognitive assessment. We also excluded respondents who moved to their current residence after 2000 (n=45), the year in which pollution was measured. We further excluded Hispanics (n=23) and those who did not identify as white or black (n=16) due to the small size of these groups, and any respondents with missing data on model covariates (n=20). In addition, 107 respondents did not live close enough to an air monitoring station to obtain data on local area pollution concentrations. Respondents who were excluded for lack of pollution data did not differ from the analytic sample on cognitive function, but were more likely to have a high school level education and less likely to be female (see Appendix Table 1). The final analytic sample consisted of 779 adults ages 55 and older.
Appendix Table 1.
Logistic Regression of Missing Monitor Data on Respondent Characteristics (n=886)
| Missing = 1
|
||
|---|---|---|
| OR | (95% CI) | |
| Cognitive Errors | 0.94 | (0.68, 1.30) |
| Age | 1.01 | (0.99, 1.04) |
| Female | 0.57* | (0.34, 0.95) |
| Black | 1.22 | (0.66, 2.25) |
| Education | ||
| College (ref) | ||
| High School | 1.90* | (1.04, 3.46) |
| Less than High School | 1.75 | (0.89, 3.45) |
| Constant | 0.06 | (0.01, 0.30) |
Note: 107 respondents could not be matched to an EPA monitor.
p<.05
The air pollution data come from the Environmental Protection Agency’s Air Quality System (AQS) and were obtained from the RAND Center for Population Health and Health Disparities Data Core Series (Escarce, Lurie, and Jewell 2011). For the purpose of this study we focus on 2000 annual average concentration of PM2.5. Air pollution data was linked to ACL respondents using census tract identifiers derived from geocoding respondent addresses in 2001/2002. We also linked tract-level 2000 Census measures of neighborhood-level socioeconomic characteristics to ACL respondents. Respondents resided in 548 census tracts across 38 states representing urban areas, suburbs, and small towns and rural areas.
Measures
Cognitive function
The ACL uses an abbreviated form of the Short Portable Mental Status Questionnaire (SPMSQ), which has been shown to be a reliable and valid instrument for differentiating older adults who are cognitively intact from those experiencing some degree of impairment (Pfeiffer 1975). The SPMSQ consists of a serial 3’s subtraction test to measure working memory and recall of the date, day of the week, and name of the president and vice-president to measure orientation. In the serial 3’s subtraction test respondents were asked to subtract from 20 by 3’s for a total of six subtractions. We created a score ranging from 0 to 5 representing the number of errors on the cognitive function assessment by summing the number of incorrect responses on the four orientation items (date, day of the week, president’s name, and vice president’s name) and whether any error was made in the subtraction test.
Fine Particulate Matter Air Pollution
We use a measure of annual average PM2.5 derived from AQS monitoring sites within a 60km radius of the respondent’s census tract centroid. It has been reported previously that the spatial distribution of fine particles is fairly uniform, with several studies finding values of PM2.5 recorded at local monitoring sites to be in good agreement with values recorded at individual residences (Monn 2001). Tract-level PM2.5 derived from local air monitors should, therefore, provide a reasonable approximation of concentrations at individual residences. Importantly, PM2.5 concentrations derived only from monitors can miss variation in particulate matter from roadway sources and ambient PM2.5 will be measured more accurately in areas where there is a denser network of monitors than in areas with a sparse network.
An annual average measure of PM2.5 was derived for each census tract in the U.S. from daily air quality monitoring reports provided by the national air monitoring network. Monitor-specific quarterly measures of PM2.5 were derived by computing an unweighted average of daily PM2.5 concentration on each day for which a 24-hour daily mean was recorded. Daily values were then aggregated to create a monitor-specific annual average. The annual average PM2.5 concentration for each census tract was interpolated at the census tract centroid using all available data from any monitors within the 60km radius. Values were interpolated using inverse distance weighting. Monitor-specific values were multiplied by the inverse distance function (1/distance from a tract centroid to a monitor), summed across all monitors within the 60km radius, and then divided by the distance function. We centered the PM2.5 measure around its sample mean.
Neighborhood Stress
Respondent reports of signs of neighborhood disorder and decay are used to characterize the level of neighborhood stress. Our measure of perceived neighborhood disorder is derived from a three-item scale consisting of respondent ratings of the physical conditions of their neighborhood. These neighborhood conditions have been used in prior studies to assess neighborhood disorder and decay (Ross and Mirowsky 1999) and reflect psychosocial stressors in the neighborhood environment (Steptoe and Feldman 2001). A neighborhood was defined by the interviewer as the area around the respondent’s residence where they engage in routine activities such as visiting with neighbors, taking a walk, shopping, or going to the park. Respondents were first asked to rate the general upkeep of their neighborhood (very good, good, adequate, poor, or very poor). They were then asked about problems they see or experience within a half mile (or about a ten minute walk) from their residence. Respondents were asked how many deteriorating or abandoned businesses and factories they see (a lot, some, a few, or none) and how much litter or trash they see in the streets, empty lots, or in properties (a lot, some, a few, or none). We reverse coded responses to the last two items so that high values represent greater levels of neighborhood stress and averaged responses across the three items (α = .63). We then created a dichotomous measure indicating high levels of neighborhood stress that was defined as having a neighborhood stress value greater than one standard deviation above the scale mean. A high-stress neighborhood is characterized as having poor upkeep and a lot of trash and deteriorating buildings.
Covariates
Individual sociodemographic characteristics include age, gender, race, education, income, marital status, and length of time at residence. Age is measured in years. We use dichotomous indicators for female gender and black race. The respondent’s highest year of educational attainment reported at the baseline (1986) interview is categorized to distinguish among those with less than 12 years of education, those with exactly 12 years of education, and those with more than 12 years of education. Annual household income is the total reported pre-tax annual income of the respondent and his/her spouse/partner. We created a dichotomous income variable where 1 = income below the sample median of $35,000 and 0 = income above the median. Marital status is a dichotomous variable where 1 = not married and 0 = married. Current employment status is a three-category variable distinguishing among those who are currently working, retired, and unemployed. We include a measure of the total number of years lived at the current residence to account for differences in length of exposure to neighborhood conditions.
We also include tract-level measures of neighborhood socioeconomic disadvantage and affluence. Neighborhood socioeconomic disadvantage is a standardized scale (α = .91) consisting of three items: percent of households on public assistance, percent of persons with below poverty level income, and the adult unemployment rate. Neighborhood affluence is a standardized scale (a = .88) consisting of two items: the percent of adults ages 25 and older with at least 16 years of education and the percent of professionals and managers.
Analytic Strategy
We first conducted bivariate analyses using Chi squared tests to examine differences in individual and neighborhood characteristics between individuals living in low versus high stress neighborhoods. We then estimated a series of negative binomial models to determine if the association between PM2.5 and cognitive function is modified by level of neighborhood stress. An inspection of the cognitive function variable showed that the variance exceeded the mean (63% of respondents had zero errors on the cognitive function assessment), suggesting the data are over-dispersed. The likelihood ratio test suggested the negative binomial model is more appropriate than the Poisson model for the outcome. There was minimal tract-level clustering in our analytic sample, 1.4 respondents per tract, so we used single-level regression models. Since standard errors can be biased downward in single-level regression models (Clarke 2008) we use a robust variance estimate that adjusts the standard errors for within-cluster correlation.
In multivariate models, we first examined the associations of PM2.5 (Model 1) and both PM2.5 and neighborhood stress (Model 2) on cognitive function. We then added controls for individual demographic and socioeconomic characteristics, length of residence, and neighborhood socioeconomic context (Model 3). Next, we examined the potential for effect modification by including a term for the interaction between PM2.5 and neighborhood stress (Model 4). As a robustness check, in the last model (Model 5) we account for differential health status by including measures of obesity (BMI>30), smoking status (current, former, never), physical activity (scale of frequency of light, moderate, and vigorous activity), and an index of 5 chronic conditions (hypertension, heart disease, cancer, diabetes, lung disease). We report the chi square difference test comparing the current model to the null model to assess model fit. Sample weights are used in all analyses to adjust for differential sampling probabilities and survey non-response. Analyses were conducted using STATA version 13.1.
RESULTS
Table 1 shows descriptive statistics for the sample. The mean number of errors on the cognitive function assessment was 0.52 (about 36.5% of respondents made at least one error on the assessment and the mean number of errors among those who made any errors was 1.42). Respondents were 68 years of age on average. Women constituted 61% of the sample and 10% of respondents were black. Most respondents reported having at least 12 years of education, with only 22% reporting less than 12 years of education. Just over half of respondents had an annual household income below $35,000. A little more than one-third of respondents reported not being married and most respondents were not employed. On average, respondents lived at their residence for nearly 23 years. The average PM2.5 concentration in areas where respondents lived was 13.8μ/m3. The national ambient air quality standard, which is determined by the EPA to be the level at which there is increased risk to human health, is 12 μg/m3 for PM2.5. Thus, the majority of ACL respondents lived in areas where PM2.5 exceeds the air quality standard. Only 12% of respondents lived in a high stress neighborhood. Neighborhood disadvantage and affluence are standardized scales and therefore have a mean near zero and standard deviation of about 1.
Table 1.
Sample Characteristics, ACL (2001/2002) Age 55+ (N = 779)
| Variables | Mean (s.d.) | Range | % |
|---|---|---|---|
| Individual-level Variables | |||
| Cognitive Errors | 0.52 (0.80) | [0 – 5] | |
| Age, years | 67.94 (9.81) | [55 – 98] | |
| Female | 61% | ||
| Black | 10% | ||
| Educational attainment | |||
| Less than High School | 22% | ||
| High School | 37% | ||
| More than High School | 41% | ||
| Household Income < $35,000 | 51% | ||
| Not Currently Married | 36% | ||
| Employment Status | |||
| Employed | 33% | ||
| Unemployed | 21% | ||
| Retired | 46% | ||
| Length of Residence | 22.68 (15.0) | [2 – 84] | |
| Neighborhood-level Variables | |||
| PM2.5 (μg/m3) | 13.78 (3.13) | [4.5 – 24.2] | |
| High Stress | 12% | ||
| Socioeconomic Disadvantage | −0.21 (0.68) | [−0.98 − 4.96] | |
| Affluence | −0.00 (0.91) | [−1.47 − 4.82] | |
s.d. = standard deviation PM2.5 = fine particulate matter
Differences in individual and neighborhood characteristics by level of perceived neighborhood stress are shown in Table 2. The number of errors on the cognitive function assessment did not differ statistically by reported level of neighborhood stress. Age was similar across neighborhood stress levels. Slightly more women reported living in high stress neighborhoods (62%) compared to low stress neighborhoods (61%). Black respondents were much more likely to report living in high stress than low stress neighborhoods (22% vs. 9%). Similarly, those with low education and income were more likely to report living in high stress neighborhoods. Those reporting living in high stress neighborhoods lived in their homes longer than those who perceived their neighborhoods to be less stressful. There was no difference in PM2.5 across neighborhoods. Those reporting high levels of neighborhood stress lived in neighborhoods that had greater than average levels of neighborhood socioeconomic disadvantage and less than average levels of neighborhood affluence.
Table 2.
Differences in Individual and Neighborhood Characteristics by Level of Neighborhood Stress, ACL (2001/2002) Age 55+ (N = 779)
| Neighborhood Stress
|
||
|---|---|---|
| Low | High | |
| Individual-level Variables | ||
| Cognitive Errors, mean score | 0.51 | 0.61 |
| Age, mean years | 68.0 | 67.7 |
| Female, % | 61 | 62* |
| Black, % | 9 | 22*** |
| Education less than 12 years, % | 20 | 33* |
| Household Income < $35,000, % | 49 | 62† |
| Not Currently Married, % | 35 | 40 |
| Unemployed/retired, % | 67 | 70 |
| Length of Residence | 22.3 | 25.7† |
| Neighborhood-level Variables | ||
| PM2.5 (μg/m3), mean | 13.8 | 13.7 |
| Socioeconomic Disadvantage, mean | −0.27 | 0.25*** |
| Affluence, mean | 0.04 | −0.34** |
p<.001;
p<.01;
p<.05;
p<.10; differences assessed using Chi square test;
PM2.5 = fine particulate matter
Results from negative binomial regression models are shown in Table 3. Incidence rate ratios (IRRs) are presented with 95% confidence intervals in parentheses. IRRs between zero and one indicate a lower error rate on the cognitive function assessment, and ratios greater than one indicate higher error rates. Model 1, which examines the main effect of PM2.5, shows a 1 − μg/m3 increase in PM2.5 was associated with an increased error rate of 1.05 (p<.05; 95% CI, 1.01–1.10). The point estimate for PM2.5 is unchanged with the inclusion of neighborhood stress in Model 2. There is a positive error rate associated with living in a high stress neighborhood, but the association is not statistically significant. The error rate for PM2.5 is slightly attenuated (IRR=1.04, p<0.10); 95% CI, 1.00–1.08) and only marginally statistically significant when controls are added for individual demographic and socioeconomic characteristics and neighborhood socioeconomic context in Model 3.
Table 3.
Negative Binomial Regression of Count of Errors on Cognitive Function, ACL (2001/2002) Age 55+ (N = 779)
| Model 1
|
Model 2
|
Model 3
|
Model 4
|
Model 5
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IRR | (95% CI) | IRR | (95% CI) | IRR | (95% CI) | IRR | (95% CI) | IRR | (95% CI) | |
| PM2.5 | 1.05 * | (1.01,1.10) | 1.05 * | (1.01,1.10) | 1.04 + | (1.00,1.08) | 1.03 | (0.98,1.07) | 1.03 | (0.99,1.07) |
| High Neighborhood Stress | 1.20 | (0.84,1.72) | 0.90 | (0.65,1.25) | 0.86 | (0.61,1.21) | 0.86 | (0.62,1.19) | ||
| PM2.5*High Neighborhood Stress | 1.09 * | (1.01,1.18) | 1.09 * | (1.00,1.18) | ||||||
| Age | 1.02 ** | (1.01,1.03) | 1.02 ** | (1.01,1.03) | 1.01 + | (1.00,1.03) | ||||
| Female | 0.95 | (0.73,1.23) | 0.97 | (0.75,1.26) | 0.97 | (0.74,1.27) | ||||
| Black | 1.67 *** | (1.29,2.16) | 1.68 *** | (1.30,2.17) | 1.66 *** | (1.27,2.17) | ||||
| Education | ||||||||||
| College (ref) | 1.00 | 1.00 | 1.00 | |||||||
| High School | 1.61 ** | (1.16,2.23) | 1.60 ** | (1.15,2.21) | 1.60 ** | (1.15,2.21) | ||||
| Less than High School | 2.14 *** | (1.49,3.08) | 2.14 *** | (1.49,3.08) | 2.19 *** | (1.54,3.11) | ||||
| Household Income < $35,000 | 1.39 * | (1.02,1.88) | 1.40 * | (1.03,1.90) | 1.38 * | (1.01,1.87) | ||||
| Not Currently Married | 1.00 | (0.75,1.32) | 1.01 | (0.76,1.34) | 1.03 | (0.78,1.36) | ||||
| Employment Status | ||||||||||
| Employed (ref) | 1.00 | 1.00 | 1.00 | |||||||
| Unemployed | 1.34 | (0.92,1.96) | 1.33 | (0.91,1.93) | 1.33 | (0.91,1.94) | ||||
| Retired | 1.35 | (0.94,1.92) | 1.33 | (0.93,1.90) | 1.32 | (0.92,1.90) | ||||
| Length of Residence | 0.99 + | (0.99,1.00) | 0.99 + | (0.99,1.00) | 0.99 | (0.99,1.00) | ||||
| Neighborhood Socioeconomic Context | ||||||||||
| Disadvantage | 0.93 | (0.80,1.08) | 0.92 | (0.79,1.06) | 0.95 | (0.82,1.09) | ||||
| Affluence | 0.80 * | (0.66,0.96) | 0.80 * | (0.67,0.96) | 0.79 * | (0.66,0.95) | ||||
| Constant | 0.52 *** | (0.45,0.59) | 0.51 *** | (0.44,0.58) | 0.06 *** | (0.02,0.15) | 0.06 *** | (0.02,0.14) | 0.09 *** | (0.03,0.28) |
|
| ||||||||||
| α (over dispersion parameter) | 0.46 | 0.45 | 0.03 | 0.02 | 0.01 | |||||
| Log Likelihood | −611.31 | −610.81 | −554.41 | −552.94 | −548.92 | |||||
| Likelihood Ratio Test χ2 | 5.71* | 6.73* | 159.14*** | 172.91*** | 190.71*** | |||||
p<.001;
p<.01;
p<.05;
p<.10;
PM2.5= fine particulate matter
Model 4 adds the interaction between PM2.5 and neighborhood stress. The interaction term can be interpreted as the increased error rate associated with a 1 − μg/m3 change in PM2.5 from a low stress to a high stress neighborhood. The interaction term is significant (p<.05) and indicates that the positive association between PM2.5 and cognitive function is stronger among those living in high stress neighborhoods. Finally, we examined the robustness of the results after adjusting for additional health and behavioral factors that may lead to poor cognitive function. As shown in Model 5, the findings were unchanged with additional covariate adjustments.
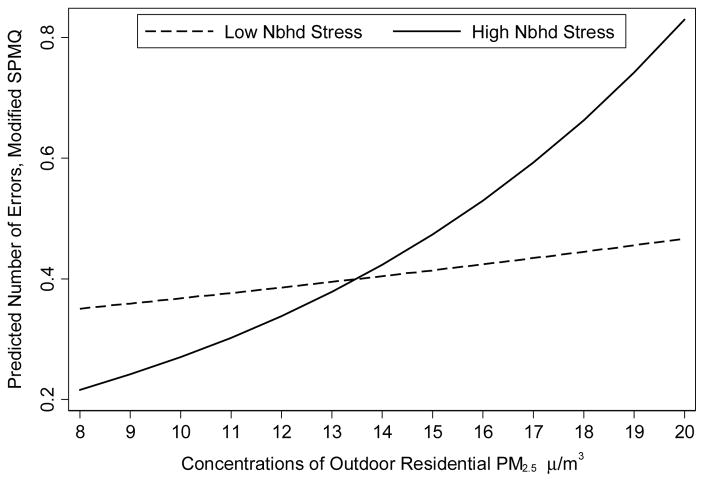
We plotted the interaction between air pollution and neighborhood stress in Figure 1. Using the estimation results from Model 4, we generated predicted values for the number of errors on the cognitive assessment for a white woman aged 70 with mean values on all other variables at different concentrations of PM2.5 and by level of perceived neighborhood stress. The predicted number of errors are plotted for concentrations of PM2.5 ranging from 8 to 20 μg/m3 (range represents approximately 95% of data). The predicted number of errors increases only slightly with increasing concentrations of PM2.5 and residence in a low stress neighborhood. This association is much stronger, however, among those living in a high stress neighborhood.
Figure 1.
Predicted Number of Errors on Cognitive Assessment by PM2.5 and Neighborhood Stress
Predicted number of errors on the modified SPMQ calculated using estimates derived from Table 3, Model 4. Predicted values shown for low neighborhood stress (dotted line) and high neighborhood stress (solid line).
CONCLUSIONS
This is the first study to evaluate whether the association of PM2.5 with cognitive function is modified by exposure to neighborhood stress. We found an inverse association between PM2.5 and cognitive function, which is consistent with what has been found in previous studies (Wueve et al. 2012; Tonne et al. 2014). However, our findings indicate the positive association between PM2.5 and cognitive function is stronger among those who are also exposed to stressful neighborhood conditions. Importantly, our findings are robust to the inclusion of a variety of socioeconomic and demographic factors, indicating that the observed differences in cognitive function are not explained by differences in these individual and community-level characteristics. Interactive effects between neighborhood stressors and environmental toxicants have previously been shown in research on lead exposure and cognitive function in older adults (Glass et al. 2009). The current study provides additional evidence for the interaction between neighborhood social stressors and environmental hazards on adult cognitive function.
This study also highlights the importance of examining the joint exposure to physical hazards and social stressors. Prior studies on air pollution and cognitive function have adjusted for measures of neighborhood socioeconomic environment, finding that inclusion of these measures does not influence the association between air pollution and cognition, but have not examined the interaction with pollution. Although social stressors may be a confounder in the pollution-cognition association, Gee and Payne-Sturges (2004) suggest exposure to social stressors increases individual susceptibility to the adverse health effects of pollutants and, therefore, represents an important vulnerability factor. Direct measurement of neighborhood stress may be particularly important. Prior studies have primarily included adjustment for socioeconomic context, but neighborhood psychosocial stressors, such as perceived disorder and decay, may have different implications for the association between pollution and cognitive function.
There are several plausible explanations for why individuals exposed to social stressors would experience worse health outcomes from air pollution exposure. Chronic exposure to social stressors can increase susceptibility to toxicants like air pollution by causing illness and disease that make it harder for the body to cope with such external challenges (McEwen and Tucker 2011). In addition, individuals exposed to stress may breathe more deeply, increasing their total exposure to outdoor air pollution. Prior research has found increased stress exposure in animals to be associated with increased respiration, suggesting an increased susceptibility to fine particulate air pollution (Clougherty and Kubzansky 2010). Finally, the neurotoxicity hypothesis suggests that prolonged exposure to stress can increase the rate at which neurons are damaged by toxic challenges (McEwen et al. 1992). Older adults living in neighborhoods in which they perceive stressful conditions, such as signs of disorder and decay, may be more susceptible to external insults to the brain, such as that posed by exposure to outdoor air pollution.
This study highlights potentially important interactions between the social and physical environment on cognition, but it has several limitations. First, the generalizability of our findings may be limited to a somewhat more cognitively intact, community-dwelling older adult population. Respondents who survived to the 2001/2002 interview are likely healthier, and possibly more cognitively intact, than the average older adult. In addition, cognitive function was not assessed among respondents who had proxy-assisted interviews, a group of older adults who may be at greater risk of poor cognitive function. In addition, respondents living in rural areas and small towns were more likely to be excluded from the analyses because we could not ascertain PM2.5 exposure for these individuals.
The ACL cognitive function measure, an abbreviated SPMSQ, also has some limitations. The SPMSQ lacks some of the assessments used in other national surveys of older adults that capture variation in cognitive function and impairment, such as word recall tasks that assess memory. The measure used in this study assesses working memory and orientation and deficits in these areas may reflect cognitive loss (Herzog and Wallace 1997)(Ashford et al. 1990). Nevertheless, it may be difficult to compare findings from this study with other studies that use more extensive cognitive screens (e.g., (Ailshire and Crimmins 2014; Weuve et al. 2012).
Although neighborhood stress was assessed using respondent reports of signs of neighborhood disorder and decay, we did not directly measure the perceived stressfulness of the neighborhood. Seeing signs of disorder and decay may not be a source of psychosocial stress for all neighborhood residents. However, the fact that respondents report seeing these signs suggests that they are likely to be bothered by the level of disorder and decay in their neighborhood. Furthermore, there is evidence that subjective assessments of neighborhood conditions have implications for physical and mental health (Wen, Hawkley, and Cacioppo 2006; Ross and Mirowsky 2001). Future research should examine both the presence of neighborhood stressors and the extent to which individuals are bothered or upset by their living conditions in the association between air pollution and cognitive function in older adults.
An additional limitation is that our measure of ambient residential air pollution may not capture total individual exposure to particulate matter. Although outdoor pollution is an important contributor to total exposure, exposure can also occur in other contexts, such as in the workplace or during daily commutes. Most of the sample was not working, however, so outdoor residential pollution concentrations may be a good approximation of their total exposure. Although prior research has found a high correlation between concentrations of outdoor PM2.5 and both indoor and personal PM2.5 (Monn 2001), our measure of ambient PM2.5 does not account for distance to the nearest major road and thus may not capture the full extent of particulate matter exposure from traffic sources. In addition, PM2.5 exposure may not be measured as reliably for respondents living in tracts with fewer reporting monitors within 60km.
Furthermore, we do not capture long-term, or lifetime, pollution exposure. Our measure may, however, reflect some degree of longer-term air pollution because pollution concentrations are highly correlated over time (Weuve et al. 2012) and many respondents reported living in the same neighborhood for at least a decade. This study was cross-sectional and we were, therefore, unable to determine if neighborhood stressors and air pollution interact to influence cognitive decline over time. Prior studies have found an association between pollution and cognitive decline (Tonne et al. 2014; Weuve et al. 2012) and future work should determine if this decline is dependent on other aspects of the residential environment such as concurrent exposure to neighborhood stressors. Finally, long-term exposure to individual-level stressors related to employment histories and lifetime socioeconomic disadvantage have been linked to compromised cognitive function in later life (Leist et al. 2013; Lyu and Burr 2015). Although we did not directly account for these exposures, we did adjust for household income and education, as well as neighborhood socioeconomic context, which may be an indirect proxy for lifetime stress.
This study highlights the importance of accounting for differential susceptibility in examination of risk factors for cognitive health and in assessing the health impacts of air pollution. This study provides evidence that neighborhood social stressors and environmental hazards operate synergistically to influence health and that social stressors should be considered a susceptibility factor in environmental health research. Examination of both social and physical environmental conditions, as well as their interaction, may offer important insights into the development of health disparities (Gee and Payne Sturges 2004). Differential exposure to physical and social stressors, which are common in low socioeconomic status and minority populations, may play an important role in producing and maintaining health disparities. This study identifies a potentially vulnerable population, those exposed to both social stressors and physical hazards, who may be at increased risk of adverse health consequences from air pollution exposure as well as cognitive aging.
Research Highlights.
Living in more polluted neighborhoods is associated with worse cognitive function
Neighborhood stress amplifies the association between PM2.5 and cognitive function
Older residents of stressful and polluted neighborhoods are a vulnerable population
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ailshire Jennifer A, Clarke Philippa. Fine Particulate Matter Air Pollution and Cognitive Function Among U.S. Older Adults. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2015;70(2):322–28. doi: 10.1093/geronb/gbu064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ailshire Jennifer A, Crimmins Eileen M. Fine Particulate Matter Air Pollution and Cognitive Function Among Older US Adults. American Journal of Epidemiology. 2014 Jun;:kwu155. doi: 10.1093/aje/kwu155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aneshensel Carol S, Ko Michelle J, Chodosh Joshua, Wight Richard G. The Urban Neighborhood and Cognitive Functioning in Late Middle Age. Journal of Health and Social Behavior. 2011;52(2):163–79. doi: 10.1177/0022146510393974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashford JW, Kolm P, Colliver JA, Bekian C, Hsu LN. Alzheimer Patient Evaluation and the Mini-Mental State: Item Characteristic Curve Analysis. Journal of Gerontology. 1989;44(5):P139–146. doi: 10.1093/geronj/44.5.p139. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay Amitava. Neurological Disorders from Ambient (Urban) Air Pollution Emphasizing UFPM and PM2.5. Current Pollution Reports. 2016;2(3):203–11. doi: 10.1007/s40726-016-0039-z. [DOI] [Google Scholar]
- Beydoun May A, Beydoun Hind A, Gamaldo Alyssa A, Teel Alison, Zonderman Alan B, Wang Youfa. Epidemiologic Studies of Modifiable Factors Associated with Cognition and Dementia: Systematic Review and Meta-Analysis. BMC Public Health. 2014;14:643. doi: 10.1186/1471-2458-14-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderón-Garcidueñas Lilian, Kavanaugh Michael, Block Michelle, D’Angiulli Amedeo, Delgado-Chávez Ricardo, Torres-Jardón Ricardo, González-Maciel Angelica, et al. Neuroinflammation, Hyperphosphorylated Tau, Diffuse Amyloid Plaques, and down-Regulation of the Cellular Prion Protein in Air Pollution Exposed Children and Young Adults. Journal of Alzheimer’s Disease: JAD. 2012;28(1):93–107. doi: 10.3233/JAD-2011-110722. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas Lilian, Mora-Tiscareño Antonieta, Ontiveros Esperanza, Gómez-Garza Gilberto, Barragán-Mejía Gerardo, Broadway James, Chapman Susan, et al. Air Pollution, Cognitive Deficits and Brain Abnormalities: A Pilot Study with Children and Dogs. Brain and Cognition. 2008;68(2):117–27. doi: 10.1016/j.bandc.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Chen Jiu-Chiuan, Wang Xinhui, Wellenius Gregory A, Serre Marc L, Driscoll Ira, Casanova Ramon, McArdle John J, Manson JoAnn E, Chui Helena C, Espeland Mark A. Ambient Air Pollution and Neurotoxicity on Brain Structure: Evidence from Women’s Health Initiative Memory Study. Annals of Neurology. 2015;78(3):466–76. doi: 10.1002/ana.24460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke Philippa. When Can Group Level Clustering Be Ignored? Multilevel Models versus Single-Level Models with Sparse Data. Journal of Epidemiology and Community Health. 2008;62(8):752–58. doi: 10.1136/jech.2007.060798. [DOI] [PubMed] [Google Scholar]
- Clarke Philippa J, Ailshire Jennifer A, House James S, Morenoff Jeffrey D, King Katherine, Melendez Robert, Langa Kenneth M. Cognitive Function in the Community Setting: The Neighbourhood as a Source of ‘cognitive Reserve?’. Journal of Epidemiology and Community Health. 2011 Apr; doi: 10.1136/jech.2010.128116. jech.2010.128116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke Philippa J, Weuve Jennifer, Barnes Lisa, Evans Denis A, Mendes de Leon Carlos F. Cognitive Decline and the Neighborhood Environment. Annals of Epidemiology. 2015;25(11):849–54. doi: 10.1016/j.annepidem.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escarce Jose J, Lurie Nicole, Jewell Adria. RAND Center for Population Health and Health Disparities (CPHHD) Data Core Series: Pollution, 1988–2004 [United States]: Version 1. 2011 http://www.icpsr.umich.edu/ICPSR/studies/27864/version/1.
- Frisoni Giovanni B, Fratiglioni Laura, Fastbom Johan, Guo Zenchao, Viitanen Matti, Winblad Bengt. Mild Cognitive Impairment in the Population and Physical Health Data on 1,435 Individuals Aged 75 to 95. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2000;55(6):M322–28. doi: 10.1093/gerona/55.6.M322. [DOI] [PubMed] [Google Scholar]
- Gatto Nicole M, Henderson Victor W, Hodis Howard N, St John Jan A, Lurmann Fred, Chen Jiu-Chiuan, Mack Wendy J. Components of Air Pollution and Cognitive Function in Middle-Aged and Older Adults in Los Angeles. NeuroToxicology. 2014;40(January):1–7. doi: 10.1016/j.neuro.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee Gilbert C, Takeuchi David T. Traffic Stress, Vehicular Burden and Well-Being: A Multilevel Analysis. Social Science & Medicine (1982) 2004;59(2):405–14. doi: 10.1016/j.socscimed.2003.10.027. [DOI] [PubMed] [Google Scholar]
- Glass Thomas A, Bandeen-Roche Karen, McAtee Matthew, Bolla Karen, Todd Andrew C, Schwartz Brian S. Neighborhood Psychosocial Hazards and the Association of Cumulative Lead Dose With Cognitive Function in Older Adults. American Journal of Epidemiology. 2009;169(6):683–92. doi: 10.1093/aje/kwn390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajat Anjum, Diez-Roux Ana V, Adar Sara D, Auchincloss Amy H, Lovasi Gina S, O’Neill Marie S, Sheppard Lianne, Kaufman Joel D. Air Pollution and Individual and Neighborhood Socioeconomic Status: Evidence from the Multi-Ethnic Study of Atherosclerosis (MESA) Environmental Health Perspectives. 2013;121(11–12):1325–33. doi: 10.1289/ehp.1206337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog A Regula, Wallace Robert B. Measures of Cognitive Functioning in the AHEAD Study. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 1997;52B(Special Issue):37–48. doi: 10.1093/geronb/52B.Special_Issue.37. [DOI] [PubMed] [Google Scholar]
- Heusinkveld Harm J, Wahle Tina, Campbell Arezoo, Westerink Remco HS, Tran Lang, Johnston Helinor, Stone Vicki, Cassee Flemming R, Schins Roel PF. Neurodegenerative and Neurological Disorders by Small Inhaled Particles. NeuroToxicology. 2016;56(September):94–106. doi: 10.1016/j.neuro.2016.07.007. [DOI] [PubMed] [Google Scholar]
- Hill Terrence D, Ross Catherine E, Angel Ronald J. Neighborhood Disorder, Psychophysiological Distress, and Health. Journal of Health and Social Behavior. 2005;46(2):170–86. doi: 10.1177/002214650504600204. [DOI] [PubMed] [Google Scholar]
- Hurd Michael D, Martorell Paco, Delavande Adeline, Mullen Kathleen J, Langa Kenneth M. Monetary Costs of Dementia in the United States. New England Journal of Medicine. 2013;368(14):1326–34. doi: 10.1056/NEJMsa1204629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang Iain A, Llewellyn David J, Langa Kenneth M, Wallace Robert B, Huppert Felicia A, Melzer David. Neighborhood Deprivation, Individual Socioeconomic Status, and Cognitive Function in Older People: Analyses from the English Longitudinal Study of Ageing. Journal of the American Geriatrics Society. 2008;56(2):191–98. doi: 10.1111/j.1532-5415.2007.01557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langa Kenneth M, Chernew Michael E, Kabeto Mohammed U, Regula Herzog A, Ofstedal Mary Beth, Willis Robert J, Wallace Robert B, Mucha Lisa M, Straus Walter L, Mark Fendrick A. National Estimates of the Quantity and Cost of Informal Caregiving for the Elderly with Dementia*. Journal of General Internal Medicine. 2001;16(11):770–78. doi: 10.1111/j.1525-1497.2001.10123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leist Anja K, Maria Glymour M, Mackenbach Johan P, van Lenthe Frank J, Avendano Mauricio. Time Away from Work Predicts Later Cognitive Function: Differences by Activity during Leave. Annals of Epidemiology. 2013;23(8):455–62. doi: 10.1016/j.annepidem.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque Shannon, Surace Michael J, McDonald Jacob, Block Michelle L. Air Pollution & the Brain: Subchronic Diesel Exhaust Exposure Causes Neuroinflammation and Elevates Early Markers of Neurodegenerative Disease. Journal of Neuroinflammation. 2011;8(1):1–10. doi: 10.1186/1742-2094-8-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien Sonia J, McEwen Bruce S, Gunnar Megan R, Heim Christine. Effects of Stress throughout the Lifespan on the Brain, Behaviour and Cognition. Nature Reviews. Neuroscience. 2009;10(6):434–45. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Lyu Jiyoung, Burr Jeffrey A. Socioeconomic Status Across the Life Course and Cognitive Function Among Older Adults An Examination of the Latency, Pathways, and Accumulation Hypotheses. Journal of Aging and Health. 2015 May; doi: 10.1177/0898264315585504. 898264315585504. [DOI] [PubMed] [Google Scholar]
- McEwen Bruce S, Tucker Pamela. Critical Biological Pathways for Chronic Psychosocial Stress and Research Opportunities to Advance the Consideration of Stress in Chemical Risk Assessment. American Journal of Public Health. 2011;101(Suppl 1):S131–39. doi: 10.2105/AJPH.2011.300270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Goudsmit E, Ravid R, Swaab DF, Mirmiran M, Kopp N, De Kloet ER, Taljaard JJF. Reexamination of the Glucocorticoid Hypothesis of Stress and Aging. Prog Brain Res. 1992;93:365–83. doi: 10.1016/S0079-6123(08)64585-9. [DOI] [PubMed] [Google Scholar]
- Monn Christian. Exposure Assessment of Air Pollutants: A Review on Spatial Heterogeneity and Indoor/Outdoor/Personal Exposure to Suspended Particulate Matter, Nitrogen Dioxide and Ozone. Atmospheric Environment. 2001;35(1):1–32. doi: 10.1016/S1352-2310(00)00330-7. [DOI] [Google Scholar]
- Nash David T, Fillit Howard. Cardiovascular Disease Risk Factors and Cognitive Impairment. The American Journal of Cardiology. 2006;97(8):1262–65. doi: 10.1016/j.amjcard.2005.12.031. [DOI] [PubMed] [Google Scholar]
- Peters Ruth, Peters Jean, Booth Andrew, Mudway Ian. Is Air Pollution Associated with Increased Risk of Cognitive Decline? A Systematic Review. Age and Ageing. 2015 Jul;:afv087. doi: 10.1093/ageing/afv087. [DOI] [PubMed] [Google Scholar]
- Pfeiffer Eric. A Short Portable Mental Status Questionnaire for the Assessment of Organic Brain Deficit in Elderly Patients†. Journal of the American Geriatrics Society. 1975;23(10):433–41. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- Power Melinda C, Adar Sara D, Yanosky Jeff D, Weuve Jennifer. Exposure to Air Pollution as a Potential Contributor to Cognitive Function, Cognitive Decline, Brain Imaging, and Dementia: A Systematic Review of Epidemiologic Research. Neurotoxicology. 2016 Jun; doi: 10.1016/j.neuro.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranft Ulrich, Schikowski Tamara, Sugiri Dorothee, Krutmann Jean, Krämer Ursula. Long-Term Exposure to Traffic-Related Particulate Matter Impairs Cognitive Function in the Elderly. Environmental Research. 2009;109(8):1004–11. doi: 10.1016/j.envres.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Rönnlund Michael, Nyberg Lars, Bäckman Lars, Nilsson Lars-Göran. Stability, Growth, and Decline in Adult Life Span Development of Declarative Memory: Cross-Sectional and Longitudinal Data From a Population-Based Study. Psychology and Aging. 2005;20(1):3–18. doi: 10.1037/0882-7974.20.1.3. [DOI] [PubMed] [Google Scholar]
- Ross Catherine E, Mirowsky John. Disorder and Decay The Concept and Measurement of Perceived Neighborhood Disorder. Urban Affairs Review. 1999;34(3):412–32. doi: 10.1177/107808749903400304. [DOI] [Google Scholar]
- Ross Catherine E, Mirowsky John. Neighborhood Disadvantage, Disorder, and Health. Journal of Health and Social Behavior. 2001;42(3):258–76. [PubMed] [Google Scholar]
- Schikowski Tamara, Vossoughi Mohammad, Vierkötter Andrea, Schulte Thomas, Teichert Tom, Sugiri Dorothee, Fehsel Karin, et al. Association of Air Pollution with Cognitive Functions and Its Modification by APOE Gene Variants in Elderly Women. Environmental Research. 2015;142(October):10–16. doi: 10.1016/j.envres.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Sheffield Kristin M, Kristen Peek M. Neighborhood Context and Cognitive Decline in Older Mexican Americans: Results From the Hispanic Established Populations for Epidemiologic Studies of the Elderly. American Journal of Epidemiology. 2009;169(9):1092–1101. doi: 10.1093/aje/kwp005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe Andrew, Feldman Pamela J. Neighborhood Problems as Sources of Chronic Stress: Development of a Measure of Neighborhood Problems, and Associations with Socioeconomic Status and Health. Annals of Behavioral Medicine. 2001;23(3):177–85. doi: 10.1207/S15324796ABM2303_5. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Repetti RL, Seeman T. Health Psychology: What Is an Unhealthy Environment and How Does It Get under the Skin? Annual Review of Psychology. 1997;48:411–47. doi: 10.1146/annurev.psych.48.1.411. [DOI] [PubMed] [Google Scholar]
- Tonne Cathryn, Elbaz Alexis, Beevers Sean, Singh-Manoux Archana. Traffic-Related Air Pollution in Relation to Cognitive Function in Older Adults. Epidemiology (Cambridge, Mass) 2014;25(5):674–81. doi: 10.1097/EDE.0000000000000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Ming, Hawkley Louise C, Cacioppo John T. Objective and Perceived Neighborhood Environment, Individual SES and Psychosocial Factors, and Self-Rated Health: An Analysis of Older Adults in Cook County, Illinois. Social Science & Medicine (1982) 2006;63(10):2575–90. doi: 10.1016/j.socscimed.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Weuve Jennifer, Puett Robin C, Schwartz Joel, Yanosky Jeff D, Laden Francine, Grodstein Francine. Exposure to Particulate Air Pollution and Cognitive Decline in Older Women. Archives of Internal Medicine. 2012;172(3):219–27. doi: 10.1001/archinternmed.2011.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wight Richard G, Aneshensel Carol S, Miller-Martinez Dana, Botticello Amanda L, Cummings Janet R, Karlamangla Arun S, Seeman Teresa E. Urban Neighborhood Context, Educational Attainment, and Cognitive Function among Older Adults. American Journal of Epidemiology. 2006;163(12):1071–78. doi: 10.1093/aje/kwj176. [DOI] [PubMed] [Google Scholar]
- Wilker Elissa H, Preis Sarah R, Beiser Alexa S, Wolf Philip A, Au Rhoda, Kloog Itai, Li Wenyuan, et al. Long-Term Exposure to Fine Particulate Matter, Residential Proximity to Major Roads and Measures of Brain Structure. Stroke. 2015;46(5):1161–66. doi: 10.1161/STROKEAHA.114.008348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Yu-Tzu, Matthew Prina A, Brayne Carol. The Association between Community Environment and Cognitive Function: A Systematic Review. Social Psychiatry and Psychiatric Epidemiology. 2014;50(3):351–62. doi: 10.1007/s00127-014-0945-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Blackwell T, Gore R, Sands L, Reus V, Browner WS. Depressive Symptoms and Cognitive Decline in Nondemented Elderly Women: A Prospective Study. Archives of General Psychiatry. 1999;56(5):425–30. doi: 10.1001/archpsyc.56.5.425. [DOI] [PubMed] [Google Scholar]