Abstract
Oritavancin is a novel glycopeptide currently being developed for the treatment of complicated skin and skin structure infections (cSSSI), including those caused by multidrug resistant gram-positive pathogens. The disposition of oritavancin in skin structures was investigated using a cantharide-induced blister fluid model. Seventeen healthy male subjects received oritavancin, but only 16 subjects were evaluated after one subject discontinued study drug. Each subject (eight per dose group) received 200 mg of oritavancin once a day for 3 days (group A) or 800 mg as one single dose (group B). Group A plasma samples and exudates from blister fluid were collected on days 3, 4, 7, 9, and 12 and on days 3, 4, 7, and 9, respectively. Group B samples and exudates were collected on days 1, 2, 5, 7, and 10 and on days 1, 2, 5, and 7, respectively. Drug concentrations were determined using a liquid chromatography-tandem mass spectrometry assay and, subsequently, pharmacokinetic analysis was performed. Differences between treatment groups in ratios for area under the concentration-time curve for blister fluid and plasma (AUCblister fluid/AUCplasma ratios) were evaluated using a t test (α = 0.05). Mean maximum concentration of drug in plasma or blister fluid was approximately 8-fold and 11-fold higher in plasma than in blister fluid following the 200- or 800-mg doses of oritavancin, respectively. Mean AUCblister fluid/AUCplasma ratios at 24 h were 0.190 (standard deviation [SD], 0.052) and 0.182 (SD, 0.062) for groups A and B, respectively (P = 0.791). To place these results in a clinical context, mean drug concentrations in blister fluid exceed the oritavancin MIC at which 90% of strains are inhibited of Staphylococcus aureus (2 μg/ml) by approximately 2- to 5.5-fold at 12 h and 1.5- to 3-fold at 24 h following administration of both dosing regimens. These results support the potential use of oritavancin for the treatment of cSSSI.
Oritavancin, a semisynthetic glycopeptide, is an intravenous antibiotic agent being developed for the treatment of gram-positive bacterial infections. It is a chemical modification of the naturally occurring glycopeptide LY264826 (5). The modification, N-alkyl-linked additions on one of the two amino sugars, confers activity against vancomycin-resistant enterococci. It has a molecular weight of 1,989.0 and a molecular formula of C86H97N10O26Cl3 · 2H3PO4.
The antibacterial spectrum of oritavancin includes Staphylococcus aureus, including methicillin-resistant strains; Staphylococcus epidermidis and other coagulase-negative staphylococci, including methicillin-resistant and some teicoplanin-resistant strains; enterococci, including vancomycin-resistant, teicoplanin-resistant (vanA) isolates of Enterococcus faecium and Enterococcus faecalis and the intrinsically vancomycin-resistant species Enterococcus gallinarum and Enterococcus casseliflavus; and streptococci, including Streptococcus pyogenes and Streptococcus pneumoniae, including penicillin-resistant isolates. Rapid bactericidal in vitro activity against most isolates of E. faecalis and E. faecium is a property of oritavancin that distinguishes it from vancomycin, ampicillin, linezolid, and quinupristin-dalfopristin.
A phase 1 study in healthy subjects has demonstrated the elimination of oritavancin to be very slow, with approximately 6% of the dose eliminated from the body within a 1-week period after single-dose intravenous infusion (1). The plasma pharmacokinetics (PK) of oritavancin have been evaluated in several single- and multiple-dose clinical pharmacology studies. Across studies, oritavancin displayed linear pharmacokinetics for weight-based doses ranging from 0.02 to 3 mg/kg of body weight and fixed doses from 100 to 600 mg. Based on population pharmacokinetic analyses, oritavancin plasma concentrations display a multiexponential decline and are well described by a three-compartment model with corresponding apparent tissue distribution (α and β) and plasma terminal (γ) half-lives of 2.4, 18, and 360 h, respectively (J. S. Owen, S. M. Bhavnani, J. Fiedler-Kelly, J. S. Loutit, S. B. Porter, and L. Phillips, Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-20, 2004).
Oritavancin has been shown to be effective in two phase 3 studies when dosed with either 200 mg or 1.5 to 3 mg/kg once daily for 3 to 7 days for the treatment of patients with complicated skin and skin structure infections (H. Giamarellou, W. O'Riordan, H. Harris, S. Owen, S. B. Porter, and J. S. Loutit, Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. L-739a, 2003; M. Wasilewski, D. Disch, J. McGill, H. Harris, W. O'Riordan, and M. Zeckel, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. UL-18, 2001). Inflammatory blister fluid models are useful for evaluating the disposition of drugs into skin tissues and provide information useful for dosage regimen decision support.
MATERIALS AND METHODS
Study design and methodology.
This was an open-label, single-center study designed to characterize the pharmacokinetic profile of oritavancin skin penetration after administration of oritavancin via intravenous infusion in normal healthy male subjects. Seventeen men ranging in age from 19 to 51 years (mean, 32 years), comprised of 13 Caucasians, 1 African-American, 2 Hispanics, and 1 subject of unknown ethnicity, were enrolled into the study. The study protocol was approved by an institutional review board and all participants provided written informed consent. Prior to the study, volunteers underwent complete physical examinations and laboratory evaluations, including blood chemistries, hematology, and urinalysis.
Blister induction and drug administration.
Oritavancin was administered to an initial group of eight subjects (group A) who received 200 mg per day infused over 60 min for 3 consecutive days (total dose, 600 mg). After the group A subjects completed all study assessments through day 12, a second group of nine subjects (group B) received a single 800-mg dose of oritavancin infused over 90 min. On the evening before the third oritavancin dose for the group A subjects and the evening before dosing for group B subjects, 0.2-ml drops of an ointment containing 0.25% cantharidin powder (Sigma Laboratories, St. Louis, Mo.) and standard ointment base were applied to the anterior forearms of the volunteers to produce a total of four blisters per volunteer (3). The integrity of the blisters was maintained by spraying them with a fast-drying plastic dressing (New-Skin liquid bandage spray; Medtech Laboratories, Inc., Jackson, Wyo.). Additionally, one blister was induced on the forearm at approximately 84 h and again at 132 h after the start of the final oritavancin infusion to allow for blister exudate collection at 96 and 144 h postdose, respectively. The 200-mg dosing scheme was implemented in this study because it was the minimum dose that patients were to receive in the phase 3 trials. The single 800-mg oritavancin dose regimen was explored as it may be considered for future studies in patients with uncomplicated skin and skin structure infections. Routine safety monitoring was conducted during and after oritavancin dosing in all subjects.
Pharmacokinetic sampling.
Blood samples were collected into heparinized Vacutainers from an indwelling catheter contralateral to that used for drug administrations at predose and at 0.5, 1, 2, 4, 8, 12, 24, 96, and 144 h after the last dose of oritavancin in each group. Simultaneously, exudate (100 to 200 μl) from cantharide-induced blisters was obtained. For plasma, additional samples were collected at 216 h postdose. All samples were stored at −80°C until assayed. Samples were analyzed by a validated procedure in which oritavancin extraction from plasma and blister fluid was accomplished by protein precipitation and detection using a high performance liquid chromatography-tandem mass spectrometry assay. The lower limit of quantification (LLOQ) for skin blister fluid and plasma was 1.25 and 0.075 μg/ml, respectively. The interday variability of the assay in plasma ranged from 3.8 to 6.3% over the concentration range of 0.075 to 2.5 μg/ml.
Pharmacokinetic analysis.
Noncompartmental pharmacokinetic analysis was performed using WinNonlin Pro, version 4.0 (Pharsight Corp., Mountain View, Calif.), to generate pharmacokinetic parameter estimates for each oritavancin dose group for both plasma and blister fluid. Parameter estimates included area under the plasma and blister fluid concentration-time curve from time zero to 24 h (AUC0-24), area under the plasma and blister fluid concentration-time curve from time zero to time t (the time of the last concentration above the LLOQ) (AUC0-t), maximum concentration of drug in plasma or blister fluid (Cmax), and tmax (time at which Cmax occurs). AUC was calculated using the linear trapezoidal rule and Cmax was obtained directly from the experimental plasma and blister concentration-time data, without interpolation.
Descriptive statistics, including the number of subjects (n), mean, geometric mean, standard deviation (SD), coefficient of variation, and range, were calculated for each of the PK parameters in each oritavancin dose group for both plasma and blister fluid. Drug concentrations at or below the LLOQ were treated as zero for all calculations. The comparison of AUCblister fluid and AUCplasma between dose groups was evaluated using a t test with a level of significance of 0.05.
RESULTS
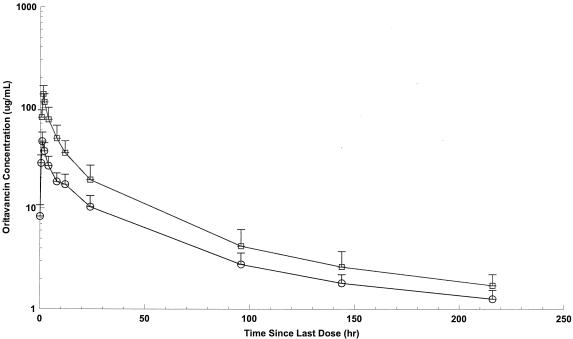
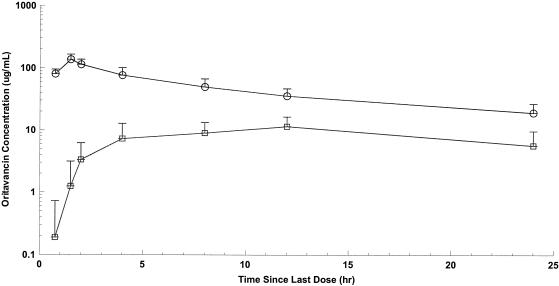
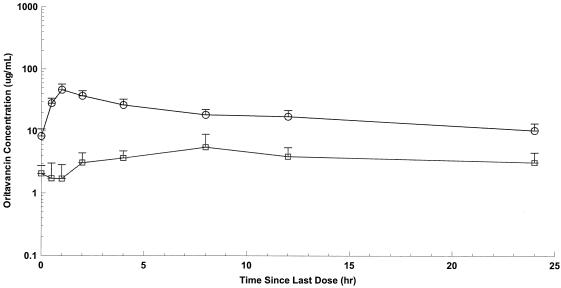
Noncompartmental pharmacokinetic parameters for plasma and blister fluid are presented in Table 1. After the third day of the 200-mg-per-day dosing regimen, the highest mean concentration of oritavancin was observed at approximately 1 and 10 h in plasma and blister fluid, respectively. At these time points, the mean maximum drug concentration was approximately eightfold higher in plasma than in blister fluid. The mean maximum plasma concentration was approximately 11-fold greater than that in blister fluid after a single 800-mg dose of oritavancin. Oritavancin levels decrease in blister fluid after 15 h and became undetectable 100 to 150 h after the last dose (regardless of regimen). The mean plasma concentration of oritavancin at 24 h was approximately 22% of the peak plasma concentration in the 200-mg dose group compared with 14% in the 800-mg dose group.
TABLE 1.
Summary statistics for noncompartmental PK parameters for oritavancin in plasma and blister fluid
| PK parameter | Mean result (SD) for plasma at dose:
|
Mean result (SD) for blister fluid at dose:
|
||
|---|---|---|---|---|
| 200 mg QD, 3 daysd | 800 mg, 1 day | 200 mg QD, 3 days | 800 mg, 1 day | |
| Cmax (μg/ml)a | 46.2 (10.7)b | 137 (28.6) | 5.85 (3.05)b | 12.2 (4.70) |
| tmax (h) | 1.00 (0.00) | 1.50 (0.00) | 10.0 (6.05) | 9.50 (3.67) |
| C12 (μg/ml) | 17.2 (4.42) | 36.2 (10.8) | 3.90 (1.53) | 11.4 (4.90) |
| C24 (μg/ml) | 10.3 (2.99) | 19.5 (7.17) | 3.12 (1.37) | 6.28 (3.31) |
| AUC0-24 (μg · h/ml)a | 457 (99.4)b | 1,111 (316) | 90.7 (35.7)b | 208 (76.7) |
| AUC0-t (μg · h/ml)a | 1,146 (277)b | 2,267 (762) | NCc | NC |
PK parameter was tested for a difference between treatment groups.
Statistically significant compared to the 800-mg dose group (P < 0.005).
NC, not calculated.
QD, once a day.
Mean AUCblister fluid/AUCplasma ratios at 24 h were 0.195 (SD, 0.053) and 0.185 (SD, 0.063) for the 200- and 800-mg dose groups, respectively (P = 0.730). Overall, the mean peak plasma and blister fluid concentrations, along with the mean values of AUC at 24 h, were greater in the 800-mg dose group than with those receiving a cumulative dose of 600 mg (200 mg for 3 days) (P of <0.0001 for plasma Cmax, P of 0.0049 for blister fluid Cmax, P of <0.0001 for plasma AUC0-24, and P of 0.0019 for blister fluid AUC0-24) (Table 1). Mean concentration-time curves of oritavancin in plasma and blister fluid following the third 200-mg dose or the single 800-mg dose are shown in Fig. 1 to 3. While no observed plasma concentrations were at or below the LLOQ, a number of concentrations for skin blister fluid were at or below the LLOQ (47/152). The majority of these concentrations were collected at 24 h postdose. However, given that certain concentrations for skin blister fluid obtained at 0 to 2 h postdose were at or below the LLOQ and thus assumed to be equal to zero, mean concentrations for these time points in Fig. 2 and 3 were lower than the LLOQ.
FIG. 1.
Mean oritavancin concentration versus time in plasma following the third 200- or one 800-mg dose. Circles, 200-mg dose; squares, 800-mg dose. Each sample represents the mean ± SD; n = 8.
FIG. 3.
Mean oritavancin plasma and blister fluid concentrations versus time during the 24 h following the 800-mg dose. Circles, oritavancin in plasma; squares, oritavancin in blister fluid. Each sample represents the mean ± SD; n = 8.
FIG. 2.
Mean oritavancin plasma and blister fluid concentrations versus time during the 24 h following the third 200-mg dose. Circles, oritavancin in plasma; squares, oritavancin in blister fluid. Each sample represents the mean ± SD; n = 8.
Eight of 17 subjects enrolled (47%) experienced at least one treatment-emergent adverse event. The incidence of adverse events was 75% (6/8) in group A and 22% (2/9) in group B. Adverse events were generally mild and transient in nature. The most frequently reported adverse events were general disorders and administration site conditions (group A, 50% [4/8]; group B, 11% [1/9]). One subject in each group experienced a moderate adverse event, one of which was injection site thrombosis, possibly related to study drug, while the other was muscle spasms, probably not related to study drug. There were no severe adverse events, study deaths, or premature study withdrawals associated with an adverse event.
DISCUSSION
Increasing antimicrobial resistance among aerobic gram-positive bacteria is a global concern. National rates of methicillin-resistant Staphylococcus aureus (MRSA) have been reported to generally range from 30 to 50%, with rates as high as 70% (2). Staphylococci remain the leading cause of complicated skin and skin structure infections. The high prevalence of MRSA has limited the utility of traditional treatment modalities, and today, relatively few treatment options remain available to the clinician when resistant pathogens are encountered.
Oritavancin, a novel glycopeptide antimicrobial agent with in vitro activity against multidrug resistant gram-positive bacteria, including MRSA (R. S. Blosser, J. S. Loutit, S. B. Porter, R. K. Flamm, and D. F. Sahm, Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. C1-1636, 2003; R. S. Blosser, S. B. Porter, D. F. Sahm, and J. S. Loutit, Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. C1-1637, 2003; D. F. Sahm, R. S. Blosser, J. S. Loutit, and S. B. Porter, Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. C1-1640, 2003), is being developed for the treatment of complicated skin and skin structure infections. This study was conducted to compare oritavancin exposure in the interstitial space fluids of soft tissues (i.e., the relevant target site) with that of plasma using a cantharidin-induced skin blister fluid model.
A key observation from this study concerns the residence time of oritavancin in skin blister fluid. Oritavancin levels are maximal in blister fluid 10 h after dosing and decrease to undetectable levels 100 to 150 h after the last dose. This is consistent with what is currently understood about the pharmacokinetics and tissue distribution of oritavancin, namely, that tissue residence time is generally shorter in tissues such as skin that are not rich in macrophages. In addition, this observation is consistent with the general lack of late-onset drug-related adverse events in clinical studies.
Our main finding was that total-drug oritavancin exposure in interstitial fluids was approximately 19% of that in plasma, regardless of dosing regimen. Since drug concentration in interstitial fluid exists in equilibrium with the unbound plasma fraction, one would expect the oritavancin exposure in interstitial fluid from noninflamed tissue to be similar to that of free-drug exposure in plasma. Approximately 13% of oritavancin is unbound to plasma proteins (P. A. Rowe and T. J. Brown, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-2193, 2001). Thus, for the 200-mg regimen, an AUCblister fluid/AUCplasma ratio (90.7/457) of 0.19 translates to an AUCblister fluid/AUCfree plasma ratio (90.7/59.4) of approximately 1.5. Given that the cantharidin-induced skin blisters are derived via chemical irritation, it appears that there is a modest accumulation of oritavancin in the presence of inflammation.
Our observations are consistent with findings from studies comparing the microdialysis (a noninflammatory model) and cantharidin-induced skin blister fluid models for estimating drug exposure in interstitial fluids. Müller and colleagues (4) compared moxifloxacin concentrations in cantharidin-induced skin blisters and interstitial fluid obtained via microdialysis in a study with a crossover design. After oral administration, the mean AUCmicrodialysate/AUCfree plasma and AUCblister fluid/AUCfree plasma ratios were approximately 0.84 and 1.3, respectively.
The mechanism of the potential preferential oritavancin distribution to inflamed tissue is unknown. The most likely hypothesis involves macrophages, as oritavancin has been shown to be concentrated in macrophages, which migrate to inflamed tissues (F. Van Bambeke, H. Chanteux, D. Tyteca, M. P. Mingeot-Leclercq, and P. M. Tulkens. Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-1169, 2003). Further study may be warranted to evaluate the potential preferential distribution of oritavancin to inflamed tissue.
Two of the most important factors that affect the adequacy of an antimicrobial regimen are the drug exposure observed in a patient and how susceptible the infecting pathogen is to the agent selected for therapy (6). Integration of these two parameters may be described pharmacodynamically. Outcomes of infection in nonclinical infection models (in vitro and animal) and human studies usually correlate with at least one of three pharmacokinetic-pharmacodynamic (PK-PD) measures: (i) proportion of the dosing interval at which concentrations of an antimicrobial agent exceeds the MIC of the agent against the pathogen (percent time>MIC); (ii) ratio of the peak concentration of the antimicrobial agent to the MIC of the agent against the pathogen (Cmax:MIC); and (iii) ratio of the AUC to the MIC of the agent against the pathogen (AUC:MIC).
The pharmacodynamic profile of oritavancin has been characterized in vitro and in animal infection models. Oritavancin displays a concentration-dependent pattern of bactericidal activity in vitro against a wide variety of pathogens commonly associated with complicated skin and skin structure infections, including methicillin-susceptible and -resistant S. aureus (D. F. Sahm, R. S. Blosser, J. S. Loutit, and S. B. Porter, Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-1170, 2003). Boylan et al. studied the PK-PD of oritavancin in a neutropenic-mouse thigh model of S. aureus infection (1a). The endpoint for efficacy was the change in bacterial density at 24 h. The results of this study demonstrated a strong association between both free-drug Cmax:MIC ratio and free-drug time>MIC and reduction in bacterial density (r2 = 0.93 and 0.84, respectively). Bacterial stasis was associated with a free-drug Cmax:MIC ratio of approximately 6 and percent free-drug time>MIC of approximately 17 to 20%. Near maximal effect associated with a greater than 90% reduction in bacterial density (∼1- to 1.5-log reduction in bacterial density) was achieved with a free-drug Cmax:MIC ratio of approximately 14 and percent free-drug time>MIC of approximately 42 to 50%.
A subsequent analysis of clinical data from a phase 3 study of oritavancin in patients with S. aureus bacteremia supported the findings of a exposure-response relationship with free-drug percent time>MIC and microbiological response. A classification and regression tree-defined breakpoint of 22% free-drug time>MIC was identified for microbiological response; the probability of success greater than or equal and less than this value was 93 and 76%, respectively (S. M. Bhavnani, J. A. Passarell, J. S. Owen, J. S. Loutit, S. B. Porter, and P. G. Ambrose, Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-1475, 2004).
Integration of the above-described PK-PD targets associated with efficacy from exposure-response analyses based on both animal and clinical data with oritavancin exposure data from the present analysis requires consideration of the MIC distribution for oritavancin against target pathogen(s) and the impact of oritavancin plasma protein binding on available drug concentrations. Given that the oritavancin MIC at which 90% of strains are inhibited (MIC90) for methicillin-susceptible and -resistant S. aureus is 2.0 μg/ml (Blosser et al., 43rd ICAAC) and that approximately 13% of oritavancin is unbound to plasma proteins (P. A. Rowe and T. J. Brown, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-2193, 2001), mean free-drug plasma concentrations associated with the 200-mg and 800-mg regimen exceeded the MIC90 over close to the entire dosing interval (percent free-drug time>MIC greater than or equal to 92%).
Although the extent of protein binding of oritavancin is understood in plasma, the extent of binding in blister fluid is not as clear. While further studies may be required to elucidate this issue, inferences may be drawn from previous studies evaluating the relationship between free-drug in plasma and blister fluid. Data from both Wise (7) and Redington et al. (6) demonstrated a close approximation between free-drug plasma and blister fluid AUC values for a number of β-lactam agents, thus suggesting comparatively less protein binding in blister fluid. If the same relationship was true for oritavancin plasma and skin blister exposures, one would expect concordance between free-drug concentrations in plasma (based on a protein binding assumption of 87%) and total-drug concentrations in blister fluid. Indeed, free-drug extrapolated concentrations in plasma are close to or less than total-drug concentrations in blister fluid. Assuming that free-drug concentrations in plasma equal that of blister fluid or that a high proportion of the total concentrations in blister fluid are free, a percent free-drug time>MIC of 22% or higher would be expected for a 200-mg regimen in most patients.
These data demonstrate that after administration of oritavancin at 200 mg once daily for 3 days or 800 mg as a single dose, blister fluid concentrations for total drug exceed the MIC90 of oritavancin against S. aureus. Moreover, for either oritavancin regimen, percent free-drug time>MICs associated with in vivo efficacy are exceeded in plasma. Together, these findings buttress the selection of the 200-mg once daily regimen and support oritavancin as a potential therapeutic modality for complicated skin and skin structure infections.
Acknowledgments
We thank Julie Passarell for statistical support.
We thank InterMune, Inc., for financial support of this study.
REFERENCES
- 1.Bhavnani, S. M., J. S. Owen, J. S. Loutit, S. B. Porter, and P. G. Ambrose. 2004. Pharmacokinetics, safety, and tolerability of ascending single intravenous doses of oritavancin administered to healthy human subjects. Diagn. Microbiol. Infect. Dis. 50:95-102. [DOI] [PubMed] [Google Scholar]
- 1a.Boylan, C. J., K. Campanale, P. W. Iversen, D. L. Phillips, M. L. Zecke, and T. R. Parr. 2003. Pharmacodynamics of oritavancin (LY333328) in a neutropenic-mouse thigh model of Staphylococcus aureus infection. Antimicrob. Agents Chemother. 47:1700-1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diekema, D. J., M. A. Pfaller, F. J. Schmitz, J. Smayevsky, J. Bell, R. N. Jones, M. Beach, and the SENTRY Participants Group. 2001. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific Region for the SENTRY Antimicrobial Survelliance Program, 1997-1999. Clin. Infect. Dis. 32:S114-S132. [DOI] [PubMed] [Google Scholar]
- 3.Maglio, D., C. H. Nightingale, and D. P. Nicolau. 2003. Production and resolution of cantharidin-induced inflammatory blisters. Antimicrob. Agents Chemother. 22:77-80. [DOI] [PubMed] [Google Scholar]
- 4.Müller, M., H. Staβ, M. Brunner, J. G. Möller, E. Lackner, and H. G. Eichler. 1999. Penetration of moxifloxacin into peripheral compartments in humans. Antimicrob. Agents Chemother. 43:2345-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagarajan, R. 1993. Structure-activity relationships of vancomycin-type glycopeptide antibiotics. J. Antibiot. (Tokyo) 46:1181-1195. [DOI] [PubMed] [Google Scholar]
- 6.Redington, J., S. C. Ebert, and W. A. Craig. 1991. Role of antimicrobial pharmacokinetics and pharmacodynamics in surgical prophylaxis. Rev. Infect. Dis. 13:S790-799. [DOI] [PubMed] [Google Scholar]
- 7.Wise, R. 1983. Protein binding of β-lactams: the effects on activity and pharmacology particularly tissue penetration II. J. Antimicrob. Chemother. 12:105-118. [DOI] [PubMed] [Google Scholar]