Abstract
Background and Aim: Autonomic symptoms in Parkinson’s disease (PD) are very common and contribute to the severity of patient’s disability. We evaluated the occurrence of autonomic symptoms in Greek patients with PD utilizing the Scales for Outcomes in Parkinson’s Disease-Autonomic questionnaire (SCOPA-AUT), a specific 23-item self-completed questionnaire for the assessment of autonomic dysfunction in patients with PD. Subjects and Methods: One hundred and sixty-one PD patients and forty matched controls were enrolled in the study. Clinical assessment was performed with the Hoehn and Yahr scale. Patients completed a demographic questionnaire, the Non-Motor Symptoms Questionnaire (NMSQuest), the Parkinson’s Disease Questionnaire (PDQ-39) and the SCOPA-AUT scale which was properly translated into Greek and validated for the study. Results: SCOPA-AUT scale showed a good reliability profile and correlated well with other measures for non-motor symptoms and health-related quality measures in PD patients. PD patients scored higher than controls in the total SCOPA -AUT score (mean score 11.9 versus 6.4). Patients reported problems in many items of the SCOPA-AUT, but the most common autonomic symptoms emerged in the Urinary and the Gastrointestinal domains. Especially sialorrhea, constipation, straining for defecation, incontinence and nocturia differentiated patients from controls. Furthermore, mean total SCOPA-AUT score correlated with duration and severity of the disease. Conclusion: Autonomic symptoms in PD are too important to remain undetected. By incorporating into everyday practice the use of suitable and reliable questionnaires, physicians will be able to adequately detect and manage these symptoms. Hippokratia 2016, 20(2):115-120
Keywords: Parkinson’s disease, autonomic function, SCOPA-AUT, non-motor symptoms
Introduction
Parkinson’s disease (PD) is a common neurodegenerative disorder characterized by motor symptomatology, such as tremor at rest, rigidity, postural instability, bradykinesia, and freezing episodes. However, the clinical spectrum of PD is more extensive covering a wide range of non-motor symptoms, including cognitive and behavioral impairment, sleep disorders, autonomic and sensory symptoms (pain, hyposmia) as well as fatigue1,2. The widespread degeneration beyond the substantia nigra in other brain regions is responsible for the various motor and non-motor symptoms3.
Autonomic symptoms, including deficits in cardiovascular, gastrointestinal, genitourinary, pupillomotor, respiratory and thermoregulatory functions, are common in PD4-6. Their reported prevalence ranges from 2 % to 80 %5,7,8. They may be present in the early stages of the disease, sometimes even before the classic motor symptoms become apparent9,10. Autonomic dysfunction in PD is a serious problem that has a great impact on daily life functioning and health-related quality of life of the patients. Therefore the identification and assessment of autonomic dysfunction seem to be of great importance in clinical practice in order to avoid complications and require appropriate treatment. Visser et al8 developed a specific patient-reported questionnaire that assesses autonomic symptoms in PD: the ‘Scales for Outcomes in Parkinson’s Disease-Autonomic questionnaire (SCOPA-AUT)’. SCOPA-AUT is an easily self-administered questionnaire for assessment of the frequency and burden of autonomic dysfunction in PD.
The purpose of our study was to evaluate the occurrence of autonomic symptoms in Greek patients with PD utilizing the SCOPA-AUT and to determine a relationship between autonomic symptoms and disease-related parameters.
Subjects and Methods
Subjects
One hundred and sixty-one patients with PD (diagnosed according to the U.K. PD Brain Bank criteria11) were included in the study. Exclusion criteria were dementia, sensorial deficit (blindness), severe concomitant illness (stroke, injury e.t.c.), pharmacological effect (dopamine antagonists e.t.c.), and inability to answer questionnaires. The study was carried out at the 3rd University Department of Neurology of the Aristotle University of Thessaloniki, between 2010 and 2015 after obtaining the approval of the Ethical Committee of G.Papanicolaou hospital (1st/19-1-2010). Forty healthy subjects served as a control population. Both patients and controls provided informed consent.
Parkinsonian patients answered a demographic questionnaire including age, gender, education, and information about the disease (duration of disease, age at onset, type of treatment). Clinical assessment was made using the Hoehn and Yarh stage12.
Methods
The SCOPA-AUT is a specific 23-item self-completed questionnaire for the assessment of autonomic dysfunction in patients with PD. The 23 items of the SCOPA-AUT are grouped into six domains: i) gastrointestinal functioning (seven items), ii) urinary functioning (six items), iii) cardiovascular functioning (three items), iv) thermoregulatory functioning (four items), v) pupillomotor functioning (one item), and vi) sexual function (two items for men and two for women). The maximum score is 69, with the score for each item ranging from 0 (never experiencing the symptom) to 3 (often experiencing the symptom)8.
Translation and Back-Translation of the SCOPA-AUT
The SCOPA-AUT was translated into Greek by two independent native Greek speakers with excellent knowledge of English. The Greek version of the SCOPA-AUT, obtained by consensus, was back- translated into English by two English natives without access to the original English version of the questionnaire. This first back-translation was compared with the original version and modifications were made to eliminate all discrepancies between the original and the back-translated versions. PD patients (with the aid of caregiver when necessary) and healthy controls completed the Greek version of the SCOPA-AUT.
Since there is no gold standard test for autonomic disturbance in PD to compare with SCOPA-AUT in order to prove its construct validity, we chose for cross-validation two well-validated tests that assess non-motor symptoms in general: a) the Non-Motor Symptoms Questionnaire (NMSQuest)13,14, and b) the Parkinson’s Disease Questionnaire-39 (PDQ-39)15,16 that captures PD patient’s perspective of his/her motor and non-motor disability in general, yielding a single index score and in particular evaluating eight specific domains (e.g. mobility, activities of daily living/ADL, emotional well-being, stigma, social support, cognition, communication, bodily discomfort). Aspects that are relevant to autonomic symptoms are embedded in both tests.
Data analysis
A reliability analysis of the total SCOPA-AUT scale and its domains (subscales) was initially performed employing the calculation of Cronbach’s alpha coefficient. A Cronbach’s alpha over 0.70 was set as the accepted satisfactory reliability level17. Inter-item correlations (IICC) and corrected item to total correlations coefficients (CITCC) of all scale domains were also calculated. Mean values of IICCs over 0.300 were regarded as satisfactory and reflected an acceptable homogeneity of the scale. CITCC satisfactory level was set above 0.40018 .
For every SCOPA-AUT subscale the standard error of measurement for single observation (SEM) was calculated [SEM=Standard Deviation X √ (1-alpha)], setting an arbitrary criterion value of SEM smaller or equal to a half of the standard deviation (SD) to indicate the precision of the scale19.
Bivariate comparisons between PD patients and controls data were performed either by the t-test for two independent samples, when the Levene’s test for homogeneity of variances showed that variances between the two groups were equal (e.g. education, age), or the Man-Whitney U test for two independent samples, when variances were not equal (Total SCOPA-AUT scale score and SCOPA-AUT domains). The Man-Whitney U test was also employed for comparisons related to treatment.
Comparison between gender frequencies of the two study groups was performed using the Pearson Chi-Square (χ2). The same test was employed for comparisons of every other frequency differences. Exploration of differences between mean SCOPA- AUT scores of PD patients, in relation to stage of the disease, was performed using the Kruskal-Wallis H test. Correlation between total SCOPA- AUT score and disease duration was calculated using the Pearson’s r correlation coefficient. Correlations between subscale scores and auxiliary tests scores were performed employing the Spearman’s rho correlation coefficient. For all tests, a p-value lower than 0.05 was set as statistically significant. Statistical analysis was performed by means of the IBM Statistical Package for Social Sciences (SPSS), version 19.0 (SPSS Inc., Chicago, IL, USA).
Results
Clinical and demographic data
Eleven patients failed to answer all SCOPA-AUT items, particularly those concerning sexual function and they were excluded from analysis. Therefore the final PD patients study group consisted of 150 subjects. There were 97 (64.7 %) men and 53 (35.3 %) women, while in the control group there were 23 (57.5 %) men and 17 (42.5 %) women. The difference in sex proportions between groups was not significant (p =0.541). PD patients were matched to controls for age. The mean age was 60.62 ± 8.35 years for the PD group, and 58.4 ± 8.9 years for the controls (p =0.840). The two groups were also matched for education (mean educational level for PD patients was 9.61 ± 4.0, and for controls was 10.9 ± 4.0 years, p =0.109). PD patients had a mean duration of the disease of 7.37 ± 4.84 years. Duration of the disease was the same in men and women (p =0.876). Distribution according to the stage of the disease was as follows: 12.7 % of PD patients were in stage 1, 74.0 % in stage 2, 10.7 % in stage 3, and 2.7 % in stage 4. Distribution of stage frequencies was the same in men and women (p =0.984).
Thirty-five PD (23.3 %) patients were untreated while the rest were receiving levodopa or/and a dopaminergic agonist or rasagiline. There was no difference between men and women regarding treatment.
SCOPA-AUT data
All subjects responded to the SCOPA -AUT test items without difficulty. Reliability analysis yielded a satisfactory Cronbach’s alpha for the total scale (alpha=0.824), showing a very good internal consistency of the scale. The same applied to most of the scale domains, except the Gastrointestinal and the Thermoregulatory ones. Results are presented in Table 1. Mean IICCs depicting the subscales item homogeneity, and CITCC of all scale domains are also presented in Table 1. Furthermore items with item to total correlation coefficient over 0.400 are summarized. It is noteworthy that although some scale items of CITCCs did not reach the set level of 0.400, this did not have a significant effect on the reliability coefficient since deletion of these items did not raise the alpha value over 0.824.
Table 1. Internal consistency of the Scales for Outcomes in Parkinson’s Disease-Autonomic questionnaire (SCOPA-AUT) domains for the 150 patients with Parkinson’s disease and the forty matched controls who were enrolled in the current study.
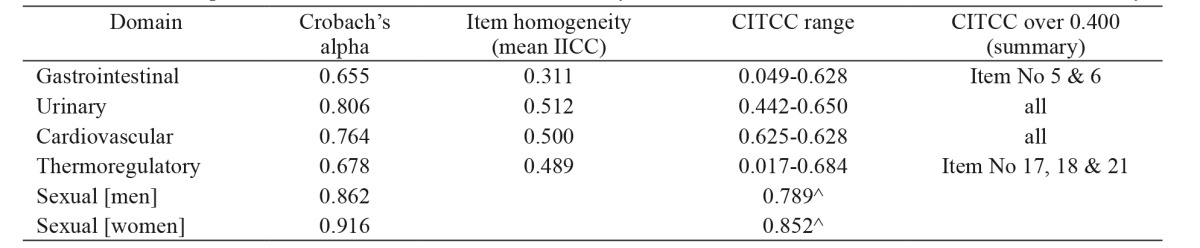
IICC: inter-item correlation coefficient, CITCC: corrected item to total correlation coefficient, ^: inter-item correlations
Correlations between SCOPA-AUT domains scores are presented in Table 2. Not all subscales correlated well with others. Particularly weak correlations were observed in the Pupillomotor and the Sexual subscale for women. Regarding precision, SEM was satisfactory in all domains, with the exception of the Thermoregulatory subscale (Table 2).
Table 2. Internal validity (rho coefficients) and precision (SEM) of the Scales for Outcomes in Parkinson’s Disease-Autonomic questionnaire (SCOPA-AUT) domains for the 150 patients with Parkinson’s disease and the forty matched controls who were enrolled in the current study.
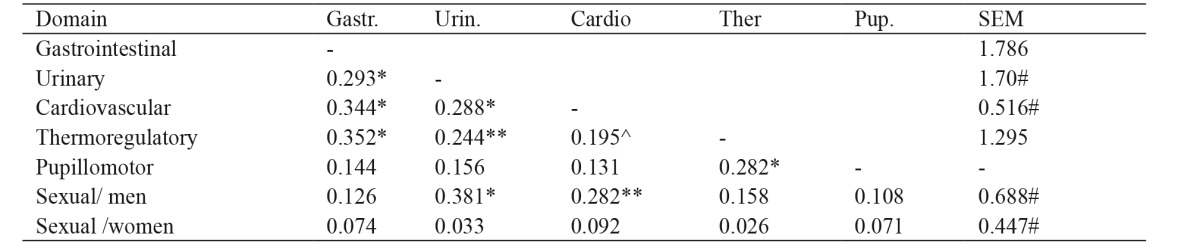
Gastr.: Gastrointestinal, Urin.: Urinary, Cardio: Cardiovascular, Ther.: Thermoregulatory, Pup.: Pupillomotor, SEM: standard error of measurement, *: p =0.000, **: p =0.003, ^: p =0.017, #: SEM =lower than ½ standard deviation, unmarked coefficients are not significant
All PD patients responded to the PDQ-39 and the NMSQest. Mean values of their performance are presented in Table 3. Correlation between SCOPA-AUT total score and NMSQest score was significant. All SCOPA-AUT domains scores, with the exception of the sexual domain for women, correlated significantly with the NMSQest. The same applied to correlations with PDQ-39 single index. Spearman’s rho correlation coefficients are presented in Table 4.
Table 3. Mean scores of auxiliary scales of the 150 patients with Parkinson’s disease in the Non-Motor Symptoms Questionnaire and Parkinson’s Disease Questionnaire-39.
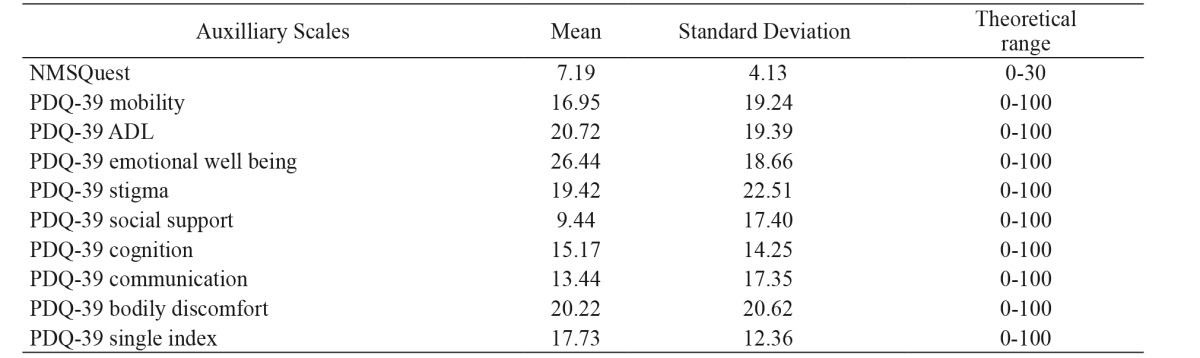
NMSQuest: Non-Motor Symptoms Questionnaire, PDQ-39: Parkinson’s Disease Questionnaire-39
Table 4. Correlations between the Scales for Outcomes in Parkinson’s Disease-Autonomic questionnaire (SCOPA-AUT) scores and the auxiliary scales of the Non-Motor Symptoms Questionnaire and Parkinson’s Disease Questionnaire-39.
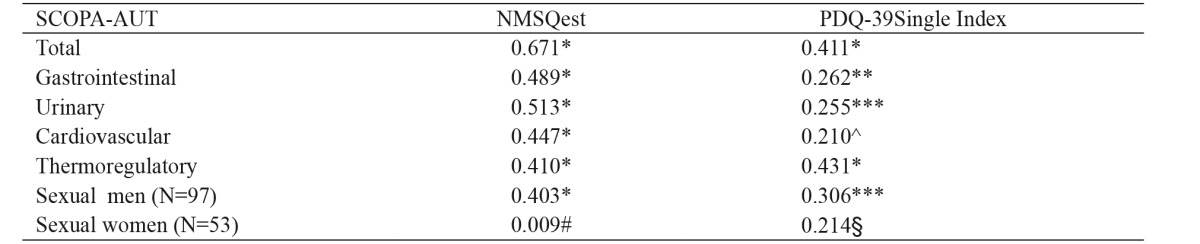
NMSQuest: Non-Motor Symptoms Questionnaire, PDQ-39: Parkinson’s Disease Questionnaire-39, *: p =0.000, **: p =0.001, ***: p =0.002, ^: p =0.010, #: p =0.949, §: p =0.124
All PDQ-39 domains, except the Social stigma subscale, correlated with total SCOPA-AUT score and rho ranged from to 0.189 (PDQ-39 social support subscale) to 0.397 (PDQ-39 mobility scale) (p range: 0.02 - 0.000).
PD patients scored higher than controls in the total SCOPA-AUT score in general and in the Gastrointestinal and Urinary scales (p <0.001) in particular, with the Urinary scale having the highest level of all. The mean values of all SCOPA-AUT subscales in PD patients and controls are shown in Table 5. Furthermore single item scores that differentiated PD patients from controls are presented in Table 6. The highest p levels were observed in items relevant to sialorrhea, constipation, straining for defecation, incontinence, and nocturia.
Table 5. Total SCOPA-AUT and subscales mean scores: comparisons between the 150 patients with Parkinson’s disease and the forty matched controls.
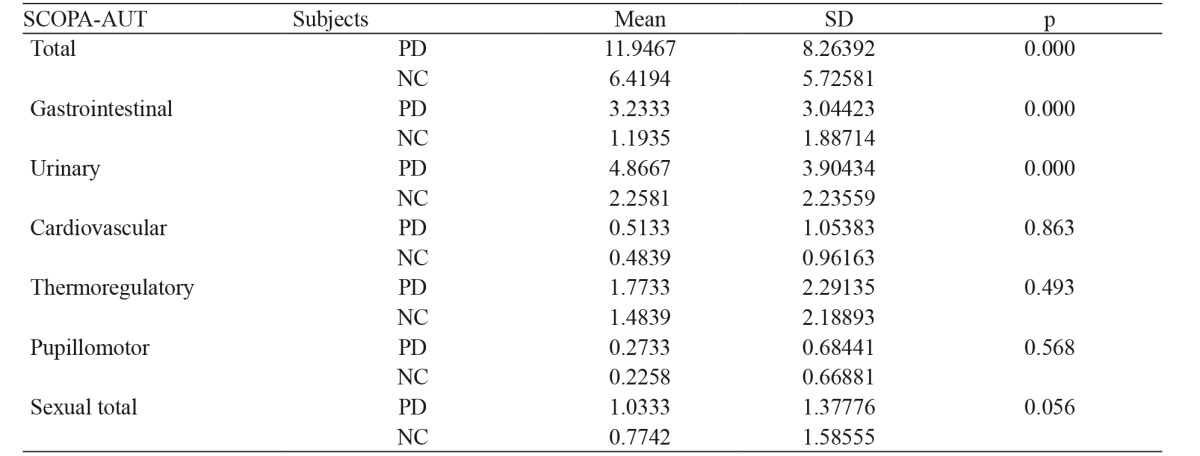
SCOPA-AUT: Scales for Outcomes in Parkinson’s Disease-Autonomic questionnaire, SD: Standard Deviation, PD: patients with Parkinson’s disease, NC: controls
Table 6. Significant single item score differences between the 150 patients with Parkinson’s disease and the forty matched controls.

A test floor effect was not demonstrated in the study groups since 0 total score had only 1.35 % of PD patients and 3.25 % of the normal controls. The highest observed SCOPA-AUT score was 50 (one PD patient) while only 3.3 % of PD patients scored over 30. The highest score in the control group was 24 (one subject). The small percentage of very high scores shows that there is not a ceiling effect. There were no differences between men and women scores in the total scale SCOPA-AUT score as well as in the subscales scores.
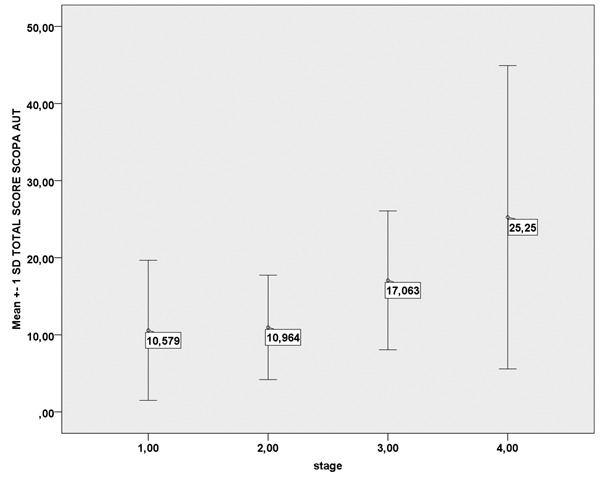
Comparison of total mean SCOPA-AUT performance in PD patients in relation to stage of the disease, showed that there was a significant increase in the score as the disease severity increased (Figure 1). Correlation between total score and disease duration was significant (r =0.221; p =0.006). Age had no effect on the total score. However, age was related to urinary symptoms, since older patients showed significantly higher scores in the Urinary domain (p =0.006).
Figure 1. Mean total SCOPA AUT scores in relation to stage of Parkinson’s disease (p =0.012). SCOPA-AUT: Scales for Outcomes in Parkinson’s Disease-Autonomic questionnaire, SD: Standard Deviation.
Comparison of mean total SCOPA-AUT score between treated and untreated PD patients showed no difference between these two groups (p =0.344). The same applied to all domains exept the Gastrointestinal domain score, where there was a significant increase in the treated patients’ group (p =0.038).
In brief, our results show that SCOPA-AUT scale has a good reliability profile and it correlates well with other measures for non-motor symptoms and health-related quality in PD patients. Its construct validity is further strengthened by its significant correlations to disease severity and duration. Regarding autonomic symptoms PD patients report a high frequency of symptoms related to gastrointestinal and urinary systems. Age and treatment may also have a role in these two domains.
Discussion
Autonomic symptoms are common in PD and occur virtually in all patients at some time during the course of the disease. Autonomic disturbances, which are often underreported, can be severely disabling associated with significant morbidity and mortality. The SCOPA-AUT is a reliable, easily self-administered questionnaire for assessing autonomic function in PD and it is validated in different languages19-22.
In our study the metric properties of SCOPA-AUT were assessed for the first time in a sample of Greek PD patients. SCOPA-AUT showed a good reliability profile, yielding an above satisfactory level Cronbach’s alpha coefficient and a good item to total correlation coefficients. Likewise, most of SCOPA-AUT subscales performed well, exept the Gastrointestinal and Thermoregulatory scale which showed a weak internal consistency. SCOPA-AUT subscales inter-correlated well with the exception of the Pupillomotor and the Sexual scale for women.
Precision based on the low SEMs in most subscales, exept the Thermoregulatory scale, was remarkable. In scales like SCOPA-AUT covering a variety of heterogeneous symptoms, some discrepancies in the statistical parameters are expected and have been observed in other studies too19. Construct validity was indirectly assessed since there is no other equivalent test to compare with. However, SCOPA-AUT, in general, showed a significant correlation with the auxiliary scales (PDQ-39 and the NMSQuest) we used for comparisons. A strong relationship to stage of the disease added more weight to the instrument validity.
Our results show that, compared with a group of matched controls, patients with PD experience significant autonomic dysfunction. The SCOPA-AUT mean score of the patients was higher than the mean score of the controls (11.9 versus 6.4). This score is a much lower compared to other studies where patients’ score ranged from 18 to 235,22. Perhaps this is due to the absence of patients in the very late stage of the disease in our study. Similarly in the study of Kim et al, where there were no patients in stage 5, the mean SCOPA-AUT score was 11.723. Patients reported problems in almost all items of the SCOPA-AUT, that is dysfunction in more than one component of the autonomic nervous system (parasympathetic cholinergic, sympathetic cholinergic, sympathetic noradrenergic and enteric)24. Scores in different SCOPA-AUT domains ranged from 4.8 to 0.2. The most common autonomic symptoms emerged in the Urinary and the Gastrointestinal domain. This applied to all studies evaluating autonomic symptoms in PD5,8,22. Furthermore, the most prominent differences in autonomic dysfunction between patients and controls were found in the Urinary and Gastrointestinal domain. Especially sialorrhea, constipation, straining for defecation, incontinence, and nocturia differentiated patients from controls. Cardiovascular and pupillomotor symptoms were less frequent in both patients and controls. Similarly, in the study of Verbaan et al5cardiovascular symptoms were the least frequent in patients with PD. It is of notice that although cardiac denervation is an early sign of the disease patients report less frequent cardiovascular symptoms. Verbaan et al5suggested that this discrepancy is due to different applied methods of assessment (objective versus subjective).
SCOPA-AUT mean score became significantly higher as the duration and the severity of disease (according to the Hoehn and Yahr scale) increased. However, dysautonomic symptoms appeared in all stages of the disease even in the first stage. These findings are in agreement with other studies5,19,22,23 showing that the SCOPA-AUT questionnaire has the discriminative ability to distinguish groups of patients in different stages. Furthermore, Kim et al23 assessed autonomic function in PD patients by means of autonomic function tests and they found that cardiovagal function was the domain most influenced by disease progression and the pattern of sudomotor impairment was similar to that in patients with peripheral autonomic neuropathy. The impact of age on the appearance of autonomic symptoms is a controversial issue. Some studies have reported an association of autonomic symptoms with age, while others did not5,19,22. In our study, no correlation was found between age and total SCOPA-AUT, but there was a relationship to the Urinary domain. Another controversial issue is the role of treatment in the appearance and aggravation of autonomic symptoms. Most studies described relations between dopaminergic treatment and worsening of the cardiovascular function4,5,24. In our study, only an increase in the gastrointestinal symptoms was observed in association with treatment. Verbaan et al5 found a correlation between gastrointestinal, urinary, thermoregulatory function and dopaminergic treatment.
Autonomic dysfunction is now recognized as a cardinal feature of PD presenting not only in advanced disease but also early in the course of it, preceding even the motor symptoms. It affects patients’ quality of life and is one of the main causes of mortality and morbidity. Therefore it is very important for physicians to recognize and treat autonomic symptoms in patients with PD during the whole course of the disease. SCOPA-AUT is a reliable, self-administered questionnaire for screening autonomic symptoms in patients with PD. Further autonomic function tests are needed for objective evaluation of the autonomic dysfunction uncovered by SCOPA-AUT.
Conflict of interest
Authors declare no conflict of interest.
References
- 1.Chaudhuri KR, Schapira AH. Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol. 2009;8:464–474. doi: 10.1016/S1474-4422(09)70068-7. [DOI] [PubMed] [Google Scholar]
- 2.Todorova A, Jenner P, Ray Chaudhuri K. Non-motor Parkinson’s: integral to motor Parkinson’s, yet often neglected. Pract Neurol. 2014;14:310–322. doi: 10.1136/practneurol-2013-000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jellinger KA. Synuclein deposition and non-motor symptoms in Parkinson disease. J Neurol Sci. 2011;310:107–111. doi: 10.1016/j.jns.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 4.Dubow JS. Autonomic dysfunction in Parkinson’s disease. Dis Mon. 2007;53:265–274. doi: 10.1016/j.disamonth.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Verbaan D, Marinus J, Visser M, van Rooden SM, Stiggelbout AM, van Hilten JJ. Patient-reported autonomic symptoms in Parkinson disease. Neurology. 2007;69:333–341. doi: 10.1212/01.wnl.0000266593.50534.e8. [DOI] [PubMed] [Google Scholar]
- 6.Asahina M, Vichayanrat E, Low DA, Iodice V, Mathias CJ. Autonomic dysfunction in parkinsonian disorders: assessment and pathophysiology. J Neurol Neurosurg Psychiatry. 2013;84:674–680. doi: 10.1136/jnnp-2012-303135. [DOI] [PubMed] [Google Scholar]
- 7.Jost WH. Autonomic dysfunctions in Parkinson’s disease. J Neurol. 2003;250 Suppl 1:I28–I30. doi: 10.1007/s00415-003-1105-z. [DOI] [PubMed] [Google Scholar]
- 8.Visser M, Marinus J, Stiggelbout AM, van Hilten JJ. Assessment of autonomic dysfunction in Parkinson’s disease: The SCOPA-AUT. Mov Disord. 2004;19:1306–1312. doi: 10.1002/mds.20153. [DOI] [PubMed] [Google Scholar]
- 9.Palma JA, Kaufmann H. Autonomic disorders predicting Parkinson’s disease. Parkinsonism Relat Disord. 2014;20 Suppl 1:S94–S98. doi: 10.1016/S1353-8020(13)70024-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Postuma RB, Gagnon JF, Pelletier A, Montplaisir J. Prodromal autonomic symptoms and signs in Parkinson’s disease and dementia with Lewy bodies. Mov Disord. 2013;28:597–604. doi: 10.1002/mds.25445. [DOI] [PubMed] [Google Scholar]
- 11.Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1988;51:745–752. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 13.Chaudhuri KR, Martinez-Martin P, Schapira AH, Stocchi F, Sethi K, Odin P, et al. International multicentre pilot study of the first comprehensive self-completed nonmotor symptoms questionnaire for Parkinson’s disease: the NMSQuest study. Mov Disord. 2006;21:916–923. doi: 10.1002/mds.20844. [DOI] [PubMed] [Google Scholar]
- 14.Bostantjopoulou S, Katsarou Z, Karakasis H, Peitsidou E, Milioni D, Rossopoulos N. Evaluation of non-motor symptoms in Parkinson’s disease: An underestimated necessity. Hippokratia. 2013;17:214–219. [PMC free article] [PubMed] [Google Scholar]
- 15.Jenkinson C, Fitzpatrick R, Peto V, Greenhall R, Hyman N. The Parkinson’s Disease Questionnaire (PDQ-39): development and validation of a Parkinson’s disease summary index score. Age Ageing. 1997;26:353–357. doi: 10.1093/ageing/26.5.353. [DOI] [PubMed] [Google Scholar]
- 16.Katsarou Z, Bostantjopoulou S, Peto V, Alevriadou A, Kiosseoglou G. Quality of life in Parkinson’s disease: Greek translation and validation of the Parkinson’s disease questionnaire (PDQ-39) Qual Life Res. 2001;10:159–163. doi: 10.1023/a:1016720400862. [DOI] [PubMed] [Google Scholar]
- 17.Bland JM, Altman DG. Cronbach’s alpha. BMJ. 1997;314:572. doi: 10.1136/bmj.314.7080.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whiston SC. Principles and applications of assessment in councelling, 4th edition. Cengage Learning, Bremont, CA , USA. 2013 [Google Scholar]
- 19.Rodriguez-Blazquez C, Forjaz MJ, Frades-Payo B, de Pedro-Cuesta J, Martinez-Martin P. Longitudinal Parkinson’s Disease Patient Study, Estudio Longitudinal de Pacients con Enfermedad da Parkinson Group. Independent validation of the scales for outcomes in Parkinson’s diseases-autonomic (SCOPA-AUT) Eur J Neurol. 2010;17:194–201. doi: 10.1111/j.1468-1331.2009.02788.x. [DOI] [PubMed] [Google Scholar]
- 20.Cervantes-Arriaga A, Rodríguez-Violante M, Villar-Velarde A, López-Gómez M, Corona T. [Metric properties of clinimetric indexes for non-motor dysfunction of Parkinson’s disease in Mexican population] Rev Invest Clin. 2010;62:8–14. [PubMed] [Google Scholar]
- 21.Mantarova SG, Velcheva IV, Georgieva SO, Stambolieva KI. Validation of the Bulgarian version of Scales for Outcomes in Parkinson’s Disease - Autonomic (SCOPA-AUT-BG) Folia Med (Plovdiv) 2013;55:56–62. doi: 10.2478/folmed-2013-0028. [DOI] [PubMed] [Google Scholar]
- 22.Carod-Artal FJ, Ribeiro Lda S, Kummer W, Martinez-Martin P. Psychometric properties of the SCOPA-AUT Brazilian Portuegese version. Mov Disord. 2010;25:205–212. doi: 10.1002/mds.22882. [DOI] [PubMed] [Google Scholar]
- 23.Kim JB, Kim BJ, Koh SB, Park KW. Autonomic dysfunction according to disease progression in Parkinson’s disease. Parkinsonism Relat Disord. 2014;20:303–307. doi: 10.1016/j.parkreldis.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein DS. Dysautonomia in Parkinson disease. Compr Physiol. 2014;4:805–826. doi: 10.1002/cphy.c130026. [DOI] [PMC free article] [PubMed] [Google Scholar]


