Abstract
Purpose: Retinopathy of prematurity (ROP) is a visual-impairing disorder of the developing retinal vasculature in premature infants. Recent advances in neonatal care have led to an increase in the vulnerable premature population. The aim of this retrospective study was to assess the incidence of ROP and its risk factors according to degree of prematurity. Methods: Data from a sequence of 1,562 infants <32 weeks of gestational age, admitted to the Jewish General Hospital Neonatal Intensive Care Unit, a tertiary care perinatal center in Montreal, Canada, were reviewed to determine the incidence and risk factors of ROP. Perinatal risk factors for ROP were analyzed using univariate and multivariate analyses in four consecutive gestational age (GA) groups (24-25+6/7weeks, 26-27+6/7 weeks, 28-29+6/7 weeks and 30-31+6/7 weeks). Results: The overall incidence in our study was 15.6 %. Severe ROP, defined as stage 3 or plus disease was detected in 5.2 % of the neonates screened. In the univariate analyses, many risk factors in each GA group were found to have a significant association with ROP. On subsequent multivariate logistic regression analysis, birth weight, small for gestational age, the presence of patent ductus arteriosus (PDA), sepsis, necrotizing enterocolitis (NEC), and mechanical ventilation >7 days were independently associated with the development of ROP. Birth weight was consistently an independent risk factor for ROP in all GA groups. Conclusion: Our study confirmed the importance of birth weight as an independent ROP risk factor. Sepsis, NEC, PDA, and prolonged mechanical ventilation have been shown to be independent risk factors in the different gestational age groups. Hippokratia 2016, 20(2): 121-126
Keywords: Retinopathy of prematurity, risk factors, ROP incidence, neonates
Introduction
Retinopathy of prematurity (ROP) is a disorder of the developing retinal vasculature in premature infants. It is a leading cause of childhood blindness and the main cause of visual impairment in premature infants, posing an increasing concern due to advances in neonatal care and increased survival of extremely preterm infants1-3. There has been accumulating evidence supporting that ROP is a multifactorial disease4,5. Low gestational age (GA), low birth weight (BW), small for gestational age (SGA), intraventricular hemorrhage (IVH), neonatal sepsis, blood transfusions, patent ductus arteriosus (PDA), respiratory distress syndrome (RDS), mechanical ventilation (MV), and oxygen therapy in preterm infants were all reported as risk factors for ROP6. The well-recognized risk factors are low BW and low GA1-3. On the other hand, a potentially protective factor for ROP is human milk feeding7.
ROP’s incidence has varied considerably over the years. It was first noted in the 1940s and 1950s mainly as a consequence of the use of unmonitored supplemental oxygen (“first epidemic”)8. As survival of extremely premature infants improved over the next decades and despite better methods of monitoring oxygen supplementation, a rising incidence of ROP reemerged (“second epidemic”)8. In the last decade or so, an increasing frequency of ROP blindness has been documented in low-income countries where neonatal care is rapidly improving. Studies suggest that ROP is becoming an important cause of blindness in China, Southeast and South Asia, Latin America, and Eastern Europe – especially in urban centers of newly industrializing countries. This is referred to as a “third epidemic”8.
Recent studies have shown that the incidence of any stage of ROP among infants weighing <1,251 g was 68 %9. Although the majority of infants who develop ROP have spontaneous resolution of the process without retinal detachment or cicatricial sequelae, approximately 6 % of low BW infants (<1,251 g) develop severe ROP that requires treatment to prevent visual loss5. Conventional treatment of ROP is retinal ablation with cryotherapy or laser therapy. Recently, intravitreal injection of antivascular endothelial growth factor (anti-VEGF) is used for the treatment of ROP.
The aim of this retrospective study was to identify the incidence of ROP and the associated risk factors in very preterm and extremely preterm neonates in our neonatal intensive care unit (NICU) where a strict protocol of saturation limits has been implemented.
Patients and Methods
Data was analyzed from the electronic information recorded at the time of discharge of each infant from the hospital. Our study included infants with GA less than 32 weeks, born at the Jewish General Hospital and admitted to NICU. All infants were screened for ROP. Exclusion criteria were i) multiple pregnancies, and ii) infants having major congenital anomalies. The examination was performed by an experienced pediatric ophthalmologist using an indirect ophthalmoscope in all infants 4-6 weeks after birth, according to GA. Infants born with GA of 27 weeks or less were first examined at 31 weeks postmenstrual age. The first examination was performed four weeks after birth for infants born beyond 27 weeks GA. In infants who developed ROP, the routine reexamination was performed every week, however, when indicated, some infants were examined as frequently as twice per week. ROP was classified according to the International Classification of ROP10,11. The highest ROP stage in either eye was recorded. In our study severe ROP was defined as stage 3 or plus disease. Our target range of oxygenation (SpO2 alarms) were set between 82 % and 93 %, for infants with birth weight <1,000 g aiming for saturation ≥ 85%, 84-95 % for infants 1,000 to 1,500 g and 85-96 % for those over 1,500 g. Demographic and predisposing factors are presented in the Tables.
Infants were assigned to four groups based on GA: Group A consisted of infants 24-256/7(24-25) weeks GA, group B 26-27+6/7(26-27) weeks GA, group C of 28-29+6/7(28-29) weeks GA, and group D of 30-31+6/7(30-31) weeks GA. This type of classification allowed us to determine risk factors associated with each GA group and to detect potential differences.
Statistical analysis
Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS) statistical software, version 20.0 (SPSS Inc., Chicago, IL, USA). Independent sample t-test was used for continuous data and χ2 test for nominal and ordinal data to evaluate the differences between the two groups (ROP, non ROP). Multivariate analysis by stepwise logistic regression was used to determine the independent risk factors for ROP.
Results
During the study period (1994-2008) 1,562 neonates with GA less than 32 weeks were admitted to our NICU, of them ROP was diagnosed in 227 (14.5 %). Among the 1,562 neonates, 1,389 (89 %) survived. The overall incidence of ROP was 15.6 % (217/1,389) among the survivors. Severe ROP was detected in 4.5 % (63/1,389) of neonates. The incidence of ROP requiring either operation or anti-VEGF treatment was 1.4 % (20/1,389) among the surviving infants and 9.2 % (20/217) in the ROP group. In our NICU, feeding protocol has prioritized human milk. Following this policy about 90% of the infants in this study had received breast milk.
Group A: 24-25 weeks GA (Table 1)
Table 1. Comparison of the demographic characteristics and morbidities of neonates with and without retinopathy of prematurity with gestational age 24-25 6/7 weeks. Of the 245 neonates, 96 died (mortality: 39 %) and 149 survived. Severe retinopathy was detected in 58 neonates (38.9 %), of whom 19 (12.7 %) required operation or anti-VEGF therapy.

Values are presented as number of neonates (with percentage in brackets), or means ± standard deviation (median with range in brackets). ROP: retinopathy of prematurity, GA: gestational age, M: male, F: Female, CS: cesarean section, SGA: small for gestational age, IVH: intraventricular hemorrhage, RDS: respiratory distress syndrome, PVL: periventricular leukomalacia, PDA: patent ductus arteriosus, NEC: necrotizing enterocolitis, MV: mechanical ventilation, TPN: total parenteral nutrition
Of the 245 neonates with GA less than 26 weeks, 149 (61 %) survived. The incidence of ROP in those that survived was 77.9 % (116/149). Severe retinopathy was detected in 38.9 % (58/149) of the neonates and 19 out of those 58 required an operation or anti-VEGF therapy. Risk factors are indicated in Table 1. The first examination was performed at 28 days of life. Multivariate analysis showed that BW, PDA and sepsis were independently associated with ROP in order of significance based on the p values. Logistic regression analysis demonstrated that the most significant risk factor for ROP in this age group was BW [odds ratio: 0.99, 95% confidence interval (CI): 0.986-0.997]. Multivariate analysis for severe ROP demonstrated that SGA (odds ratio: 0.131, 95% CI: 0.035-0.483), and BW (odds ratio: 0.991, 95% CI: 0.986-0.997) were independently associated with severe ROP.
Group B: 26-27 weeks GA (Table 2)
Table 2. Comparison of the demographic characteristics and morbidities of neonates with and without retinopathy of prematurity with gestational age 26-27 6/7 weeks. Of the 290 neonates, 39 died (mortality: 13.4 %) and 251 survived. Severe retinopathy was detected in 12 neonates (4.8 %), of whom 1 (0.4 %) required treatment.

Values are presented as number of neonates (with percentage in brackets), or means ± standard deviation (median with range in brackets). ROP: retinopathy of prematurity, GA: gestational age, M: male, F: Female, CS: cesarean section, SGA: small for gestational age, IVH: intraventricular hemorrhage, RDS: respiratory distress syndrome, PVL: periventricular leukomalacia, PDA: patent ductus arteriosus, NEC: necrotizing enterocolitis, MV: mechanical ventilation, TPN: total parenteral nutrition
This group consisted of 290 neonates. The incidence of ROP was 30.7 % (77/251) among the survivors, and severe retinopathy was detected in 4.8 % (12/251) of the neonates. Risk factors are indicated in Table 2. Multivariate analysis showed that the presence of PDA (odds ratio: 3.52, 95% CI: 1.57-7.91), and BW (odds ratio: 0.995, 95% CI: 0.992-0.997) were independently associated with the development of ROP.
Group C: 28-29 weeks GA (Table 3)
Table 3. Comparison of the demographic characteristics and morbidities of neonates with and without retinopathy of prematurity with gestational age 28-29 6/7 weeks. Of the 444 neonates, 18 died (mortality: 4 %) and 426 survived. Severe retinopathy was detected in 3 neonates (0.7 %), of whom none required treatment.

Values are presented as number of neonates (with percentage in brackets), or means ± standard deviation (median with range in brackets). ROP: retinopathy of prematurity, GA: gestational age, M: male, F: Female, CS: cesarean section, SGA: small for gestational age, IVH: intraventricular hemorrhage, RDS: respiratory distress syndrome, PVL: periventricular leukomalacia, PDA: patent ductus arteriosus, NEC: necrotizing enterocolitis, MV: mechanical ventilation, TPN: total parenteral nutrition
This cohort included 444 neonates and the mortality rate was 4 % (18/444). The incidence of ROP was 4.3 % (18/426) and severe retinopathy was detected in 0.7 % (3/426) among the survivors. Risk factors are indicated in Table 3. On multivariate analysis, BW and days on MV were the only statistically significant independent risk factors for developing ROP and severe ROP, respectively.
Group D: 30-31 weeks GA (Table 4)
Table 4. Comparison of the demographic characteristics and morbidities of neonates with and without retinopathy of prematurity with gestational age 30-31 6/7 weeks. Of the 579 neonates, 16 died (mortality: 2.7 %) and 426 survived. Severe retinopathy was not detected in any neonate.
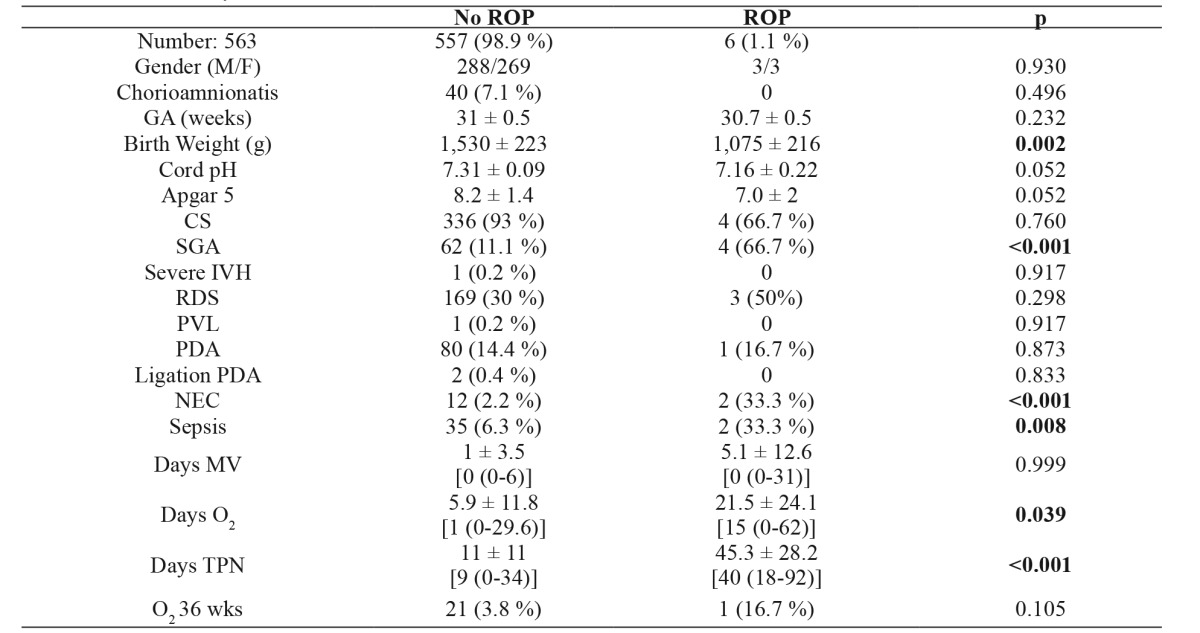
Values are presented as number of neonates (with percentage in brackets), or means ± standard deviation (median with range in brackets). ROP: retinopathy of prematurity, GA: gestational age, M: male, F: Female, CS: cesarean section, SGA: small for gestational age, IVH: intraventricular hemorrhage, RDS: respiratory distress syndrome, PVL: periventricular leukomalacia, PDA: patent ductus arteriosus, NEC: necrotizing enterocolitis, MV: mechanical ventilation, TPN: total parenteral nutrition
Of the 579 neonates in this GA group, 97.3 % (563/579) survived. The incidence of ROP was 1.1 % (6/563) among the survivors, and severe retinopathy was not detected in any neonate. In group D, GA did not have statistical significance. However, BW was significantly lower in the ROP group (Table 4). Finally, days of oxygen exposure and days of total parenteral nutrition (TPN) were significantly higher in the ROP group, clearly indicating sicker infants. Out of all these, on subsequent multiple logistic regression analysis, BW, sepsis and NEC were found to be independent risk factors for developing ROP.
Discussion
Our study regards a rather long chronological period, but with the same management protocol and large numbers of premature infants in each category. In a period of scrupulous oxygen administration monitoring and lower target ranges of oxygen saturation levels, this retrospective study attempted to examine the possible risk factors of ROP in four consecutive GA groups of very and extremely premature infants. ROP cases have been documented in gestation >32 weeks, however the incidence of ROP in babies 32-34 weeks GA is negligible. Optimal oxygen saturation levels for premature infants are still debatable. Low oxygen saturation levels (82-88 %) were related to increased mortality, whereas higher saturation levels with increased risk of ROP12,13. Our policy, over the years, was to aim for SpO2 target range according to BW as indicated in the section of patients and methods. With this practice, we have achieved low mortality rates based on Canadian multicenter statistics produced annually by the Canadian Neonatal Network as well as low incidence of ROP. The overall ROP incidence in our study was 15.6 %. Severe ROP was documented in 5.2 % (73/1,389) of infants. This incidence is one of the lowest reported so far for infants less than 32 weeks of GA1,2,14-18. The incidence of ROP varies in different studies mainly due to the mean GA of the included infants and the percentage of extremely low birth weight (ELBW) in each study. For this reason, we decided to divide our patients to subgroups according to GA, indicative of the importance of GA, a well-known predisposing factor.
In particular, according to GA, the incidence of ROP displays a gradual dramatic decrease from 77.9 % in the 24-25 GA to 1.1 % in the 30-31 GA, highlighting the fact that prematurity is directly related to the occurrence of ROP. Our findings are in complete agreement with all published data in the literature1,2,14-18 and also with the known association of low BW and low GA.
SGA infants have been consistently associated with the development of ROP. In our cohort, BW was an independent risk factor in all GA groups, and BW was found to be inversely related to ROP. Additionally, on subsequent multivariate logistic regression analysis, SGA, the presence of PDA, sepsis, MV >7 days, and NEC were independently associated with the development of ROP.
Specifically, we are reporting one of the lowest incidences of ROP in the literature for infants born 24-25 weeks of gestation (Group A), a GA with the highest incidence2,15. Moreover, severe ROP requiring treatment was also low for this age group (12.7 % among the survivors). Follow-up of these neonates showed no deterioration of the ROP. Noticeable is the fact that the independent aggravating factor for this age group was SGA. This is probably due to the increased morbidity and the appearance of pathophysiological mechanics favorable to the development of ROP19,20. The same problem applies for sepsis in the younger and the older GA groups. Several studies have implicated infection in the development of ROP21,22.
Also, infants with a gestational age of 26-27 weeks had one of the lowest incidences of ROP reported both in terms of incidence as well as severity1,15. The study by Isaza and Arora1 reports ROP stage 3 or worse at 17.3 % (36/207) in newborns with GA ≤27 weeks while in our study for the same age group the percentage is at 17 % (70/400). Treatment was required in only one neonate. Apart from BW, independent risk factors were found to be PDA and the duration of MV; findings that are consistent with several previous studies2,23-25. It is speculated that changes in perfusion of the retinal vessels, brought about by open ductus arteriosus, and the deterioration of lung function, are both involved as pathophysiological mechanisms in the occurrence of ROP2,23.
The incidence of the disease was also very low for group C (GA 28-29 weeks), 4.3 % (18/426), and only 0.7 % (3/426) developed severe ROP, but none of them required treatment. In this group, independent risk factors for the onset of the disease were the SGA neonates, the duration of MV, and length of TPN. We assume that the TPN and the MV were not the causes of ROP, but the expression of the severity of their condition.
The older GA group (GA 30-31 weeks) of neonates in our study, showed extremely rarely ROP and in fact none of the neonates had a severe form of the disease. And likewise in this group, SGA was an independent risk factor [66.7 % (4/6 neonates) were SGA]. Although NEC has been associated with the development of ROP in many studies2,24,25, in our cohort, we found a statistically significant correlation between NEC and ROP only in this group [33.3 % (2/6 neonates)]. We assume that NEC, as well as TPN, were only indicative of the severity of the neonate’s condition.
Blood transfusions have been reported in the development of ROP26. In our multivariate analysis, we found that blood transfusions were not independently associated with ROP, possibly due to the fact that other concurrent risk factors placed these babies in the high-risk group. In most of the recent studies, using the multivariate logistic regression, blood transfusions are no longer associated with the development of ROP.
In summary, in our cohort, there were no significant changes in survival rates and demographics during the period of the study. Additionally, all infants less than 32 weeks GA were examined, up to the time of discharge and at the follow-up clinic. The incidence and severity of ROP in our study were one of the lowest reported. We hypothesize that the high incidence of human milk feedings (90 %) may have played a protective role in the development of ROP. Also, we reconfirm the fact that SGA is an independent risk factor for ROP in all GA groups. Sepsis has being associated with ROP, in the younger (GA: 24-25 weeks) and the oldest (GA: 30-31 weeks) groups. Duration of MV was an independent risk factor for ROP in all GA groups <30 weeks gestation while the presence of PDA was an independent risk factor only in infants with GA <28 weeks. Our study had some limitations. One limitation is its retrospective nature. However, data was routinely recorded for all infants in the computer system. Another limitation is the inclusion of only inborn infants; hence it may not represent the real incidence and severity of ROP when outborn infants are analyzed at the time of their departure from the hospital and also during their follow-up visits. Finally, we are not reporting any specific information about the zone, clock hours of ROP and plus disease.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Isaza G, Arora S. Incidence and severity of retinopathy of prematurity in extremely premature infants. Can J Opthalmol. 2012;47:296–300. doi: 10.1016/j.jcjo.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 2.Hwang JH, Lee EH, Kim EA. Retinopathy of Prematurity among Very-Low-Birth-Weight Infants in Korea: Incidence, Treatment, and Risk Factors. J Korean Med Sci. 2015;30 Suppl 1:S88–S94. doi: 10.3346/jkms.2015.30.S1.S88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinkuller PG, Du L, Gilbert C, Foster A, Collins ML, Coats DK. Childhood blindness. J AAPOS. 1999;3:26–32. doi: 10.1016/s1091-8531(99)70091-1. [DOI] [PubMed] [Google Scholar]
- 4.Thomas K, Shah PS, Canning R, Harrison A, Lee SK, Dow KE. Retinopathy of prematurity: Risk factors and variability in Canadian neonatal intensive care units. J Neonatal Perinatal Med. 2015;8:207–214. doi: 10.3233/NPM-15814128. [DOI] [PubMed] [Google Scholar]
- 5.Schaffer DB, Palmer EA, Plotsky DF, Metz HS, Flynn JT, Tung B, et al. Prognostic factors in the natural course of retinopathy of prematurity. The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Ophthalmology. 1993;100:230–237. doi: 10.1016/s0161-6420(93)31665-9. [DOI] [PubMed] [Google Scholar]
- 6.Hoogerwerf A, Schalij-Delfos NE, van Schooneveld MJ, Termote JU. Incidence of retinopathy of prematurity over the last decade in the Central Netherlands. Neonatology. 2010;98:137–142. doi: 10.1159/000280386. [DOI] [PubMed] [Google Scholar]
- 7.Zhou J, Shukla VV, John D, Chen C. Human Milk Feeding as a Protective Factor for Retinopathy of Prematurity: A Meta-analysis. Pediatrics. 2015;136:e1576–e1586. doi: 10.1542/peds.2015-2372. [DOI] [PubMed] [Google Scholar]
- 8.Gilbert C. Retinopathy of prematurity: a global perspective of the epidemics, population of babies at risk and implications for control. Early Hum Dev. 2008;84:77–82. doi: 10.1016/j.earlhumdev.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 9.Good WV, Hardy RJ, Dobson V, Palmer EA, Phelps DL, Quintos M, et al. Early Treatment for Retinopathy of Prematurity Cooperative Group. Early treatment for retinopathy of prematurity cooperative group. The incidence and course of retinopathy of prematurity: findings from the early treatment for retinopathy of prematurity study. Pediatrics. 2005;116:15–23. doi: 10.1542/peds.2004-1413. [DOI] [PubMed] [Google Scholar]
- 10.The Committee for the Classification of Retinopathy of Prematurity An international classification of retinopathy of prematurity. Arch Ophthalmol. 1984;102:1130–1134. doi: 10.1001/archopht.1984.01040030908011. [DOI] [PubMed] [Google Scholar]
- 11.The Committee for the Classification of Retinopathy of Prematurity An international classification of retinopathy of prematurity. Arch Ophthalmol. 2005;123:991–999. doi: 10.1001/archopht.123.7.991. [DOI] [PubMed] [Google Scholar]
- 12.Saugstad OD. Oxygen in newborns: How much is too much? J Perinatol. 2005;25 Suppl 2:S45–S49. doi: 10.1038/sj.jp.7211321. [DOI] [PubMed] [Google Scholar]
- 13.Wright KW, Sami D, Thompson L, Ramanathan R, Joseph R, Farzavandi S. A physiologic reduced oxygen protocol decreases the incidence of threshold retinopathy of prematurity. Trans Am Ophthalmol Soc. 2006;104:78–84. [PMC free article] [PubMed] [Google Scholar]
- 14.Gunn DJ, Cartwright DW, Gole GA. Incidence of retinopathy of prematurity in extremely premature infants over an 18-year period. Clin Exp Ophthalmol. 2012;40:93–99. doi: 10.1111/j.1442-9071.2011.02724.x. [DOI] [PubMed] [Google Scholar]
- 15.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Austeng D, Källen K, Hellström A, Jakobsson P, Lundgren P, Tornqvist K, et al. Regional differences in screening for retinopathy of prematurity in infants born before 27 weeks of gestation in Sweden--the EXPRESS study. Acta Ophthalmol. 2014;92:311–315. doi: 10.1111/aos.12165. [DOI] [PubMed] [Google Scholar]
- 17.Xu Y, Zhou X, Zhang Q, Ji X, Zhang Q, Zhu J, et al. Screening for retinopathy of prematurity in China: a neonatal units-based prospective study. Invest Ophthalmol Vis Sci. 2013;54:8229–8236. doi: 10.1167/iovs.13-12297. [DOI] [PubMed] [Google Scholar]
- 18.Cerman E, Balci SY, Yenice OS, Kazokoglu H, Celiker H, Eraslan M. Screening for retinopathy of prematurity in a tertiary ophthalmology department in Turkey: incidence, outcomes, and risk factors. Ophthalmic Surg Lasers Imaging Retina. 2014;45:550–555. doi: 10.3928/23258160-20141118-10. [DOI] [PubMed] [Google Scholar]
- 19.Dhaliwal CA, Fleck BW, Wright E, Graham C, McIntosh N. Retinopathy of prematurity in small-for-gestational age infants compared with those of appropriate size for gestational age. Arch Dis Child Fetal Neonatal Ed. 2009;94:F193–F195. doi: 10.1136/adc.2008.143552. [DOI] [PubMed] [Google Scholar]
- 20.Bardin C, Piuze G, Papageorgiou A. Outcome at 5 years of age of SGA and AGA infants born less than 28 weeks of gestation. Semin Perinatol. 2004;28:288–294. doi: 10.1053/j.semperi.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Chen M, Citil A, McCabe F, Leicht KM, Fiascone J, Dammann CE, et al. Infection, oxygen, and immaturity: interacting risk factors for retinopathy of prematurity. Neonatology. 2011;99:125–132. doi: 10.1159/000312821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dammann O, Brinkhaus MJ, Bartels DB, Dördelmann M, Dressler F, Kerk J, et al. Immaturity, perinatal inflammation, and retinopathy of prematurity: a multi-hit hypothesis. Early Hum Dev. 2009;85:325–329. doi: 10.1016/j.earlhumdev.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 23.Kumar P, Sankar MJ, Deorari A, Azad R, Chandra P, Agarwal R, et al. Risk factors for severe retinopathy of prematurity in preterm low birth weight neonates. Indian J Pediatr. 2011;78:812–816. doi: 10.1007/s12098-011-0363-7. [DOI] [PubMed] [Google Scholar]
- 24.Tsui I, Ebani E, Rosenberg JB, Lin J, Angert RM, Mian U. Patent ductus arteriosus and indomethacin treatment as independent risk factors for plus disease in retinopathy of prematurity. J Pediatr Ophthalmol Strabismus. 2013;50:88–92. doi: 10.3928/01913913-20130108-03. [DOI] [PubMed] [Google Scholar]
- 25.Hakeem AH, Mohamed GB, Othman MF. Retinopathy of prematurity: a study of prevalence and risk factors. Middle East Afr J Ophthalmol. 2012;19:289–294. doi: 10.4103/0974-9233.97927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dani C, Reali MF, Bertini G, Martelli E, Pezzati M, Rubaltelli FF. The role of blood transfusions and iron intake on retinopathy of prematurity. Early Hum Dev. 2001;62:57–63. doi: 10.1016/s0378-3782(01)00115-3. [DOI] [PubMed] [Google Scholar]


