Abstract
In this study, we describe a multiplex PCR to detect a AGC→ACC (serine to threonine) mutation in the katG gene and a −15 C-to-T substitution (inhAC−15T) at the 5′ end of a presumed ribosome binding site in the promoter of the mabA-inhA operon. These mutations have been reported in the majority of previous studies as the most frequent mutations involved in the resistance to isoniazid (INH) of Mycobacterium tuberculosis clinical strains with high levels of resistance. The method was optimized and validated after an analysis of 30 M. tuberculosis clinical isolates with known sequences of the relevant part of the katG gene and the regulatory region of the mabA-inhA operon. We analyzed 297 INH-resistant M. tuberculosis isolates collected in Spain from 1996 to 2003 by PCR-restriction fragment length polymorphism (using the katG gene), DNA sequencing, and the newly developed multiplex PCR. The results were concordant for all 297 isolates tested. The analysis revealed that 204 (68.7%) of the isolates carried one or both of the mutations. This finding suggests that with further development this multiplex PCR will be able to detect the majority of the INH-resistant M. tuberculosis clinical isolates from Spain and other countries where a high frequency of similar mutations occur.
Currently, throughout the world, isoniazid (INH) and rifampin (RIF) together represent the backbone of short-course chemotherapy for Mycobacterium tuberculosis infections. The number of multidrug-resistant strains of M. tuberculosis, defined as resistant to INH and RIF, has been increasing over the years, and several outbreaks have been reported (5, 9, 24). The development of resistance to these two drugs reduces the efficacy of standard antituberculosis (anti-TB) treatment to 77%. It is very important, therefore, to identify these strains as soon as possible to allow for adjustments in treatment and to minimize the transmission of drug-resistant strains. Phenotypic drug susceptibility testing by conventional methods on solid media (6, 8) requires 10 to 30 days after the primary culture has been isolated. This time can be reduced by the use of rapid methods such as BACTEC, which requires 5 to 10 days.
Resistance to RIF has been shown to be caused by an alteration of the β subunit of RNA polymerase, which is encoded by the rpoB gene. More than 95% of RIF-resistant strains are associated with mutations within an 81-bp region of the rpoB gene (encoding the RNA polymerase β subunit). Specific mutations, insertions, and deletions have been described in several countries by several authors, and this 81-bp region has been termed the rifampin resistance determinant region (7, 14, 20, 26, 27, 30). Numerous methods exist to detect resistance to rifampin (10, 12, 18, 23).
In contrast, resistance to INH is more complicated, as mutations in several genes can lead to drug resistance. For most INH-resistant strains, mutations have been found in two genes, i.e., the katG gene, encoding catalase-peroxidase (31), and the mabA-inhA regulon (4), encoding a target of activated prodrug, enoyl-acyl carrier protein reductase (1-3, 11, 13, 15, 17, 21, 27). For some other INH-resistant strains, however, mutations in the ahpC promoter region (located in the 105-bp oxyR-ahpC intergenic region) or within the β-ketocyl acyl carrier protein synthase gene kasA have also been reported (19, 25). Most studies have examined the mutations present in these genes by DNA sequencing or analyses of a portion of the katG gene after PCR amplification and digestion with the restriction enzyme MspI or SatI (2, 3, 17).
Molecular methods have been developed to detect resistance to INH and RIF as an alternative to conventional tests because of their ability to provide results rapidly. Upon the elucidation of the genes involved in resistance to RIF and INH, several studies describing various PCR-based molecular genetic techniques for the detection of resistance were published (12).
In the present study, we report a simple, rapid, and inexpensive assay based on allele-specific PCR methodology targeting an AGC→ACC mutation in the katG gene and an inhAC−15T mutation in the regulatory region of the mabA-inhA operon to detect INH-resistant M. tuberculosis strains and to identify the M. tuberculosis complex in the same PCR tube for each sample.
MATERIALS AND METHODS
Description of study samples. (i) Set one.
Thirty M. tuberculosis strains with known sequences for the relevant part of the katG gene and the regulatory region of the mabA-inhA operon served as samples for optimization of the PCR assay conditions. The strains had the following distribution of katG and mabA-inhA operon alleles: wild type, 6 strains; AGC→ACC mutation, 10 strains; inhAC−15T mutation, 10 strains; and both the AGC→ACC and inhAC−15T mutations, 4 strains.
(ii) Set two.
A total of 297 isolates collected from 1996 to 2003 in Spain and identified as INH-resistant M. tuberculosis strains by standard biochemical methods served to validate the developed multiplex PCR assay. Susceptibility testing with INH (0.2 and 1.0 μg/ml) was performed with Lowenstein-Jensen medium by the proportional method of Canetti et al. (6). A total of 50 M. tuberculosis strains that were susceptible to isoniazid were used as controls.
Extraction of DNAs for PCR.
DNAs were extracted from cultures grown in Lowenstein-Jensen medium. A loop of culture was suspended in 500 μl of distilled water and boiled for 5 min, followed by centrifugation for 5 min at 12,000 × g, and 5 μl of the supernatant was used as a source of genomic DNA for amplification of the katG gene and the mabA-inhA operon.
PCR-RFLP.
katG restriction fragment length polymorphism (RFLP) analysis to detect codon 315 mutations was performed by the use of previously described protocols and reaction components (23). A 620-bp region of the katG gene was amplified by use of the following primer set described by Uhl et al. (28): katG904, 5′-AGCTCGTATGGCACCGGAAC; and katG1523, 5′-TTGACCTCCCACCCGACTTG. After amplification, 10 μl of the product was digested with MspI (MBI Fermentas, Madrid, Spain) and analyzed by 2% MS8 agarose (8066; Pronadisa, Madrid, Spain) gel electrophoresis. Ten microliters of the same katG-specific PCR product was digested with SatI (MBI Fermentas) and analyzed in the same run with products generated by restriction with MspI (Fig. 1). SatI contains an overlapping GC sequence at a single recognition site of the triplet AGC at position 315, and any mutation at this position eliminates cleavage of the katG fragment.
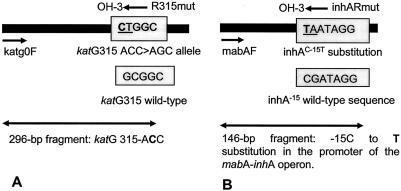
FIG. 1.
Schematic presentation of multiplex allele-specific PCR to detect the AGC→ACC mutation in codon 315 of the katG gene (A) and the −15 C-to-T substitution (inhAC−15T) at the 5′ end of a presumed ribosome binding site in the promoter of the mabA-inhA operon (B). Short arrows indicate the primers, and long double-headed arrows depict the resulting PCR fragments. The targeted sequences are shown in shaded boxes. The mutated bases are underlined and in bold, and the mutated bases are underlined.
PCR and DNA sequencing.
For PCRs and DNA sequencing, specially designed primers (for regions in the katG gene and the regulatory region of the mabA-inhA operon that are known to confer resistance to M. tuberculosis) were used to amplify genomic DNAs. These primers were designed from a published sequence of the M. tuberculosis H37Rv genome (GenBank) by the use of PCGENE software (IntelliGenetics, Mountain View, Calif.).
PCRs were performed with a pureTaq Ready-To-Go PCR bead kit (Amersham Biosciences, Piscataway, N.J.). PCRs were performed in a 9600 thermocycler (Perkin-Elmer) under the same PCR conditions for both katG and mabA-inhA (an initial denaturation step of 5 min at 94°C, followed by 30 cycles of 1 min at 94°C, 1 min at 65°C, and 90 s at 72°C and ending with a final elongation step of 10 min at 72°C). Efficient PCR amplifications were confirmed by gel electrophoresis in 0.7% MS8 agarose gels (8066; Pronadisa). PCR products were purified by use of a GFX PCR DNA and gel band purification kit (Amersham Biosciences) and were sequenced by use of a Big Dye Terminator cycle sequencing ready reaction kit (Applied Biosystems Inc., Foster City, Calif.) in an ABI Prism 377 automated DNA sequencer (Applied Biosystems). The following thermocycler parameters were used: denaturation at 94°C for 3 min, followed by 25 cycles of denaturation at 96°C for 10 s and primer annealing at 60°C for 4 min. The primer sequences are shown in Table 1.
TABLE 1.
Primer sequences
| Primer target or type | Primer designation | Sequence |
|---|---|---|
| Sequencing primers | ||
| katG | katGF | GTGCCCGAGCAACACCCACC |
| katGR | CGCACGTCGAACCTGTCGAGG | |
| mabA-inhA | mabAF | CGAAGTGTGCTGAGTCACACCG |
| inhAR | CCCCACCGAAATGCAGGTCG | |
| Multiplex PCR primers | ||
| gyrB | MTUBf | TCGGACGCGTATGCGATATC |
| MTUBr | ACATACAGTTCGGACTTGCG | |
| katG | katg0F | GCAGATGGGGCTGATCTACG |
| R315mut | TCCATACGACCTCGATGCCAG | |
| mabA-inhA | mabAF | CGAAGTGTGCTGAGTCACACCG |
| inhARmut | AGTCACCCCGACAACCTATTA |
All sequence data were assembled and edited electronically with the EDITSEQ, ALIGN, and MEGALIGN programs (DNASTAR, Madison, Wis.) and were compared with the published sequences for the katG gene and the mabA-inhA operon (GenBank accession number NC_000962).
Multiplex PCR.
Specially designed primers were used to detect the AGC→ACC and inhAC−15T mutations. The reverse primer R315mut was positioned so that its 3′-OH end paired with two changed bases, i.e., a GC-to-CT substitution (Fig. 1A). Consequently, in the presence of the AGC→ACC mutation at this position in katG, a 296-bp fragment was amplified by the forward primer katg0F (described previously by Mokrousov et al. [22]). On the other hand, the reverse primer inhARmut was positioned so that its 3′-OH end paired with two changed bases, a −15 C-to-T substitution (inhAC−15T) and a −14 G-to-A substitution at the 5′ end of a presumed ribosome binding site in the promoter of the mabA-inhA operon (Fig. 1B). If the inhAC−15T mutation occurred, a 146-bp fragment was amplified by the forward primer mabAF. In the absence of a mutation, the result would be two mismatches at the 3′ end of the reverse primers (R315mut and inhARmut). Thus, under appropriate PCR conditions, resistant strains with these two mutations resulted in 296-bp and/or 146-bp PCR products. The MTUBf and MTUBr primers (previously reported by Kasai et al. [16]) produced a 1,020-bp fragment by amplification of a partial sequence of the gyrB gene. This fragment was the individual positive control and needed to be present in every sample. The absence of this amplification product would show that the PCR was inhibited and that the result should be ignored.
The PCR mix consisted of 10 mM Tris-HCl (pH 9), 50 mM KCl, 1.5 mM MgCl2, a 200 μM concentration of each deoxynucleoside triphosphate, 2.5 U of pureTaq DNA polymerase and reaction buffer, which were included in the pureTaq Ready-To-Go PCR bead kit (Amersham Biosciences), and PCR primers to a final volume of 25 μl. The concentrations of the primers were 200 mM for katG0F and 400 mM for R315mut (to detect the AGC→ACC mutation), 400 mM for both mabAF and inhARmut (to detect the inhAC−15T mutation), and 400 mM for both MTUBf and MTUBr. All primer sequences are shown in Table 1.
For PCRs, a GenAmp PCR system 9600 thermocycler (Applied Biosystems) was used, beginning with a 5-min denaturation at 95°C and followed by 30 cycles of 1 min at 95°C, 1 min at 68°C, and 45 s at 72°C. The final cycle was followed by an extension time of 10 min at 72°C. Efficient PCR amplifications and the sizes of the products were estimated after electrophoresis in 2% agarose gels by staining with ethidium bromide.
RESULTS AND DISCUSSION
An analysis of 297 INH-resistant and 50 INH-susceptible M. tuberculosis clinical isolates from TB cases by katG PCR-RFLP and automated DNA sequencing showed that none of the 50 INH-susceptible strains had the AGC→ACC mutation (S315T) at codon 315 of katG or the less frequent mutations in that codon. The AGC→ACC missense mutation was observed in 130 (43.8%) INH-resistant strains by PCR-RFLP with an MspI endonuclease treatment (Fig. 2, lanes 2 and 3), and 17 INH-resistant strains showed a different mutation in codon 315 by PCR-RFLP with a SatI endonuclease treatment (Fig. 2, lanes 10 and 11). The use of the MspI endonuclease only detected the AGC→ACC mutation, whereas SatI was able to detect any mutation in codon 315 that affected the GC nucleotides at that position. Of the 17 strains with different mutations, 12 (4.0%) showed an AGC→AAC mutation at codon 315 of katG and 5 (1.7%) showed an AGC→ACA mutation by automated DNA sequencing. The results are summarized in Table 2.
FIG. 2.
Profiles generated by PCR-restriction digestion with MspI and SatI endonucleases. Lanes 1 to 6, strains digested with MspI; lane 1, wild-type strain; lane 2, AGC→ACC mutation (S315T); lane 3, S315T mutation and R463L polymorphism; lane 4, AGC→AAC mutation at katG codon 315; lane 5, AGC→ACA mutation at katG codon 315; lane 6, wild type; lanes 7 to 12, the same strains digested with the SatI endonuclease; M, 50-bp DNA ladder (Amersham Biosciences).
TABLE 2.
Comparison of INH susceptibility test results obtained by the different methods used in this study
| No. of strains | Susceptibility of strains by indicated methoda
|
||||
|---|---|---|---|---|---|
| Proportional methodb | PCR-RFLP
|
DNA sequencingc | Multiplex PCR | ||
| MspI | SatI | ||||
| 130 | R | AGC > ACC | R | AGC > ACC | AGC > ACC |
| 12 | R | S | R | AGC > AAC | S |
| 5 | R | S | R | AGC > ACA | S |
| 70 | R | S | S | inhAC−15T | inhAC−15T |
| 4 | R | AGC > ACC | R | AGC > ACC + inhAC−15T | AGC > ACC + inhAC−15T |
| 76 | R | S | S | S | S |
| 50 | S | S | S | S | S |
R, resistant; S, susceptible. When indicated, mutations were detected by the various methods.
Performed on Lowenstein-Jensen medium.
DNA sequencing of the katG gene and the promoter of the mabA-inhA operon.
The inhAC−15T mutation was found in 70 (23.6%) INH-resistant isolates, with 4 of these strains showing the AGC→ACC mutation as detected by PCR-RFLP, and in none of the 50 INH-susceptible strains (Table 2).
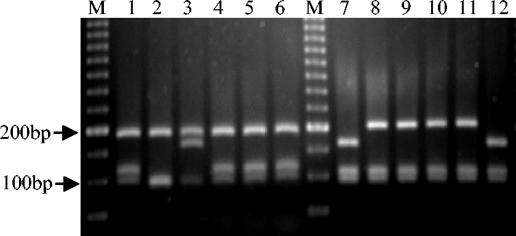
The multiplex PCR that we developed was evaluated for its detection of the AGC→ACC mutation in codon 315 of the katG gene and the inhAC−15T mutation in the promoter of the mabA-inhA operon in the same PCR tube for each sample. The 130 INH-resistant strains with the AGC→ACC mutation produced a 296-bp band (Fig. 3, lanes 1 to 4), while the 70 strains with the inhAC−15T mutation produced a 146-bp band (Fig. 3, lanes 5 to 12). The four strains with both mutations produced two bands, one at 296 bp and the other at 146 bp (Fig. 3, lanes 13 and 14). All of the strains, including the susceptible strains (Fig. 2, lanes 16 to 18), produced a 1,020-bp band by amplification of a partial sequence of the gyrB gene. The multiplex PCR results were concordant with those generated by PCR-RFLP analysis and DNA sequencing for all of the strains tested (Table 2). The specificity of the multiplex PCR for the detection of INH resistance in resistant strains carrying the AGC→ACC mutation at codon 315 of katG and/or the inhAC−15T mutation in our setting was 100%. The sensitivity of the multiplex PCR for the detection of INH resistance depended on the prevalence of these two mutations among the INH-resistant strains in each geographic area. In our setting, the sensitivity was 68.7%. The negative predictive value was 35%, and the positive predictive value was 100%. In Spain, the prevalence rates of the AGC→ACC allele at codon 315 of katG and of the inhAC−15T mutation are 47.2 and 25%, respectively (unpublished data), whilst other mutations in codon 315 are found in 6.6% of INH-resistant strains. Consequently, 72.2% of the INH-resistant strains in our country could be detected with the multiplex PCR.
FIG. 3.
Profiles generated by multiplex PCR assay. Lanes 1 to 4, strains with the AGC→ACC mutation at katG codon 315; lanes 5 to 13, strains with the −15C→T substitution in the promoter of the mabA-inhA operon; lanes 14 to 15, strains with both mutations; lanes 16 to 18, INH-susceptible strains; M, 100-bp DNA ladder (Bio Tools).
Mutations at the Ser315 codon of katG and at the regulatory region of the mabA-inhA operon occur most frequently in INH-resistant isolates (1-3, 11, 13, 15, 17, 21, 27). These mutations, AGC→ACC and inhAC−15T, respectively, may be responsible for 70% of INH-resistant M. tuberculosis cases.
The prevalence of mutations in codon 315 of katG varies depending on the geographical region studied, with percentages ranging from 35% in Beirut (2) to 91% in Latvia and Russia (21, 27), while the prevalence of the inhAC−15T mutation varied from 32.7% in a study by Bakonyte et al. (3) to 3.3% in a study by Van Rie et al. (29), with several studied strains being similar to those in Bakonyte et al.'s study. Torres et al. (26) reported that this mutation was found with a frequency of 4.3% in strains isolated from Seville, Spain, but the present study shows a much higher frequency of 23.6%. The frequency of other mutations in codon 315 (5.7%) was found to be lower than those in other studies (1, 11).
The high prevalence of the AGC→ACC mutation in the katG gene and of the inhAC−15T mutation in the promoter of the mabA-inhA operon in the strains isolated from Spain suggests that the multiplex PCR technique can be used as a single, rapid tool for detecting INH resistance in M. tuberculosis clinical strains with a high probability. The assay is easy to perform and interpret and could be implemented as part of the routine practices of clinical laboratories in areas with a high prevalence of multidrug-resistant TB strains. For easier and unambiguous interpretations of multiplex PCR profiles, each run should include a wild-type strain (as a positive control for no amplification due to mutation) and one strain with a known mutation (as a positive control of amplification due to mutation). Furthermore, the procedure is inexpensive and requires only standard PCR and electrophoresis equipment. However, all negative results should be confirmed by conventional methods based on cell culture.
Acknowledgments
We thank A. Valverde and M. A. García-Aranda for excellent technical assistance and A. Echeita and S. Herrera for their time and advice. We are indebted to Malcolm Yates for revisions of the English language in the manuscript.
This work was supported by Instituto de Salud Carlos III (0017/99).
REFERENCES
- 1.Abate, G., S. E. Hoffner, V. Ostergaard Thomsen, and H. Miöiner. 2001. Characterization of isoniazid-resistant strains of Mycobacterium tuberculosis on the basis of phenotypic properties and mutations in katG. Eur. J. Clin. Microbiol. Infect. Dis. 20:329-333. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad, S., E. Fares, G. F. Araj, T. D. Chugh, and A. S. Mustafa. 2002. Prevalence of S315T mutation within the katG gene in isoniazid-resistant clinical Mycobacterium tuberculosis isolates from Dubai and Beirut. Int. J. Tuberc. Lung Dis. 6:920-926. [PubMed] [Google Scholar]
- 3.Bakonyte, D., A. Baranauskaite, J. Cicenaite, A. Sosnovskaya, and P. Satakenas. 2003. Molecular characterization of isoniazid-resistant Mycobacterium tuberculosis clinical isolates in Lithuania. Antimicrob. Agents Chemother. 47:2009-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banerjee, A., E. Dubnau, A. Quemard, U. Balasubramanian, K. S. Um, T. Wilson, D. Collins, G. Lisle, and W. R. Jacobs, Jr. 1994. inhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis. Science 263:227-230. [DOI] [PubMed] [Google Scholar]
- 5.Bifani, P. J., B. Plikaytis, V. Kapur, K. Stockbauer, X. Pan, M. L. Lutfey, S. L. Moghazeh, W. Eisner, T. M. Daniel, M. H. Kaplan, J. T. Crawford, J. M. Musser, and B. N. Kreiswirth. 1996. Origin and interstate spread of New York City multidrug-resistant Mycobacterium tuberculosis clone family. JAMA 275:452-457. [PubMed] [Google Scholar]
- 6.Canetti, G., N. Rist, and J. Grosset. 1963. Mesure de la sensibilité du bacilli tuberculeux aux drogues antibactériens port le méthode des proportions. Rev. Tuberc. Pneumol. 27:217-272. [PubMed] [Google Scholar]
- 7.Cavusoglu, C., S. Himioglu, S. Guneri, and A. Bilgic. 2002. Characterization of rpoB mutations in rifampin-resistant clinical isolates of Mycobacterium tuberculosis from Turkey by DNA sequencing and line probe assay. J. Clin. Microbiol. 40:4435-4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins, C. H., J. M. Grange, and M. D. Yates. 1997. Tuberculosis bacteriology: organization and practice. Butterworth-Heinemann, Oxford, United Kingdom.
- 9.Davies, P. D. 2003. The worldwide increase in tuberculosis: how demographic changes, HIV, infection and increasing numbers in poverty are increasing tuberculosis. Ann. Med. 35:235-243. [DOI] [PubMed] [Google Scholar]
- 10.De Beenhouwer, H., Z. Liang, G. Jannes, W. Mijs, L. Machtelinckx, R. Rossau, H. Traore, and F. Portaels. 1995. Rapid detection of rifampin resistance in sputum and biopsy specimens from tuberculosis patients by PCR and line probe assay. Tuberc. Lung. Dis. 76:425-430. [DOI] [PubMed] [Google Scholar]
- 11.Dobner, P., S. Rüsch-Gerdes, G. Bretzel, K. Felsmann, M. Rifai, T. Löscher, and H. Hinder. 1997. Usefulness of Mycobacterium tuberculosis genomic mutations in the genes katG and inhA for the prediction of isoniazid resistance. Int. J. Tuberc. Lung Dis. 1:365-369. [PubMed] [Google Scholar]
- 12.García de Viedma, D. 2003. Rapid detection of resistance in Mycobacterium tuberculosis: a review discussing molecular approaches. Clin. Microbiol. Infect. 9:349-359. [DOI] [PubMed] [Google Scholar]
- 13.Haas, W. H., K. Schilke, J. Brand, B. Amthor, K. Weyer, P. Fourie, G. Bretzel, V. Sticht-Groh, and H. Bremer. 1997. Molecular analysis of katG gene mutations in strains of Mycobacterium tuberculosis complex from Africa. Antimicrob. Agents Chemother. 41:1601-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herrera, L., M. S. Jiménez, A. Valverde, M. A. García-Aranda, and J. A Sáez-Nieto. 2003. Molecular analysis of rifampin-resistant Mycobacterium tuberculosis isolated in Spain (1996-2001). Description of new mutations in the rpoB gene and review of the literature. Int. J. Antimicrob. Agents 21:403-408. [DOI] [PubMed] [Google Scholar]
- 15.Herrera, L., A. Valverde, P. Saíz, J. A. Saéz-Nieto, J. L. Portero, and M. S. Jiménez. 2004. Molecular characterization of isoniazid-resistant Mycobacterium tuberculosis clinical strain isolates in The Philippines. Int. J. Antimicrob. Agents 23:572-576. [DOI] [PubMed] [Google Scholar]
- 16.Kasai, H., T. Ezaki, and S. Harayama. 2000. Differentiation of phylogenetically related slowly growing mycobacteria by their gyrB sequences. J. Clin. Microbiol. 38:301-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiepiela, P., K. S. Bishop, A. N. Smith, L. Roux, and D. F. York. 2000. Genomic mutations in the katG, inhA and ahpC genes are useful for the prediction of isoniazid resistance in Mycobacterium tuberculosis isolates from Kwazululu Natal, South Africa. Tuberc. Lung Dis. 80:47-56. [DOI] [PubMed] [Google Scholar]
- 18.Kim, B., K. Lee, B. Park, S. Kim, E. Park, Y. Park, G. Bai, S. Kim, and Y. Kook. 2001. Detection of rifampin-resistant Mycobacterium tuberculosis in sputa by nested PCR-linked single-strand conformation polymorphism and DNA sequencing. J. Clin. Microbiol. 39:2610-2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee, A. S. G., I. H. K. Lim, L. L. H. Tang, A. Telenti, and S. Y. Wong. 1999. Contribution of kasA analysis to detection of isoniazid-resistant Mycobacterium tuberculosis in Singapore. Antimicrob. Agents Chemother. 43:2087-2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mani, C., N. Selvakumar, S. Narayanan, and P. R. Narayanan. 2001. Mutations in the rpoB gene of multidrug-resistant Mycobacterium tuberculosis clinical isolates from India. J. Clin. Microbiol. 39:2987-2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martilla, H., H. Soini, E. Eerola, E. Vyshnevskaya, B. Vyshnevskiy, T. Otten, A. Vasilyef, and M. Viljanen. 1998. A Ser315Thr substitution in KatG is predominant in genetically heterogeneous multidrug-resistant Mycobacterium tuberculosis isolates originating from the St. Petersburg area in Russia. Antimicrob. Agents Chemother. 42:2443-2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mokrousov, I., T. Otten, A. Vyazovaya, E. Limeschenko, M. L. Filipenko, C. Sola, N. Rastogi, L. Steklova, B. Vyshnevskiy, and O. Narvskaya. 2003. PCR-based methodology for detecting multidrug-resistant strains of Mycobacterium tuberculosis Beijing family circulating in Russia. Eur. J. Clin. Microbiol. Infect. Dis. 22:342-348. [DOI] [PubMed] [Google Scholar]
- 23.Nachamkin, I., C. Kang, and P. Weinstein. 1997. Detection of resistance to isoniazid, rifampin and streptomycin in clinical isolates of Mycobacterium tuberculosis by molecular methods. Clin. Infect. Dis. 24:894-900. [DOI] [PubMed] [Google Scholar]
- 24.Narvskaya, O., T. Otten, E. Limeschenco, N. Sapozhnikova, N. Graschenkova, L. Steklova, A. Nikonova, M. L. Filipenko, I. Mokrousov, and B. Vyshnevskiy. 2002. Nosocomial outbreak of multidrug-resistant tuberculosis caused by a strain of Mycobacterium tuberculosis W-Beijing family in St. Petersburg, Russia. Eur. J. Clin. Microbiol. Infect. Dis. 21:596-602. [DOI] [PubMed] [Google Scholar]
- 25.Sreevatsan, S., X. Pan, Y. Zhang, V. Deretic, and J. M. Musser. 1997. Analysis of the oxyR-ahpC region in isoniazid-resistant and -susceptible Mycobacterium tuberculosis complex organisms recovered from diseased humans and animals in diverse localities. Antimicrob. Agents Chemother. 41:600-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torres, M. J., A. Criado, N. Gónzalez, J. C. Palomares, and J. Aznar. 2002. Rifampin and isoniazid resistance associated mutations in Mycobacterium tuberculosis clinical isolates in Sevilla, Spain. Int. J. Tuberc. Lung Dis. 6:160-163. [PubMed] [Google Scholar]
- 27.Tracevska, T., I. Jansone, L. Broka, O. Marga, and V. Baumanis. 2002. Mutations in the rpoB and katG genes leading to drug resistance in Mycobacterium tuberculosis in Latvia. J. Clin. Microbiol. 40:3789-3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uhl, J. R, B. Kline, and L. Abu-Khader. 1995. Association of two point mutation in the catalase-peroxidase (katG) gene of Mycobacterium tuberculosis with isoniazid resistance, abstr. U46, p. 125. Abstr. 95th Gen. Meet. Am. Soc. Microbiol. 1995. American Society for Microbiology, Washington, D.C.
- 29.Van Rie, A., R. Warren, I. Mshanga, A. M. Jordaan, G. D. Van der Spuy, M. Richardson, J. Simpson, R. P. Gie, D. A. Enarson, N. Beyers, P. D. Van Helden, and T. C. Victor. 2001. Analysis for a limited number of gene codons can predict drug resistance of Mycobacterium tuberculosis in a high-incidence community. J. Clin. Microbiol. 39:636-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yue, J., W. Shi, J. Xie, Y. Li, E. Zeng, and H. Wang. 2003. Mutation in the rpoB gene of multidrug-resistant Mycobacterium tuberculosis isolates from China. J. Clin. Microbiol. 41:2209-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang, Y., B. Heym, B. Allen, D. Young, and S. T. Cole. 1992. The catalase peroxidase gene and isoniazid resistance in Mycobacterium tuberculosis. Nature 358:501-593. [DOI] [PubMed] [Google Scholar]