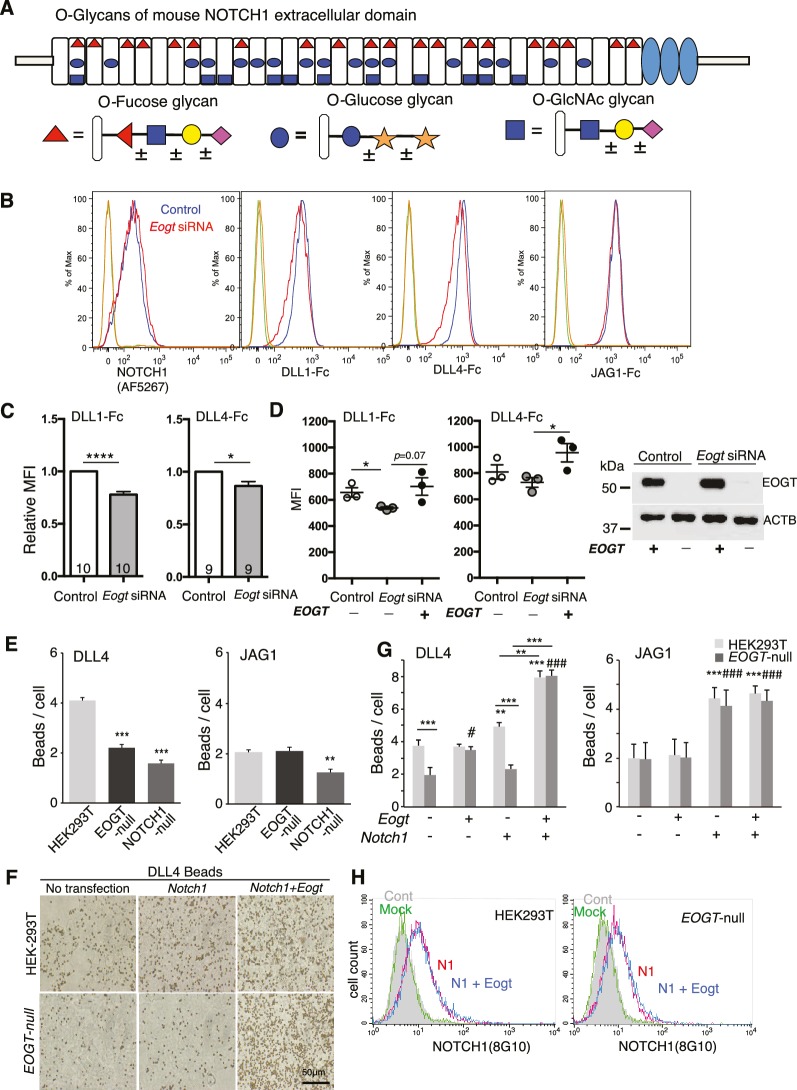
Figure 1. EOGT promotes NOTCH1 binding to Delta ligands.
(A) Diagram of predicted O-glycans on mouse NOTCH1: red triangle, O-fucose glycans; blue circle, O-glucose glycans; blue square, O-GlcNAc glycans. Individual sugar residues that may extend O-fucose, O-glucose or O-GlcNAc to varying degrees are: yellow circle, galactose; pink diamond, sialic acid; orange star, xylose. (B) Flow cytometry of Lec1 CHO cells expressing vector control or Eogt siRNA with NOTCH1 mAb, DLL1-Fc, DLL4-Fc or JAG1-Fc. (C) Relative mean fluorescence index (MFI) for binding of DLL1-Fc and DLL4-Fc to control and Eogt-siRNA Lec1 cells. Concentrations of ligand varied from 100 to 750 ng/ml. MFI values for binding to control cells taken as 1.0 were 455 ± 55 (DLL1-Fc) and 1215 ± 49 (DLL4-Fc). Data from 9 to 10 independent experiments are average, normalized MFI ± SEM; significance determined by paired, two-tailed Student’s t-test, *p<0.05, ****p<0.0001. (D) MFI values obtained for binding of DLL1-Fc or DLL4-Fc (750 ng/ml) to control and Eogt knockdown Lec1 CHO cells, before and after transfection of a human EOGT cDNA. Data are mean ± SEM from three independent experiments. Significance determined by unpaired, two-tailed Student’s t-test, *p<0.05. Western blot analysis of transfectants. (E) DLL4 and JAG1 beads bound to wild-type, EOGT-null, or NOTCH1-null HEK293T cells were observed by microscopy and counted (n = 50). Data are mean ± S.D. from three independent experiments. Statistical analysis was by Welch's t-test. **p<0.01; ***p<0.001. (F) Wild-type or EOGT-null HEK293T cells were transfected with Notch1 alone or together with Eogt followed by incubation with DLL4 beads. The number of DLL4 beads bound to cells was markedly increased by co-transfection of Eogt and Notch1. (G) Wild-type or EOGT-null HEK293T cells or cells transiently transfected with Notch1 with or without Eogt, were incubated with DLL4 or JAG1 beads. The number of Dynabeads bound per transfected cell marked by GFP expression was determined (n = 50). Data are mean ± S.D. from three independent experiments. *p<0.05; **p<0.01; ***p<0.001; #p<0.05; ###p<0.001 compared with the left-most wild type (*) or EOGTnull (#) bar by Welch's t test. (H) Wild-type or EOGT-null HEK293T cells were transfected with Notch1 with or without Eogt and subjected to flow cytometry using 8G10 NOTCH1 Ab. Mock transfectants were analyzed with (Mock) or without primary antibody (Cont).
DOI: http://dx.doi.org/10.7554/eLife.24419.003