Summary
It is widely assumed that structured exercise causes an additive increase in physical activity energy expenditure (PAEE) and total daily energy expenditure (TDEE). However, the common observation that exercise often leads to a less than expected decrease in body weight, without changes in energy intake, suggests that some compensatory behavioral adaptations occur. A small number of human studies have shown that adoption of structured exercise can lead to decreases in PAEE, which is often interpreted as a decrease in physical activity (PA) behavior. An even smaller number of studies have objectively measured PA, and with inconsistent results. In animals, high levels of imposed PA induces compensatory changes in some components of TDEE. Recent human cohort studies also provide evidence that in those at the highest levels of physical activity, TDEE is similar when compared to less physically active groups. The objective of this review is to summarize the effects of structured exercise training on PA, sedentary behavior, PAEE, and TDEE. Using models from ecological studies in animals and observational data in humans, an alternative model of TDEE in humans is proposed. This model may serve as a framework to investigate the complex and dynamic regulation of human energy budgets.
Keywords: Energy Metabolism, Basal Metabolism, Exercise Physiology, Humans, Female, Male
Introduction
The health benefits of exercise training are well-established.1 The worldwide trends of increasing rates of overweight and obesity has increased the focus on understanding how structured exercise impacts energy balance and thus body weight regulation. The prevailing view has been based on the assumption that exercise will impact energy expenditure (EE) in a dose response manner; that is, that the adoption of exercise will lead to an increase in EE in an additive manner. However, this assumption has been challenged in recent years.2, 3 Evidence has slowly accumulated suggesting that adoption of structured exercise training may induce compensatory changes in behavior or physiology that could attenuate or completely offset the intended effects on EE.4–10 In terms of behavior, adoption of exercise has been shown in some cases to lead to increases in energy intake or decreases in physical activity (PA), including spontaneous PA (SPA) which is often subconscious such as fidgeting behavior and posture maintenance.11 The objective of this review is to summarize studies that have examined effects of exercise training on PA levels and SPA, sedentary behavior, and EE. I will also discuss some of the challenges and methodological considerations associated with measuring changes in behavior and physiology, and propose some frameworks which could guide study designs to better address how structured exercise impacts EE in humans.
Components of energy expenditure
Traditionally, total daily EE (TDEE) in humans has been viewed as the sum of energy allocated to the maintenance of basal metabolic rate (BMR), the thermic effect of food (TEF), and physical activity EE (PAEE) (Figure 1). In humans, except at the extreme levels of endurance performance,12, 13 BMR comprises the largest proportion of TDEE (~60–70%). BMR is measured after a period of rest and fasting (10–12 hr.), with subjects awake, lying down, and resting at thermoneutrality,14 and represents the basal energy requirements of the body’s organs (i.e. brain, gut, kidneys, heart, liver, muscle).15 When these conditions are not met, the term resting energy expenditure (REE) or resting metabolic rate (RMR) is used. TEF is the EE associated with digestion, absorption, and assimilation of food and accounts for 6–12% of TDEE.16 It is worth noting that differences in TEF between lean and obese subjects, where found, are small,17 and there is little evidence that defects in TEF play a major role in the development of obesity.18 In most studies of free-living humans, TEF is not measured and is assumed to be static at 10% of TDEE.8,11,15,20 PAEE can be divided into exercise EE (i.e., the EE associated with planned, structured PA) and non-exercise PAEE. Non-exercise PAEE has an enormous variety of constituents such as the EE of occupation, leisure, posture allocation (sitting, standing), ambulation, talking, and fidgeting.19
Figure 1.
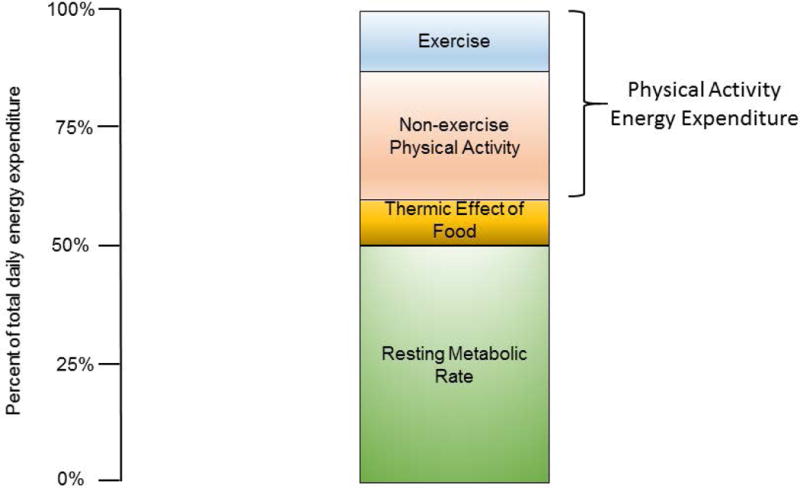
“Traditional” model of total daily energy expenditure (TDEE) in humans. In this model, non-exercise energy expenditure is associated with the energy expended in non-exercise physical activity.
In this review, I will discuss the compensatory changes in PAEE that may result from the adoption of structured exercise. These compensatory changes may result from changes in behavior (e.g. PA), EE, or both. This should not be confused with adaptive reduction in thermogenesis, which refers to regulatory changes in EE associated with weight loss. When an individual loses weight, TDEE and REE will also decrease. Although the decreases in these components is proportional to the amount of weight loss, the elegant studies by Leibel et al.20 demonstrated that TDEE and RMR decrease more than would be expected based on changes in body composition (particularly fat-free mass). This adaptive decrease also occurs in lean individuals following periods of prolonged energy restriction, such as occurred in participants in Biosphere 2.21 In these settings, the term adaptive thermogenesis is used to describe the unexplained mass-adjusted decreases in the various components of TDEE.22 Mechanistically, adaptive thermogenesis is believed to represent a defensive mechanism to prevent excess weight loss during periods of prolonged or dramatic energy deficits.22 In the current review, the focus is on the effect of structured exercise training on non-exercise PA and PAEE. In many of the studies reviewed below, body weight at the group and individual level did not change. Thus, in this context, the term ‘adaptive thermogenesis’ may be misleading. As will be discussed later, it may be more appropriate to consider the allocation of the overall energy budget. Although some studies of human physiology have clearly demonstrated that bodily functions such as reproductive function are compromised when EE is increased substantially above energy intake,23 studies of the chronic effects of exercise have typically ignored these other components of EE.
Methodological considerations
Determining whether a behavioral or physiological compensation occurs in response to acute or chronic exercise requires accurate measurement of the major components of TDEE. However, as discussed in a previous review,11 obtaining accurate measurements of TDEE and its components in free-living humans is challenging. The gold standard approach for measuring TDEE is the doubly labeled water (DLW) method, but its widespread application in research is limited by its relatively high cost. Furthermore, the DLW method does not provide information on the type or pattern of PA performed. The DLW approach can be used to provide an estimate of PAEE, provided that BMR or REE has been measured, but it only provides an average estimate of both TDEE and PAEE over the period of measurement; the DLW method does not distinguish the variability in TDEE and PAEE from day-to-day. An alternative approach is to use activity monitors to obtain an objective measure of PA and estimate PAEE and/or TDEE,4, 6, 24 but this approach has lower precision than the DLW method.25 The DLW approach has been used in several studies to determine the effects of structured exercise on non-exercise PA. In most of these studies, it has been presumed that changes in non-exercise PAEE observed in response to exercise represent changes in PA behavior.5, 26 This conclusion can only be supported with careful measures of both the amount of PA (using objective methods such as accelerometry) and the energy cost of PA. Although PA is often measured in studies examining the effects of exercise, the energy cost of PA is rarely measured. However, it has been observed that the energy cost of walking in a highly physically active population of hunter-gatherers is similar when compared to data from Western societies.27
Some of the evidence cited to support a compensatory decline in non-exercise EE has been obtained from studies designed to induce weight loss. In these studies, a lesser than expected weight loss is used as evidence that there was some degree of compensation (expenditure, intake, or both) that offset the intended weight loss. For example, Church et al.28 compared predicted and actual weight loss in overweight and obese women during a 6 month exercise intervention. Participants were randomized to a non-exercise control group, or 1 of 3 different exercise arms with incrementally higher exercise EEs (low, moderate, and high training volumes). Predicted weight loss was estimated using the “3500 kcal rule”, i.e., an energy deficit of 3500 kcal (14.6 MJ) is required to induce a 1 pound weight loss (0.4 kg).29 Using this approach, predicted and actual weight loss did not differ in women in the low and moderate training volume groups, but actual weight loss (−1.5 kg) was significantly less than predicted (−2.7 kg) in women in the high volume training group. However, this approach assumes that the composition of weight loss is consistent across individuals (70% as fat mass), and does not consider the compensatory changes in EE resulting from weight loss.30 As a result, the predicted weight loss is likely an overestimate of the true expected weight loss. Nonetheless, using models to adjust the expected weight loss based on changes in body composition has shown that exercise-induced weight loss is still less than the expected weight loss,31 suggesting that structured exercise does lead to some compensation that offsets the intended weight loss. What cannot be determined from these studies, however, is whether the compensation was behavioral (changes in intake, PA, or both) or physiological (change in some component of non-exercise EE, independent of changes in PA).
Effects of programmed exercise on non-exercise physical activity and sedentary behavior
Short term studies (up to 16 days)
A limited number of studies have examined the acute effects of exercise on non-exercise PA. Some of the earliest studies in this area were performed by Stubbs et al.,32–34 who examined the effects of different doses of exercise performed for 7–10 days on both energy intake and TDEE in healthy, lean, young men (6–8 subjects per study). In these studies, TDEE was estimated using heart rate (HR) monitors (these studies were performed before accelerometry-based activity monitors were widely available). The HR method requires an individual laboratory calibration session to develop a regression between HR and EE across a range of exercise intensities, and then extrapolating HR measured in the free-living setting to obtain an estimate of TDEE. In the studies when medium (2 × 40 min, 1.6 MJ·d−1) and high (3 × 40 min, 3.2–4.0 MJ·d−1) levels of exercise were performed, TDEE tended to decrease, thereby suggesting that either a decline in PA, PAEE, or a decrease in some other component of TDEE occurred. Conversely, a more recent study from this same group reported no changes in non-exercise PAEE (measured using DLW) in 12 young, lean men and women over 16 days using a similar exercise protocol.35 Results from this latter study are consistent with another study in young, lean men and women (N=16) that showed no changes in PAEE estimated by HR over 16 days when exercise (~2.1 MJ·d−1) was performed every other day.36 In a more recent study, Alahmadi et al. determined the effect of acute bouts of moderate-continuous (60 min of walking at 6 km·h−1 at 0% grade) and high-intensity interval (60 min, alternating 5 min intervals at 6 km·h−1 at 0% and 10% grade) on non-exercise PA (measured using accelerometers) in 16 overweight and obese young men.37 Non-exercise PA was measured for 3 days before, on the day of, and 3 days after the acute exercise trial. Compared to the pre-exercise period, non-exercise PA remained the same on the exercise day after both moderate-continuous and high-intensity interval exercise. In the post-exercise period, non-exercise PA remained stable for the first two days and then tended to increase on the third day after both the moderate-intensity continuous (16%) and high-intensity interval (25%) bouts. The reason(s) for this delayed increase are not entirely clear; nonetheless, this study does not provide evidence of a decrease in PA after an acute bout of exercise. Thus, results of these short-term studies do not provide convincing evidence of behavioral or physiological adaptation to exercise. However, these studies are limited by their small sample sizes, and by the methodological approaches used to estimate TDEE and PAEE.
Long-term studies (6 weeks up to 16 months)
Few studies have reported the effects of long-term exercise training on changes in PA and PAEE. In most of these studies, PA and PAEE were secondary outcomes. Two studies that employed the DLW approach both provide evidence that long-term participation in high volumes of exercise lead to decreases in non-exercise EE. In the first study, Westerterp at al. measured TDEE in 16 men and 16 women (28–41 yrs., BMI 19.4–26.5 kg·m2) who participated in a 44 week training program to train for a half-marathon competition.10 All participants were previously untrained. Training was performed 4 days·wk−1, and daily training duration increased from 10–30, 20–60, and 30–90 mins at weeks 8, 20, and 40, respectively. At baseline and week 40, TDEE was measured using DLW and sleeping metabolic rate (SMR), measured using whole-room indirect calorimetry, was used as a proxy for RMR. In the entire sample who completed the training program (13 men, 9 women), TDEE increased (from 11.6 to 14.5 MJ·d−1 in men and 9.9 to 11.7 MJ·d−1 in women). SMR decreased slightly in the men, and remained stable in the women. In a subset of subjects (4 men and 3 women), TDEE and SMR were also measured at weeks 8 and 20. Despite an increase in training volume during this period, TDEE plateaued in both men (14.6 vs. 13.9 MJ·d−1) and women (10.4 vs. 10.2 MJ·d−1). SMR decreased in two of the men but remained stable in the remaining subjects. In the second study, Donnelly and colleagues performed a long-term randomized control trial (Midwest Exercise Trial, MET) to determine the effects of a supervised and verified exercise regime on EE, energy intake, and body weight in previously sedentary, overweight and moderately obese young adults.26 Energy intake was ad libitum. Subjects exercised 4–5 days per week, starting at 20 mins per session and increasing to 45 mins per session, at ~55–70% of VO2max for 16 months. The goal was to expend 1.6 kJ per session (8.4 MJ·wk−1). At 16 months, men averaged 2.8 kJ per exercise session, but TDEE increased only by 1.6 kJ.d−1. Women averaged 1.8 kJ per session, but TDEE increased only by 0.9 kJ.d−1. These data suggest that non-exercise EE decreased in both men and women. However, because these studies only measured TDEE, it is could not be determined if the reduction in non-exercise EE was due to changes in behavior, physiology, or both.
Studies in older adults
The effects of exercise on non-exercise PA have important implications for health beyond weight management. If exercise causes a decrease in non-exercise PA, this may have the unintended effect of increasing sedentary time, which is inversely associated with health risks independent of PA.38, 39 This is particularly a concern in older adults, who spend larger portions of the day engaged in sedentary behavior.40 For example, Diblasio et al.4 studied post-menopausal women during a 13 week walking intervention. Half of the women had increases and half decreases in estimated PAEE and TDEE. The women that increased PAEE and TDEE had measurable improvements in lipid profiles, whereas no changes were observed in the women who decreased. Few studies have considered the effects of exercise on PA or PAEE in older adults. Goran et al. measured TDEE and PAEE in 11 elderly men (56–78 yrs.) who performed endurance training (3 days/wk.) for 8 weeks.5 Training progressed from a net EE of 0.6 kJ per session at 60% of VO2max to 1.2 kJ per session at 85% of VO2max. TDEE was unchanged, but PAEE decreased by 62% (from 2.4 to 1.4 kJ·d−1), which was interpreted as a compensatory decline in PA during the non-exercise portions of the day. Similar results were observed in two other studies that used different methods to estimate PAEE. Morio et al. studied the effects of a 14 week progressive endurance training program in 13 elderly subjects (63 ± 2 yrs.) using 7 day activity records.9 Estimated TDEE did not change, but the energy expended during free living activities significantly decreased by 7.7%. Interestingly, time spent walking also significantly decreased. In another study of elderly individuals (59 ± 4 yrs.), Meijer et al. reported that non-exercise PA (measured using accelerometry) was significantly lower on training compared to non-training days after 12 weeks of exercise training.7 The training program consisted of both endurance and weight-lifting activities, although the exercise intensity was not reported. Evidence from these studies suggests that adoption of regular endurance exercise in older adults may lead to decreases in non-exercise PA. However, a recent study conducted in older adults (≥ 65 yrs.) in a primary care setting reported that a program aimed at increasing walking resulted in increases in steps and decrease in sedentary time.41 Thus, it is possible that lower intensity training programs may be more beneficial in older adults in terms of preventing declines in non-exercise PA.
Sedentary behavior
As described above, using an approach combining DLW and accelerometry measurements would permit one to gain insight into whether any observed changes in TDEE could be attributed to changes in behavior. However, few studies have done so, and in the few studies where this was done, PA was assessed using accelerometers that are well-suited to measuring intensity of PA, but are not well-suited to measuring sedentary behavior. Sedentary behavior is defined as activities that require minimal movement, resulting in a very low level of EE (i.e. <1.5 metabolic equivalent units; METs) and is typically associated with sitting, reclining, or lying down during waking hours.39 It is likely that any behavioral compensation that occurs in response to an exercise training program is due to reallocation of time spent engaged in light intensity (e.g. 1.5 – 3.0 METs) activities to more time spent engaged in sedentary activities. However, hip-worn accelerometers are ill-suited for this purpose, and are prone to high rates of misclassification of light and sedentary activities.42 The advent of positional accelerometers, such as the activPal accelerometer (PAL Technologies, Glasgow, Scotland) has overcome this limitation, and these devices have been shown to quantify sedentary time with a high degree of accuracy.42, 43 Only one study has employed this approach to measure changes in sedentary time in response to exercise training.44 In that study, sedentary time remained stable in overweight and obese adults who completed a 12 week supervised aerobic exercise training program that gradually progressed in volume (5 × 30 mins·d−1 in weeks 1–4 to 5 × 40 mins·d−1 in weeks 7–12) and intensity (from 50% to 55–65% of heart rate reserve). However, sedentary time did increase in approximately half of the participants in the supervised exercise training arm, highlighting the variability in response. Given this variability, it would be interesting and clinically relevant to understand what factor(s) contribute to this response. Identifying patients who are more likely to increase sedentary time in response to exercise training could allow for more targeted interventions to target both behaviors. Indeed, in this same study, the groups that received instructions to reduce sedentary time (both with and without exercise) reduced sedentary time by ~5%.44 This study also provides evidence that exercise and sedentary behavior are distinct domains of the PAEE spectrum with different attributes and determinants.45
Allocation models of energy expenditure
The EE of free-living animals is often limited by the total energy that can be expended or stored over a given period of time, and this may influence the animals’ behavior patterns.46–48 For example, how does animal behavior change when energy demands increase due to decreases in ambient temperature, which requires an increase in EE to invoke the appropriate thermoregulatory responses? It is posited that some animals have a limit to their energy budgets, and thus, their behavior will be modified to accommodate the individual energy budget. Mathot and Dingemanse have proposed three models by which this may occur (Figure 2).46 In the allocation model, the total energy budget is constrained; thus an increase in the energy cost related to maintaining basal functioning will reduce the amount of energy that is available to support other functions such as foraging, hunting, etc., altering the animal’s behavior. In contrast, the independent model predicts that changes in basal EE have no impact on the energy budgeted for behavior. This model is analogous to the factorial model of exercise in humans that assumes that exercise has an additive effect on TDEE.49 Finally, the performance model suggests that an increase in the basal energy metabolism reflects an increase in the capacity of the organism to mobilize energy stores.
Figure 2.
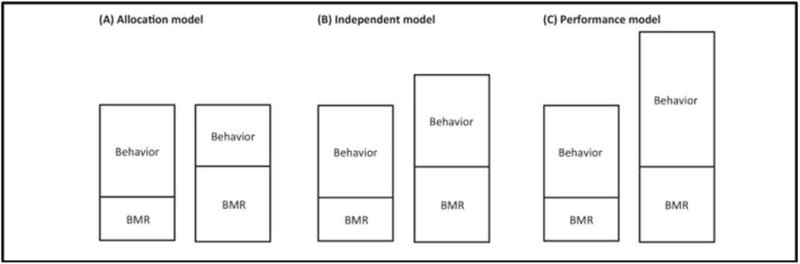
Allocation models for balancing energy budgets in animals. See text for description. Adopted from ref 46: “Energetics and behavior: unrequited needs and a new direction”, K.J. Mathot and N.J. Dingemanse, Trends in Ecology and Evolution: 30(40), 199–206, 2015, with permission from Elsevier Limited (License Number: 3834971229174).
These three models can be used as a foundation for models of human energy budgets as it relates to exercise (Figure 2). For example, the independent model would predict that exercise would increase TDEE in an additive manner, whereas the allocation model would predict that exercise would lead to a reduction in some component of non-exercise EE (for example, non-exercise PAEE). There is evidence that each of these models is evident in different human populations. For example, some studies have shown that exercise, particularly lower volume and intensity exercise,10 leads to an additive increase in TDEE, suggesting that the energy budgets are regulated in an additive manner. In contrast, as described above, several studies have shown that TDEE remains either unchanged,5 or increases less than expected,26, 28, 50 suggesting that in some situations, human energy budgets are constrained by an allocation model. Moreover, results from these studies suggest that allocation may be affected by confounders such as age (older more likely to reallocate),5 sex (males more likely to reallocate),26 and exercise volume (allocation more likely with higher volumes).28 Finally, there is even evidence for a performance model in humans. For example, Hunter et al.51 reported that both TDEE and BMR were increased in older men and women (61–77 yrs.) following a 26 week progressive resistance exercise program. Thus, in contrast to the study of Goran,5 an intervention which was targeted at increasing strength increased the basal energy requirement via an increase in lean (muscle) mass, but also increased TDEE due to the increase in the capacity of the older adults to mobilize energy stores, most likely mediated by increases in physical function.
These allocation models can serve as a conceptual framework for designing human studies. Using these models to establish a priori hypotheses will guide proper experimental design and selection of appropriate measurement tools. For example, if the hypothesis to be tested is that a particular exercise intervention in a target population will adversely impact non-exercise PA behavior, then an objective measure of non-exercise behavior should be used (and as discussed above, one that is capable of accurately measuring sedentary behavior).
Is total energy expenditure regulated in humans?
Most, if not all, human studies that have determined either the acute or chronic effects of exercise on PAEE and/or TDEE have assumed an additive model, i.e. increases in PA will lead to proportional increases in PAEE and TDEE, without consideration of potential reallocation of the energy, as described above. Recently Pontzer has posited that TDEE is a constrained variable.2 According to this model, rather than increasing with PA in a dose-dependent manner, TDEE is constrained such that with increasing PA, TDEE increases and eventually plateaus. Thus, at some threshold level, increases in PA have little or no effect on TDEE (Figure 3). In this model, high levels of PAEE lead to compensatory changes in other components of TDEE. The foundation for this model is based primarily on empirical studies in animals that have manipulated the amount of PA and measured the effects on EE. For example, Perrigo and colleagues performed studies in rodents that manipulated the amount of PA required to obtain food (i.e. revolutions on an exercise wheel).52 These and other studies demonstrated that in several species, there was a diminishing effect of PA on TDEE.2 Consistent with these empirical results, observational studies in primates53 and giant pandas54 have shown that TDEE is similar in captive and wild populations. The constrained model is similar to the allocation model in Figure 2, in that both models predict that in the face of increasing energy demands, the allocation of EE, rather than TDEE, is altered. The models differ in that the allocation model suggests that changes in BMR cause changes in behavior, whereas the constrained EE model suggests that changes in behavior (PA) causes changes in one or more of the components of TDEE.
Figure 3.
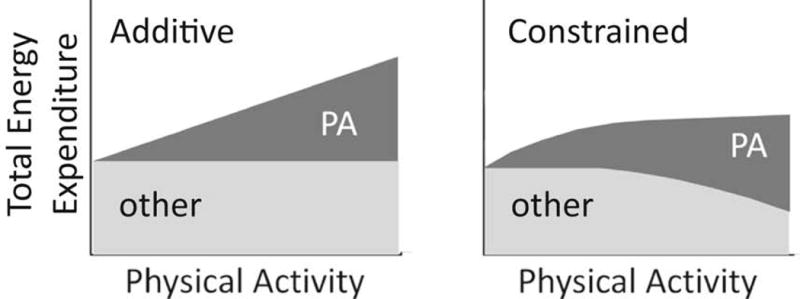
Additive and constrained models of total daily energy expenditure. See text for description. Adopted from ref 3: “Constrained total energy expenditure and metabolic adaptation to physical activity in adult humans”, H. Pontzer et al., Current Biology: 26(3), 410–417, 2016, with permission from Elsevier Limited (License Number: 3837790768211).
Although there are few studies that have directly tested this constrained model in humans, several lines of evidence support the hypothesis that TDEE is constrained in humans. First, as discussed above, in humans training to compete in a half-marathon, TDEE plateaued in the latter part of the training program despite additional increases in training volume.10 Second, cross-sectional studies comparing cohorts of American women to Nigerian women,55 hunter-gatherers to European and Americans27 reported no differences in TDEE (adjusted for body size) despite dramatic differences in PA. These results are supported by a meta-analysis of 98 different studies representing 183 cohorts (4972 individuals) from developing and industrialized societies that showed no differences in TDEE (after adjustment for age and weight), where daily PA levels would presumably be higher in the developing societies.56 Finally, Pontzer and colleagues recently tested a constrained EE model using data from several different studies of adults (n=332) from five different populations.3 In these studies, TDEE was measured using DLW, and PA was assessed using accelerometry. After adjustment for body size and composition, TDEE showed a non-linear association with PA; TDEE plateaued above the 6th or 7th decile of PA (Figure 4). Based on these results, they have proposed that high levels of PA lead to compensatory declines in energy spent on other physiological functions, but the processes affected are not known. However, the validity of the constrained model requires further empirical investigation in a prospective study, perhaps with measurement of biomarkers to provide insight into how compensation in TDEE is occurring. Nevertheless, the assumption that increases in PA have an additive effect on TDEE can be questioned.
Figure 4.
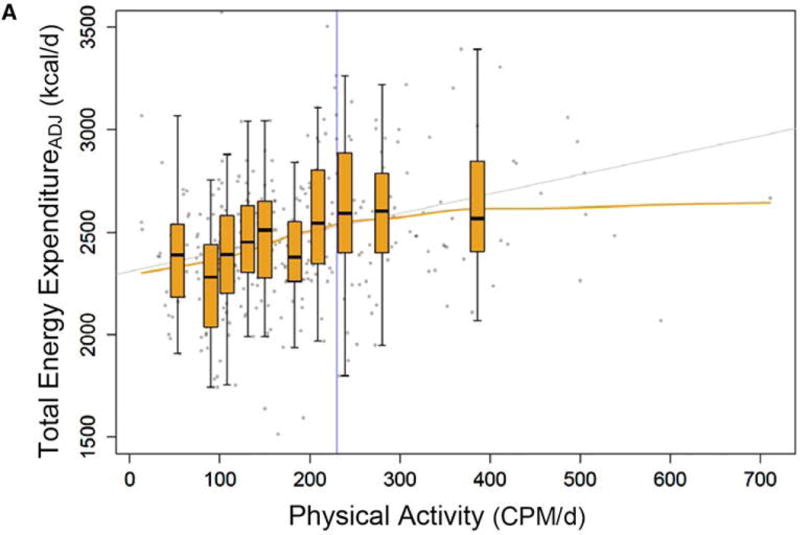
The association between physical activity (expressed as the average daily physical activity per day from accelerometer counts per minute, CPM·d−1) and total daily energy expenditure (ADJ) adjusted for fat mass, fat free mass, age, height, sex, and study site (N=331 individuals from 5 different cohort studies). Each boxplot (median and quartiles of TDEE) represents decile of physical activity. Adopted from ref 3: “Constrained total energy expenditure and metabolic adaptation to physical activity in adult humans”, H. Pontzer et al., Current Biology: 26(3), 410–417, 2016, with permission from Elsevier Limited (License Number: 3837790768211).
If TDEE is constrained in humans, how can this hypothesis be reconciled with the findings of Hunter,51 where the total energy budget in older adults increased after a 26 week resistance exercise program? One explanation could be that these individuals were below their total energy budget ceiling at baseline. Indeed, the TDEE at baseline (~7.8 MJ·d−1) is substantially lower than that observed in younger, physically inactive adults of similar body mass index (~9.9 MJ·d−1)10. This conclusion is supported by the observation of Pontzer and colleagues that TDEE increased linearly with increasing level of PA up to a critical point, and then plateaued at the highest levels of PA (Figure 4).3 However, as described by Pontzer et al.,3 the mechanisms and factors that determine this threshold remain unknown. Understanding how TDEE and its components are regulated in humans could improve public health strategies related to obesity and metabolic diseases.3
An alternative model of Total Daily Energy Expenditure
Although the traditional model of TDEE (Figure 1) is widely accepted in studies of human physiology, based on the studies described in the previous sections, it may be appropriate to consider an alternative model (Figure 5). In this model, one or more of the four “adaptable” components may be reduced with high levels of structured exercise. The processes that contribute to the “other” component are not known at this time, but may reflect the energy cost associated with other bodily functions such as somatic repair, reproduction, immune function, the energy cost of locomotion, and thermoregulation.2 For example, emerging evidence indicates that the energy cost associated with thermoregulation even at temperatures slightly below the thermoneutral zone is substantial.57 At this time, however, it would be difficult to accurately quantify the EE associated with this other component, but incorporating biomarkers (e.g. sex steroid hormone concentrations, inflammatory markers) may provide an indication of whether these physiological functions were impacted by the intervention.2
Figure 5.
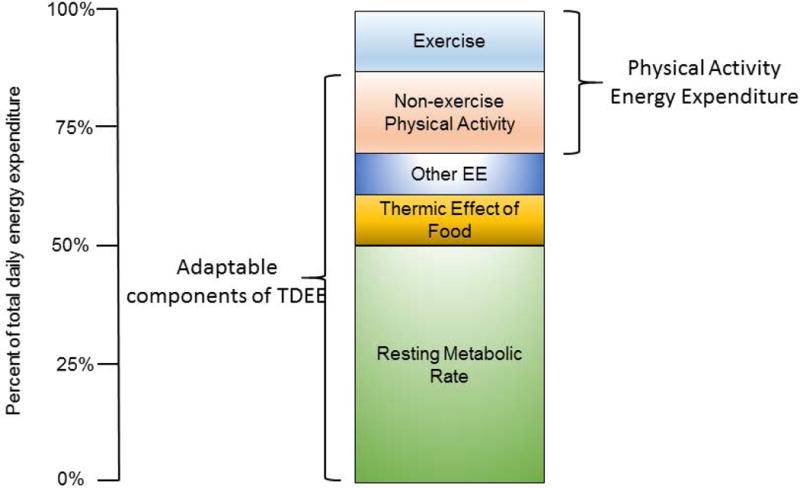
An alternative model of total daily energy expenditure (TDEE) in humans. In this model, one or more of the four “adaptable” components may be reduced with high levels of structured exercise. The processes that contribute to the “other” component are not well-understood, but may encompass physiological processes such as reproductive function, inflammation, and thermoregulation.
Conclusions, outstanding questions, and future direction
Current public health models and obesity prevention strategies assume that increasing daily PA will lead to increases in TDEE in an additive manner.2, 3, 49 As summarized in this review, emerging evidence suggests that adoption of regular, structured exercise leads to compensatory changes in behavior and/or physiology that may attenuate the expected increases in TDEE, particularly at higher levels of PA. However, there remain a number of unanswered questions and priorities for future research:
Foremost would be empirical studies to test the constrained EE hypothesis. These studies would require careful measurements of all components of TDEE using DLW and indirect calorimetry, coupled with objective measures of PA, to decipher changes in physiology from changes in behavior, as well as select biomarkers related to reproductive, somatic, and immunologic function.
If TDEE is constrained and regulated, how is this achieved? Homeostatic models are characterized by a set point, one or more sensors, and a control center that integrates the signals from the sensor(s), and generates responses targeted at effector organ(s) to maintain the regulated variable within physiologic limits.58 Before the constrained EE model can be fully accepted, it will be important to identify these components of the homeostatic control system.
If TDEE is constrained, what is the ceiling for TDEE in different populations? Does this ceiling change with weight loss or with aging? Is the ceiling different in lean vs. obese individuals? Is the ceiling stable, or can it change over time?
Do individual differences in the TDEE ceiling differentiate responders and non-responders to an exercise program that is intended to induce weight loss?26
How can elite endurance athletes develop exceptionally high energy budgets without compromising health?12 How long can these high energy budgets be sustained?
What is the impact of different types, intensity, duration, frequency, and volume of exercise on allocation of TDEE and its components? Indeed, some evidence suggests that the impact on the components of TDEE is greatest at higher exercise intensities.3
Are there circadian influences, i.e., does exercise performed in the morning and evening have the same effect on TDEE?
It is clear the regulation of human EE and management of total energy budgets are complex and dynamic processes that are poorly understood. It is hoped that the models summarized in this review (Figures 2,46 3,3 and 5) will provide a new, integrative framework from which the effects of exercise on health and body weight can be investigated.
Acknowledgments
The author wishes to acknowledge Kong Chen and Herman Pontzer for their insightful conversations on this topic. This work was supported by an NIH award to Dr. Melanson (R01 DK 077088), as well as support from the Colorado Nutrition and Obesity Research Center (P30 DK048520) and the Colorado Clinical and Translational Science Institute (UL1 RR025780)
Funding sources: This work was supported by an NIH award to Dr. Melanson (R01 DK 077088), as well as support from the Colorado Nutrition and Obesity Research Center (P30 DK048520) and the Colorado Clinical and Translational Science Institute (UL1 RR025780)
Footnotes
Conflict of Interest Statement:
The author has no conflicts to declare
References
- 1.Pedersen BK, Saltin B. Exercise as medicine - evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports. 2015;25(Suppl 3):1–72. doi: 10.1111/sms.12581. [DOI] [PubMed] [Google Scholar]
- 2.Pontzer H. Constrained Total Energy Expenditure and the Evolutionary Biology of Energy Balance. Exerc Sport Sci Rev. 2015;43:110–6. doi: 10.1249/JES.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 3.Pontzer H, Durazo-Arvizu R, Dugas LR, et al. Constrained Total Energy Expenditure and Metabolic Adaptation to Physical Activity in Adult Humans. Curr Biol. 2016;26:410–7. doi: 10.1016/j.cub.2015.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Blasio A, Ripari P, Bucci I, et al. Walking training in postmenopause: effects on both spontaneous physical activity and training-induced body adaptations. Menopause. 2012;19:23–32. doi: 10.1097/gme.0b013e318223e6b3. [DOI] [PubMed] [Google Scholar]
- 5.Goran MI, Poehlman ET. Endurance training does not enhance total energy expenditure in healthy elderly persons. Am J Physiol. 1992;263(5 Pt 1):E950–7. doi: 10.1152/ajpendo.1992.263.5.E950. [DOI] [PubMed] [Google Scholar]
- 6.Manthou E, Gill JM, Wright A, Malkova D. Behavioral compensatory adjustments to exercise training in overweight women. Med Sci Sports Exerc. 2010;42:1121–8. doi: 10.1249/MSS.0b013e3181c524b7. [DOI] [PubMed] [Google Scholar]
- 7.Meijer EP, Westerterp KR, Verstappen FT. Effect of exercise training on total daily physical activity in elderly humans. Eur J Appl Physiol Occup Physiol. 1999;80:16–21. doi: 10.1007/s004210050552. [DOI] [PubMed] [Google Scholar]
- 8.Meijer GA, Janssen GM, Westerterp KR, Verhoeven F, Saris WH, ten Hoor F. The effect of a 5-month endurance-training programme on physical activity: evidence for a sex-difference in the metabolic response to exercise. Eur J Appl Physiol Occup Physiol. 1991;62:11–7. doi: 10.1007/BF00635626. [DOI] [PubMed] [Google Scholar]
- 9.Morio B, Montaurier C, Pickering G, et al. Effects of 14 weeks of progressive endurance training on energy expenditure in elderly people. Br J Nutr. 1998;80:511–9. doi: 10.1017/s0007114598001603. [DOI] [PubMed] [Google Scholar]
- 10.Westerterp KR, Meijer GA, Janssen EM, Saris WH, Ten Hoor F. Long-term effect of physical activity on energy balance and body composition. Br J Nutr. 1992;68:21–30. doi: 10.1079/bjn19920063. [DOI] [PubMed] [Google Scholar]
- 11.Melanson EL, Keadle SK, Donnelly JE, Braun B, King NA. Resistance to exercise-induced weight loss: compensatory behavioral adaptations. Med Sci Sports Exerc. 2013;45:1600–9. doi: 10.1249/MSS.0b013e31828ba942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper JA, Nguyen DD, Ruby BC, Schoeller DA. Maximal sustained levels of energy expenditure in humans during exercise. Med Sci Sports Exerc. 2011;43:2359–67. doi: 10.1249/MSS.0b013e31822430ed. [DOI] [PubMed] [Google Scholar]
- 13.Saris WH, van Erp-Baart MA, Brouns F, Westerterp KR, ten Hoor F. Study on food intake and energy expenditure during extreme sustained exercise: the Tour de France. Int J Sports Med. 1989;10(Suppl 1):S26–31. doi: 10.1055/s-2007-1024951. [DOI] [PubMed] [Google Scholar]
- 14.Henry CJ. Basal metabolic rate studies in humans: measurement and development of new equations. Public Health Nutr. 2005;8:1133–52. doi: 10.1079/phn2005801. [DOI] [PubMed] [Google Scholar]
- 15.Dulloo AG, Jacquet J, Solinas G, Montani JP, Schutz Y. Body composition phenotypes in pathways to obesity and the metabolic syndrome. Int J Obes (Lond) 2010;34(Suppl 2):S4–17. doi: 10.1038/ijo.2010.234. [DOI] [PubMed] [Google Scholar]
- 16.D’Alessio DA, Kavle EC, Mozzoli MA. Thermic effect of food in lean and obese men. J Clin Invest. 1988;81:1781–9. doi: 10.1172/JCI113520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Jonge L, Bray GA. The thermic effect of food and obesity: a critical review. Obes Res. 1997;5:622–31. doi: 10.1002/j.1550-8528.1997.tb00584.x. [DOI] [PubMed] [Google Scholar]
- 18.Weinsier RL, Nelson KM, Hensrud DD, Darnell BE, Hunter GR, Schutz Y. Metabolic predictors of obesity. Contribution of resting energy expenditure, thermic effect of food, and fuel utilization to four-year weight gain of post-obese and never-obese women. J Clin Invest. 1995;95:980–5. doi: 10.1172/JCI117807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levine JA. Non-exercise activity thermogenesis. Proc Nutr Soc. 2003;62:667–79. doi: 10.1079/PNS2003281. [DOI] [PubMed] [Google Scholar]
- 20.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332:621–8. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 21.Weyer C, Walford RL, Harper IT, Milner M, MacCallum T, Tataranni PA, Ravussin E, et al. Energy metabolism after 2 y of energy restriction: the biosphere 2 experiment. Am J Clin Nutr. 2000;72:946–53. doi: 10.1093/ajcn/72.4.946. [DOI] [PubMed] [Google Scholar]
- 22.Dulloo AG, Jacquet J, Montani JP, Schutz Y. Adaptive thermogenesis in human body weight regulation: more of a concept than a measurable entity? Obes Rev. 2012;13(Suppl 2):105–21. doi: 10.1111/j.1467-789X.2012.01041.x. [DOI] [PubMed] [Google Scholar]
- 23.Loucks AB, Thuma JR. Luteinizing hormone pulsatility is disrupted at a threshold of energy availability in regularly menstruating women. J Clin Endocrinol Metab. 2003;88:297–311. doi: 10.1210/jc.2002-020369. [DOI] [PubMed] [Google Scholar]
- 24.Hollowell RP, Willis LH, Slentz CA, Topping JD, Bhakpar M, Kraus WE. Effects of exercise training amount on physical activity energy expenditure. Med Sci Sports Exerc. 2009;41:1640–4. doi: 10.1249/MSS.0b013e31819c71a4. [DOI] [PubMed] [Google Scholar]
- 25.Westerterp KR. Assessment of physical activity: a critical appraisal. Eur J Appl Physiol. 2009;105:823–8. doi: 10.1007/s00421-009-1000-2. [DOI] [PubMed] [Google Scholar]
- 26.Herrmann SD, Willis EA, Honas JJ, Lee J, Washburn RA, Donnelly JE. Energy intake, nonexercise physical activity, and weight loss in responders and nonresponders: The Midwest Exercise Trial 2. Obesity (Silver Spring) 2015;23:1539–49. doi: 10.1002/oby.21073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pontzer H, Raichlen DA, Wood BM, Mabulla AZ, Racette SB, Marlowe FW. Hunter-gatherer energetics and human obesity. PLoS One. 2012;7(7):e40503. doi: 10.1371/journal.pone.0040503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Church TS, Martin CK, Thompson AM, Earnest CP, Mikus CR, Blair SN. Changes in weight, waist circumference and compensatory responses with different doses of exercise among sedentary, overweight postmenopausal women. PLoS One. 2009;4(2):e4515. doi: 10.1371/journal.pone.0004515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wishnofsky M. Caloric equivalents of gained or lost weight. Am J Clin Nut. 1958;6:542–6. doi: 10.1093/ajcn/6.5.542. [DOI] [PubMed] [Google Scholar]
- 30.Hall KD, Sacks G, Chandramohan D, et al. Quantification of the effect of energy imbalance on bodyweight. Lancet. 2011;378:826–37. doi: 10.1016/S0140-6736(11)60812-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dhurandhar EJ, Kaiser KA, Dawson JA, Alcorn AS, Keating KD, Allison DB. Predicting adult weight change in the real world: a systematic review and meta-analysis accounting for compensatory changes in energy intake or expenditure. Int J Obes (Lond) 2015;39:1181–7. doi: 10.1038/ijo.2014.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stubbs RJ, Hughes DA, Johnstone AM, et al. Rate and extent of compensatory changes in energy intake and expenditure in response to altered exercise and diet composition in humans. Am J Physiol Regul Integr Comp Physiol. 2004;286:R350–8. doi: 10.1152/ajpregu.00196.2003. [DOI] [PubMed] [Google Scholar]
- 33.Stubbs RJ, Sepp A, Hughes DA, et al. The effect of graded levels of exercise on energy intake and balance in free-living men, consuming their normal diet. Eur J Clin Nutr. 2002;56:129–40. doi: 10.1038/sj.ejcn.1601295. [DOI] [PubMed] [Google Scholar]
- 34.Stubbs RJ, Sepp A, Hughes DA, et al. The effect of graded levels of exercise on energy intake and balance in free-living women. Int J Obes Relat Metab Disord. 2002;26:866–9. doi: 10.1038/sj.ijo.0801874. [DOI] [PubMed] [Google Scholar]
- 35.Whybrow S, Hughes DA, Ritz P, et al. The effect of an incremental increase in exercise on appetite, eating behaviour and energy balance in lean men and women feeding ad libitum. Br J Nutr. 2008;100:1109–15. doi: 10.1017/S0007114508968240. [DOI] [PubMed] [Google Scholar]
- 36.McLaughlin R, Malkova D, Nimmo MA. Spontaneous activity responses to exercise in males and females. Eur J Clin Nutr. 2006;60:1055–61. doi: 10.1038/sj.ejcn.1602417. [DOI] [PubMed] [Google Scholar]
- 37.Alahmadi MA, Hills AP, King NA, Byrne NM. Exercise intensity influences nonexercise activity thermogenesis in overweight and obese adults. Med Sci Sports Exerc. 2011;43:624–31. doi: 10.1249/MSS.0b013e3181f7a0cb. [DOI] [PubMed] [Google Scholar]
- 38.Owen N, Sparling PB, Healy GN, Dunstan DW, Matthews CE. Sedentary behavior: emerging evidence for a new health risk. Mayo Clin Proc. 2010;85:1138–41. doi: 10.4065/mcp.2010.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Owen N, Healy GN, Matthews CE, Dunstan DW. Too much sitting: the population health science of sedentary behavior. Exerc Sport Sci Rev. 2010;38:105–13. doi: 10.1097/JES.0b013e3181e373a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matthews CE, Chen KY, Freedson PS, et al. Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am J Epidemiol. 2008;167:875–81. doi: 10.1093/aje/kwm390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mutrie N, Doolin O, Fitzsimons CF, et al. Increasing older adults’ walking through primary care: results of a pilot randomized controlled trial. Fam Pract. 2012;29:633–42. doi: 10.1093/fampra/cms038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kozey-Keadle S, Libertine A, Lyden K, Staudenmayer J, Freedson PS. Validation of wearable monitors for assessing sedentary behavior. Med Sci Sports Exerc. 2011;43:1561–7. doi: 10.1249/MSS.0b013e31820ce174. [DOI] [PubMed] [Google Scholar]
- 43.Grant PM, Dall PM, Mitchell SL, Granat MH. Activity-monitor accuracy in measuring step number and cadence in community-dwelling older adults. J Aging Phys Act. 2008;16:201–14. doi: 10.1123/japa.16.2.201. [DOI] [PubMed] [Google Scholar]
- 44.Kozey-Keadle S, Libertine A, Staudenmayer J, Freedson P. The Feasibility of Reducing and Measuring Sedentary Time among Overweight, Non-Exercising Office Workers. J Obes. 2012;2012:282303. doi: 10.1155/2012/282303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Owen N, Sugiyama T, Eakin EE, Gardiner PA, Tremblay MS, Sallis JF. Adults’ sedentary behavior determinants and interventions. Am J Prev Med. 2011;41:189–96. doi: 10.1016/j.amepre.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 46.Mathot KJ, Dingemanse NJ. Energetics and behavior: unrequited needs and new directions. Trends Ecol Evol. 2015;30:199–206. doi: 10.1016/j.tree.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 47.Scantlebury DM, Mills MG, Wilson RP, et al. Mammalian energetics. Flexible energetics of cheetah hunting strategies provide resistance against kleptoparasitism. Science. 2014;346:79–81. doi: 10.1126/science.1256424. [DOI] [PubMed] [Google Scholar]
- 48.Zub K, Szafranska PA, Konarzewski M, Speakman JR. Effect of energetic constraints on distribution and winter survival of weasel males. J Anim Ecol. 2011;80:259–69. doi: 10.1111/j.1365-2656.2010.01762.x. [DOI] [PubMed] [Google Scholar]
- 49.FAO/WHO/UNU. Human energy requirements. 2001. (Food and Nutrition Technical Report Series 1). [Google Scholar]
- 50.Donnelly JE, Hill JO, Jacobsen DJ, et al. Effects of a 16-month randomized controlled exercise trial on body weight and composition in young, overweight men and women: the Midwest Exercise Trial. Arch Intern Med. 2003;163:1343–50. doi: 10.1001/archinte.163.11.1343. [DOI] [PubMed] [Google Scholar]
- 51.Hunter GR, Wetzstein CJ, Fields DA, Brown A, Bamman MM. Resistance training increases total energy expenditure and free-living physical activity in older adults. J Appl Physiol. 2000;89:977–84. doi: 10.1152/jappl.2000.89.3.977. [DOI] [PubMed] [Google Scholar]
- 52.Perrigo G, Bronson FH. Foraging effort, food intake, fat deposition and puberty in female mice. Biol Reprod. 1983;29:455–63. doi: 10.1095/biolreprod29.2.455. [DOI] [PubMed] [Google Scholar]
- 53.Pontzer H, Raichlen DA, Gordon AD, et al. Primate energy expenditure and life history. Proc Natl Acad Sci (USA) 2014;111:1433–7. doi: 10.1073/pnas.1316940111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nie Y, Speakman JR, Wu Q, et al. Exceptionally low daily energy expenditure in the bamboo-eating giant panda. Science. 2015;349:171–4. doi: 10.1126/science.aab2413. [DOI] [PubMed] [Google Scholar]
- 55.Luke A, Dugas LR, Ebersole K, et al. Energy expenditure does not predict weight change in either Nigerian or African American women. Am J Clin Nutr. 2009;89:169–76. doi: 10.3945/ajcn.2008.26630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dugas LR, Harders R, Merrill S, et al. Energy expenditure in adults living in developing compared with industrialized countries: a meta-analysis of doubly labeled water studies. Am J Clin Nutr. 2011;93:427–41. doi: 10.3945/ajcn.110.007278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen KY, Brychta RJ, Linderman JD, et al. Brown fat activation mediates cold-induced thermogenesis in adult humans in response to a mild decrease in ambient temperature. J Clin Endocrinol Metab. 2013;98:E1218–23. doi: 10.1210/jc.2012-4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Modell H, Cliff W, Michael J, McFarland J, Wenderoth MP, Wright A. A physiologist’s view of homeostasis. Adv Physiol Educ. 2015;39:259–66. doi: 10.1152/advan.00107.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]


